Abstract
The European eel migrates 5,000–6,000 km to the Sargasso Sea to reproduce. Because they venture into the ocean in a pre-pubertal state and reproduce after swimming for months, a strong interaction between swimming and sexual maturation is expected. Many swimming trials have been performed in 22 swim tunnels to elucidate their performance and the impact on maturation. European eels are able to swim long distances at a cost of 10–12 mg fat/km which is 4–6 times more efficient than salmonids. The total energy costs of reproduction correspond to 67% of the fat stores. During long distance swimming, the body composition stays the same showing that energy consumption calculations cannot be based on fat alone but need to be compensated for protein oxidation. The optimal swimming speed is 0.61–0.67 m s−1, which is ~60% higher than the generally assumed cruise speed of 0.4 m s−1 and implies that female eels may reach the Sargasso Sea within 3.5 months instead of the assumed 6 months. Swimming trials showed lipid deposition and oocyte growth, which are the first steps of sexual maturation. To investigate effects of oceanic migration on maturation, we simulated group-wise migration in a large swim-gutter with seawater. These trials showed suppressed gonadotropin expression and vitellogenesis in females, while in contrast continued sexual maturation was observed in silver males. The induction of lipid deposition in the oocytes and the inhibition of vitellogenesis by swimming in females suggest a natural sequence of events quite different from artificial maturation protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spawning migration of European eels to the Sargasso
Eels have a complex life cycle that includes the crossings of the Atlantic Ocean both as larvae and as adults. It is difficult to comprehend that this migration pattern developed in the past, as it seems like an enormous waste of energy. Possibly this pattern of going out to sea and the returning of the larvae back to the coast drifting on the current is an innate character of the species as all 16 species (excluding subspecies) of the Anguilla genus show this pattern. However, there are only a few of them that migrate really far into the ocean. Those species are the Atlantic species European eel Anguilla anguilla and American eel A. rostrata, Japanese eel A. japonica and furthermore A. australis, A. dieffenbachii and A. marmorata (Aoyama 2009). The shortest distance among these six species is covered by A. australis and the longest by A. anguilla, respectively 2,000 and 5,000–6,000 km. Likely the two Atlantic species were split off from the Anguilla tree about 30 million years ago, when the Tethys sea was still open and the eels could move from the Indian Ocean through the Tethys Sea into the Atlantic Ocean (Aoyama 2009). At that time, the Atlantic Ocean was still a small ocean, less than 2,000 km wide. In the eras after the Eocene, the Tethys Sea slowly closed and the two continents Europe and America drifted apart, taking the Sargasso Sea with it to the western side of the Atlantic. This implies that the migration distance for the European eels increased continuously over millions of years. Due to the increasing distance, a strong selection pressure must have been on long distance swimming as well as on low energy cost of transport.
At the beginning of the previous century, Johannes Schmidt (1923) found the smallest eel larvae (leptocephali) of the European eel near the Sargasso Sea. This is still the only evidence to date that locates the spawning grounds in the Sargasso Sea, neither eggs nor mature adults have ever been found in this area. Tucker (1959) opposed the conclusion of Johannes Schmidt (1923) that the Sargasso Sea could be the spawning ground of the European eel. He simply stated that it is very unlikely that eels would be able to cross the ocean and asked the scientific community to come up with proof that eels were able to do so. He suggested that European eels were in fact the same species as the American eels coming from the same parents, and that the adults were simply lost at sea due to starvation. The small differences in number of vertebrae could have been caused by the longer exposure to cold water. Tucker’s “new solution to the Atlantic eel problem” provoked a long debate (D’Ancona and Tucker 1959; Deelder and Tucker 1960) and was finally rejected when a distinction could be made between the two Atlantic eel species based on allozymes (Williams and Koehn 1984), enzymes (Comparini and Rodino 1980), mitochondrial DNA (Avise et al. 1986, 1990; Tagliavini et al. 1995) and genomic DNA (Nieddu et al. 1998). However, it is remarkable that the topic of energy requirements for long distance swimming of European eel was left open for so long, particularly since eel swimming was assumed by biomechanics to be very inefficient (Videler 1993). As eels have been swimming across the Atlantic for millions of years, one would expect the opposite: an extreme strong selection pressure on swimming efficiency and long distance migration. So, the major question still remained: How do they do it?
Every year at the end of the growth season in autumn, a part of the population ceases feeding, becomes restless, starts to mature and changes into migrant silver eels. Likely only those with sufficient lipid stores (Larsson et al. 1990; Svedäng and Wickström 1997) will start their reproductive migration to the spawning grounds in the Sargasso. They leave in a prepubertal condition with less than 2% relative gonad mass (GSI). The smaller males (on average 40 cm) leave already in August (Usui 1991). The much larger females (on average 80 cm) leave between October and December (Dutch situation, eels in the Mediterranean need to swim a shorter distance and leave later) to arrive an estimated 3.5 months later (Palstra et al. 2008a) at the spawning grounds. When fully mature, in early spring, males reach maximal gonad masses of 10% and females of 60% of their body mass (Palstra et al. 2005). Silver eels thus must swim across the Atlantic Ocean within 6 months, as this is the difference between the time they leave and the time the first larvae are observed in the Sargasso Sea. Obviously long-term swimming capacity is a major requirement for successful reproduction. Migrating eels do not feed; therefore, they rely for their energy mostly on fat stores (Tesch 2003), which can be as large as 30% of their body weight. The long distance migration provokes two major questions: (1) Do they have enough energy reserves? (2) Are they built to swim 5,000–6,000 km within the required period?
Swimming and sexual maturation
The continental phase in the lifecycle of European eel can be considered as a feeding phase to attain the lipid reserves to fuel migration but also to provide the future offspring with sufficient energy. In order to store the required amount of lipid before maturing, this phase is characterised by a severely depressed lipid mobilisation (EELREP 2005) and blockage of maturation. It appears that this is above all a requirement for an extended female growth stage. The postponement of reproductive migration allows the storage of high amounts of fat during life, a strategy optimising reproductive output.
Pre-pubertal blockage of maturation is due to a deficient gonadotropin releasing hormone (GnRH) release and a dopaminergic inhibition of the pituitary production of gonadotropins FSH and LH by dopamine (Weltzien et al. 2009). These gonadotropins control gonad development directly or indirectly by acting on steroid metabolism in the production of oestrogens and androgens in both male and female eels. This dual neuroendocrine control is extreme for eels but also occurs in various adult teleosts (Vidal et al. 2004). However, dopamine only counteracts regulation of the last steps of gametogenesis in other teleosts, whilst in eel dopamine seems to play a role in earlier stages (Vidal et al. 2004). The extreme blockage of maturation is the main reason why eel still cannot be bred naturally in captivity.
Silvering marks the start of lipid mobilisation and sexual maturation. During this physiological process, numerous changes occur in external appearance, sensory organs, alimentary tract, swim-bladder, osmoregulatory system and gonads (recently reviewed by Durif et al. 2009). Most noticeable is the enlargement of the eyes, strongly correlated to gonad development (Pankhurst 1982) probably through increased testosterone (T) levels (Boëtius and Larsen 1991). It appears that silvering is not a true metamorphosis, e.g., a marked and abrupt developmental change in the form or structure of an animal, but a mere initiation of maturation; the start of puberty (Roussea et al. 2009). Silvering is more flexible than generally presumed (Svedäng and Wickström 1997) and may well be arrested at various stages such as for Atlantic salmon Salmo salar (Mills 1989). Durif et al. (2005) identified intermediate phases and found that they were correlated to migration. The most advanced stages of maturation are from migrating silver eels Anguilla spp. caught closest to the spawning grounds. Moreover, a negative correlation seems to exist between migration distance to the spawning grounds and GSI at the start of oceanic migration of the various Anguilla species (Todd 1981). However, eels have only been incidentally observed during their oceanic spawning migration (Ernst 1977; Robins et al. 1979; Bast and Klinkhardt 1988).
Mature eels of species A. japonica and A. marmorata have recently been caught at the spawning grounds. Over the last two decades, Tsukamoto and colleagues have been able to pinpoint the time (new moon) and place (seamounts west of the Mariana Islands) of spawning of A. japonica and A. marmorata with such precision (Tsukamoto 1992, 2006, 2009) that they recently caught three mature male eels in the open ocean (resp. n = 2 and 1; Chow et al. 2009). These eels had considerable larger eye size than the early silver eels from coastal waters, they were dark brown or dark blackish grey which is entirely different from early white-bellied silver eels (and which resembles final maturation stages after hormonal treatment: Palstra et al. 2005 and personal observations), and GSI was comparable or even higher than after hormonal treatment (Chow et al. 2009). Several days after their catch, 200 pre-leptocephali were caught in the same area indicating that these males were already very close to spawning. Besides some reports on mature males occurring far off the spawning grounds, for instance of A. anguilla in the Mediterranean (Grassi 1896) and of A. japonica even on the farm (Matsubara et al. 2008), it shows that in the field we only know the fully mature situation from just before the act of spawning.
To summarise, we only know that European eels leave the continent in a prepubertal condition, swim for months and are only then fully mature and able to reproduce. Therefore, we assume that lipid mobilisation and early maturation are linked to migration and that swimming itself may be a natural trigger for these processes. Indeed, no change in lipid mobilisation was found between yellow and silver eels from the same location without having engaged in sustained swimming (EELREP 2005). We therefore hypothesised that lipolysis becomes activated during and due to sustained swimming. Furthermore, we hypothesised that swimming triggers silvering, the start of maturation, but that blockage of more advanced female stages, including vitellogenesis, is likely required in order to allow the long spawning migration.
Swimming performance
Measuring swimming performance with 22 swim-tunnels
The principle of the Blazka swim-tunnel was explained in earlier publications (Blazka et al. 1960; Smith and Newcomb 1970; Van Dijk et al. 1993). The advantage of this swim-tunnel is the compact design, which allowed us to construct 22 swim-tunnels in an air-conditioned room of 100 m2. This type consists of two concentric tubes, with the inner circle and outer ring having the same surface area resulting in the same flow rate. The propellor pushes the water into the outer ring and further into a bundle of flow streamers to reduce the size of the vortices generating a semi lamellar flow (Fig. 1).
Swim tunnels for long distance migration studies. a The set up consists of 22 2-m-long Blazka-type swim tunnels. b The tunnels consist of two concentric perspex tubes of 2 m and two PVC endcaps. The propeller pushes water into the outer ring and ‘sucks it’ out from the inner tube. The cross-sectional area of the inner tube and the outer ring has the same surface area to obtain equal flow rates at both sides. The water is pushed through streamers with internal diameters of ~10 mm resulting in a semi laminar flow in the inner tube. Flow rates through different transects were found homogeneous as determined by Laser-Doppler tests. c One of the tunnels with a 72-cm silver female eel swimming at 0.5 BL s−1 and d an eel swimming under experimental conditions of red light which is invisible for silver eels. Source b: van den Thillart et al. 2004
Flow characteristics of swim-tunnels of the Blazka-type were to our knowledge never described before. In a recent study, we applied the very accurate Laser-Doppler system to demonstrate the homogeneity of the flow in the swim-tunnels at 3 different cross sections, (Van den Thillart et al. 2004). The actual flow in a 2-m-long swim-tunnel was measured at 3 different cross-sections (11, 61, and 110 cm from the inflow) and at different distances from the wall (0.5, 1.0, 2.0, 4.0 and 9.5-cm). A linear relationship was observed between the number of revolutions per minute and the measured water velocity. The linearity existed up to 0.9 m s−1, while the calibration allowed running the tunnels up to flows of 1.5 m s−1.
