Abstract
Haematological parameters are often used as health status and stress indicators in fish. However, information on the effects of artificial photoperiods on these parameters is scarce and ambiguous. The consequences of three different artificial photoperiod regimes [light/day 12 h:12 h (LD 12:12) for 150 days as controls; LD 14:10 and LD 24:0, both for an initial 60 days, and then LD 12:12 for the remaining 90 days] were evaluated in trout. In all fish the haemogram and the erythrocyte production index (EPI) were assessed at days 7, 14, 30 and 60 (phase 1) and at days 90, 120 and 150 (phase 2). At the end of phase 1, photoperiods LD 14:10 and 24:0 induced higher production of immature (late basophilic) erythrocytes (0.06–0.08 × 1012 cells/l, P < 0.05) and elevated EPI (young erythrocytes = 1.6 in LD 14:10, 2.25 in LD 24:0; late basophilic erythrocytes = 4.1–4.9 in LD 14:10, 3.3 in LD 24:0) than in controls (late basophilic erythrocytes = 0.02–0.04 × 1012 cells/l; EPI young erythrocytes = 0.2–1.0; EPI late basophilic erythrocytes = 1.4–2.7). During phase 2, only reduced numbers of lymphocytes and thrombocytes were observed at day 150 in the LD 14:00 group. An increase in the EPI (1.9) of control young erythrocytes was found at day 150.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many biological activities are regulated in a seasonal manner by the interaction of organisms with these environmental factors that translate into internal changes, which, in turn, affect behaviour, feeding and reproduction (Sumpter 1997; Cerda-Reverter et al. 1998; Herrero et al. 2005). Biological rhythms are typically synchronized with ambient oscillations of photoperiod, temperature and moon phases (Bolliet et al. 2001; Rouff and Rensing 2004), photoperiod being the key “Zeitgeber” (Bromage et al. 2001), usually in close relationship with temperature (Pankhurst and Porter 2003). Seasonality in day length induces fluctuations in the amount of melatonin produced by the pineal gland in most fish. Receptors for this hormone exist in the central nervous system and other tissues whose activation influences various enzymatic activities (Vanecek 1998; Witt-Enderby et al. 2003).
The immune system of poikilothermic vertebrates also exhibits seasonal changes (Zapata et al. 1992; Yellon et al. 1999; Kaattari and Ottinger 2000; Leu et al. 2001; Boivin et al. 2003; Bilbo et al. 2003). These changes are probably related to temperature fluctuations; nevertheless, Nakanishi (1986) and Slater and Schreck (1993) have shown seasonal variations in immune system parameters in fish kept at constant temperature, suggesting that, in addition, other factors such as photoperiod could induce these changes. Thus, these variations could be controlled by a neuro-endocrine endogenous rhythm (Zapata et al. 1992).
References to photoperiod effects on haematological parameters in fish are scarce and can be inferred mainly from seasonality studies (Bridges et al. 1976; Collazos et al. 1995). Additionally, since the majority of these reports are field studies, they were also influenced by other factors, such as temperature, which would complicate deductions on how light affects these blood parameters. Furthermore, laboratory controlled studies of the effects of artificial photoperiod on haematological aspects show different results, presumably due to differences in the illumination protocols utilized, species, and duration of exposure to light (Melingen et al. 2002; Leonardi and Klempau 2003; Biswas et al. 2004).
In fish, as in mammals, haematological parameters are frequently used as health or disease indicators (Duthie and Tort 1985; Gallardo et al. 2003; Ballarin et al. 2004). These indicators are susceptible to age and nutrition (Silveira-Coffigny et al. 2004) and also to a variety of environmental conditions such as temperature, dissolved oxygen concentration and contamination (Person-Le Ruyet et al. 2004; Affonso et al. 2002; Orrego et al. 2005). Haematological parameters are recognized as secondary stress indicators when an increase in erythrocyte numbers, haematocrit, haemoglobin concentration and leukopenia are observed (Wedemeyer et al. 1990; Wojtaszek et al. 2002; Pierson et al. 2004). In this respect, Leonardi and Klempau (2003) have shown that a light/day 24 h:0 h (LD 24:0) regime under laboratory conditions is capable of inducing stress in trout, and that this stress extends up to 2 months after the application of the artificial photoperiod.
