Abstract
Regulation of arterial partial pressure of O2 (PaO2) in Atlantic salmon (Salmo salar) was investigated during resting conditions in normoxic and hyperoxic water. Dorsal aorta cannulated adult Atlantic salmon (1.2–1.6 kg, n = 8) were exposed to 2 week sequential periods of normoxia [16.7 ± 1.1 kPa (mean ± SD)] and hyperoxia (34.1 ± 4.9 kPa) in individual tanks containing seawater (33.7 ± 0.2 ppt) at stable temperature conditions (8.7 ± 0.7°C) and a light regime of L:D = 12:12. Tank design and sampling procedures were optimized to provide suitable shelter and current for the fish, and to allow repeated, undisturbed sampling of blood from free-swimming fish. Fish were sampled regularly through the experimental period. PwO2, PaO2, blood ion composition (Na+, K+, Cl−), acid–base status (pH, PCO2, HCO3 −), haematocrit and glucose were measured. The most frequently observed PaO2 values were in the range of 60–80% of PwO2, both during normoxia and hyperoxia, and PaO2 values were significantly lower during normoxia than during hyperoxia. Blood pH, PCO2 and HCO3 − were significantly elevated during hyperoxia, while, Na+, Cl− and Hct were significantly lower. K+ and glucose showed no significant differences. This study demonstrates a lack PaO2 regulation in Atlantic salmon to low partial pressures, in contrast to previous reports for many aquatic gill breathing animals. Both during normoxia and hyperoxia, PaO2 reflects PwO2, and alterations in external PO2 consequently result in proportional arterial PO2 changes. Physiological adaptation to hyperoxia, as illustrated by changes in several blood parameters, does not include down-regulation of PaO2 in Atlantic salmon. The lack of PaO2 regulation may make Atlantic salmon vulnerable to the oxidative stress caused by increased free radical formation in hyperoxic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eukaryotes evolved during a period where atmospheric PO2 was much lower than present levels, and many animals utilize respiratory, morphological and behavioural strategies to reduce PO2 tension towards and inside cells to the approximate level of the atmosphere of the time of origin of eukaryotic cells (Massabuau 2003). Active down-regulation of arterial oxygen partial pressure (PaO2) during resting conditions through respiratory adaptations to a set-point of about 1–3 kPa has been documented in a number of aquatic phyla (see review by Massabuau 2003), including fish (Silurus glanis: Forgue et al. 1989; Cyprinis carpio: Takeda 1990; Soncini and Glass 2000). This has been termed the low PaO2 strategy (Massabuau 2001). The PaO2 regulation seems independent of ambient PO2 up to 40 kPa (Massabuau 2001). Similar strategies have been described in terrestrial insects (Hetz and Bradley 2005), spiders (Angersbach 1978) and air-breathing crabs (Farrelly and Greenaway 1994). Salmonids generally inhabit cool, well-oxygenated waters throughout their lifecycle and display an actively swimming lifestyle with a high capacity for oxygen uptake and high metabolic rates. Previous reports on in rainbow trout (Oncorhynchus mykiss) show PaO2 levels consistently at about 80% of PwO2 both during normoxia (Powell and Perry 1997; Aota and Randall 2004; Leef et al. 2007) and hyperoxia (Wood and Jackson 1980). Rainbow trout has also been shown to maintain a higher PO2 in red muscle compared to mammals during active swimming, indicating specialized adaptations to ensure adequate oxygen supply (McKenzie et al. 2004). However, Thomas et al. (1988) documented chronic presence of brown trout (Salmo trutta) in low oxygen (7 kPa) waters, indicating physiological adaptation to arterial PO2 < 7 kPa. While high PaO2 values are consistently reported for salmonids, these results are obtained from relatively short-term experiments not directly targeted at investigating the possible presence of a low PaO2 strategy during resting, unstressed, conditions.