Likely due to the semi lamellar flow type the drop-off at the wall was very steep; at 2.0 cm the flow was about 70% and at 4.0 cm about 90% of the flow in the middle. So, fish with a width of >4.0 cm cannot swim in the boundary layer. Furthermore as far as eels are concerned, this species needs an even wider space because of the large amplitude of their tail beat (anguilliform swimming mode). In addition, we observed that the head of the tested swimming eels remained between 5 and 10 cm from the wall (Fig. 1). Eels were rather easy with swimming, just placing them in streaming water is apparently sufficient to activate their swimming mode. The only crucial point is to avoid stress when forcing them to swim. Changing the flow rate up and down slowly during the first hour of starting up was almost always sufficient. Once they were swimming, eels could swim for months as long as the speed remained low (0.5–0.6 BL s−1).
Recently, we published results from a 10-day swimming trial with European silver eels swimming at 0.5 BL s−1 in the same set up in sea water (van Ginneken and van den Thillart 2000). In another experiment (Van den Thillart et al. 2004), we tested the suitability of the set-up for long distance migration. Seven farmed silver eels were swum for several months in fresh water (19°C) at 0.5 BL s−1. Two eels stopped swimming during the first 2 months, and the experiment was stopped after 3 months. Every day the oxygen consumption rate was measured during several hours by automatic control of the water inlet between 85 and 75% air saturation. Oxygen consumption data of the five eels was measured continuously during swimming over 95 days at 0.5 BL s−1, corresponding to a distance of 2,850 km. These first long distance swimming results with European silver eels show their impressive endurance.
Optimal swimming speed and cost of transport
The capacity of individual fish to swim can be tested in swim-tunnels as described and used by many authors (Brett 1964) more or less in the same way as mammals can be tested on runways. The fish are usually trained to prevent interference by handling stress, followed by stepwise increase of swimming speed. At each speed, the individual fish are usually left swimming for 1–2 h, as it takes 30–60 min to establish a new stable condition with respect to circulation and ventilation (Jones and Randall 1978). During this start-up period, the animal reaches a certain rhythm that helps to optimise its swimming efficiency. With each increase of speed, the drag increases to the 2-nd power, and therefore the oxygen consumption increases also to the 2-nd power with speed. When the swimming speed comes above ca 80% of the maximal speed, anaerobic metabolism is activated. There is not a complete metabolic switch, but the anaerobic processes are activated to supply the extra energy required for muscle contraction. Both anaerobic glycolysis and the creatine kinase reaction are triggered by a low ATP/ADP ratio (in fact by the reduced phosphorylation potential). Initially, the lactic acid is buffered by the alkaline reaction of phospho-creatine hydrolysis (van den Thillart and van Waarde 1996). Metabolic acidosis develops when most of the PCr is depleted; the low pH together with the high inorganic phosphate causes muscle fatigue, which ultimately results in collapse. The collapse point is a reproducible parameter, as is the oxygen consumption rate at the collapse point. Likely the maximal oxygen consumption rate determines the maximal sustained swimming speed, which is the speed where the animal does not fatigue when swimming for a few hours. The maximal sustained swimming speed is usually around 80% of Umax.
Somewhere between resting and maximal oxygen consumption, the optimal swimming speed (Uopt) can be found. This is (by definition) the speed where the cost of transport is the lowest. The cost of transport (COT in mg O2 kg−1 km−1) is the ratio of oxygen consumption rate over swimming speed, which is high at low speed as well as at high speed. At low speed because the standard metabolic rate is a large part of energy consumption, which is not used for swimming, and at high speeds it is high because of the drag which increases with 2nd power of the speed. So, the lowest COT is found at an intermediate speed, which is then called the optimal swimming speed. Particularly for migrating animals the cost of transport is crucial as it determines the maximal distance that can be covered for the available energy, when swimming at the optimal speed. A low cost of transport is certainly a prerequisite for high endurance. Therefore, maximal endurance should be expected at the optimal swimming speed. According to biomechanical criteria, the best endurance is the maximal speed where the oxygen consumption rate remains stable and the mode of swimming does not change (Videler 1993). These two criteria are based on different assumptions, either lowest energy cost or stable swimming mode. It is likely though that both will come together at the same speed, as such would be selected out in the population over several generations. To determine maximal endurance conditions for eels, they have to be swum for extended periods at different swimming speeds.
Swimming fitness can be described by a number of parameters such as maximal swimming speed, optimal swimming speed, and minimal cost of transport. These were recently determined for European eel in a single day protocol, using the previously described swim-tunnels. In this study (Palstra et al. 2008a), eels were swum 2 h at each speed from 0.5 to 1.0 m s−1 in steps of 0.1 m s−1. At each speed, the oxygen consumption was measured continuously for 90 min. The maximal aerobic speed was interpolated according to the method of Brett (1964). A group of 40 farmed eels were tested twice (test 1 and test 2) with 2-h intervals, and in between at the same speeds with 12-h intervals (endurance). The endurance test lasted 5 days, so each series with three tests took 7 days; speed test 2 was a way to test whether the results were repeatable and to see whether training effects would occur. Results are summarised in Table 1 showing the relation between oxygen consumption and swimming speed for each of the 3 tests. The results show also that the two different tests were similar; a 12 h run gave the same result as the two 2 h speed tests. This implies that the 2-h speed test can be used for testing endurance as well. This corresponds with our observations; once the eels are swimming they do not change their mode of swimming nor their oxygen consumption at that speed. As these two remain the same then also the ratio remains the same, which is the cost of transport (mg O2 kg−1 km−1). When considering the COT at the different swimming speeds, we clearly see that the values remain almost the same, eels swim over 0.5–0.9 m s−1 at almost the same COT. As the results were the same for the 2- and 12-h speed tests in this series with farmed eels, three other eel groups were tested only in a 1 day speed test with 2-h intervals (Fig. 2).
Swimming endurance tests. Swimming parameters (means ± SE) of experimental eels: farmed small eels (60–70 cm) in seawater (SW) (light-grey dots), farmed large eels (70–80 cm) in SW (light-grey vertical stripes), Lake Grevelingen eels in SW (light-grey horizontal stripes), farmed large eels in FW (dark-grey vertical stripes), Loire eels in FW (dark-grey horizontal stripes). Indicated are statistical significant differences (P ≤ 0.05) between groups. For Ucrit and Uopt were the absolute values in m s−1 compared because ANCOVA showed no effects of BL and BW. For COTmin were the relative values in mg kg−1 km−1 because ANCOVA showed an effect of BW. Source: Palstra et al. (2008a)
For all five groups, the fitness parameters were calculated (Table 1; Fig. 2). The average COT remained similar while fish were swimming at the different speeds. The optimal swimming speeds, were very similar for all 4 groups and were found 0.61–0.67 m s−1, which is ~60% higher than the generally assumed cruise speed of 0.4 m s−1. This would imply that female eels may reach the Sargasso Sea within 3.5 months instead of 6 months.
The costs of migration
During long-distance migration, all animals are likely to maximise the distance covered per given fuel unit, thus they will try to migrate at the lowest cost of transport. The migration distance of the different eel species varies: the European eel (A. anguilla) 5,000–6,000-km (Schmidt 1923), the American eel (A. rostrata) 4,000-km (Tucker 1959; McCleave et al. 1987); the Japanese eel (A. japonica) 4,000-km (Tsukamoto 1992) and the Australian eel (A. australis) 2,000-km (Jellyman 1987). These eel species migrate impressive distances, but European eels need to be the most efficient swimmers among eels.
The long-term swimming experiments with 5 eels of about 0.9-kg as discussed earlier indicate that eels can be stimulated to swim under laboratory conditions for a very long period without resting. Five out of seven eels were able to swim 3 months at 0.5 BL s−1, covering a distance of 2,850 km (Van den Thillart et al. 2004). In the literature, limited data are available on swimming performance of eels or other anguilliform swimming teleosts (Webb 1975; McCleave 1980). It is even suggested that the swimming movement of eel is less efficient than that of for example salmonids (Videler 1993; Bone et al. 1995). However, biomechanical efficiency of propulsion is different from overall swimming efficiency. The first relates to the transfer of kinetic energy from muscle to environment and the resulting displacement of the body. The overall swimming efficiency, however, is the total energy required by the animal to transport itself over a certain distance. This determines the amount of fuel stores i.e., grams of fat, that the eel needs to swim across the Atlantic Ocean.
Based on a 10-day swimming trial with European silver eel, we demonstrated earlier that the energy cost of transport of those eels was extremely low: 0.137 cal g−1 km−1 (van Ginneken and van den Thillart 2000). This is 2.4–3.0 times lower than values reported in literature for other species (Schmidt-Nielsen 1972). In a more recent study, we exposed female yellow eels of about 900-g to a 6-month swimming trial at a mean swimming speed of 0.5 BL s−1 (Van Ginneken et al. 2005b). The eels swam in this experiment a complete simulated migration run of 5,500 km. Oxygen consumption data demonstrate that the swimming eels have a twofold higher O2 consumption than the resting eels, while over a period of almost 6 months the energy consumption remained almost constant. The slope, expressed in mg O2 kg−1 h−1, shows an increase of about 10%, which was mainly due to weight loss. As the length of the animal did not change, the drag must have remained the same, so the energy cost of transport for the whole animal did not change.
From the oxygen consumption, the energy consumption can be calculated based on fat combustion data given by Brafield and Llewellyn (1982). Thus, we can estimate how much fat the eels would have used, assuming that fat is the only energy source, based on the oxicalorimetric data for fat combustion. The values calculated for the two long-term swimming trials gave about the same oxygen consumption per km (28 vs. 31 mg O2 kg−1 km−1), corresponding with a cost of transport of about 0.4 kJ km−1 and 10 mg fat km−1, respectively. As for a trans-Atlantic journey of 5,500 km, this would then require about 60 g fat for an eel of 1 kg. The variance between individuals was in experiment I 7% and in experiment II 14%; meaning that the fat consumption ranged between 55 and 74 g fat kg−1 over 6,000 km. The calculated values for the cost of transport (COT) correspond rather well for the two experiments. On the other hand, the COT values for eel are some four to fivefolds lower than those obtained for salmonids (Schmidt-Nielsen 1972). Recent data on salmonid swimming including from our own experiments confirmed earlier results from Schmidt-Nielsen that salmonids swim indeed at much higher cost of transport. Lee et al. (2003) swam different salmons species at 0.5–2.2 BL s−1 (12–16°C), the salmon were about 64 cm (fork length) and 2.65 kg. At 0.5 BL s−1, the COT was 150–200 mg kg−1 km−1 falling to 100 mg kg−1 km−1 at 1.2 BL s−1. These data are between four and seven times higher than those observed with the female eels in our swim trials, which clearly indicate again that eels are very efficient swimmers.
When eels would swim at the same energy consumption rate as salmonids, they would need 300 g fat kg−1 for crossing the ocean instead of 60 g of fat kg−1. As most silver eels have less than 200 fat kg−1, evidently none of the migrating eels would be able to cross the ocean.