The effects of artificial photoperiod regimes on reproductive patterns have been studied in several species and with varied artificial photoperiods (Bromage et al. 2001). On the other hand, it has been reported in mammals that sex hormones can affect cells of the immune system (Slater et al. 1995; Johnson et al. 1997; Beagley and Gockel 2003; Wira et al. 2003). This has also been observed in some fish (Saha et al. 2004; Takafumi et al.2001).
In Chile, LD 24:0 is commonly used to inhibit grilsing and manage smoltification timing. In previous reports it has been shown that LD 24:0 can induce alterations in the haematological parameters of fish under field conditions compared with control fish kept under natural photoperiod (Valenzuela et al. 2006a, 2006b). This observation might be influenced by natural temperature variations. Thus, one the objectives of the present report was to assess the effect of artificial photoperiods on haematological parameters at constant temperature. Three regimes were selected: LD 12:12 (closest to natural at that timepoint), LD 14:10 and LD 24:0. We chose LD 14:10 to avoid the possible stress observed by Leonardi and Klempau (2003). The different effects were assessed during and after artificial illumination protocols, considering after-effect observations when the normal photoperiod was resumed (Leonardi and Klempau 2003, Valenzuela et al. 2006a, 2006b) and the possible relationships between haematological parameters and gonadal development (Valenzuela et al. 2006b).
Materials and methods
Juvenile trout (Oncorhynchus mykiss) close to the “smolt” stage (70 ± 8 g), purchased at a local fish farm in the VIII Region, Chile (37°17′ S 71°43′ W), were acclimatized for 1 month in 300 l white plastic tanks, at the Bioassays Laboratory of the University of Concepción, at a controlled temperature (11 ± 1°C) and under a LD 12:12 photoperiod. Afterwards, they were divided into three groups (with two replicates each). Each group was kept at a maximum density of 20 kg/m3 and was fed commercial pellets at a daily 0.5% rate (Vextra Alpha, Ewos, Chile). An independent recirculation system was provided for each tank. During the experiments, the pH (6.0–6.8), dissolved oxygen (7.1–8.7 mg O2/l) and ammonia (<0.1 mg/l) were recorded twice a day.
Artificial illumination was provided by two fluorescent tubes (18 W Luminex Interna) per tank suspended 30 cm above the water surface and controlled by Ticino timers. Each tank was covered externally with black PVC sheets to avoid light influences on neighbouring tanks.
No water temperature increase from the effect of the lamps was observed during three daily readings throughout the experiment: water and animal room temperatures remained constant at 11 ± 1°C.
Experiment design
To assess the effect of artificial photoperiods without the influence of temperature, we kept the fish in the animal room at 11 °C ± 1°C. Control trout were kept at the same conditions used for acclimatization. After acclimatization (June 29th to October 30th) the control protocol (LD 12:12) was applied during the entire experimental period (150 days), whereas the LD 14:10 and LD 24:0 protocols were applied for 2 months (phase 1, July 31st to October 30th, following acclimatization) and then LD 12:12 until January 29th (phase 2). Ten fish were sampled from each group at the beginning of phase 1 and a similar number on the following days: 7, 14, 30, 60, 90, 120 and 150 (30 fish per sampling). Sampling was performed without the reposition of the fish. During sampling on day 120, no fish were examined from the LD14:10 group, because we kept them for the next sampling to evaluate their reproductive status. During each sampling a visual inspection was made to assess gonadal development and the emergence of secondary sexual features.
Blood analysis
Blood samples were obtained by puncture of the caudal venous sinus with 3 ml disposable syringes of randomly chosen and anaesthetized fish (ethyl p-amino benzoate, BZ-20, Veterquímica, Chile). Blood was transferred to Eppendorf tubes containing EDTA and analysed according to the procedures of Campbell and Murru (1990) and Klontz (1994). The haematocrit was determined immediately after the blood had been drawn by centrifugation at 12,000 r.p.m. for 5 min, and haemoglobin concentration was assessed by the cyano-metahaemoglobin method using a haemoglobin standard of 18 g/l and reading absorbance at 546 nm in a Shimadzu UV 1601 spectrophotometer.
