Abstract
An 8-week growth trial was conducted to assess the effect of dietary protein on growth, feed utilization, protein retention efficiency, and body composition of young Heteropneustes fossilis (10.02 ± 0.09 g; 9.93 ± 0.07 cm). Isocaloric (4.15 kcal g−1, GE) diets with varying levels of protein (25, 30, 35, 40, 45, and 50% of the diet) were fed near to satiation to triplicate groups of fish. Optimum dietary protein was determined by analyzing live weight gain (LWG%), feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR%), and protein retention efficiency (PRE%) data. Maximum LWG% (167), best FCR (1.42), PER (1.75), SGR (1.76), and PRE (31.7%) were evident in fish fed 40% protein diet (Diet 4). Body protein data also supported the above level. However, second-degree polynomial regression analysis of the above data indicated that inclusion of dietary protein in the range of 40–43% is optimum for the growth of young H. fossilis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensification of fish culture has led to dependence on artificial feeds. Protein is the most expensive component in fish feeds and also the most important factor affecting growth performance of fish and feed cost (Lovell 1989; Luo et al. 2004). Reducing the feeding costs could be key factor for successful development of aquaculture. Fish have high dietary protein requirement (Deng et al. 2006). The significance of qualitative and quantitative feeds is well recognized (Jauncey 1982; Mohanty and Samantary 1996; Gunasekera et al. 2000; Yang et al. 2002; Giri et al. 2003; Deepak and Garg 2003; Yang et al. 2003; Sales et al. 2003; Kalla et al. 2004; Islam and Tanaka 2004; Luo et al. 2004; Cortes-Jacinto et al. 2005; Kim and Lee 2005; Tibbetts et al. 2005; Sa et al. 2006). Level of dietary protein is of fundamental importance, because it significantly influences growth, survival, and yield of fish as well as economics of a farming industry by determining the feed cost which is typically the largest operational cost. Increase in dietary protein has often been associated with higher growth rate in many species. However, there is a certain level beyond which further growth is not supported, and may even decrease (Mohanty and Samantary 1996; Shiau and Lan 1996; McGoogan and Gatlin 1999; Gunasekera et al. 2000; Kim and Lall 2001; Yang et al. 2002; Abbas et al. 2005; Debnath et al. 2007; Kvale et al. 2007). Considerable research effort has been expended to determine the quantity and quality of dietary protein necessary to achieve optimum performance of fish.
Heteropneustes fossilis is an important tropical freshwater food fish (Mohamed and Ibrahim 2001) and its culture has gained attention because of its ability to efficiently utilize animal and plant origin feedstuffs and withstand adverse environmental conditions, in addition to its medicinal value and market potential for intensive culture (Pillay 1990; Jhingran 1991; Thakur 1991). Apart from these factors, it has exceptional tolerance to high ammonia and low oxygen for several months, inhabits derelict and stagnant, slow-flowing, water bodies, agricultural fields, or swamps and wetlands (Saha and Ratha 1998, 2007) which make it a successful candidate for aquaculture. Information on the basic nutritional requirements and feeding of H. fossilis is needed in view of emphasis on catfish culture in the country (Tripathi and Das 1976; Dehadrai and Thakur 1980). Protein requirements are generally higher for smaller fish. As fish grow larger, their protein requirements usually decrease. Information on some aspects of nutrient requirement of H. fossilis is available (Sing and Srivastava 1984; Akand et al. 1991; Anwar and Jafri 1992; Firdaus et al. 1994, 2002; Firdaus and Jafri 1996; Firdaus 1993; Jhingran 1991; Niamat and Jafri 1984; Mohamed 2001; Mohamed and Ibrahim 2001; Usmani and Jafri 2002; Usmani et al. 2003). Although information on effect of dietary protein level on growth, feed conversion, and body composition of Singhi H. fossilis fry (Akand et al. 1989) is available, no published data is available on this aspect of the young stage of H. fossilis. The present study was therefore conducted to optimize the dietary protein level for growth, protein retention efficiency, and body composition of young H. fossilis. The information will be useful in developing protein balanced diet for the culture of this fish species.
Materials and methods
Preparation of experimental diets
Six casein-gelatin based isocaloric (414.83 kcal/100 gross energy) diets containing graded levels of protein (25, 30, 35, 40, 45, and 50% crude protein) were formulated (Table 1). Diets were prepared taking into account the amount of protein contributed by casein and gelatin and made isocaloric by adjusting the dextrin. Calculated quantities of dry ingredients were thoroughly mixed and stirred in a volume of hot water (80°C) in a steel bowl attached to a Hobart electric mixer (Hobart, Troy, OH, USA). Gelatin powder was dissolved separately in a volume of water with constant heating and stirring and then transferred to the above mixture. Other dry ingredients and oil premix, except carboxymethyl cellulose, were added to the lukewarm bowl one by one with constant mixing at 50°C temperature. Carboxymethylcellulose was added last and the speed of the blender was gradually increased as the diet started to harden. The final diet, with the consistency of bread dough, was poured into a Teflon-coated pan and placed in a refrigerator to gel. The prepared diets were in the form of semi-moist cake (50% dry matter) from which cubes were cut and stored at −4°C in sealed polythene bags until used.