Long-term swimming experiments with eels have been published for eels swimming at 0.5 BL s−1 for 3 months and for 5.5 months (van den Thillart et al. 2004; Van Ginneken et al. 2005b).
During long-term swimming the eels lost weight, which can be due to diminished energy stores, but also due to water loss. Therefore, the total body composition was determined of the eels after swimming 5,500 km. The body composition of the three eel groups in this experiment (control, resting, and swimming) remained the same in all three conditions, which is remarkable (Table 2). The weight fractions of water, fat, protein, carbohydrate, and ash remained constant; the fat and protein content of the dry weight were 68 and 28%, respectively. This implies in the first place that the buoyancy of the animal did not change and that therefore no volume compensation is required by the swim-bladder during swimming or resting. Another implication is that the energy consumption calculated from oxygen consumption and weight loss cannot be based on fat alone, but needs to be compensated for protein oxidation as well. Since body composition does not change, it is possible to calculate the energy consumption from weight loss as well. Therefore, we need to recalculate the energy content of the dry weight according to the composition given in Table 2. The recalculated data are presented in Table 3. The energy content of the dry weight was found to be 33.7 kJ g−1, which is only 15% lower than that of fat combustion (39.5 kJ g−1), indicating that fat is by far the main energy source (Table 3).
From the oxygen consumption per hour and the swimming speed (1.34 km h−1), the cost of transport was found: 31.43 mg O2 km−1. From this, the total oxygen consumed must have been 188.58 g O2 (over 6,000-km), which corresponds to 188.58 × 13.6 = 2569.2 kJ. When dividing by 33.7 kJ g dry weight−1, we find an estimate for the dry weight loss of 76.3, or 152.6 g wet weight. The observed wet weight loss over 5,533-km was 180.2 ± 38.2 g (Van Ginneken et al. 2005b). The calculated figure based on oxygen consumption is 84% of the observed value. Considering the errors occurring in the different measurements we can conclude that the respirometry data (oxygen consumption) corroborate well with the calorimetric data (weight loss). It is important to emphasise that these two techniques for measuring the energy consumption are fully independent from each other.
Our new calculations based on 33.7 kJ g dry weight−1 and 13.6 kJ gO2 −1 (see Table 3) provide an estimation of the total energy consumption over 6,000-km of 2569.2 kJ based on oxygen consumption and 3294.5 kJ based on weight loss. The resultant COT for both methods is respectively, 0.43 and 0.55 kJ km−1. A break down of the fuel usage for a simulated migration over 6,000 km provides the following results: fat 52.1 g, protein 21.6 g, and carbohydrate 0.6 g. Thus, the minimal fat requirement for migration is about 5.2% of total body weight. This value is lower than the first estimate of 6.5%, which is due to the fact that also proteins are being used as fuel for energy generation.
Since we recently found that optimal swimming speeds of European silver eels are about 0.8 BL s−1 (Palstra et al. 2008b), two migration trials were performed at these speeds: one with large farmed silver eels in fresh water at 18 degrees, and one with wild silver eels that were about to start their oceanic migration in seawater at decreasing temperatures from 18 down to 10°C over 15 days swimming (Palstra et al. 2006a). We found that the farmed eels in fresh water swam at a COT of 34 ± 5 mg O2 kg−1 km−1 during 2,173 ± 305 km migration, while the wild silver eels in seawater swam at a higher COT of 52 ± 5 mg O2 kg−1 km−1 during 1,232 ± 305 km migration. The COT remained constant and very similar to the minimal COT (COTmin) found at 2-h swim tests (Palstra et al. 2008a) giving these tests a high prediction value. By comparing farmed eels that swam in either fresh water (FW) or seawater (SW) we found that 20% of this difference can be explained by higher oxygen consumption in SW than in FW (Palstra et al. 2008a). Remarkably, oxygen consumption rates of the wild silver eels remained similar during the induced temperature profile (Fig. 3). For the wild silver eels we extrapolated that they would spend 78 ± 4 g fat kg−1 on fuelling a complete 5,500 km spawning migration, taking into account that 79.8% of the fuel costs comes from burning fats (van Ginneken et al. 2005b). Table 4 presents an overview of the costs of migration for all experiments.
Oxygen consumption profiles during simulated migration. The swimming speed was increased the first 4 days from 0.5 to 0.8 body-length per second (BL s−1) with increments of 0.1 BL s−1 per day. a Daily mean of oxygen consumption rate (\( \dot{M}{\text{O}}_{2} \)) of farmed eels (n = 6) in 18°C fresh water (FW) swimming 27 days at 0.8 BL s−1. b Mean O2 of Lake Grevelingen eels (n = 6) in salt water (SW) swimming 26 days. Starting at day 4, the water temperature was lowered with 0.5°C per day from 18 to 10°C. Source: Palstra et al. (2006b)
Swimming in the ocean: diel vertical migration and pressure effects
The Uopt for wild European female silver eels swimming in SW is 0.62 m s−1 (or 0.77 BL s−1; Palstra et al. 2008a) which agrees well with the sustained cruise speeds found for 11 silver eels (69–96 cm) tracked in the North Sea by Tesch (1974 and reviewed by Beamish 1978) which ranged between 0.6 and 0.9 BL s−1. Other tracking studies revealed speeds of 0.50–2.09 km h−1 (reviewed by Tesch 2003; McCleave and Arnold 1999; Jellyman and Tsukamoto 2002) corresponding with 0.14–0.58 m s−1 or 0.18–0.73 BL s−1 for eels of 80 cm. Thus, Uopt corresponds only to the fastest of these migration speeds. Besides the already discussed temperature effects on swimming behaviour, other environmental conditions—like depth, currents and predators—experienced during oceanic migration may also affect the swimming speed. Hence, the actual ground speeds may differ significantly from the Uopt.
The exact route of migration of European eels is still largely unknown (reviewed by Tesch and Rohlf 2003). Trackings of considerable numbers of eels in the North Sea and on the east Atlantic shelf have shown that eels swim uninterrupted in a compass direction geographically north and west. With decreasing latitude, directional preference turns over farther northward and attains in a NW swimming direction. The NW course must lead them to the continental slope where they start to swim in a SW direction. Eels that were released and tracked in the East Atlantic swam in a WSW direction. This kind of navigational ability could be based on magnetic sensing of the inclination or strength of the magnetic field. Furthermore, by using currents like the North Equatorial Current, ground speeds in the field may be even higher than the optimal swimming speed.
Eels use all depth zones, except for bottom layers, during all tidal phases (reviewed by Tesch and Rohlf 2003). A diel vertical migration during deep-sea migration has been shown repeatedly during tracking experiments using pop-up tag methodology (reviewed by Tesch and Rohlf 2003 and very recently by Tsukamoto 2009; recent research papers by Jellyman and Tsukamoto 2005 and Aarestrup et al. 2009). Eels were ascending during dusk and descending during dawn indicating that they do this to escape predators. They migrated at depths between 200 and 700 m both in continental and deep-sea waters which probably persists as far as the spawning grounds. Fricke and Käse (1995) artificially induced maturation and released these eels at the supposed Sargasso spawning grounds. They preferred a depth of about 300 m which is in accordance with the depth range of newly hatched yolk sac larvae (Kleckner and McCleave 1988). A point of discussion remains however, that none of these studies has tried to determine the effects of the pop-up tag on swimming behaviour. Since these tags are relatively large for eels (van Ginneken and Maes 2005), they may increase drag, decrease swimming speed and efficiency, influence route choice and diel vertical migration itself. Therefore, we believe it is of utmost importance that potential effects are investigated to validate the results of pop-up tag methodology.
Experiments with male eels swimming in FW at 14.7 ± 0.5°C, in a Blazka type swim tunnel and under pressure (101 ATA corresponding with conditions at 1,000-m depth) in a hyperbaric chamber, revealed that pressure decreases oxygen consumption and thus increases the energetic efficiency at any swimming speed in the range 0.3–1.0 BL s−1 (Sébert et al. 2009). This corresponds to a 60% decrease in COT which would spare about half of the fat stores when a 6,000 km migration is considered. These experiments were performed in FW, SW would increase oxygen consumption with 20% (Palstra et al. 2008a). The small male silver eels that were used have a 23% higher respiration rate than females (Scaion and Sébert 2008). Diel vertical migration in the field (200–700 m) may result in higher efficiency at higher depth and pressure during the day. On the other hand temperatures at 700 m in the open ocean are around 6°C. It remains a major question whether silver eels are capable to swim at those extreme low temperatures. Possibly, they hide in the dark during day time, and continue to swim at lower depths and higher temperatures at night. Obviously this aspect of their migration can only be solved by more advanced tracking experiments.
Interaction between swimming and sexual maturation
Swimming stimulates silvering and early oocyte development
The 22 Blazka-type calibrated swimming flumes at Leiden University have also been used for swimming trials elucidating aspects of swimming induced silvering and maturation. In 2006, a new 6,000-L swimming gutter was built to allow group-wise swimming of males and females, expected to lead to lower stress levels thus avoiding possible negative effects on maturation.
Swimming induces an increase of eye diameter (Table 5). This has been observed repeatedly in different fresh water (FW) swimming trials. Continuous swimming at 0.5 body-lengths per second of eels from the landlocked Lake Balaton (aged 13–21 years; otolith analysis by Palstra et al. 2006a, 2007a) resulted in an increase of the eye index (EI) that was already apparent after 2 weeks of swimming and occurred in all eels exposed to the swimming trial (Palstra et al. 2007a). The observed changes appeared even stronger after 6 weeks of swimming. Significant increases in EI were also apparent in FW swimming trials with migrating eels from the river Loire (aged 10–28; otolith analysis by Palstra et al. 2006b) and with older farmed eels (Table 5). Since younger farmed eels did not show swimming-induced enlargement of the eyes, age-dependent maturation sensitivity is suggested. Arguments for age-dependent maturation also come from other observations: (1) older eels showed increased capacity to incorporate more lipid from the muscle into the oocytes (Palstra et al. 2006c), and (2) older eels were more sensitive to hormonal stimulation (Palstra 2006; also Durif et al. 2006). Repeated yearly silvering and subsequent regression (Durif et al. 2005) might unlock the strong inhibition of sexual maturation in eels. Age might thus be a key factor for successful maturation. Eels may nowadays compromise in this perspective and leave to the Sargasso at a younger age, since a long life-time increases the impact of anthropogenic factors that negatively interfere with reproduction capacity (PCBs: van Ginneken et al. 2009b, reviewed by van Ginneken et al. 2009a; swim-bladder parasites: Palstra et al. 2007b, reviewed by Székely et al. 2009; eel viruses: reviewed by Haenen et al. 2009).
It appears that the increase of the eye diameter occurs solely in freshwater, since no changes were detected in salt-water (SW) trials (Table 5), neither in males nor females. Since the enlargement of the eyes is used for discriminating between the yellow and silver phase (Pankhurst 1982), it can thus be stated that FW-swimming induces silvering. Changes in the length (Durif et al. 2005) and shape (Tesch 2003) of the pectoral fins are also considered as indicative for the degree of silvering. However, in none of the swimming trials were such changes detected (Palstra et al. 2007a).