Leukocytes and erythrocytes were counted using Neubauer chambers diluting the blood in a Rees–Ecker solution at a proportion of 1:100. Polychromatophil (immature erythrocytes) numbers were obtained from 1,000 erythrocytes per fish, counted in May–Grünwald Giemsa-stained blood extensions. Absolute numbers were calculated on the basis of total erythrocyte counts in Neubauer chambers. We applied similar procedures to obtain differential and total leukocyte (including thrombocytes) counts on the basis of 500 counted cells in the stained extensions.
The qualitative description of erythrocytes was adopted from Fijan (2002) and Valenzuela et al. (2006a) (Figure 1).
The erythrocytic production index (EPI) for immature erythrocytes (types 1 and 2) was calculated according to:
This index is a general indicator of the rate of erythrocyte production and was adapted for trout, where % iE is the percentage of immature erythrocytes (type 1 or 2), Hto is the fish haematocrit, 0.27 is a constant corresponding to mean trout haematocrit in litre by litre (L/L) according to Klontz (1994), and bMT is maturation time for blood erythrocytes (according to Rodak 1992 for humans, since no data exist for trout).
The secondary erythrocyte indices [mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC)] were obtained from the haematocrit, haemoglobin and erythrocyte count data for each fish.
Statistical analysis
We used factorial analysis of variance to investigate interactions and to compare the effects of photoperiod and time; Tukey’s multiple comparison test was used for determination of significant differences between treatments. All analyses were performed with the STATISTICA (version 6.0) packet program. Significative differences were accepted as P < 0.05.
Results
Phase 1
Erythrocyte series
Artificial photoperiods did not induce significant changes in erythrocyte counts (Fig. 2A), haematocrit, haemoglobin concentration or secondary erythrocyte indices (Table 1). However, all fish showed a gradual and constant diminishment of haemoglobin concentration, MCH and MCHC until day 60. In most parameters, changes were observed with time and strong relationships between time and artificial photoperiods.
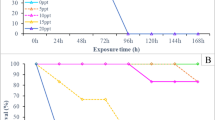
Changes induced by artificial photoperiods on (A) total erythrocyte counts and (B) total immature erythrocyte counts. * indicate significant differences with respect to controls (P < 0.05). Lines under the figure show time lapse of phase 1 (solid line) and phase 2 (under LD 12:12, broken line). LD 12:12 = control
Although no changes in total erythrocyte numbers could be observed, immature erythrocyte numbers varied in fish under artificial photoperiods (Fig 2B). In the control group (LD 12:12) these cells remained unchanged (P > 0.05), while, in fish under LD 24:0 and LD 14:10, immature erythrocytes increased their numbers towards the end of this phase. Fish under LD 14:10 exhibited more late basophilic erythrocytes, with two peaks, one at day 7 and the other at day 60, whereas fish under LD 24:0 had only one peak, at day 60 (Fig. 3A). Young erythrocytes did not change (Fig. 3B)
Changes induced by artificial photoperiods on immature erythrocytes. (A) Late basophilic–polychromatic erythrocytes. (B) Young erythrocytes. * indicate significant differences with respect to controls (P < 0.05). Lines under the figure show time lapse of phase 1 (solid line) and phase 2 (under LD 12:12, broken line). LD 12:12 = control
The iEPI of late basophilic—polychromatic erythrocytes controls showed rather stable behaviour, oscillating between 1.4 and 2.7, while the LD 14:10 group evidenced a peak of 4.9 at day 7 and another of 4.1 at day 60, coincident with the unique erythrocyte production peak of the LD 24:0 group (Fig. 4A). The iEPI for young erythrocytes (Fig. 4B) resulted in an oscillation between 0.3 and 0.9 for controls, whereas for LD 14:10 a peak of 1.6 at day 7 and for LD 24:0 a peak of 2.2 at day 30 were evident.