In the aquatic environment, hyperoxia may occur periodically as a result of algal photosyntehsis, being most pronounced in enclosed areas with little circulation (e.g., Dejours 1981). In commercial land-based salmonid aquaculture, pure oxygen is added to the water to increase the production potential of limited water supplies. Thus, chronic or periodic exposure to hyperoxia may occur. Acute toxicity of hyperoxia in Atlantic salmon has been documented. Lygren et al. (2000) found acute mortality after 1 week exposure to 59 kPa (280% saturation) at 5–8°C and 1–2‰ salinity, while Brauner et al. (2000) documented mortalities of Atlantic salmon smolts during 96 h exposure to 82 kPa (390% saturation) at 10°C in freshwater. The observed mortalities were highest in combination with hypercapnia, and more pronounced in the following seawater challenge tests than during exposure. Impaired osmoregulatory capacity after seawater transfer has been reported in coho salmon (Onchorhyncus kisutch) smolts exposed to approximately 37 kPa for 6 h to 1 week (Brauner 1999). A number of studies report small effects of hyperoxia on growth, and while most studies report no effect (Edsall and Smith 1990; Caldwell and Hinshaw 1994; Lygren et al. 2000), Dabrowski et al. (2004) reported a significant growth increase in juvenile rainbow trout exposed to 38 kPa (180% saturation) at 18°C for 18 weeks, and Hosfeld et al. (2008) reported increased growth in Atlantic salmon parr exposed to 25.8 kPa (123% saturation) for 42 days at 9.5–9.5°C in freshwater. Bæverfjord et al. (in preparation) report reduced growth in parr/smolts of Atlantic salmon exposed to hyperoxia (27 kPa, 130%) compared to mild hyperoxia (22 kPa, 105%) and normoxia (17 kPa, 80%) at 7–10°C. Reduced ventilation rate is commonly observed during hyperoxia, resulting in increased blood PCO2 (e.g., Hosfeld et al. 2008). The respiratory acidosis is compensated by elevation of HCO3 − (Wood and Jackson 1980), and HCO3 −/Cl− exchange in the gill (reviewed by Evans et al. 2005). Morphological adaptation to hyperoxia in the respiratory tissues includes increased diffusion distance across respiratory surfaces in seabass (Dicentrarchus labrax; Saroglia et al. 2000).
Effects of environmental oxygen levels on oxidative damage and responses in fish is reviewed by Lushchak and Bagnyukova (2006). Lygren et al. (2000) observed reduced levels of α-tocopherol and ascorbate concentrations in Atlantic salmon reared under hyperoxic conditions (140–150% saturation for 12 weeks at 1–2‰ salinity), and oxidative damage occurred measured as an increased levels of thiobarbituric-reactive substances (TBARS) in liver, while Olsvik et al. (2005) found no oxidative damage or increased mRNA expression of antioxidant enzymes in liver of parr/smolts exposed to 130% saturation at 7–9°C in freshwater for 18 weeks. In the same experiment, Olsvik et al. (2005) also report changes in status of the glutatione antioxidant system in blood in response to hyperoxia, with decreased oxidized glutathione (GSSG) concentrations, stable total glutathione (tGSH) and a resulting lower oxidative index (OSI = 100 × 2 GSSG/tGSH). Ritola et al. (2002) report increased catalase (CAT) activity in liver and gills, as well as increased selenium-dependant glutathione peroxidase (Se-GPX) in liver in a 48 h time course after short term hyperoxia exposure (47 mg/l for 4 h, freshwater, 15°C). Increased GSH levels in blood cells, and decreased GSSG levels in gills and liver were found in the same experiment, indicating increased detoxification of free radicals. Short-term exposure (5 h) of rainbow trout to 16.9 and 21.1 mg/l O2 at 10°C in freshwater showed a significant increase in DNA double strand breaks (dssb) in gills, comparable at the highest oxygen level to effects of the potent carcinogen N-methyl-N′-nitro-N-nitrosoguanidide (MNNG, 200 μM; Liepelt et al. 1995).
The respiratory strategy used by Atlantic salmon both during normoxia and hyperoxia will probably impact both the sensitivity to hyperoxia, the observed effects on growth and the responses of biochemical antioxidant systems. The aim of the experiment was to determine whether Atlantic salmon employ regulatory strategies to reduce PaO2, and if so to what extent, during unstressed conditions.