Feeding trial
Induced bred H. fossilis were procured from a local fish hatchery. These were transported to the wet laboratory, given a prophylactic dip in KMnO4 solution (1:3,000), and stocked in circular aluminium plastic lining [Plastic Crafts, Mumbai, India; 4 × 3 × 3 (c. 1.2 × 0.9 × 0.9 m)] fish tanks (water volume 600 l) for 2 weeks. During this period, the fish were fed to satiation a mixture of soybean, mustard oil cake, rice bran, and wheat bran in the form of moist cake twice a day at 0700 and 1700 hours. These were then acclimatized for 1 week on casein-gelatin based (40% CP) H-440 diet (Halver 2002) near to satiation.
Young H. fossilis (10.02 ± 0.09 g; 9.93 ± 0.07 cm) were stocked randomly in triplicate groups in 70-l circular polyvinyl troughs (water volume 55 l) fitted with a continuous flow-through system at the rate of 10 fish per troughs for each dietary treatment levels. The fish were fed experimental diets to apparent satiation divided over two feeding schedule at 0700 and 1700 hours. No feed was offered to the fish on the day they were weighed. The feeding trials lasted for 8 weeks. Initial and weekly body weights were recorded on a top loading balance (Precisa 120A; PAG Oerlikon, Zurich, Switzerland). Troughs were siphoned off to remove fecal matter before feeding daily. Any uneaten feed was siphoned off immediately, dried in a hot air oven and reweighed to measure the amount of feed consumed.
Water quality assessment
Water quality indices were monitored daily during the feeding trial. Water in each trough was sampled and analyzed for the average water temperature, dissolved oxygen, free carbon dioxide, pH, and total alkalinity, and were recorded following standard methods (APHA 1992). The average water temperature, dissolved oxygen, free carbon dioxide, pH, and total alkalinity based on daily measurements were 27.5–28.9°C, 67–7.1, 5.5–10.7, 7.5–7.8, and 65.7–80.5 mg l−1, respectively.
Statistical analyses
All growth data were subjected to analysis of variance (Snedecor and Cochran 1968; Sokal and Rohlf 1981). Differences among treatment means were determined by Duncan’s Multiple Range Test at a P < 0.05 level of significance (Duncan 1955). To predict more accurate responses to the dietary protein intake, the optimum level was estimated using second-degree polynomial regression analysis (Y = aX2 + bX + c) as described by Zeitoun et al. (1976). Statistical analysis was done using Matlab (version 7.1) and SPSS (version 13.0).
Chemical analyses
Proximate composition of casein, gelatin, experimental diets, and initial and final carcass was analyzed using standard methods (AOAC 1995) for dry matter (oven drying at 105 ± 1°C for 22 h), crude protein (N-Kjeldahl X 6.25 using Kjeltec Tecator™; Technology 2300, Foss, Hoeganaes, Sweden), crude fat (solvent extraction with petroleum ether B.P. 40–60°C using Socs Plus; Pelican equipments, Chennai, India, for 2–3 h) and ash (oven incineration at 650°C for 4–6 h using muffle furnace; S.M. Scientific Instrument (p), Jindal Company, India). Gross energy content was determined on a Gallenkamp ballistic bomb calorimeter-CBB 330 010L (Gallenkamp, Loughbrough, UK). Six subsamples of a pooled sample of 10 fish were analyzed for initial and final body composition. At the end of the experiment, five fish from each replicate of dietary treatments were pooled separately and three subsamples of each replicate (n = 3 × 3) analyzed for final body composition.
Results
Growth parameters of young H. fossilis fed diets containing graded levels of protein are presented in Table 2. Live weight gain (LWG%), feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR%), and protein retention efficiency (PRE%) were sensitive to the levels of protein in the diet and improved significantly (P < 0.05) as dietary protein level increased from 25 to 40% whereas at 45% dietary protein growth responses were almost constant or slightly reduced. However, a significant fall in growth and conversion efficiencies was noted at 50% protein of the diet. Maximum LWG (167%), best FCR (1.42), PER (1.75), SGR (1.76%), and PRE (31.7%) were obtained for the fish fed diet with 40% protein. Fish fed lower levels of protein in the diet exhibited significantly less (P < 0.05) growth and reduced feed utilization efficiency. Poorest FCR (3.52), PER (1.14), SGR (1.04) and PRE (14%) were observed for fish fed diet containing 25% protein (Diet 1).
At the end of the experiment, significant differences (P < 0.05) in body composition were noted among the groups (Table 3). Moisture content showed a continuous decrease with the increase in the levels of dietary protein. Maximum body protein was recorded for fish fed diet containing 40% protein (Diet 4). Fish fed diets containing 45% protein diet did not show any significant change (P > 0.05) in growth parameters and body protein content. However, significant falls in growth parameters and body protein were recorded in fish fed diet containing 50% protein (Diet 6). Similar trend was evident for protein retention efficiency (PRE%). However, a significant and continuous increase in body fat of the fish was noted with the increase in protein content of the diet from 25% (Diet 1) to 50% (Diet 6). Significant differences (P > 0.05) in ash content were evident in fish fed 25% (Diet 1) and 30% (Diet 2) protein diets whereas it remained non-significant (P > 0.05) for the groups receiving other diets (Diets 3–6).