Changes induced by artificial photoperiods on the erythrocyte production index (EPI) in (A) late basophilic–polychromatic erythrocytes and (B) young erythrocytes. * indicate significant differences with respect to controls (P < 0.05). Lines under the figure show time lapse of phase 1 (solid line) and phase 2 (under LD 12:12, broken line). LD 12:12 = control
Thrombocytes and leukocytes
Total leukocyte numbers were unaffected by artificial photoperiods (Fig. 5A, P > 0.05) as were polymorphonuclear cells and monocytes in particular (Table 3). All groups tended to increase their lymphocytes and thrombocytes with time (Fig. 5B and C)
Changes induced by artificial photoperiods on (A) total leukocyte counts, (B) lymphocyte counts and (C) thrombocytes. * indicates significant differences with respect to controls (P < 0.05). Lines under the figure show time lapse of phase 1 (solid line) and phase 2 (under LD 12:12, broken line). LD 12:12 = control
Gonadal development
No significant changes or differences in gonadal development or secondary sexual characteristics were observed in the different photoperiod groups (Fig. 6).
Changes in gonads of trout under artificial photoperiods. (A) Initial conditions of the experiment. (B) Gonadal development at day 150 in males under phase 1 LD 14:10. (C) Gonadal development at day 150 in control females (LD 12:12). (D) Gonadal development at day 150 in females under phase 1 LD 14:10. (E) Gonadal development at day 150 in females under phase 1 LD 24:0. Arrows show gonads
Phase 2
Erythrocyte series
As opposed to phase 1, time did not influence haematological parameter values, excepting immature erythrocytes. No significant differences were observed in erythrocyte numbers (Fig. 2A), haematocrit, haemoglobin concentration or secondary indices (Table 2) between the three protocols. Similarly, the number of immature erythrocytes and the iEPI did not show changes related to the different photoperiods. Only the young erythrocytes of the control group showed a 1.9-fold increase in the rate of production at day 150 (Fig. 4B).
Thrombocytes and leukocytes
Total numbers of leukocytes had diminished by day 150 in the LD 14:10 group, affecting neither polymorphonuclear cells nor monocytes (Table 3). Nevertheless, a significant effect of photoperiod could be observed in the number of lymphocytes: whilst controls and LD 24:0 remained above 7 × 109cell/l, the LD 14:10 group dropped to fewer than 4 × 109/l at day 150 (Fig. 5B). Similar results were found for thrombocytes (Fig. 5C).
Gonadal development
Towards the end of this phase, an important increase in gonadal size was observed in trout exposed to LD 14:10 and LD 24:0 as compared to controls (LD12:12). Microscopic changes were more evident in males, some of which even spermiated spontaneously when manipulated (Fig. 6).
Discussion
Results show that photoperiod alterations did not affect the majority of haematological parameters, excepting lymphocytes and immature erythrocytes. Even though variations in parameters existed with time, these were probably related to the (LD14:10 and LD 24:0) photoperiod-associated increase in gonadal development (unpublished results).
Erythrocytes
In general, this type of research is scarce; Leonardi and Klempau (2003) showed that a LD 24:0 photoperiod does not significantly change erythrocyte numbers in trout, and, similarly, Biswas et al. (2004) did not find alterations in the haematocrit of tilapia (Oreochromis niloticus) under short pulses of LD 6:6. Our results are consistent in that no changes were found in trout haematocrit or erythrocyte numbers during or after artificial lighting. Furthermore, no effect could be shown on our haemoglobin concentration data and secondary erythrocyte indices. On the other hand, these results differ from those of field studies after LD 24:0 was used, where significant changes were found in erythrocytes (Valenzuela et al. 2006a). This could be due to interactions between artificial lighting and temperature under natural conditions.
When our constant temperature controlled results—which did not show significant differences—are compared with those of seasonal haematology studies by others (Bridges et al. 1976; Collazos et al. 1998), where, during the fall–winter seasons, a lowering of haematocrit and erythrocyte numbers (and an increase during spring–summer) could be observed, it can be concluded that these changes were mostly temperature dependent. Furthermore, differences in haematocrit and erythrocyte numbers have also been documented between sexes (Lane 1979), although others did not report these dissimilar observations (Korzhuev et al. 1982). Pickering (1986) suggests that these gender-dependent differences observed in salmon are common occurrences for mature fish, this based on the observation that, in mammals, testosterone stimulates erythropoiesis.