Materials and methods
Experimental approach
To obtain reliable data on resting PaO2 levels in fish, minimization of stress due to experimental conditions and sampling is essential. An approach using dorsal aorta (DA)-cannulated fish in an experimental setup allowing unrestrained movement of the fish, as well as a sampling procedure allowing sampling without the fish detecting the presence of the sampler, was applied. Most reported studies using DA-cannulated salmonids are relatively short-term studies focussing on various aspects of physiological regulation (e.g., Wood and Jackson 1980; Gilmour and Perry 1994; McKenzie et al. 2004) or uptake and metabolism of various foodstuffs (e.g., Hamre et al. 2001; Sunde et al. 2003). Recently, improvements in surgical procedures (Kiessling et al. 2003; Olsen et al. 2005) combined with novel experimental tank design (Djordjevic et al. 2009) have been documented to give stable haematological values and background cortisol levels in Atlantic salmon during long-term experiments.
Experimental facilities and fish
The experiment was carried out at the Norwegian Institute for Water Research (NIVA) research station at Solbergstrand (Akershus County, Norway). Atlantic salmon (Aquagen strain, n = 30) were kept in a round 4-m3 holding tank supplied with seawater from 60 m depth prior to the experiment. Temperature and salinity for a 2-month period prior to experiment 9.2 ± 0.8°C and 33.7 ± 0.4 g l−1, respectively, and Oxygen concentration was kept >80% saturation. A 12 h light/12 h dark regime was used both before and during the experiments. Fish were fed 5 days a week 1% body mass ration of standard commercial diet, 9-mm pellets (Optiline V 2000–50 A 9.0-mm pellets, Skretting, Norway). Three days prior to cannulation, fish (n = 8), ranging in body weight from 1.2 to 1.6 kg, were moved from the holding tank to 8 individual experimental tanks. The fish were not fed during the experimental period. Salinity (33.7 ± 0.2 g l−1) and temperature (8.7 ± 0.7°C) were kept stable during the experimental period. Experimental tanks (1 × 1 × 0.3 m with water, Fig. 1) were equipped with an individual light source and a shelter consisting of a 0.3 × 0.5 m shelf attached to the tank wall 0.05 m above the water surface. Water flow rate was adjusted to 2 l min−1; each tank was equipped with an aquarium pump (375 l h−1, 50 Hz) to create an adjustable directed current independent of incoming water flow. The resulting water current was approximately 10 cm s−1 along the perimeter of the tank. Tanks were physically separated from each other, and vibration isolated by consecutive layers of Styrofoam and gravel beneath the tank feet to avoid any transmission of vibrations from the external environment. All other possible disturbances in the experimental facilities were minimized. Both during normoxic and hyperoxic exposure, water was passed through a low pressure oxygenation unit consisting of a cylindrical pvc tube (height: 1.62 m, diameter 0.26 m, Fig. 1).
Cannulation procedure
All fish were cannulated during the same day. Dorsal aorta cannulation was performed following the procedure of Soivio et al. (1975) as modified and described by Kiessling et al. (1995; 2003) using 0.5 mg kg−1 Metomidate (metomidate hydrochloride; Syndell Ltd, Victoria, B.C. Canada) as pre anaesthetic sedation (Kreiberg and Powell 1991) for 15 min in the experimental tanks followed by 60 mg l−1 Metacanium (MS 222, tricaine methane sulphonate, Norwegian Medical Depot, Bergen, Norway) anaesthetics in a separate bath (40 l). This bath was equipped with an aquarium pump connected to a silicone hose that was kept in the mouth of the fish in order to ventilate the gills during cessation of respiratory movements. Cessation of the coughing reflex was used as a criterion for surgical anaesthesia. The fish was then transferred to a V-shaped surgical table protected with a soft wet cloth. During surgery, the fish were also covered by wet cloth, and aerated seawater with 40 mg l−1 MS 222 was constantly pumped over the gills as recommended by Kiessling et al. (2009). The surgical procedure was performed within 2 min for each fish, and all fish regained normal swimming behaviour within 10 min after the procedure.