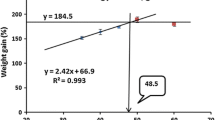
On subjecting the LWG% data to second-degree polynomial regression analysis (Zeitoun et al. 1976), the optimum dietary protein level was found to be at 42.75% protein of the diet. The relationship was described by the following equations:
The FCR of young H. fossilis fed 40 and 45% protein was significantly lower than those of the other dietary protein levels. The FCR (Y) to dietary protein level (X) relationship was estimated by the following second-degree polynomial regression equation:
Based on the above equation, the estimated FCR occurred at approximately 41.5% protein of the diet.
The PER of young H. fossilis fed 40% protein diet differed significantly from the other levels of dietary protein inclusion. The PER (Y) to dietary protein level (X) relationship was estimated by the second-degree polynomial regression equation:
Based on the above equation, the estimated PER occurred at a dietary protein level of approximately 40.0% of the diet.
Also, the SGR of young H. fossilis fed 40 and 45% protein diet were significantly higher than those of the other levels of dietary protein inclusion. The SGR (Y) to dietary protein level (X) relationship was estimated by the following second-degree polynomial regression equation:
Based on the above equation, the estimated SGR occurred at a dietary protein level of approximately 42.3% of the diet.
Similarly, the PRE% of young H. fossilis fed 40% protein diet differed significantly from the other levels of dietary protein inclusion. The PRE% (Y) to dietary protein level (X) relationship was estimated by the following second-degree polynomial regression equation:
Based on the above equation, the estimated PRE% occurred at a dietary protein level of approximately 40.3% of the diet.
On the basis of the above polynomial equations, the maximum LWG%, best FCR, PER, SGR, and highest PRE% occurred at 42.75, 41.5, 40.0, 42.3, and 40.3% protein in the diet, respectively.
Discussion
In the present study, growth and conversion efficiencies increased with increasing dietary protein levels from 25 to 40%. Although growth of the fish levelled off when reared on 45% protein in the diet, the decline in growth was statistically insignificant (P > 0.05) and, hence, feeding fish with 45% protein in the diet would be uneconomical. Therefore, inclusion of 40% protein in the diet for young H. fossilis is appropriate. However, a significant fall in growth and conversion efficiencies was noted at 50% protein of the diet indicating that 40% protein diet (Diet 4) satisfied the requirement and is considered optimum for achieving maximum growth and excellent conversion efficiency. A similar trend has been observed in many other fish species irrespective of culture strategies (Jauncey 1982; Cho et al. 1985; Khan and Jafri 1991; Vergara et al. 1996; Bai et al. 1999; Ng et al. 2001; Kim et al. 2002; Kim and Lee 2005; Wang et al. 2006). The decline in growth performance at protein level above 40% can be attributed to the fact that the fish body cannot utilize dietary protein once the optimum level has been reached (Phillips 1972). Excess protein in the diet could reduce the performance due to higher energy requirement for catabolism rather than for protein deposition. Once protein is catabolized, the nitrogen fraction of the amino acids are excreted by deamination which leads to the release of amino groups that cannot be recycled through metabolic processes. The amino groups, therefore, must be excreted which is done at the expense of energy resulting to the lesser utilization of energy for growth purposes and more towards excretion and deamination of the excess amino nitrogen resulting from the excess amount of dietary protein intake. Therefore, from a metabolic point of view, it could be said that dietary protein beyond 40% could not be used for body protein synthesis or tissue building in fish and was being oxidized to produce energy to deaminate and excrete the extra nitrogenous load from the body. However, the amount of NH3 in the surrounding water of the fish fed high protein diet might have not affected the growth and health status of the fish, as the feeding trial was conducted in troughs fitted with water flow-through system at 1–1.5 l/min. The decrease in weight gain at protein levels above the optimum level may also be because of a reduction in available energy for growth and due to inadequate non-protein energy necessary to deaminate and excrete excess absorbed amino acids (Jauncey 1982; Cho et al. 1985; Vergara et al. 1996; Kim et al. 2002) Decrease in the protein utilization beyond requirement level of dietary protein is a well-documented phenomenon (Jobling and Wandshik 1983; Daniels and Robinson 1986; Tibbetts et al. 2000; Catacutan et al. 2001; Ng et al. 2001; Kim et al. 2002; Lee et al. 2002; Yang et al. 2002; Deepak and Garg 2003; Sales et al. 2003; Yang et al. 2003; Islam and Tanaka 2004; Kalla et al. 2004; Luo et al. 2004; Cho et al. 2005; Jacinto et al. 2005; Kim and Lee 2005; Tibbetts et al. 2005; Sa et al. 2006). In the present study, PER and protein retention efficiency increased with the increase in dietary protein content up to 40% and then decreased with further elevation of dietary protein level at 45 and 50% (Diets 5 and 6) which is also evident in other studies (Lee and Putnam 1973; Bromley 1980; Pongmaneerat and Watanabe 1991; Lee et al. 2002). Body protein was also found to increase with the increase in dietary protein up to 40% and declined thereafter. Body fat content increased with the increase in dietary protein levels which is in accordance with the findings of Khan et al. (1993). Higher body lipid content beyond the optimum protein level in the diet may be due to the fact that excess dietary protein gets deaminated and stored as body fat. In the present study, a negative correlation between moisture and fat content is evident. Feed intake was significantly different among the treatments during this study and ranged from 207 g at the highest dietary protein level to 279 g at the lowest dietary protein level. The variation in the feed intake may be due to the fact that fish eat to satisfy their energy requirement (NRC 1993) and they stop feeding once the dietary energy need is met. The low feed intake (207 g) at 50% protein diet may be as a result of this.