In our investigation, during phase 1, no changes were evident in any of the haematological parameters studied, excepting immature erythrocyte numbers (peaks at days 7 and 60 for LD 14:10 and one peak at day 60 for LD 24:0). Towards the end of phase 2 (at day 150), when LD 14:10 and 24:0 fish began to show evident sexual maturation, (Fig. 6), no significant changes in haematocrit, haemoglobin concentration or total erythrocyte numbers could be observed. In this respect it must be considered that, in mammals, oestrogens are erythropoiesis inhibitors (Pololi-Anagnostou et al. 1981) and androgens the contrary (Ohno and Fisher 1978; Hero et al. 2005). This aspect is important to consider, as evidence exists that similar effects occur in fish (Álvarez et al. 1994). This suggests that during artificial lighting erythropoietic stimulation occurred, probably due to androgen production. This is supported by the iEPI data, which show a 4–5-fold erythropoiesis stimulation of late basophilic or polychromatic erythrocytes and almost a twofold stimulation of young erythrocytes during the first 60 days.
The use of iEPI is a good indicator of erythrocyte production; however, this index must be used with caution, since no fish erythrocyte maturation timing data support these results, being merely based on comparisons with information on human red blood cell (RBC) counts (Rodak 1992). Considering the temperature-dependent physiological activities of fish, these values might be actually underestimated; therefore, trout iEPI values might be even higher. Furthermore, the standardized haematocrit (0.27) can also vary in function of environmental conditions; thus, if the mean human haematocrit (0.45) is taken into consideration, the same tendencies are noticeable in the iEPI, but the magnitude of erythrocyte production diminishes. Any modification of parameters considered by the iEPI will result in the same tendencies, and the increase of cell production will stay constant. The improved use of this index will be dependent on perfecting fish erythrocyte life span measurements in the future. As of now, the index can be used with relative precision.
Bridges et al. (1976) show an increase in summer in the percentage of immature erythrocytes in the winter flounder (Pseudopleuronectes americanus). Härdig and Höglund (1984) also report an important elevation in the percentages of immature erythrocytes in salmon during summer and relate this to the observation that these fish cease feeding during reproduction, which, in turn, retards erythrocyte maturation and their accumulation. Those authors also suggest that seasonal changes in haematological parameters depend more on photoperiod than on water temperature variations. In view of that, in the present investigation, trout were kept at a constant 11°C and were fed equally, and no food rejection was observed during the whole experiment; the changes shown in the number of immature erythrocytes confirm the suggestion of Härdig and Höglund (1984) in that photoperiods might be regulating erythropoiesis, specifically the protocols LD 14:10 (summer) and LD 24:0 (constant light).
Considering that: (1) fish under LD 24:0 and LD 14:10 showed a peak in immature erythrocyte production approximately 3 months before an increase was seen in the gonadal somatic index at the end of phase 2, (2) secondary sexual characteristics were also evident by the end of phase 2 and (3) the normal spawning time for salmonids is in June–August in the southern hemisphere, it is interesting to note that the increase in the production rate of young erythrocytes in the control group at the end of the experiment (approximately at day 150) occurs in the summer 5 months before spawning time. Thus, the increase of iEPI in fish under LD 14:10 and 24:0 may correspond to sex hormone-driven erythropoietic preparation for spawning.
One important aspect to underline is that, although erythropoietic stimulation could be revealed, no significative increases in erythrocyte numbers were evident. This could be explained by considering that the spleen is an erythrocyte storage organ (Fange 1992) and that, in fish, it can contract under adrenergic stimulation (Kita and Itazawa 1989, 1990), rapidly releasing erythrocytes into circulation upon demand or in response to stressors.
Leukocytes
The effects of artificial photoperiods on leukocytes and thrombocytes are poorly documented. Melingen et al. (2002) exposed Atlantic salmon to photoperiods and immunization and established that fish exposed to continuous light had diminished numbers of B lymphocyte and increased numbers of polymorphonuclear cells, relating these findings to seawater transfer. Leonardi and Klempau (2003) found diminishment only in thrombocyte numbers in salmon under LD 24:0. Biswas et al. (2004) determined that tilapia under short-pulse LD 6:6 photoperiods presented more lymphocytes than LD 12:12 controls did but invariable numbers of polymorphonuclear cells. Those authors could not establish stress effects by photoperiod application as measured by cortisol levels.