Sampling procedure and analysis
Fish were sampled at 1–2 day intervals during the 45 day experimental period. Sampling was performed in two steps to minimize the disturbance for the fish. The free floating cannulae (0.6–0.7 m) was obtained using a hooked steel wire, and about 0.2 m was put through the hole in the tank wall (4 mm) situated 0.04 m above the water surface just behind the shelter. Blood samples were obtained at least 10 min later by cutting the end of the cannulae, thus emptying the cannulae of saline and allowing blood to flow through by gravity. Sampling was aborted, or samples discarded, at any indication of the fish detecting being sampled during the procedure. The first drop of blood was discarded, and samples for PaO2 determination was obtained directly from the cannula into a 50-ml micropipette (Assistent, Glaswarenfabrik Karl Hecht, 97647 Sondheim, Germany). Approximately, 0.1 ml of blood was then obtained directly into a i-STAT EC8+ cartridge (i-STAT Corporation, Windsor Center Drive, NJ 08520, USA). PaO2 and PwO2 was analysed immediately, using a microx TX3 oxygen metre with an micro-optode sensor (PSt1) (PreSens, Precision Sensing, GmbH, Josef-Engert-Str 11, D-93053, Regensburg, Germany). A portable i-STAT clinical analyser was used to determine plasma Na+, K+, Cl−, glucose, pCO2, HCO3 − and pH in whole-blood (Jacobs et al. 1993; Pidetcha et al. 2000; Harrenstien et al. 2005). pH, pCO2 and HCO3 − values were corrected for the temperature difference between ambient water temperature and the temperature-adjusted (37°C) values displayed by the instrument in accordance with the i-STAT procedure (Eliason et al. 2007). Samples for haematocrit were obtained from the cannula in 10-μl capillary tubes and analysed in duplicate on a portable microhaematocrit centrifuge (Compur Microspin, Bayer Diagnostics, GmbH, 35463, Fernwald, Germany).
Statistics
Data were analysed by the main factorial model (general linear model, Statistical Analysis System (SAS) for PC (ver. 8.2), ANOVA for unbalanced data). Included in the model as main factor was treatment (normoxia or hyperoxia). Fish was included as a discrete variable. All pairwise comparisons were made by variance (F-test, Proc-GLM procedure) using the least-squares means procedure when significant effects were found in the main model. The level of statistical significance was set to P < 0.05. All data were tested for normality by a normal probability plot (proc univariate).
Results
Arterial oxygen levels
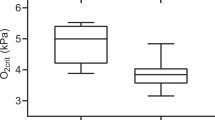
The results demonstrate a strong relationship between PwO2 and PaO2 both during normoxia and hyperoxia (Fig. 2a, b), where PaO2 = 12.1 ± 1.7 and PwO2 = 16.8 ± 1.1 during normoxia. During hyperoxia (PwO2: 35.4 ± 3.0), a significantly higher PaO2 (24.5 ± 4.2) was observed (Fig. 2a, b). PwO2 − PaO2 tension difference was significantly larger during hyperoxia (Fig. 2c), and the most frequently observed PwO2 − PaO2 differences were in the range of 4–5.5 and 8–13 kPa during normoxia and hyperoxia, respectively. While the tension difference was higher, the percentage difference between PwO2 and PaO2 was not significantly different between normoxia and hyperoxia periods (Fig. 2d), possibly demonstrating reduced oxygen diffusion across the gills during hyperoxia. No PaO2 values in the range of 1–3 kPa were observed.