From the second-degree polynomial regression analysis of the growth and body composition data, the optimum dietary protein level for growth of young H. fossilis is found to be in the range of 40.0–42.75% of the diet. The value obtained during the present study is higher than the values reported (as percentages) for young grey mullet, Mugil capito, 24 (Papaparaskera-Papoutsoglou and Alexis 1986), Nile tilapia, Oreochromis niloticus, 25 (El-Saidy and Gaber 2005), Rohu, Labeo rohita, 25 (Khan et al. 2005), juvenile greenlip abalone, Haliotis laevigata, 27 (Coote et al. 2000), young tilapia, Oreochromis mossambicus, 28 (De Silva et al. 1989), Notemigonus crysoleucas, 29 and goldfish, Carassius auratus, 32 (Lochmann and Phillips 1994), walking catfish, Clarias batrachus, 30 (Chuapoehuk 1987). Nile tilapia, O. niloticus, 30 (Siddiqui et al. 1988), Shingi, H. fossilis, 27.73–35.43 (Akand et al. 1989), big head carp, Aristichthys nobilis, 30 (Santiago and Reyes 1991), juvenile silver perch, Bidynus bidynus, 31 (Yang et al. 2002), juvenile freshwater crayfish, Cherax quadricarinatus, (Cortes-Jacinto et al. 2003), juvenile silver perch, Spinibarbus hollandi, 32.7 (Yang et al. 2003), Catla, Catla catla, 30–35 (Seenappa and Devaraj 1995), South African abalone, Haliotis midae, 35.87 (Sales et al. 2003), and rohu, L. rohita, 35 (Satpathy et al. 2003), and is lower than the requirement reported for pike perch, Sander lucioperca, 43 (Nyina-wamwiza et al. 2005), African catfish, Clarias gariepinus, 43 (Ali and Jauncey 2005), bagrid catfish Mystus nemurus, 44 (Ng et al. 2001), grouper, Epinephelus malabaricus, 44 (Shiau and Lan 1996), Catla, C. catla, 47% (Singh and Bhanot 1988), Nile tilapia, O. niloticus, 45 (El-Sayed and Teshima 1992), juvenile Florida pompano, Trachinotus carolinus, 45 (Lazo et al. 1998), juvenile spotted sand bass, Paralabrax maculatofascinatus, 45 (Alvarez-Gonzalez et al. 2001), Catla, C. catla, 47 (Singh and Bhanot 1988), American eel, Anguilla rostrata, 47 (Tibbetts et al. 2000), juvenile haddock, Melanogrammus aeglefinus, 49.9 (Kim et al. 2001), discus, Symphysodon spp., 44.9–50.1 (Chong et al. 2000), Mahseer, Tor putitora, 45–50 (Islam and Tanaka 2004), juvenile olive flounder, Paralichthys olivaceus, 46.4–51.2 (Kim et al. 2002), juvenile haddock, M. aeglefinus, 54.6 (Tibbetts et al. 2005), juvenile turbot, Scophthalmus maximus, 55 (Cho et al. 2005), and Salmo trutta, 57 (Arzel et al. 1995) and comparable to the requirement for African catfish, C. gariepinus, 40 (Degani et al. 1989), C. batrachus, 40 (Khan and Jafri 1990), Catla, C. catla, 40 (Khan and Jafri 1991), mangrove red snapper, Lutjanus argentimaculatus, 40 (Catacutan et al. 2001), juvenile masu salmon, Oncorhynchus masuo, 40 (Lee and Kim 2001), Mahseer, T. putitora , 40 (Hossain et al. 2002), juvenile blackspot seabream, Pagellus bogaraveo, 40 (Silva et al. 2006), cuneate drum, Nibea miichthioides, 40 (Wang et al. 2006), Chanos chanos, 40 (Jana et al. 2006), Persian sturgeon, Acipenser persicus, 40 (Mohseni et al. 2007), Mexican silverside, Menidia estor, 40.9 (Martinez-Palacios et al. 2007), juvenile sunshine bass, Morone chrysops × M. saxatilis, 41 (Webster et al. 1995) and Malaysian catfish, M. nemurus, 42 (Khan et al. 1993). The differences in protein requirements among these fish species may be due to different dietary formulations, fish sizes, and different methodologies applied (Akiyama et al. 1997; Luo et al. 2004; Tibbetts et al. 2005; Sa et al. 2006). Some of these discrepancies may be attributed to differences in experimental design, e.g., feeding level and frequency, in the experimental conditions, e.g., water quality, water flow rate, biomass rate, reference protein used, or energy density of the diet (Luzzana et al. 1998). Digestibility and energy content bring about variable effects (Simmons et al. 1999; De Silva et al. 2000). Variations may also be attributed to differences between phylogenetically distinct families or species (Akiyama et al. 1997).