Ample evidence exists in fish, as well as in other vertebrates, that, in spring (September–November in the southern hemisphere), the immune system is activated (Zapata et al. 1992), which explains the general increase of leukocytes during phase 2 in our experiments. Pickering (1986) describes lymphopenia in sexually mature fish towards spawning time, coinciding with our results in that the LD 14:10 group showed the strongest development of sexual maturation and the lowest numbers of leukocytes (and lymphocytes in particular). Although water temperature plays an important role in the number of lymphocytes (Pickering 1986), in the present investigation, temperature was kept constant during the whole experiment.
Our observed diminishment of thrombocytes is coincident with the findings of Leonardi and Klempau (2003); thrombocytopenia has been associated with high cortisol levels (Wiik et al. 1989). On the other hand, Pickering (1986) describes low numbers of these cells during spawning, again being consistent with the present results, since the LD 14:10 group showed the strongest development of sexual maturation and the lowest numbers of thrombocytes.
Low numbers of both lymphocytes and thrombocytes are generally associated with stress (Wedemeyer et al. 1990) by means of the immunosuppressive effect of cortisol (Pulsford et al. 1994). In addition, Pickering and Cristie (1981) show elevated cortisol levels in sexually mature fish; furthermore, it must be considered that sexual hormones also play an immunosuppressive role (Slater and Schreck 1993; Harris and Bird 2000).
Results indicate that application of LD 14:10 and LD 24:0 induces advancement of gonadal development as well as a heightened rate of erythrocyte production, the latter probably being a consequence of the former. Although no plasmatic levels of sex hormones were actually measured, the evidence that these hormones might act on haematological parameters (erythrocytes, lymphocytes) is shown by the manifest gonadal development under LD 14:10. Future measurements of plasmatic levels of sex hormones will clearly complement these studies to confirm their effect on lymphocytes and erythrocytes. This aspect is particularly important when it is considered that the diminishment of lymphocyte numbers in trout under LD 14:10 could be explained as a photoperiodic effect on the hypothalamus–hypophysis–gonad axis and not necessarily as an altered photoperiod stressor effect, additionally supported by the fact that no stress evidence was found in the present experiments, either by application or termination of artificial lighting regimes, as shown by plasmatic cortisol levels (unpublished data).
References
Affonso EG, Polez VLP, Correa CF, Mazon AF, Araujo MRR, Moraes G, Rantin FT (2002) Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp Biochem Physiol 133(C):375–382
Álvarez F, Flaño E, Villena AJ, Zapata A, Razquin BE (1994) Seasonal intrathymic erythropoietic activity in trout. Dev Comp Immunol 18(5):409–420
Ballarin L, Dall’Oro M, Bertotto D, Libertini A, Francescon A, Barbaro A (2004) Haematological parameters in Umbrina cirrosa (Teleostei, Sciaenidae): a comparison between diploid and triploid specimens. Comp Biochem Physiol 138(A):45–51
Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Nelson RJ (2003) Photoperiod affects the expression of sex and species differences in leukocyte number and leukocyte trafficking in congeneric hamsters. Psychoneuroendocrinology 28:1027–1043
Biswas AK, Maita M, Yoshizaki G, Takeuchi T (2004) Physiological responses in Nile tilapia exposed to different photoperiod regimes. J Fish Biol 65:811–821
Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS (2003) Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102:4143–4145
Bolliet V, Aranda A, Boujard T (2001) Demand-feeding rhythm in rainbow trout and European catfish. Synchronisation by photoperiod and food availability. Physiol Behav 73:625–633
Bridges D, Cech Jr W, Pedro DH (1976) Seasonal hematological changes in the winter flounder Pseudopleuronectes americanus. Trans Am Fisheries Soc 5:596–600