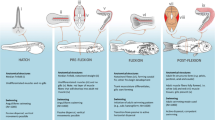
The ovaries of European silver eels show oocytes after transformation of the oogonia in the first developmental stages (stage 1–2; Adachi et al. 2003). Further progression requires incorporation of lipids (stage 3) and vitellogenin (stage 4). Already after 1 week of FW- swimming of Lake Balaton eels (Palstra et al. 2007a), the GSI increased and oocytes became larger with large numbers of lipid droplets (Fig. 4). After 6 weeks of swimming, changes were much more pronounced than after 2 weeks of swimming: both GSI and oocyte diameter were significantly higher. In contrast to resting eels, the swimming eels had oocytes in the lipid droplet stage 3. These results indicate that a high level of lipid mobilisation induced by FW- swimming is required not only for fuel but also for a natural incorporation of lipid droplets in the oocytes. Oocytes of eels that had swum contained more than 100 large droplets. Most developed oocytes had lipid droplets that covered >50% of the cytoplasm and formed a complete ring around the circumference of the developing oocyte (Couillard et al. 1997), which is typical for previtellogenic oocytes (Colombo et al. 1984). We can thus conclude that swimming activates lipid metabolism. This may represent a crucial step in oocyte maturation, since the amount of lipid droplets influences the subsequent developmental events before and after fertilisation (Palstra et al. 2005), and provides the necessary reserves for the offspring. In artificially matured eels, in total 57 ± 22 g kg body weight−1 lipid is incorporated into the oocytes corresponding to 28% of the lipid reserves of the average silver eel (Palstra et al. 2006b). However, we did not observe any yolk globuli in the oocytes of swimming eels. The oocytes also did not reach sizes that are characteristic for vitellogenesis (stage 4). Adachi et al. (2003) showed for A. japonica that ‘ovarian’ vitellogenesis (uptake of vitellogenins) begins when oocytes are about 250 μm in diameter. In our studies, we found maximum oocyte diameters of 236 μm, which are quite close to the onset of vitellogenesis.
Induced changes by swimming in fresh water with a differences (averages ± standard error) in pituitary LH and 11-KT (top to bottom) in young farmed eels that were sampled at the start of the experiment (control), that rested during the experimental period (rest) or swam 5,500 km (swim). Pituitary LH is increased in swimmers and 11-KT tends to increase but individual variation is high. Based on data from van Ginneken et al. 2007a. b Increase in eye size, c oocyte growth and development in the same eels, and in particular d deposition of fat droplets (top to bottom) occur within 2 weeks of swimming in old Lake Balaton eels and were more pronounced after 6 weeks of swimming. Yolk deposition however remained absent. Based on data from Palstra et al. (2007a)
After swimming 5,500 km in freshwater, young farmed eels showed increased LH levels in the pituitary (Fig. 4; van Ginneken et al. 2007a). FW-swimming thus stimulates gonadotropin production by the pituitary but it is still unclear whether secretion is also stimulated. After swimming 5,500 km in freshwater, the young farmed eels also showed tendencies for increased levels of plasma 17β-estradiol (E2) and 11-ketotestosteron (11-KT; Fig. 4), while plasma vitellogenin (Vtg), pituitary-adrenocorticotropic hormone (ACTH), plasma-ACTH, pituitary- melanophore-stimulating hormone (MSH) α and plasma-αMSH were unaffected (van Ginneken et al. 2007a). 11-KT is considered as the major hormonal mediator of silvering in female eels (Lokman et al. 2003), as evidenced by the numerous silvering effects, like enlargement of the eyes, that Rohr et al. (2001) observed after implanting 11-KT in female A. australis. Just recently, Lokman et al. (2007) and Endo et al. (2008) showed that 11-KT has an important role in growth of previtellogenic oocytes and lipid transfer and deposition which agrees with our histological observations. E2 did not show these effects in vitro (Lokman et al. 2007) but in vivo it did (Olivereau and Olivereau 1979) although effects were not only attributable to E2 but probably also to gonadotropins (Lokman et al. 2007). A significant role for 11-KT is thus clear, but a role for E2 cannot be excluded. The young farmed eels in van Ginneken’s study, however, did not show an increase in eye size but older swimming eels did after swimming in freshwater (Table 5).
The next stage: female swimming in seawater suppresses vitellogenesis
Plasma Vtg levels in FW-swimming Lake Balaton eels were still below detection limits (Palstra and Dufour unpublished data). Wild migratory silver eels swimming in SW even showed decreased levels of plasma Vtg after swimming 1,000 km in salt water while testosterone (T) and E2 were unaffected (Palstra et al. unpublished data). Recently, we measured blood plasma E2 and estimated Vtg indirectly through plasma calcium (Ca) concentration (Palstra et al. unpublished data). Swimming, but also resting, increased E2 levels but only in first instance. Ca levels were found to be lower in SW-swimming eels. Results thus show that swimming does not stimulate vitellogenesis which corresponds with histological findings; the absence of yolk globuli in the oocytes of swimmers.
Recently, we have partially cloned 4 different genes from A. anguilla tissue extracts (Palstra et al. 2009c): the oestrogen receptor 1 (esr1, formerly known as E2 receptor α), vitellogenin 1 (vtg1), vitellogenin 2 (vtg2) and β-actin (Genbank accession numbers EU073125 (esr1), EU073127 (vtg1), EU073128 (vtg2)). Additionally, we have cloned the gonadotropins; the common glycoprotein α subunit (gpα: Genbank accession number EU078899), fshβ, lhβ, and more recently their receptors fshr and lhr (resp. Genbank accession numbers EU191610 and EU348825). We have applied the developed molecular primers for housekeeping gene β-actin and targeted genes for esr1-, vtg1- and vtg2-expression on liver samples of female silver eels that swam for 1.5 or 3 months in salt water. In swimming eels, the expression of the esr1 was lower than in resting eels for both timepoints (Palstra et al. 2010). This reduction in expression probably resulted in the reduced expression of vtg1 and vtg2 in swimmers. From this, we can conclude that hepatic vitellogenesis is indeed reduced in swimming silver eels in salt water.
On the basis of evidence from different angles, it can be concluded that swimming inhibits the whole process of vitellogenesis, at least in the first instance. Firstly, esr1-, vtg1- and vtg2-expression were reduced in the livers of swimming females (Palstra et al. 2010). Secondly, plasma Vtg was repeatedly determined as not detectable and plasma Ca as not elevated in swimming females (van Ginneken et al. 2007a and Palstra et al. 2010). Thirdly, oocytes of swimming females from Lake Balaton (Palstra et al. 2007a), Lake Grevelingen (Palstra et al. unpublished data) and the river Loire (Palstra et al. unpublished data) did not contain any yolk globuli and were all smaller than 250 μm, the border for switching to stage 4 vitellogenic oocytes. In a recent experiment, we have injected eels that swam or rested for 3 months with carp pituitary extract up to full maturation. The swimming-induced suppression of vitellogenesis resulted in delayed ovulation of 2–3 weeks.
Male swimming in seawater stimulates spermatogenesis
Female eels stay 7–30 years in the freshwater before migration, in contrast to just 4–9 years for males. As a consequence, females reach a tenfold larger size than males at the onset of migration (resp. 1,000 g vs. 100 g body-weight on average). As the energy requirements for males are far less than those for females, it is possible that the observed dopaminergic inhibition is sex-specific. We have tested this hypothesis by subjecting male and female eels to a GnRH-agonist (GnRHa), specifically the commercial product Gonazon For Fish (Intervet), as well as to stimulation by long-term swimming in seawater (SW) that is supposed to stimulate GnRH excretion by the hypothalamus (Palstra et al. 2008b). Males that were either stimulated by 3 months SW-swimming or by GnRHa-injection showed a two- to three-fold higher LHβ expression level than the male starters and resters (Fig. 5). Both treatments also caused a three- to five-fold increase in GSI (Fig. 5) and an induced spermatogenesis (>80% presence of spermatogonia late type b; Fig. 5). One male swimmer even showed the formation of spermatocytes. In contrast, females were not stimulated by SW-swimming nor by GnRHa, and even showed regression of maturation over time as demonstrated by lower LHβ expression, GSI and oocyte diameters in all groups after 3 months (Fig. 5). The expression of FSHβ did not significantly change under the different treatments in both males and females. Thus, in contrast to the response of the females, we observed sexual maturation in males upon GnRHa-injection, indicating that dopaminergic inhibition is not effective in males. Males were also stimulated after 3 months SW-swimming, suggesting that swimming acts via a similar mechanism. Since LHβ-expression was not enhanced in females that either swam or received a GnRHa-injection, their pituitaries were considered as not sensitised and still under dopaminergic control. Females can therefore only be stimulated by circumventing dopaminergic control (Dufour et al. 1988; Vidal et al. 2004) through injection of pituitary homogenates (Fontaine et al. 1964) that do increase LHβ subunit expression (Schmitz et al. 2005; Jeng et al. 2007). What remains to be tested are stimulation by hormone producing cell implants that take over the role of the pituitary (Palstra and van den Thillart 2009) or by swimming/GnRHa injection in combination with a dopamine antagonist, for instance metaclopramide (like used in combination with mGnRHa in OVOPEL, Interfish), domperidone (like used in combination with sGnRH in OVAPRIM, Syndell), reserpine or pimozide (Mylonas and Zohar 2007).
Expression (Q-RT-PCR) of luteinising hormone subunit (LHβ) in the pituitary, the gonadosomatic index (GSI) and gonad development in male and female eels. Eels were sampled at the start, after 3 months rest, after 3 months of SW-swimming and 3 months after a single GnRHa injection (Gonazon For Fish, Intervet). a male LHβ expression (ng relative to total RNA), b female LHβ expression, c male GSI, d female GSI, e oocyte diameters, f male testis stage with frequency distribution of spermatogonia type a, spermatogonia early type b, spermatogonia late type b and spermatocytes; g testis containing mainly spermatogonia type a typical for unstimulated fish, and h testis containing mainly spermatogonia late type b typical for stimulated fish. The scale bar represents 100 μm. In females, regression occurred during the experimental period, an effect which was more pronounced in the resting group than those stimulated by SW-swimming and GnRHa. In males, however, SW-swimming and GnRHa activated maturation (student t-tests with *P < 0.05; **P < 0.01. LHβ and GSI: 3 m rest vs. start, 3 m swimming or 3 m GnRHa vs. 3 m rest. Gonad development: 3 m rest, 3 m swimming or 3 m GnRHa vs. start). Source: Palstra et al. (2008b)
So while gonadotropin expression and vitellogenesis in females during SW-swimming are suppressed, full spermiation can be expected in males after longer swimming trials. Our latest results show that male eels that swam for 3 months in salt water and that were subsequently treated with human chorionic gonadotropin started their spermiation earlier, and they produced more sperm of higher density.
Sexual maturation during riverine and oceanic migration
When results from the laboratory are extrapolated to the field situation, neglecting effects of other possible triggers, it can be assumed that migratory eels do not necessarily silver before their freshwater migration rather than, or especially, during it. Old swimming silver eels showed increase of eye size but it appears that this occurs only in freshwater trials. As far as we know, it is unknown if eels from salt or brackish water generally have larger eyes than eels from freshwater leading to only the latter showing this swimming-induced change in eye size.
During freshwater migration, lipid mobilisation occurs. Extensive lipid incorporation in the oocytes was apparent during freshwater swimming trials. In the field, Cottril et al. (2001) found that the concentration of total plasma non-esterified fatty acids (NEFA) appeared to follow the trend of E2 and GSI, increasing with sexual maturity in migrating A. rostrata.