The study indicates that the dietary protein levels influences the growth, conversion efficiency, and body composition of the fish, and that the inclusion of dietary protein in the range of 40–43% of the diet is optimum for the growth and efficient feed utilization of protein for growth of young H. fossilis. Data generated during the present study would be useful in developing protein balanced diets for the intensive culture of the young H. fossilis.
References
Abbas G, Rukhsana KJ, Akhtar, Hong L (2005) Effects of dietary protein level on growth and utilization of protein and energy by juvenile mangrove red snapper (Lutjanus argentimaculatus). J Ocean Univ China 4:49–55
Akand AM, Miah MI, Haque MM (1989) Effect of dietary protein level on growth, feed conversion and body composition of shingi (Heteropneustes fossilis Bloch). Aquaculture 50:175–180
Akand AM, Hasan MR, Habib MBA (1991) Utilization of carbohydrate and lapis as dietary energy source by stinging catfish, Heteropneustes fossilis (Bloch). In: De Silva SS (ed) Fish nutrition research in Asia. Proceedings of the fourth Asian fish nutrition workshop. Asian Fisheries Society special publication, Asian Fish Soci Manila, Philippines, pp 93–100
Akiyama T, Oohara I, Yamamoto T (1997) Comparison of essential amino acid requirements with A/E ratio among fish species. Fish Sci 63:963–970
Ali MZ, Jauncey K (2005) Approaches to optimizing dietary protein to energy ratio for African catfish Clarias gariepinus (Burchell, 1822). Aquac Nutr 11:95–101
Alvarez-Gonzalez CA, Civera-Cerecedo R, Ortiz-Galindo JL, Dumas S, Moreno-Legorreta M, Grayeb-Del Alamo T (2001) Effect of dietary protein level on growth and body composition of juvenile spotted sand bass, Paralabrax maculatofascinatus, fed practical diets. Aquaculture 194:151–159
Anwar MF, Jafri AK (1992) Preliminary observation on the growth food conversion body composition of catfish, Heteropneustes fossilis Bloch, fed varying levels of dietary lipids. J Inland Fish Soc India 24:45–49
AOAC (1995) In: Cunniff P (ed) Official methods of analysis of the association of official analytical chemist, 16th edn. Arlington, Virginia
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn. APHA, Washington DC
Arzel J, Metailler R, Kerleguer C, Delliou HL, Guillaume J (1995) The protein requirement of brown trout (Salmo trutta) fry. Aquaculture 130:67–78
Bai SC, Wang XJ, Cho ES (1999) Optimum dietary protein level for maximum growth of juvenile yellow puffers. Fish Sci 65:380–383
Bromley PJ (1980) Effect of dietary protein, lipid, and energy content on the growth of turbot (Scophthalmus. maximus L.). Aquaculture 19:359–369
Catacutan MR, Pagador GE, Teshima S (2001) Effect of dietary protein and lipid level and protein to energy ratio on growth, survival and body composition of the mangrove red snapper, Lutjanus argentimaculatus (Forsskal 1775). Aquac Res 32:811–818
Cho CY, Cowey CB, Watanabe T (1985) Fish nutrition in Asia: methodological approaches to research and development. International Development Research Centre, Ottawa, Canada
Cho SH, Lee SM, Lee JH (2005) Effect of dietary protein and lipid levels on growth and body composition of juvenile turbot (Scophthalmus maximus L.) reared under optimum salinity and temperature conditions. Aquac Nutr 11:235–240
Chong ASC, Hashim R, Ali AB (2000) Dietary protein requirement for discus (Symphysodon spp.). Aquac Nutr 6:275–278
Chuapoehuk W (1987) Protein requirements of walking catfish, Clarias batrachus (Linnaeus), fry. Aquaculture 63:215–219
Coote TA, Hone PW, Van Barneveld RJ, Maguire GB (2000) Optimal protein level in a semipurified diet for juvenile greenlip abalone Haliotis laevigata. Aquac Nutr 6:213–220
Cortes-Jacinto EC, Colmenares HV, Ceracedo RC, Cordova RM (2003) Effect of dietary protein level on growth and survival of juvenile freshwater crayfish Cherax quadricarinatus (Decapoda: Parastacidae). Aquac Nutr 9:207–213
Cortes-Jacinto EC, Villarreal-Colmenares H, Cruz-Suárez LE, Civera-Cerecedo R, Nolasco-Soria H, Hernandez-Llamas A (2005) Effect of different dietary protein and lipid levels on growth and survival of juvenile Australian redclaw crayfish, Cherax quadricarinatus (von Martens). Aquac Nutr 11:283–291
Daniels WH, Robinson EH (1986) Protein and energy requirement of juvenile red drum (Sciaenops ocellatus). Aquaculture 53:243–252
Debnath D, Pal AK, Sahu NP, Yengkokpam S, Baruah K, Choudhury D, Venkateshwarlu G (2007) Digestive enzymes and metabolic profile of Labeo rohita fingerlings fed diets with different crude protein levels. Aquaculture 146:107–114