Median, 25–75 percentile (box) and 5–95 percentile (bars) values of normoxia and hyperoxia periods (N = 108 and 54, respectively). a Arterial O2 tension (PaO2), b water O2 tension (PwO2), c water-arterial tension difference (PwO2 − PaO2) and d water-arterial tension difference in %. Values denoted with * (P < 0. 05), ** (P < 0.01) and *** (P < 0.001) differ significantly from normoxia
Haematological parameters
Na+ and Cl− levels were significantly lower during hyperoxia, while K+ did not change significantly (Fig. 3a–c). The changes in Na+ and Cl−, were relatively small, with mean values varying only by 2–3 mmol/l between periods of normoxia and hyperoxia. Glucose was not significantly different (normoxia: 3.5 ± 0.9, hyperoxia: 3.2 ± 0.5 mmol/l), but a higher variation, both with lower and higher observations was observed during normoxia (Fig. 3d). pH, PCO2 and HCO3 − were all significantly increased during hyperoxia compared to normoxic levels (Fig. 3e–g, respectively). The pH increase was small (7.87 ± 0.04 to 7.89 ± 0.05 during normoxia and hyperoxia, respectively), while PCO2 mean values increased by about 40% during hyperoxia (normoxia: 0.44 ± 0.07; hyperoxia: 0.72 ± 0.14 kPa), and HCO3 − also showed a large increase (normoxia: 6.1 ± 1.2; hyperoxia: 10.4 ± 2.4 mmol/l). Haematocrit (Fig. 3h) was significantly lower during hyperoxia (normoxia: 18.0 ± 3.1; hyperoxia: 17.3 ± 3.3%), but as for pH and ion levels, the changes were relatively small.
Median, 25–75 percentile (box) and 5–95 percentile (bars) values of blood parameters during normoxia and hyperoxia periods. a Sodium (Na+), b potassium (K+), c Chloride (Cl−), d glucose (Glu), e pH, f carbon dioxide tension (PCO2), g Bicarbonate (HCO3 −) and h hematocrit. For a through g, n = 55–57 and 34–37 for the normoxia and hyperoxia periods, respectively, while for h, n = 79 and 50, respectively. Values denoted with * (P < 0. 05) and ** (P < 0.01) differ significantly from normoxia
Discussion
The PO2 measurements did not demonstrate low PaO2 regulation in Atlantic salmon and do not support the hypothesis of low PaO2 regulation in this species. The results support previous, more short-term, experiments on salmonids (Wood and Jackson 1980; Gilmour and Perry 1994) and contrasts reported work on other fish species (Silurus glanis: Forgue et al. 1989; Cyprinis carpio: Takeda 1990). If not consistent low values, at least large variations in PaO2, as reported for lemon shark (Negaprion brevirostris, Bushnell et al. 1982), should be expected if PaO2 regulation was present. The complete lack of PaO2 values in the predicted low range of 1-3 kPa despite a large number of samples (N = 108 and 64 during normoxia and hyperoxia, respectively) indicate absence of low PaO2 regulation.
Experimental conditions can never be completely excluded as a factor causing stress and abandonment of naturally occurring regulation. However, the experimental conditions and sampling was optimized to avoid stressful conditions and sampling disturbance, and extensive evaluation of the suitability of the experimental setup has been conducted (Djordjevic et al. 2009). Similar experimental setups have demonstrated the presence of low PaO2 regulation in other fish species (Forgue et al. 1989). The aquaculture-strain background of the fish in this experiment may have influenced the result, as 7–8 generations of selective breeding with increased growth rate as the main selection criteria may have resulted in changes in respiratory patterns. This issue warrants future investigation.