Vitellogenesis seems to be inhibited during freshwater migration. Vtg and Ca levels are low in wild silver eels. Versonnen et al. (2004) measured Ca as indicator for Vtg levels at 20 locations and found very low levels. In a recent study (Palstra et al. unpublished data), we measured E2 and Ca in blood plasma in migrating silver eels in the River Rhine (Germany) during the migratory season in August, September and October 2005 and investigated the gonads histologically. E2 levels were higher in October but Ca levels stayed low over the months and oocytes were still smaller than 250 μm and without yolk globuli. Silver eels exhibit large variability in plasma Vtg levels as demonstrated by homologous radioimmuno- and immunoenzymatic assays (Burzawa-Gerard and Dumas-Vidal 1991; Sbaihi et al. 2001). Van Ginneken and Dufour (unpublished data) measured plasma Vtg in 104 large female silver eels from the brackish Lake Grevelingen (The Netherlands). Of these eels, 96% still showed low Vtg levels <0.5 μg ml−1.
Salt water swimming trials revealed no changes in eye diameter (Table 5). The question remains whether silver eels migrating in the ocean continue the increase in eye size. Probably, eyes will increase during progressed maturation, since the eye diameter of migratory silver eels from Lake Grevelingen, caught near the North Sea sluice, increases further when stimulated by hormonal injections (Palstra 2006).
Also at least during the first part of oceanic migration, vitellogenesis seems to be suppressed in migrating silver eels: eels did not show an increase in GSI and still showed reduced esr1-, vtg1- and vtg2-expression after swimming for 3 months in salt water. This may be because (1) raising the severely depressed lipid mobilisation and stimulating the extensive lipid incorporation requires long-term swimming exercise and (2) undesired effects of vitellogenesis during swimming are prevented. Vitellogenesis induces muscle atrophy (Salem et al. 2006a, b) and is associated with mobilisation of phospho-calcium reserves coming from resorption of vertebrae (Sbaihi 2002; Sbaihi et al. 2007), which are obviously undesired processes during swimming. Growth of oocytes is most pronounced during vitellogenesis and subsequent maturation. The oocyte diameter will increase twofold up to 400 μm during vitellogenesis and again twofold up to 800 μm due to hydration during final maturation (Palstra et al. 2005). These increases result in similar increases of gonad mass and with that the body diameter. This then increases drag during swimming and with that increases the cost of transport (reviewed by van Ginneken and Maes 2005). Also this situation is considered as undesirable during migration.
When we extrapolate lab results to the field, it can be hypothesised that vitellogenesis and maturation occur near or at the spawning grounds. Spawning ground-specific triggers may be involved during vitellogenesis and final maturation. Spawning ground-specific triggering of the final stages of maturation has also been considered for other homing fishes (Palstra et al. 2004). These triggers may involve area-specific odour, intersex pheromonal communication (Liley and Stacey 1983; Lam 1983; van Ginneken et al. 2005a; Huertas et al. 2006) or triggering by an increase in water temperature by rising in the water column. A rise in water temperature is known to increase the responsiveness of the liver to oestrogen in the production of vitellogenins (Yaran et al. 1980). The first studies on combinations of stimulators started recently investigating swimming exercise with high pressure treatment in hyperbaric chambers (Sébert et al. 2009), swimming at daily alternating temperature profiles (Tsukamoto K, presented at the Aquaculture Europe 2008 symposium organised by the European Aquaculture Society in Krakow, Poland) and hormonal stimulation at different temperature profiles (Pérez et al. 2008).
Linking metabolism with maturation
Several candidates may play a decisive role in linking the metabolic status with the onset of sexual maturation, as induced by exercise at the start of migration. Swimming may up-regulate Kisspeptin-levels, a peptide involved in processes leading to puberty (van Aerle et al. 2008; Filby et al. 2008). Kisspeptin is expressed by the appropriate exercise tissues (adipose tissue, intestine; van Aerle et al. 2008), although expression in fish muscle does not seem to occur in zebrafish (Biran et al. 2008). Kisspeptin signals the hypothalamus to release GnRH or may even work directly on the pituitary, since the isoform receptor kiss1rb in zebrafish was shown to be strongly expressed in the pituitary (Biran et al. 2008). These direct effects of kisspeptin on the pituitary are a subject of great controversy (a.o. discussed at the 2nd Int. Symp. For Fish Growth and Reproduction, June 20–21 2009, Hong Kong S.A.R., China) but in rainbow trout we have found expression of the kiss1-receptor in the pituitary (Palstra et al. 2009b). Swimming may indirectly through Kisspeptin, or directly up-regulate GnRH-levels (in silver eels the mammalian-type GnRH—mGnRH) or down-regulate dopamine levels that subsequently lead to positive effects on gonadotropin production in the pituitary (e.g., Montero et al. 1995). However, studies in eel species, as well as fish species in general, regarding exercise effects on hypothalamic–pituitary interaction are still lacking. Kiss1peptin-, mGnRH- and dopamine-action may be exerted through swimming-induced alterations in cortisol that binds to glucocorticoid receptor—expressing neurons (Teitsma et al. 1999). Silver eels have higher cortisol levels (van Ginneken et al. 2007b) and higher cortisol levels have been measured in swimming eels of Lake Grevelingen and Lake Balaton although individual variance is very high (Palstra et al. 2009a). Cortisol is known to be involved in mobilisation of lipids. Cortisol peaks lead to lipolysis of muscle and hepatic lipids (Freeman and Idler 1973; Davis et al. 1985; Barton et al. 1987; Mommsen et al. 1999) releasing fatty acids into the blood. Cortisol has a less defined role in maturation of fish. DiBattista et al. (2005) showed that cortisol treatment in rainbow trout significantly decreased dopaminergic activity in the telencephalon. Epstein et al. (1971) and Fontaine (1994) showed that successive high concentrations of plasma cortisol, lasting for at least 7 days, triggers silvering in eel. Cortisol is also known as a stimulator of LH synthesis in vitro and in vivo (Huang et al. 1999; Dufour et al. 2003) and as an inhibitor of vitellogenin synthesis (Sbaihi 2001). Cortisol was shown to inhibit E2-induced vitellogenin synthesis in the rainbow trout, an effect mediated by a decrease in ERα mRNA levels (Lethimonier-Desdoits et al. 2000). These observations perfectly match our observations on swimming eels.
Some other candidates that have a metabolic function and may exhibit positive effects on gonadotrope function are insulin (Dufour et al. 2000), leptin (Dufour et al. 2003) and steroidogenic acute regulatory protein (StAR; Li et al. 2003) in eels. Blood-plasma levels of fatty acids and their metabolites produced from cyclooxygenase and lipoxygenase pathways rise before and during migration of eel (Cottril et al. 2001; van Ginneken et al. 2007a, b) and salmon (Sasaki et al. 1989) and can modulate gonadal steroid production in a manner similar to that observed in mammals (Sorbera et al. 2001). Other interesting candidates are ghrelin and insulin-like growth factors (IGFs). Like leptin, ghrelin may be an important neuroendocrine integrator of somatic growth, energy balance and reproduction in mammals (Tena-Sempere 2005) and in fish (Amole et al. 2009). Its role in eel reproduction is still unknown. Insulin-like growth factor I in teleosts, like in mammals, is mainly produced by the liver under the stimulatory control of GH and together they play an important role in regulating body growth (Duguay et al. 1996). Studies in mammals show that IGF-I could be a good candidate for a link between somatic development and pubertal activation of reproduction (Cohick and Clemmons 1993). In the European eel, IGFs exert a direct effect at the pituitary level by stimulating LH synthesis (Huang et al. 1998). IGFs may also have other direct targets, such as a stimulatory effect on all stages of 11-ketotestosterone-induced spermatogenesis (Nader et al. 1999).
Discussion and conclusions
Simulated migration
Our respiratory measurements as well as the carcass analyses suggest that eels have a much higher swimming efficiency than trout. In eel, the COT values obtained from oxygen consumption data and carcass analyses are 0.42 and 0.61 kJ kg−1 km−1 (Table 4), respectively, whereas trout has a much higher COT value of around 2.7 kJ kg−1 km−1. The COT in trout matches the value measured by Webb (1971), and is similar to other salmonids (Brett and Glass 1973) and many adult fish species (Videler 1993). This means that eel swim 4–6 times more efficiently than other fish species, even across swimming styles. European eel is able to swim 5,500 km, a distance corresponding to their supposed spawning area in the Sargasso Sea at a remarkably low energy costs. The amount of energy for swimming 5,500 km corresponds to 60–80 g fat. Remarkably, as eels consume proteins and fat in the same ratio as present as in their body the energy losses have no effect on buoyancy. So, we can conclude that healthy well-fed eels are able to reach the Sargasso leaving enough reserves for reproduction.
Speed tests with 4 groups of eels showed that the swimming performance between the groups were rather similar. There were no differences between 2- and 12-h speed tests, suggesting that eels swim at the same low efficiency from the start. COT values changed little with speed; the lowest COT (COTmin) over 0.5–1.0 m s−1 was found at ~0.65 m s−1, a swimming speed ~60% higher than the minimal cruising speed. At that speed (0.8 BL s−1) female silver eels can reach the spawning site within 3.5 months, leaving ample time for final maturation and finding mates.
As discussed, several oceanic conditions might influence the swimming speed and COT either positively or negatively. Eels could benefit from currents to increase the ground speed or decrease the COT, for instance by entering the south- and west-flowing currents from North west Africa to the Caribbean (Aarestrup et al. 2009). Diel vertical migration increases the travel distance about 7% (based on data of Aarestrup et al. 2009) but decreases COT in negative relation to pressure (Sébert et al. 2009). Since the latter gain in energetic efficiency was highest at the supposed Uopt, no consequences for swimming speed are expected. However, potential effects of tags on swimming efficiency, speed and behaviour need to be investigated to validate the diel vertical migration as found by pop-up tag methodology. Our studies indicate that the daily variations in temperature that result from vertical migration (from 7 to 12°C: Aarestrup et al. 2009) do not affect swimming efficiency (Fig. 3b). With respect to sexual maturation, diel vertical migration can be expected to have effects through the alternation in pressure (Sébert et al. 2007), temperature (Tsukamoto K, presented at the Aquaculture Europe 2008 symposium organised by the European Aquaculture Society in Krakow, Poland; Pérez et al. 2008) and avoidance of light (Sébert et al. 2008).