Deepak, Garg SK (2003) Effect of different dietary protein levels on growth performance and digestibility in the catfish, Heteropneustes fossilis (Bloch) when processed soybean is used as the protein source. J Natcon 15:128–133
Degani G, Yigal BZ, Levanon D (1989) The effect of different protein levels and temperatures on feed utilization, growth and body composition of Clarias gariepinus (Burchell 1822). Aquaculture 76:293–301
Dehadrai PV, Thakur NK (1980) Magur and singhi culture. Central Inland Fisheries Research Institute, Barrackpore, India
Deng J, Mai K, Ai Q, Zhang W, Wang X, Xu W, Liufu Z (2006) Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 258:503–513
De Silva SS, Gunasekera RM, Atapattu D (1989) The dietary protein requirements of young tilapia and an evaluation of the least cost dietary protein levels. Aquaculture 80:271–284
De Silva SS, Gunasekera RM, Gooley G (2000) Digestibility and amino acid availability of three protein rich ingredient incorporated diets by Murray cod Maccullochella peelii peelii (Mitchell) and Australian shortfin eel Anguilla australis Richardson. Aquac Res 31:195–205
Duncan DB (1955) Multiple range and multiple ‘F’ Tests. Biometrics 11:1–42
El-Saidy DMSD, Gaber MMA (2005) Effect of dietary protein levels and feeding rates on the growth performance, production traits and body composition on Nile tilapia, Oreochromis niloticus (L.) cultured in concrete tanks. Aquac Res 36:163–171
El-Sayed AFM, Teshima S (1992) Protein and energy requirements of Nile tilapia, Oreochromis niloticus fry. Aquaculture 103:55–63
Firdaus S (1993) On relative efficiency of purified diets, with variable protein levels, in young catfish Heteropneustes fossilis Bloch. Indian J Fish 40:162–165
Firdaus S, Jafri AK (1996) Growth and haematological response of young catfish Heteropneustes fossilis Bloch, to dietary phosphorous supplementation. J Aquac Trop 11:263–269
Firdaus S, Jafri AK, Rahman N (1994) Effect of iron deficient diet on the growth and haematological characteristics of the catfish, Heteropneustes fossilis Bloch. J Aquac Trop 9:179–185
Firdaus S, Jafri AK, Khan MA (2002) Effect of dietary levels of mineral mixture on the growth, body composition and haematology of catfish, Heteropneustes fossilis Bloch. J Aquac Trop 17:273–281
Giri SS, Sahoo SK, Sahu AK, Mehe PK (2003) Effect of dietary protein level on growth, survival, feed utilization and body composition of hybrid Clarias catfish (Clarias batrachus x C. gariepinus). Anim Feed Sci Technol 104:169–178
Gunasekera RM, De Silva SS, Collins RA, Gooley G, Ingram BA (2000) Effect of dietary protein level on growth and food utilization in juvenile Murray cod Maccullochella peelii peelii (Mitchell). Aquac Res 31:181–187
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish Nutrition, 3rd edn. Academic press, San Diego, CA, pp 61–141
Hossain MA, Hasan N, Azad Shah AKM, Hussain MG (2002) Optimum dietary protein requirement of Mahseer, Tor putitora (Hamilton) fingerlings. Asian Fish Soci Manila, Philippines. Asian Fish Sci 15:203–214
Islam MS, Tanaka M (2004) Optimization of dietary protein requirement for pond-reared mahseer Tor pittitora Hamilton (Cypriniformes: Cyprinidae). Aquac Res 35:1270–1276
Jana SN, Garg SK, Patra BC (2006) Effect of inland water salinity on growth performance and nutritional physiology in growing milkfish, Chanos chanos (Forsskal): field and laboratory studies. J Appl Ichthyol 22:25–34
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung S, Huang Q, Qin J, Su B (2005) Maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 7:125–137
Jauncey K (1982) The effect of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Jhingran SJ (1991) Culture of air breathing fishes and non air breathing predatory carnivorous fishes. In: Jhingran VG (ed) Fish and fisheries of India. Hindustan Publishing Corporation, Delhi, India, pp 498–503
Jobling M, Wandshik A (1983) Quantitative protein requirement of Artic Charr, Salvelinus alpines (L.). J Fish Biol 22:705–712
Kalla A, Bhatnagar A, Garg SK (2004) Further studies on protein requirements of growing Indian major carps under field conditions. Asian Fish Sci 17:191–200
Khan MA, Jafri AK (1990) On the dietary protein requirement of Clarias batrachus Linnaeus. J Aquac Trop 5:191–198
Khan MA, Jafri AK (1991) Dietary protein requirement of two size classes of the Indian major carp, Catla catla Hamilton. J Aquac Trop 6:79–88