The reported data suggest that Atlantic salmon continuously experience large internal PO2 gradients, and thus a high diffusion gradient between blood and cells. Lack of a low PaO2 regulatory strategy in Atlantic salmon may render Atlantic salmon more susceptible to the effects of hyperoxia. On the other hand, Atlantic salmon antioxidant defences, adapted to PaO2 > 10 kPa during normoxia, may provide sufficient antioxidant capacity also during hyperoxia. Long-term hyperoxia experiments on Atlantic salmon (Olsvik et al. 2005) and Atlantic cod (Gadus morhua) (Olsvik et al. 2006) using comparable methodology and hyperoxia levels indicate some differences in responses between species, with changes in the levels and red-ox state of the glutathione system (in blood) as the prominent adaptation to hyperoxia in Atlantic salmon. No significant changes in m-RNA expression of these enzymes appeared in liver of Atlantic salmon, while increased expression of antioxidant enzymes [Glutathine peroxidase (GSH-Px) and Superoxide dismutase (SOD)] were observed in liver of Atlantic cod. High Atlantic salmon tissue-levels of carotenoids (e.g., Bjerkeng and Berge 2000), in particular astaxanthin, with antioxidant properties may also provide protection against high PO2 tissue levels. Mortalities of Atlantic cod occurred at lower hyperoxia levels (Toften et al., in preparation) than those reported as acutely toxic to Atlantic salmon (Brauner et al. 2000; Lygren et al. 2000). Despite the lack of directly comparative experiments, it may seem as if Atlantic salmon is not among the most sensitive species in terms of acute toxicity.
Alterations in haematological parameters were observed during hyperoxia (Fig. 3), demonstrated compensation for a metabolic acidosis caused by reduced ventilation (e.g., Perry and Gilmour 2006). The decrease in plasma Cl− coincides with significantly increased HCO3 −, indicative of regulation of blood buffering capacity during hyperoxia. (e.g., Wheatly et al. 1984) The HCO3 − value during normoxia is somewhat higher than reported in the literature (Perry and Gilmour 2006), but most of these reported studies are based on short duration experiments and/or acute sampling. Slightly elevated pH and PCO2 along with the increased HCO3 − are indicative of compensation to hyperoxic conditions (Wood and Jackson 1980). The significantly lower haematocrit may indicate regulatory responses in red blood cell production, possibly through reduced erythropoietin levels (Fandrey et al. 1994).
In conclusion, the reported experiment did not demonstrate the presence of a low PaO2 strategy in Atlantic salmon. The increased PwO2 − PaO2 gradient during hyperoxia, and alterations in ion and acid–base parameters, indicates respiratory adjustments consistent with the literature on salmonids. The presence of low PaO2 regulation cannot be ruled out on the basis of a single experiment. However, in the production schemes of modern aquaculture, including intensive feeding, high biomasses, extensive use of artificial lighting and a number of potential stressors occurring as a part of normal production procedures, the feasibility of such a strategy may be limited. It may also be the case that salmonid fish, with high activity rates and inhabiting well oxygenated environments, apply oxygen regulatory strategies more similar to that of mammals and birds (Massabuau 2003).
References
Angersbach D (1978) Oxygen transport in the blood of the tarantula Eurypelma californicum: PO2 and pH during rest, activity and recovery. J Comp Physiol B 123:113–125
Aota S, Randall DJ (2004) The effect of exogenous catecholamines on the ventilatory and cardiac responses of normoxic and hyperoxic rainbow trout, Oncorhynchus mykiss. J Comp Physiol Biochem B 163:138–146
Bjerkeng B, Berge GM (2000) Apparent digestibility coefficients and accumulation of astaxanthin E/Zisomers in Atlantic salmon (Salmo salar L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Physiol B 27(3):423–432
Brauner CJ (1999) The effect of diet and short duration hyperoxia exposure on seawater transfer in coho salmon smolts (Oncorhynchus kisutch). Aquaculture 177:257–265