Swimming effects on sexual maturation
During their continental phase, yellow eels have depressed lipid mobilisation and pre-pubertal blockage of maturation. Since the start of spawning migration marks the onset of lipid mobilisation and maturation, swimming may be a crucial trigger of these processes. Swimming trials of older female eels in freshwater show that eyes were enlarged in all swimmers and that swimming thus induced silvering. FW-swimming stimulates early oocyte development and deposition of lipids in the oocytes of female eels, probably regulated by increased 11-ketotestosteron (11-KT) levels. Increased LH levels in the pituitary show that FW-swimming stimulates gonadotropin production. Swimming also increases plasma 17β-estradiol (E2) but hepatic vitellogenesis is however not initiated: plasma vitellogenin (Vtg) levels remain low and yolk globuli do not appear in the oocytes. Swimming trials of female silver eels in salt water show that swimming inhibits gonadotropin expression and vitellogenesis; expression of LHβ in the pituitary and of the esr1, vtg1 and vtg2 in the liver were lower in female swimmers and plasma Vtg levels were reduced. Female eels are probably still under dopaminergic control in contrast to male silver eels that continue maturing by SW-swimming. Both the induction of lipid deposition in the oocytes and the inhibition of vitellogenesis by swimming in females may allow a natural sequence of events leading to a higher gamete quality in contrast to stimulation with pituitary extract injections. The effect of long-term swimming on gamete quality is subject of our current research. When these results are extrapolated and linked to observations in the field, it appears that freshwater migration triggers silvering and lipid mobilisation. Vitellogenesis is inhibited by swimming to allow the migration and probably occurs only near or at the spawning grounds. The sex-specific differences in stimulation during SW-swimming lead to a tempting ecological hypothesis. The large females may migrate in 3.5 months having 2.5 months left to mature at the spawning grounds. The twofold smaller males however, cannot swim that fast and need a twofold longer migration time of 7 months. They leave earlier (1 month) and they fully mature during the trip being about ready to spawn at arrival.
In general, fine-tuning between migration and maturation of fishes is a non-elucidated research topic that deserves much more attention. It is peculiar that the influence of swimming exercise on maturation has never been thoroughly investigated, especially since fatty migrant fish like tuna and eel are of major commercial interest but very difficult or even impossible to reproduce in captivity.
The eel is a very appropriate model to study metabolic and hormonal switches that trigger the sexual maturation. However, not many of the required molecular tools exist for this species that are available for salmonid species that could also serve as a model. Because of this, our attention has currently expanded to rainbow trout which allows us to identify new players by using a salmonid cDNA microarray platform (GEO GPL6154). The near future of high throughput sequencing will allow us to sequence fish genomes in a fast and affordable way, and analyse transcriptomic changes on the largest possible scale.
The fate of the fat: lipid costs of female reproduction
As described, wild European silver eels spend 78 ± 4 g fat kg−1, or 39% of the fat stores with average fat percentages of 20%, on fuelling the complete spawning migration to the Sargasso (Table 4). Artificially matured eels from the same batch by hormonal injections incorporated 57 ± 22 g fat kg−1, or 28% of the fat stores, in the oocytes. The amount of fats that is deposited is positively related to the age of the eel (Palstra et al. 2006c). Thus, in total 67% of the fat stores are spent on the spawning migration and oocyte maturation, and because body composition stays the same this would correspond to 67% of the total energy stores. When a boundary between iteroparous and semelparous is considered of 60–70% total energy depletion range (Wootton 1990), the European eel exhibits a probable semelparous life style. For comparison: iteroparous trouts spend 40–50% energy on spawning with 3–4% of gonadal energy (Jonsson 2005) and semelparous Pacific salmons spend 75–82% energy on spawning with 10% of gonadal energy.
Perspectives for aquacultural applications
Although current protocols for artificial reproduction of eels by hormonal injections are successful to a certain extent (Kagawa et al. 2005; Palstra and van den Thillart 2009), the induced process of maturation can be considered abnormal in many aspects. Abnormality of maturation is evidenced by limited reproductive success (Pedersen 2003, 2004; Palstra et al. 2005), and observed phenomena like variations in yolk accumulation, egg membrane formation, differences in the process of oocyte maturation and plasma hormone levels (Adachi et al. 2003; Kagawa et al. 2005). Oocytes of non-exercised silver eels are probably still too premature for hormonal stimulation by pituitary extract of spawnable salmons or carps containing mainly LH, but also FSH, TSH, GH and prolactin. A probable cause of abnormality; incomplete lipid incorporation may be prevented by swimming. During artificial induction by hormonal injections, hepatic vitellogenesis is immediately induced (Palstra et al. 2009c). Lipid and Vtg incorporation occur simultaneously in artificially matured Japanese eel (Adachi et al. 2003) and European eel (Palstra et al. 2009c), which suggests an unnatural situation. By stimulating incorporation of lipids in the oocytes and inhibiting vitellogenesis, swimming may optimise the natural sequence of these processes. Recently, Patterson et al. (2004) reported on probable beneficial effects in salmon. They found that non-exercised females had delayed maturity, lower egg deposition rates, and were more likely to die prior to egg ovulation than exercised females and natal spawners. Pre-treatment by swimming in current protocols for artificial reproduction will likely result in higher gamete quality and general reproductive success. These are topics of our current investigations.
References
Aarestrup K, Økland F, Hansen MM, Righton D, Gargan P, Castonguay M, Bernatchez L, Howey P, Sparholt H, Pedersen MI, McKinley R (2009) Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325:1660
Adachi S, Ijiri S, Kazeto Y, Yamauchi K (2003) Oogenesis in the Japanese Eel, Anguilla japonica. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 502–518
Amole N, Gonzalez R, Mensah E, Unniappan S (2009) Novel neuroendocrine factors regulating reproduction in fish. In: Abstract book 2nd International Symposium for Fish Growth and Reproduction, June 20–21, Hong Kong S.A.R., China, pp 9
Aoyama J (2009) Life history and evolution of migration in catadromous eels (Genus Anguilla). Aqua-BioSci Monogr 2:1–42
Avise JC, Helfman GS, Saunders NC, Hales LS (1986) Mitochondrial DNA differentiation in North Atlantic eels: population genetics consequences of an unusual life history pattern. Proc Natl Acad Sci USA 83:4350–4354
Avise JC, Nelson WS, Arnold J, Koehn RK, Williams GC, Thorsteinsson V (1990) The evolutionary genetic status of Icelandic eels. Evolution 44:1254–1262
Barton BA, Schreck CB, Barton LD (1987) Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis Aquat Organ 2:173–185
Bast HD, Klinkhardt MB (1988) Fang eines Silberaales (Anguilla anguilla (L. 1758)) im Iberischen Becken (Nordostatlantik) (Teleostei: Anguillidae). Zool Anz 221:386–398
Beamish FWH (1978) Swimming capacity. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic press, London, pp 101–189
Biran J, Ben-Dor S, Levavi-Sivan B (2008) Molecular identification and functional characterization of the Kisspeptin/Kisspeptin receptor system in lower vertebrates. Biol Reprod 79:776–786
Blazka P, Volf M, Ceplea M (1960) A new type of respirometer for determination of the metabolism of fish in an active state. Physiol Bohemoslov 9:553–560
Boëtius I, Larsen LO (1991) Effects of testosterone on eye size and spermiation in silver eels. Gen Comp Endocrinol 82:238
Bone Q, Marshall NB, Blaxter JH (1995) Biology of fishes, 2nd edn. Chapman & Hall, London. ISBN 0-7514-022. Branchus, photographed on the floor of the deep Atlantic in the Bahamas. Bull Mar Sci, vol 29, pp 401–405
Brafield AE, Llewellyn MJ (1982) Animal energetics. Blackie, Glasgow
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21:1183–1226
Brett JR, Glass NR (1973) Metabolic rates and critical swimming speeds of sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. J Fish Res Board Can 30:379
Burzawa-Gerard E, Dumas-Vidal A (1991) Effects of 17 beta-estradiol and carp gonadotropin on vitellogenesis in normal and hypophysectomised European silver female eel (Anguilla anguilla L.) employing a homologous radioimmunoassay for vitellogenin. Gen Comp Endocrinol 84:264–276
Chow S, Kurogi H, Mochioka N, Kaji S, Okazaki M, Tsukamoto K (2009) Discovery of mature freshwater eels in the open ocean. Fish Sci 75:257–259
Cohick WS, Clemmons DR (1993) The insulin-like growth factors. Annu Rev Physiol 55:131–153
Colombo G, Grandi G, Rossi R (1984) Gonad differentiation and body growth in Anguilla anguilla L. J Fish Biol 24:215–228
Comparini A, Rodino E (1980) Electrophoretic evidence for two species of Anguilla leptocephali in the Sargasso Sea. Nature 287:435–437
Cottril RA, McKinley RS, van der Kraak G, Dutil J-D, Reid KB, McGrath KJ (2001) Plasma non-esterified fatty acid profiles and 17β-oestradiol levels of juvenile immature and maturing adult American eels in the St Lawrence River. J Fish Biol 50:364–379
Couillard CM, Hodson PV, Castonquay M (1997) Correlation between pathological changes and chemical contamination in American eels, Anguilla rostrata, from the St Lawrence River. Can J Fish Aquat Sci 54:1916–1927
D’Ancona U, Tucker DW (1959) Old and new solutions to the eel problem. Nature 183:1405–1406
Davis K, Torrance P, Parker NC, Shuttle MA (1985) Growth, body composition and hepatic tyrosine aminotransferase activity in cortisol-fed channel catfish, Ictalurus punctatus. J Fish Biol 29:177–184
Deelder CL, Tucker DW (1960) The Atlantic eel problem. Nature 185:589–592
DiBattista JD, Anisman H, Whitehead M, Gilmour KM (2005) The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout Oncorhynchus mykiss. J Exp Biol 208:2707–2718
Dufour S, Lopez E, Le Menn F, Le Belle N, Baloche S, Fontaine YA (1988) Stimulation of gonadotropin release and of ovarian development, by the administration of a gonadoliberin agonist and of dopamine antagonists, in female silver eel pretreated with estradiol. Gen Comp Endocr 70:20–30
Dufour S, Huang YS, Rousseau K, Sbaihi M, Le Belle N, Vidal B, Marchelidon J, Querat B, Burzawa-Gerard E, Chang CF, Schmitz M (2000) Puberty in teleosts: new insights into the role of peripheral signals in the stimulation of pituitary gonadotropins. In: Norberg B, Kjesbu OS, Taranger GL, Anderson E, Stefansson SO (eds) Reproductive physiology of fish. University of Bergen/Institute of Marine Research, Bergen, pp 455–461