Khan MS, Ang KJ, Ambak MA, Saad CR (1993) Optimum dietary protein requirement of a Malaysian catfish, Mystus nemurus. Aquaculture 112:227–235
Khan MA, Jafri AK, Chadha NK (2005) Effects of varying dietary protein levels on growth, reproductive performance, body and egg composition of rohu, Labeo rohita (Hamilton). Aquac Nutr 11:11–17
Kim JD, Lall SP (2001) Effects of dietary protein level on growth and utilization of protein and energy by juvenile haddock (Melanogrammus aeglefinus). Aquaculture 195:311–319
Kim LO, Lee SM (2005) Effects of dietary protein and lipid levels on growth and body composition of bagrid catfish, Pseudobagrus fulvidraco. Aquaculture 243:323–329
Kim JD, Lall SP, Milley JE (2001) Dietary protein requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquac Res 32:1–7
Kim KW, Wang XJ, Bai SC (2002) Optimum dietary protein level for maximum growth of juvenile olive flounder, Paralichthys olivaceus (Temminck et Schlegel). Aquac Res 33:673–679
Kvale A, Nordgreen A, Tonheim SK, Hamre K (2007) Problem of meeting dietary protein requirements in intensive aquaculture of marine fish larvae, with emphasis on Atlantic halibut (Hippoglossus hippoglossus L.). Aquac Nutr 13:170
Lazo JP, Allen Davis D, Arnold CR (1998) Effects of dietary protein level on growth, feed efficiency and survival of juvenile Florida pompano (Trachinotus carolinus). Aquaculture 169:225–232
Lee SM, Kim KD (2001) Effects of dietary protein and energy levels on the growth, protein utilization and body composition of juvenile masu salmon (Oncorhynchus masou Brevoort). Aquac Res 32:39–45
Lee DJ, Putnam GB (1973) The response of rainbow trout to varying protein/energy ratios in a test diet. J Nutr 103:916–922
Lee SM, Kim DJ, Cho SH (2002) Effects of dietary protein and lipid level on growth and body composition of juvenile ayu (Plecoglossus altivelis) reared in seawater. Aquac Nutr 8:53–58
Lochmann RT, Phillips H (1994) Dietary protein requirement of juvenile golden shiners (Notemigonus crysoleucas) and goldfish (Carassius auratus) in aquaria. Aquaculture 128:277–285
Lovell T (1989) Diet and fish nutrition husbandry. In: Halver JE (ed) Fish Nutrition. Academic Press, California, London, pp 549–604
Luo Z, Liu YJ, Mai KS, Tian L, Liu D, Tian XY (2004) Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquac Nutr 10:247–252
Luzzana U, Hardy RW, Halver JE (1998) Dietary arginine requirement of the fingerling Coho salmon (Oncorhynchus kisutch). Aquaculture 163:137–150
Martinez-Palacios CA, Rios-Duran MG, Ambriz-Cervantes L, Jauncey KJ, Ross LG (2007) Dietary protein requirement of juvenile Mexican silverside (Menidia estor Jordan 1879), a stomachless zooplanktophagous fish. Aquac Nutr 13:304–310
McGoogan BB, Gatlin DMIII (1999) Dietary manipulations affecting growth and nitrogenous waste production of red drum (Sciaenops ocellatus)-I. Effects of dietary protein and energy levels. Aquaculture 178:333–348
Mohamed SJ (2001) Dietary pyridoxine requirement of the Indian catfish Heteropneustes fossilis. Aquaculture 194:327–335
Mohamed SJ, Ibrahim II (2001) Quantifying the dietary niacin requirement of the Indian catfish, Heteropneustes fossilis (Bloch), fingerling. Aquac Res 32:157–162
Mohanty SS, Samantary K (1996) Effect of varying levels of dietary protein on the growth performance and feed conversion efficiency of snakehead Channa striata fry. Aquac Nutr 2:89–94
Mohseni M, Sajjadi M, Pourkazemi M (2007) Growth performance and body composition of sub-yearling Persian sturgeon, (Acipenser persicus, Borodin, 1987), fed different dietary protein and lipid levels. J Appl Ichthyol 23:204–208
Ng W-K, Soon SC, Hashim R (2001) The dietary protein requirement of a bagrid catfish, Mystus nemurus (Cuvier & Valenciennes), determined using semi purified diets of varying protein level. Aquac Nutr 7:45–51
Niamat R, Jafri AK (1984) Growth response of the siluroid, Heteropneustes fossilis Bloch, fed pelleted feed. Aquaculture 37:223–229
Nyina-wamwiza L, Xu LX, Blanchard G, Kestemont P (2005) Effect of dietary protein, lipid and carbohydrate ratio on growth, feed efficiency and body composition of pikeperch Sander lucioperca fingerlings. Aquac Res 36:486–492
Papaparaskera-Papoutsoglou E, Alexis MN (1986) Protein requirement of young grey mullet, Mugil capito. Aquaculture 52:105–115
Phillips AM Jr (1972).Calories and energy requirement. In: Halver JE (ed) Fish nutrition. New York: Academic press, pp 1-28.