Brauner CJ, Seidelin M, Madsen SS, Jensen FB (2000) Effects of fresh water hyperoxia and hypercapnia exposures and their influences on subsequent seawater transfer in Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 57:2054–2064
Bushnell PG, Lutz PL, Steffensen JF, Oikarj A, Gruber SH (1982) Increases in arterial blood oxygen during exercise in the lemon shark (Negaprion brevirostris). J Comp Physiol 147:41–47
Caldwell CA, Hinshaw J (1994) Physiological and hematological responses in rainbow-trout subjected to supplemental dissolved-oxygen in fish culture. Aquaculture 126:183–193
Dabrowski K, Lee K-J, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392
Dejours P (1981) Principles of comparative respiratory physiology, 2nd edn. Amstedam, North Holland, 153 pp
Djordjevic B, Kristensen T, Øverli Ø, Rosseland BO, Kiessling A (2009) Effect of nutritional status and sampling intensity on recovery after dorsal aorta cannulation in free-swimming Atlantc salmon (Salmo salar L.). Fish Physiol Biochem. doi:10.1007/s10695-009-9362-2
Edsall DA, Smith CE (1990) Performance of rainbow trout and Snake river cutthroat trout reared in oxygen supersaturated water. Aquaculture 90:251–259
Eliason EJ, Kiessling A, Karlsson A, Djordjevic B, Farrell AP (2007) Validation of the hepatic portal vein cannulation technique using Atlantic salmon Salmo salar L. J Fish Biol 71:290–297
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fandrey J, Frede S, Jelkmann W (1994) Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem J 303:507–510
Farrelly C, Greenaway P (1994) Gas exchange through the lungs and gills in air-breathing crabs. J Exp Biol 187:113–130
Forgue J, Burtin B, Massabuau J-C (1989) Maintenance of oxygen consumption in resting Silurus glanis at different levels of ambient oxygenation. J Exp Biol 143:305–319
Gilmour KM, Perry SF (1994) The effects of hypoxia, hyperoxia or hypercapnia on the acid-base disequilibrium in the arterial blood of rainbow trout. J Exp Biol 192:269–284
Hamre K, Kolås K, Sandnes K, Julshamn K, Kiessling A (2001) Feed intake and absorption of lipid oxidation products in Atlantic salmon (Salmo salar) fed diets coated with oxidised fish oil. Fish Physiol Biochem 25:209–219
Harrenstien LA, Tornquist SJ, Miller-Morgan TJ, Fodness BG, Clifford KE (2005) Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. J Am Vet Med Assoc 226:255–265
Hetz SK, Bradley TJ (2005) Insects breathe discontinuously to avoid oxygen toxicity. Nature 433:516–519
Hosfeld CD, Engevik A, Mollan T, Lunde TM, Waagbø R, Olsen AB, Breck O, Stefansson S, Fivelstad S (2008) Long-term separate and combined effects of environmental hypercapnia and hyperoxia in Atlantic salmon (Salmo salar L.) smolts. Aquaculture 280:146–153
Jacobs E, Vadasdi E, Sarkozi L, Coman N (1993) Analytical evaluation of i-STAT portable clinical analyzer and use by nonlaboratory health-care professionals. Clin Chem 39:1069–1074
Kiessling A, Dosanjh B, Higgs D, Deacon G, Rowshandeli N (1995) Dorsal aorta cannulation; a method to monitor changes in blood levels of astaxanthin in voluntarily feeding Atlantic salmon. Aquac Nutr 1:43–50
Kiessling A, Olsen RE, Buttle L (2003) Given the same dietary inclusion Atlantic salmon, Salmo salar (L.) display higher blood levels of canthaxanthin than astaxanthin. Aquac Nutr 9:253–262
Kiessling A, Johansson D, Zahl IH, Samuelsen OB (2009) Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture 286:301–308
Kreiberg H, Powell J (1991) Metomidate sedation reduces holding stress in chinook salmon. World Aquac 22:58–59
Leef MJ, Harris JO, Powell MD (2007) The respiratory effects of chloramine-T exposure in seawater acclimated and amoebic gill disease-affected Atlantic salmon Salmo salar L. Aquaculture 266:77–86
Liepelt A, Karbe L, Westendo J (1995) Induction of DNA strand breaks in rainbow trout, Oncorhynchus mykiss, under hypoxic and hyperoxic conditions. Aquat Toxicol 33:177–181
Lushchak VI, Bagnyukova TV (2006) Effects of different environmental oxygen levels on free radical processes in fish. Comp Biochem Physiol B 144(3):283–289