Dufour S, Burzawa-Gerard E, Le Belle N, Sbaihi M, Vidal B (2003) Reproductive endocrinology of the European eel, Anguilla anguilla. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 373–386
Duguay SJ, Lai-Zhang J, Streiner DF, Funkenstein B, Chan SJ (1996) Developmental and tissue-regulated expression of IGF-I and IGF-II mRNA in Sparus aurata. J Mol Endocrinol 16:123–132
Durif C, Dufour S, Elie P (2005) The silvering process of the eel: a new classification from the yellow resident stage to the silver migrating stage. J Fish Biol 66:1–19
Durif CMF, Dufour S, Elie P (2006) Impact of silvering stage, age, body size and condition on the reproductive potential of the European eel. Mar Ecol Prog Ser 327:171–181
Durif CMF, van Ginneken V, Dufour S, Müller T, Elie P (2009) Seasonal evolution and individual differences in silvering eels from different locations. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 13–38
EELREP (2005) Estimation of the reproduction capacity of European eel. Final report. Available via http://www.fishbiology.net/eelrepsum.html. Accessed 13 Oct 2009
Endo T, Todo T, Lokman PM, Ijiri S, Adachi S, Yamauchi K (2008) In vitro induction of oil droplet accumulation into previtellogenic oocytes of Japanese eel, Anguilla japonica. Cybium 32:239–240
Epstein FH, Cynamon M, McKay W (1971) Endocrine control of Na-K-ATPase in seawater adaptation in Anguilla rostrata. Gen Comp Endocrinol 16:323–328
Ernst P (1977) Catch of an eel (Anguilla anguilla) northeast of the Faroe Islands. Ann Biol 32:175
Filby AL, van Aerle R, Duitman JW, Tyler CR (2008) The Kisspeptin/Goandotropin-Releasing hormone pathway and molecular signaling of puberty in fish. Biol Reprod 78:278–289
Fontaine YA (1994) L’argenture de l’anguille: métamorphose, anticipation, adaptation. B Fr Peche Piscic 335:171–186
Fontaine M, Bertrand E, Lopez E, Callamand O (1964) Sur la maturation des organes genitaux de l’anguille femelle (Anguilla anguilla L.) et l’émission spontanée des oeufs en aquarium. Comp Rend Acad Sci 259:2907–2910
Freeman HC, Idler DR (1973) Effects of corticosteroids on liver transaminases in two salmonids, the rainbow trout (Salmo gairdneri) and the brook trout (Salvelinus fontinalis). Gen Comp Endocr 20:69–76
Fricke H, Kaese R (1995) Tracking of artificially matured eels (Anguilla anguilla) in the Sargasso Sea and the problem of the Eel’s Spawning site. Naturwissenschaften 83:32–36
Grassi GB (1896) The reproduction and metamorphosis of the common eel (Anguilla vulgaris). Proc R Soc Lond 60:262–271
Haenen O, van Ginneken V, Engelsma M, van den Thillart G (2009) Impact of eel viruses on the recruitment of European eel. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 387–400
Huang YS, Rousseau K, Le Belle N, Vidal B, Burzawa-Gerard E, Marchelidon J, Dufour S (1998) Insulin-like growth factor-I stimulates gonadotropin production from eel pituitary cells: a possible metabolic signal for induction of puberty. J Endocrinol 159:43–52
Huang Y, Rousseau K, Sbaihi M, Le Belle N, Schmitz M, Dufour S (1999) Cortisol selectively stimulates pituitary gonadotropin β-subunit in a primitive teleost, Anguilla anguilla. Endocrinology 130:1228–1235
Huertas M, Scott AP, Hubbard PC, Canario AV, Cerda J (2006) Sexually mature European eels (Anguilla anguilla L.) stimulate gonadal development of neighbouring males: possible involvement of chemical communication. Gen Comp Endocrinol 147:304–313
Jellyman D (1987) Review of the marine life history of Austral-asian temperate species of Anguilla. Am Fish Soc Symp 1:276–285
Jellyman DJ, Tsukamoto K (2002) First use of archival transmitters to track migrating freshwater eels Anguilla dieffenbachii at sea. Mar Ecol Prog Ser 233:207–215
Jellyman DJ, Tsukamoto K (2005) Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii. Mar Ecol Prog Ser 286:261–267
Jeng S-R, Yueh W-S, Chen G-R, Lee Y-H, Dufour S, Chang C-F (2007) Differential expression and regulation of gonadotropins and their receptors in the Japanese eel, Anguilla japonica. Gen Comp Endocrinol 154:161–173
Jones DR, Randall DJ (1978) The respiratory and circulatory systems during exercise. In: Hoar WS, Randall DJ (eds) Fish physiology VII: locomotion. Academic Press, New York, pp 425–502
Jonsson B (2005) Lipid energy reserves influence life history decisions of anadromous Atlantic salmon and brown trout. In: Fish & Diadromy in Europe. Ecology, Management, Conservation. Bordeaux 29 March–1 April 2005, pp 52
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Kleckner RC, McCleave JD (1988) The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to the thermal fronts and surface water masses. J Mar Res 46:647–667
Lam TJ (1983) Environmental influences on gonadal activity in fish. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology. Volume IX. Reproduction. Part B behaviour and fertility control. Academic Press, New York, pp 65–116
Larsson P, Hamrin S, Okla L (1990) Fat content as a factor inducing migratory behaviour in the eel (Anguilla anguilla L.) to the Sargasso Sea. Naturwissenschaften 77:488–490
Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251
Lethimonier-Desdoits C, Flouriot G, Valotaire Y, Kah O, Ducouret B (2000) Transcriptional interference between glucocorticoid receptor and estradiol receptor mediates the inhibitory effect of cortisol on fish vitellogenesis. Biol Reprod 62:1763–1777
Li Y-Y, Inoue K, Takei Y (2003) Steroidgenic acute regulatory protein in eels: cDNA cloning and effects of ACTH and seawater transfer on its mRNA expression. Zool Sci 20:211–219
Liley NR, Stacey NE (1983) Hormones, pheromones, and reproductive behaviour in fish. In: Hoar WS, Randall DJ, Donaldson EM (eds) Volume IX. Reproduction. Part B Behaviour and fertility control. Academic Press, New York, pp 1–64
Lokman PM, Rohr DH, Davie PS, Young G (2003) The physiology of silvering in anguillid eels: androgens and control of metamorphosis from the yellow to silver stage. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 331–350
Lokman PM, George KAN, Divers SL, Algie M, Young G (2007) 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from the shortfinned eel, Anguilla australis, in vitro. Reproduction 133:955–967
Matsubara H, Tanaka T, Nomura K, Kobayashi T, Murashita K, Kurokawa T, Unuma T, Kim S-K, Lokman MP, Matsubara T, Kagawa H, Ohta H (2008) Occurrence of spontaneously spermiating eels in captivity. Cybium 32(2 suppl):174–175
McCleave JD (1980) Swimming performance of European eel (Anguilla anguilla L.) elvers. J Fish Biol 16:445–452
McCleave JD, Arnold GP (1999) Movements of yellow- and silver-phase European eels (Anguilla anguilla L.) tracked in the western North Sea. ICES J Mar Sci 56:510–536
McCleave JD, Kleckner RC, Castonguay M (1987) Reproductive sympatry of American en European eels and implications for migration and taxonomy. Am Fish Soc Symp 1:286–297
Mills D (1989) Ecology and management of Atlantic Salmon. Chapman & Hall, London
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Montero M, Le Belle N, King JA, Mîllar RP, Dufour S (1995) Differential regulation of the two forms of gonadotropin-releasing hormone (mGnRH and cGnRH-II) by sex steroids in the European female silver eel (Anguilla anguilla L.). Neuroendocrinology 61:525–535
Mylonas CC, Zohar Y (2007) Promoting oocyte maturation, ovulation and spawning in farmed fish. In: Babin PJ, Cerda J, Lubzens E (eds) The fish oocyte. From basic studies to biotechnological applications. Springer, Heidelberg, pp 437–474
Nader MR, Miura T, Ando N, Miura C, Yamauchi K (1999) Recombinant human insulin-like growth factor I stimulates all stages of 11-ketotestosterone-induced spermatogenesis in the Japanese eel, Anguilla japonica, in vitro. Biol Reprod 61:944–947
Nieddu M, Pichiri G, Coni P, Salvadori S, Deiana AM, Mezzanotte R (1998) A comparative analysis of European and American eel (Anguilla anguilla and Anguilla rostrata) genomic DNA: 5S rDNA polymorphism permits the distinction between the two populations. Genome 41:728–732
Olivereau M, Olivereau J (1979) Effects of estradiol-17β on the cytology of the liver, gonads and pituitary, and on plasma electrolytes in the female freshwater eel. Cell Tissue Res 199:431–454
Palstra A (2006) Energetic requirements and environmental constraints of reproductive migration and maturation of European silver eel (Anguilla anguilla L.). Dissertation, University of Leiden
Palstra A, van den Thillart G (2009) Artificial maturation and reproduction of the European eel. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 309–332
Palstra AP, de Graaf M, Sibbing FA (2004) Riverine spawning and reproductive segregation in a lacustrine cyprinid species flock, facilitated by homing? Anim Biol 54:393–416
Palstra AP, Cohen EGH, Niemantsverdriet PRW, van Ginneken VJT, van den Thillart GEEJM (2005) Artificial maturation and reproduction of European silver eel: development of oocytes during final maturation. Aquaculture 249:533–547
Palstra A, van Ginneken V, van den Thillart G (2006a) Swim performance of European silver eels (Anguilla anguilla). In: Palstra AP (ed) Energetic requirements and environmental constraints of reproductive migration and maturation of European silver eel (Anguilla anguilla L.). Dissertation, University of Leiden
Palstra AP, Antonissen E, Clavero ME, Nieveen M, Niemantsverdriet P, van Ginneken VJT, van den Thillart GEEJM (2006b) The fate of fat in silver eels: lipid requirements for spawning migration. In: Palstra AP (ed) Energetic requirements and environmental constraints of reproductive migration and maturation of European silver eel (Anguilla Anguilla L.). Dissertation, University of Leiden
Palstra AP, van Ginneken VJT, Murk AJ, van den Thillart GEEJM (2006c) Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93:145–148
Palstra A, Curiel D, Fekkes M, de Bakker M, Székely C, van Ginneken V, van den Thillart G (2007a) Swimming stimulates oocyte development in European eel (Anguilla anguilla L.). Aquaculture 270:321–332
Palstra AP, Heppener DFM, van Ginneken VJT, Székely C, van den Thillart GEEJM (2007b) Swimming performance of silver eels is severely impaired by the swim-bladder parasite Anguillicola crassus. J Exp Mar Biol Ecol 352:244–256
Palstra A, van Ginneken V, van den Thillart G (2008a) Cost of transport and optimal swimming speeds in farmed and wild European silver eels (Anguilla anguilla). Comp Biochem Physiol A 151:37–44
Palstra AP, Schnabel D, Nieveen MC, Spaink HP, van den Thillart GEEJM (2008b) Male silver eels mature by swimming. BMC Physiol 8:14
Palstra AP, van Ginneken V, van den Thillart G (2009a) Effects of swimming on silvering and maturation. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 309–332
Palstra AP, van den Thillart GEEJM, Planas JV (2009b) Swimming for reproduction of anguillids and salmonids. Abstract book 16th International Congress of Comparative Endocrinology, 22–26 June 2009, Hong Kong S.A.R., China
Palstra A, Schnabel D, Nieveen M, Spaink H, van den Thillart G (2009c) Temporal expression of hepatic estrogen receptor 1, vitellogenin1 and vitellogenin2 in European silver eels. Gen Comp Endocrinol 166:1–11
Palstra AP, Schnabel D, Nieveen M, Spaink H, van den Thillart G (2010) Swimming suppresses hepatic vitellogenesis in European silver eel as shown by quantitative RT-PCR of the estrogen receptor 1, vitellogenin1 and vitellogenin2 in the liver. Reprod Biol Endocrinol 8:27