Pillay TVR (1990) Nutrition and feeds. In: Aquaculture principles and practices. Catfishes. pp 333–350
Pongmaneerat J, Watanabe T (1991) Nutritive value of protein of feed ingredients for carp Cyprinus carpio . Bull Jpn Soc Sci Fish 57:503–510
Sa R, Pousao-Ferreira P, Oliva-Teles A (2006) Effect of dietary protein and lipid levels on growth and feed utilization of White Sea bream (Diplodus sarus) juveniles. Aquac Nutr 12:310–321
Saha N, Ratha BK (1998) Ureogenesis in Indian air-breathing teleosts: adaptation to environmental constraints. Comp Biochem Physiol 120A:195–208
Saha N, Ratha BK (2007) Functional ureogenesis and adaptation to ammonia metabolism in Indian freshwater air-breathing catfishes. Fish Physiol Biochem 33:283–295
Sales J, Truter PJ, Britz PJ (2003) Optimum dietary crude protein level for growth in South African abalone (Haliotis midae L.). Aquac Nutr 9:85–89
Santiago CB, Reyes OS (1991) Optimum dietary protein level of growth of bighead carp (Aristichthys nobilis) fry in a static system. Aquaculture 93:155–165
Satpathy BB, Mukherjee D, Ray AK (2003) Effects of dietary protein and lipid level on growth, feed conversion and body composition in rohu, Labeo rohita (Hamilton), fingerlings. Aquac Nutr 9:17–24
Seenappa D, Devaraj KV (1995) Effect of different levels of protein, fat and carbohydrate on growth, feed utilization and body carcass composition of fingerlings in Catla catla (Ham.). Aquaculture 129:243–249
Shiau SY, Lan CW (1996) Optimum dietary protein level and protein to energy ratio for growth of grouper (Epinephelus malabaricus). Aquaculture 145:259–266
Siddiqui AQ, Howlander MS, Adam AA (1988) Effects of dietary protein levels on growth, feed conversion and protein utilization in fry and young Nile Tilapia, Oreochromis niloticus. Aquaculture 70:63–73
Silva P, Andrade CAP, Timoteo VMFA, Rocha E, Valente LMP (2006) Dietary protein, growth, nutrient utilization and body composition of juvenile blackspot seabream, Pagellus bogaraveo (Brunnich). Aquac Res 37:1007–1014
Simmons L, Moccia RD, Bureau DP, Sivak JG, Herbert K (1999) Dietary methionine requirement of juvenile Arctic charr, Salvelinus alpinus (L.). Aquac Nutr 5:93–100
Sing RP, Srivastava AK (1984) Effect of feeding frequency on growth, consumption and gross conversion efficiency in the siluroid catfish, Heteropneustes fossilis (Bloch). Isr J Aquac 36:80–91
Singh BN, Bhanot KK (1988) Protein requirement of the fry of Catla catla (Ham.). In: Mohan M, Joseph M (eds) Proceedings, the First Indian Fisheries Forum. AFS, India
Snedecor GW, Cochran WG (1968) Statistical methods. Iowa State University Press, Iowa
Sokal RR, Rohlf FJ (1981) Biometry. Freeman, New York
Thakur NK (1991) Possibilities and problem of catfish culture in India. J Inland Fish Soc India 23:80–90
Tibbetts SM, Lall SP, Anderson DM (2000) Dietary protein requirement of juvenile American eel (Anguilla rostrata) fed practical diets. Aquaculture 186:145–155
Tibbetts SM, Lall SP, Milley JE (2005) Effects of dietary protein and lipid levels and DPDE−1 ratio on growth, feed utilization and leptosomatic index of juvenile haddock, Melanogrammus aeglefinus L. Aquac Nutr 11:67–75
Tripathi SD, Das SR (1976) Production potential of Clarias with carps in ponds. Third workshop on all india coordinated research project on air-breathing fishes for culture in swamps, Bangalore (Karnataka), 24–25 September, 7 pp
Usmani N, Jafri AK (2002) Effect of fish size and temperature on the utilization of different protein source in two catfish species. Aquac Res 33:959–967
Usmani N, Jafri AK, Khan MA (2003) Nutrient digestibility studies in Heteropneustes fossilis (Bloch), Clarias batrachus (Linnaeus) and C. gariepinus (Burchell). Aquac Res 34:1247–1253
Vergara JM, Robaina I, de Izquierdo MM, La Higuera (1996) Protein sparing effect of lipids in diets for fingerlings of gilthead sea bream. Fish Sci 62:624–628
Wang Y, Guo J, Bureau DP, Cui Z (2006) Effects of dietary protein and energy levels on growth, feed utilization and body composition of cuneate drum, Nibea miichthioides. Aquaculture 252:421–428
Webster CD, Tiu LG, Tidwell JH, Wyk PV, Howerton RD (1995) Effects of dietary protein and lipid levels on growth and body composition of sunshine bass (Morone chrysops × Morone saxatilis) reared in cages. Aquaculture 131:291–301
Yang SD, Liou CH, Liu FG (2002) Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 213:363–372
Yang SD, Lin TS, Liou CH, Peng HK (2003) Influence of dietary protein levels on growth performance, carcass composition and liver lipid classes of juvenile Spinibarbus hollandi (Oshima). Aquac Res 34:661–666
Zeitoun IH, Ullrey DE, Magee WT, Gill JL, Bergen WG (1976) Quantifying nutrient requirements of fish. J Fish Res Board Can 33:167–172
Acknowledgements
The authors are grateful to the chairman of the Department of Zoology of Aligarh Muslim University, Aligarh, India, for providing the necessary laboratory facilities, and to Prof. John E. Halver for supporting the fish nutrition research program at this laboratory. We gratefully acknowledge the financial assistance generous funding received under DST-FIST Programme, New Delhi in the priority area of Fish Nutrition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqui, T.Q., Khan, M.A. Effects of dietary protein levels on growth, feed utilization, protein retention efficiency and body composition of young Heteropneustes fossilis (Bloch). Fish Physiol Biochem 35, 479–488 (2009). https://doi.org/10.1007/s10695-008-9273-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9273-7