Lygren B, Hamre K, Waagbø R (2000) Effect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquac Res 31:401–407
Massabuau J-C (2001) From a low arterial to low tissue oxygenation strategy, an evolutionary strategy. Respir Physiol 128:249–261
Massabuau J-C (2003) Primitive, and protective, our cellular oxygenation status? Mech Ageing Dev 124:857–863
McKenzie DJ, Wong S, Randall DJ, Egginton S, Taylor EW, Farrell AP (2004) The effects of sustained exercise and hypoxia upon oxygen tensions in the red muscle of rainbow trout. J Exp Biol 207:3629–3637
Olsen RE, Kiessling A, Milley JE, Ross NW, Lall SP (2005) Lipid source, not bile acids, affects absorption of astaxanthin in Atlantic salmon, Salmo salar L. Aquaculture 250:804–812
Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Bæverfjord G, Berntssen MHG (2005) SOD, CAT and GSH-Px mRNA expression and lipid peroxidative stress in liver of Atlantic salmon Salmo salar exposed to hyperoxic conditions during smoltification. Comp Biochem Physiol C 141(3):314–323
Olsvik PA, Kristensen T, Waagbø R, Tollefsen KE, Rosseland BO, Toften H (2006) Effects of hypo- and hyperoxia on transcription levels of five stress genes and the glutathione system in liver of Atlantic cod (Gadus morhua). J Exp Biol 209:2893–2901
Perry SF, Gilmour KM (2006) Acid-base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir Physiol Neurobiol 154:199–215
Pidetcha P, Ornvichian S, Chalachiva S (2000) Accuracy and precision of the i-STAT portable clinical analyzer: an analytical point of view. J Med Assoc Thai 83(4):445–450
Powell MD, Perry SF (1997) Respiratory and acid-base pathophysiology of hydrogen peroxide in rainbow trout (Oncorhynchus mykiss Walbaum). Aquat Toxicol 37:99–112
Ritola O, Tossavainen K, Kiuru T, Lindström-Seppä P, Mölsä H (2002) Effects of continuous and episodic hyperoxia on stress and hepatic glutathione levels in one-summer-old rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 18:159–164
Saroglia M, Cecchini S, Terova G, Caputo A, De Stradis A (2000) Influence of environmental temperature and water oxygen concentration on gas diffusion distance in sea bass (Dicentrarchus labrax, L.). Fish Physiol Biochem 23:55–58
Soivio A, Nyholm K, Westman K (1975) A technique for repeated sampling of the blood of individual resting fish. J Exp Biol 63:207–217
Soncini R, Glass ML (2000) Oxygen and acid-base status related drives to gill ventilation in carp. J Fish Biol 56:528–541
Sunde J, Kiessling A, Higgs D, Opstvedt J, Venturini G, Rungruangsak-Torrissen K (2003) Evaluation of feed protein quality by measuring plasma free amino acids in Atlantic salmon (Salmo salar L.) after dorsal aorta cannulation. Aquac Nutr 9:351–360
Takeda T (1990) Ventilation, cardiac output and blood respiratory parameters in the carp, Cyprinus carpio, during hyperoxia. Respir Physiol 81:227–239
Thomas S, Fievet B, Claireaux G, Motais R (1988) Adaptive respiratory responses of trout to acute hypoxia. I—Effects of water ionic composition on blood acid–base status response and gill morphology. Respir Physiol 74:77–90
Wheatly MG, Hòbe H, Wood CM (1984) The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia, II: the role of the kidney. Respir Physiol 55:155–173
Wood CM, Jackson EB (1980) Blood acid–base regulation during environmental hyperoxia in the rainbow trout (Salmo gairdneri). Respir Physiol 42:351–372
Acknowledgments
The authors would like to thank the staff of NIVA Research station at Solbergstrand for technical assistance. Carlos Salas kindly supplied drawings of the experimental setup. Dr. Carolyn Knight is acknowledged for improving the language of the manuscript. Financial support for the project was provided by the Norwegian Research Council (NRC) grant no. 153202/120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristensen, T., Rosseland, B.O., Kiessling, A. et al. Lack of arterial PO2 downregulation in Atlantic salmon (Salmo salar L.) during long-term normoxia and hyperoxia. Fish Physiol Biochem 36, 1087–1095 (2010). https://doi.org/10.1007/s10695-010-9386-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9386-7