Pankhurst NW (1982) Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla L. J Fish Biol 21:127–140
Patterson DA, MacDonald JS, Hinch SG, Healey MC, Farrell AP (2004) The effect of exercise and captivity on energy partitioning, reproductive maturation and fertilization success in adult sockeye salmon. J Fish Biol 64:1039–1059
Pedersen BH (2003) Induced sexual maturation of the European eel Anguilla anguilla and fertilisation of the eggs. Aquaculture 224:323–338
Pedersen BH (2004) Fertilisation of eggs, rate of embryonic development and hatching following induced maturation of the European eel Anguilla anguilla. Aquaculture 237:461–473
Pérez L, Peñeranda DS, Gallego V, Dufour S, Palstra AP, van den Thillart GEEJM, Jover M, Asturiano JF (2008) Temperature effect during the hormonal induced maturation in European eel females. World Aquaculture Society, May 19–23, Busan
Robins CR, Cohen DM, Robins CH (1979) The eels Anguilla and Histiobranchus, photographed on the floor of the deep Atlantic in the Bahamas. Bull Mar Sci 29:401–405
Rohr DH, Lokman PM, Davie PS, Young G (2001) 11-Ketotestosterone induces silvering-related changes in immature female short-finned eels, Anguilla australis. Comp Biochem Physiol A 130:701–714
Roussea K, Aroua S, Schmitz M, Elie P, Dufour S (2009) Silvering: metamorphosis or puberty? In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 39–64
Salem M, Kenney PB, Rexroad CE, Yao J (2006a) Micro-array gene expression analysis in atrophying rainbow trout muscle: a unique nonmammalian muscle degradation model. Physiol Genomics 28:33–45
Salem M, Kenney PB, Rexroad CE, Yao J (2006b) Molecular characterization of muscle atrophy and proteolysis associated with spawning in rainbow trout. Comp Biochem Physiol D 1:227–237
Sasaki S, Ota T, Takagi T (1989) Compositions of fatty acids in the lipids of chum salmon during spawning migration. Nippon Suisan Gakk 55:2191–2197
Sbaihi M (2001) The interaction of sex steroids and cortisol on the control of reproduction and calcium metabolism in a migratory teleost, the eel (Anguilla anguilla). Dissertation, University of Paris
Sbaihi M (2002) Interaction des steroïdes sexuels et du cortisol dans le contrôle de la fonction de reproduction et du métabolisme calcique chez un Téléostéen migrateur, l’anguille (Anguilla anguilla L.). Cybium 26:64
Sbaihi M, Fouchereau-Peron M, Meunier F, Elie P, Mayer I, Burzawa-Gerard E, Vidal B, Dufour S (2001) Reproductive biology of the conger eel from the south coast of Brittany, France and comparison with the European eel. J Fish Biol 59:302–319
Sbaihi M, Kacem A, Aroua S, Baloche S, Rousseau K, Lopez E, Meunier F, Dufour S (2007) Thyroid hormone-induced demineralisation of the vertebral skeleton of the eel, Anguilla anguilla. Gen Comp Endocrinol 151:98–107
Scaion D, Sébert P (2008) Glycolytic fluxes in European silver eel, Anguilla anguilla: sex differences and temperature sensitivity. Comp Biochem Physiol A 151:687–690
Schmidt J (1923) Breeding places and migration of the eel. Nature 111:51–54
Schmidt-Nielsen K (1972) Locomotion: energy cost of swimming, flying and running. Science 177:222–228
Schmitz M, Aroua S, Vidal B, Le Belle N, Elie P, Dufour S (2005) Differential regulation of luteinizing hormone and follicle stimulating hormone expression during ovarian development and under sexual steroid feedback in the European eel. Neuroendocrinology 81:107–119
Sébert M-E, Amerand A, Vettier A, Weltzien FA, Pasqualini C, Sébert P, Dufour S (2007) Effects of high hydrostatic pressure on the pituitary-gonad axis in the European eel, Anguilla anguilla (L.). Gen Comp Endocrinol 153:289–298
Sébert M-E, Legros C, Weltzien F-A, Malpaux B, Chemineau P, Dufour S (2008) Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. J Neuroendocrinol 20:917–929
Sébert P, Scaion D, Belhomme M (2009) High hydrostatic pressure improves the swimming efficiency of European migrating silver eel. Resp Physiol Neurobiol 165:112–114
Smith LS, Newcomb TW (1970) A modified version of the Blazka respirometer and exercise chamber for large fish. J Fish Res Board Can 27:1321–1324
Sorbera LA, Asturiano JF, Carrillo M, Zanuy S (2001) Effects of polyunsaturated fatty acids and prostaglandins on oocyte maturation in a marine teleost, the European Sea Bass (Dicentrarchus labrax). Biol Reprod 64:382–389
Svedäng H, Wickström H (1997) Low fat contents in female silver eels: indications of insufficient energetic stores for migration and gonadal development. J Fish Biol 50:475–486
Székely C, Palstra A, Molnár K, van den Thillart G (2009) Impact of the swim-bladder parasite on the health and performance of European eels. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 201–228
Tagliavini J, Harrison IJ, Gandolfi G (1995) Discrimination between Anguilla anguilla and Anguilla rostrata by polymerase chain reaction-restriction fragment length polymorphism analysis. J Fish Biol 47:741–743
Teitsma CA, Anglade I, Lethimonier C, Le Dréan G, Saliguat D, Ducouret B, Kah O (1999) Glucocorticoid receptor immunoreactivity in neurons and pituitary cells implicated in reproductive functions in rainbow trout: a double immunohistochemical study. Biol Reprod 60:642–650
Tena-Sempere M (2005) Exploring the role of ghrelin as novel regulator of gonadal function. Growth Horm IGF Res 15:83–88
Tesch FW (1974) Speed and direction of silver and yellow eels, Anguilla anguilla, released and tracked in the open North Sea. Ber Dtsch Wiss Komm Meeresforsch 23:181–197
Tesch FW (2003) The eel. Blackwell Science Ltd, Oxford
Tesch FW, Rohlf N (2003) Migration from continental waters to the spawning grounds. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, New York, pp 223–236
Todd PR (1981) Morphometric changes, gonad histology, and fecundity estimates in migrating New Zealand fresh-water eels (Anguilla spp.). N Z J Mar Freshw Res 15:155–170
Tsukamoto K (1992) Discovery of the spawning area for Japanese eel. Nature 356:789–791
Tsukamoto K (2006) Spawning of eels near a seamount. Nature 439:929
Tsukamoto K (2009) Oceanic migration and spawning of anguillid eels. J Fish Biol 74:1833–1852
Tucker DW (1959) A new solution to the Atlantic eel problem. Nature 183:495–501
Usui A (1991) Eel culture. Blackwell Scientific Publications Ltd, London
van Aerle R, Kille P, Lange A, Tyler CR (2008) Evidence for the existence of a functional Kiss1/Kiss1 receptor pathway in fish. Peptides 29:57–64
van den Thillart G, van Waarde A (1996) Nuclear magnetic resonance spectroscopy of living systems: applications in comparative physiology. Physiol Rev 76:799–837
van den Thillart G, van Ginneken V, Körner F, Heijmans R, van der Linden R, Gluvers A (2004) Endurance swimming of European eel. J Fish Biol 65:1–7
van Dijk PL, Van den Thillart G, Balm P, Wendelaar Bonga S (1993) The influence of gradual water acidification on the acid/base status and plasma hormone levels in the carp. J Fish Biol 42:661–671
van Ginneken V, van den Thillart G (2000) Eel fat stores are enough to reach the Sargasso. Nature 403:156–157
van Ginneken VJT, Maes GE (2005) The European eel (Anguilla anguilla Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev Fish Biol Fish 15:367–398
van Ginneken V, Vianen G, Muusze B, Palstra A, Verschoor L, Lugten O, Onderwater M, van Schie S, Niemantsverdriet P, van Heeswijk R, Eding E, van den Thillart G (2005a) Gonad development and spawning behaviour of artificially-matured European eel (Anguilla anguilla L.). Anim Biol 55:203–218
van Ginneken V, Antonissen E, Muller UK, Booms R, Eding E, Verreth J, van den Thillart G (2005b) Eel migration to the sargasso: remarkably high swimming efficiency and low energy costs. J Exp Biol 208:1329–1335
van Ginneken V, Dufour S, Sbaihi M, Balm P, Noorlander K, de Bakker M, Doornbos J, Palstra A, Antonissen E, Mayer I, van den Thillart G (2007a) Does a 5, 500-km swim trial stimulate early sexual maturation in the European eel (Anguilla anguilla L.)? Comp Biochem Physiol A 147:1095–1103
van Ginneken V, Durif C, Balm SP, Boot R, Verstegen MWA, Antonissen E, van den Thillart G (2007b) Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Anim Biol 57:63–77
van Ginneken V, Bruijs M, Murk T, Palstra A, van den Thillart G (2009a) The effect of PCBs on the spawning migration of European silver eel (Anguilla anguilla L.). In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 201–228
van Ginneken V, Palstra A, Leonards P, Nieveen M, van den Berg H, Flik G, Spannings T, Niemantsverdriet P, van den Thillart G, Murk T (2009b) PCBs and the energy costs of migration in European silver eel (Anguilla anguilla). Aquat Toxicol 92:213–220
Versonnen BJ, Goemans G, Belpaire C, Janssen CR (2004) Vitellogenin content in European eel (Anguilla anguilla) in Flanders, Belgium. Environ Pollut 128:363–371
Vidal B, Pasqualini C, Le Belle N, Claire M, Holland H, Sbaihi M, Vernier P, Zohar Y, Dufour S (2004) Dopamine inhibits luteinizing hormone synthesis and release in the juvenile European eel: a neuroendocrine lock for the onset of puberty. Biol Reprod 71:1491–1500
Videler JJ (1993) Fish swimming. Chapman & Hall, London
Webb PW (1971) The swimming energetics of trout. II. Oxygen consumption and swimming efficiency. J Exp Biol 55:521–540
Webb PW (1975) Hydrodynamics and energetics of fish propulsion. J Fish Res Board Can 190:77–105
Weltzien F-A, Sébert M-E, Vidal B, Pasqualini C, Dufour S (2009) Dopamine inhibition of eel reproduction. In: van den Thillart G, Dufour S, Rankin C (eds) Spawning migration of the European eel. Springer, Netherlands, pp 201–228
Williams GC, Koehn K (1984) Population genetics of North Atlantic catadromous eels (Anguilla). In: Turner BJ (ed) Evolutionary genetics of fishes. Plenum Press, New York, pp 529–560
Wootton RJ (1990) The ecology of teleost fishes. Chapman and Hall, London
Yaran Z, Cocos M, Salzer H (1980) Effects of temperature and photoperiod on ovarian recrudescence in the cyprinid fish Mirogrex terrae-sanctae. J Fish Biol 16:371–382
Acknowledgments
The authors would like to thank V.J.T. van Ginneken, J.V. Planas, H.P. Spaink, D. Schnabel, C. Székely, C. Durif, F. Daverat, J. Klein-Breteler, G. van der Laak, M. Nieveen, M. de Bakker, P. Niemantsverdriet, M. Fekkes, W. Spoor, E. Antonissen, M. Casteleijn, R. van der Linden, R. Heijmans, E. de Kuyper, J. Bij, M. Brittijn, S. van Schie, L. Wagenaar, D. Curiel, E. Clavero, J. van Rijssel, M.A. Guerrero, G. Romano, A. Dumadag, D. de Jong and W. Enright. The research was funded by the Dutch Technology Foundation (STW-project #LBI66.4199), the European Union (EELREP #Q5RS-2001-01836) and the Dutch Ministry of Agriculture, Nature and Food Quality (projects #320187, #TRCviss/2006/6204). AP is currently a Marie Curie Fellow (project #219971: Swimming for reproduction).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palstra, A.P., van den Thillart, G.E.E.J.M. Swimming physiology of European silver eels (Anguilla anguilla L.): energetic costs and effects on sexual maturation and reproduction. Fish Physiol Biochem 36, 297–322 (2010). https://doi.org/10.1007/s10695-010-9397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9397-4