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Campbell TW, Murru F (1990) An introduction to fish hematology. Comp Cont Ed Vet Sci 12:525–533
Cerda-Reverter JM, Zanuy S, Carrillo M, Madrid JA (1998) Time-course studies on plasma glucose, insulin, and cortisol in Sea Bass (Dicentrarchus labrax) held under different photoperiodic regimes. Physiol Behav 64(3):245–250
Collazos ME, Barriga C, Ortega E (1995) Seasonal variations in the immune system of the cyprinid Tinca tinca. Phagocytic function. Comp Immunol Microbiol Infect Dis 18(2):105–113
Collazos ME, Ortega E, Barriga C, Rodríguez B (1998) Seasonal variation in haematological parameters in male and female Tench Tinca tinca. Mol Cell Biochem 114:432–435
Duthie GG, Tort L (1985) Effects of dorsal aortic cannulation on the respiration and haematology of Mediterranean living Scyliorhinus canicula. Comp Biochem Physiol 81(A):879–885
Fange R (1992) Fish blood cells. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology: the cardiovascular system, vol XII (part B) Academic, London
Fijan N (2002) Morphogenesis of blood cell lineages in channel catfish. J Fish Biol 60:999–1014
Gallardo MA, Sala-Rabanal M, Ibarz A, Padro´s F, Blasco J, Fernández-Borra J, Sánchez J (2003) Functional alterations associated with ‘‘winter syndrome’’ in gilthead sea bream (Sparus aurata). Aquaculture 223:15–27
Härdig J, Höglund LB (1984) Seasonal variation in blood components of reared Baltic salmon, Salmo salar L. J Fish Biol 24:565–679
Harris J, Bird DJ (2000) Modulation of the fish immune system by hormones. Vet Immunol Immunopathol 77:163–176
Hero M, Wickman S, Hanhijärvi R, Siimes MA, Dunkel L (2005) Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146(2):245–252
Herrero MJ, Pascual M, Madrid JA, Sánchez-Vázquez FJ (2005) Demand-feeding rhythms and feeding-entrainment of locomotor activity rhythms in tench (Tinca tinca). Physiol Behav 84:595–605
Johnson RW, Arkins S, Dantzer R, Kelley KW (1997) Hormones, lymphohemopoietic cytokines and the neuroimmune axis. Comp Biochem Physiol 116(A):183–201
Kaattari SL, Ottinger CA (2000) Seasonality of trout leukocytic sensitivity to toxicity: implications for immunotoxic biomonitoring. Mar Environ Res 50:465–472
Kenneth W, Beagley, Christine, Gockel M (2003) Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 38(1):13–22
Kita J, Itazawa Y (1989) Release of erythrocytes from the spleen during exercise and splenic constriction by adrenaline infusion in the Rainbow trout. Jpn J Ichthyol 36(1):48–52
Kita J, Itazawa Y (1990) Effects of adrenaline on the blood flow through the spleen of rainbow trout (Salmo gairdneri). Comp Biochem Physiol 95A(4):591–595
Klontz G W (1994) Fish hematology. In: Stolen JD, Rowley AF, Zelikoff JT, Kaatari SL, Smith SA (eds) Techniques in fish immunology. FITC 3 SOS Publications, Fair Haven, USA, pp 121–131
Korzhuev PA, Alyakrinskava IO, Dolgova SN (1982) Characteristics of the blood in young and adult Salmo salar (Salmonidae). J Ichthyol 22:112–1120
Lane HC (1979) Progressive changes in the haematology and tissue water of sexually mature trout, Salmo gairdneri Richardson during the autumn and winter. J Fish Biol 15:425–436
Leonardi MO, Klempau AE (2003) Artificial photoperiod influence on the immune system of juvenile rainbow trout (Oncorhynchus mykiss) in the southern hemisphere. Aquaculture 221:581–591
Leu SL, Shiah IS, Yatham LN, Cheu YM, Lam RW (2001) Immune-inflammatory markers in patients with seasonal affective disorder: effects of light therapy. J Affect Disorders 63:27–34
Melingen GO, Pettersen EF, Wergeland HI (2002) Leucocyte populations and response to immunization and photoperiod manipulation in Atlantic salmon (Salmo salar L) 0 + smolt. Aquaculture 214:381–396
Nakanishi T (1986) Seasonal changes in the humoral immune response and the lymphoid tissues of the marine teleost Sebastiscus marmoratus. Vet Immunol Immunopathol 12:213–221
Ohno Y., Fisher JW (1978) Effects of androgens on burst forming units (BFU-E) in normal rabbit bone marrows. Life Sci 22:2031–2036
Orrego R, Moraga G, Gonzalez M, Gavilán JF, Valenzuela A, Burgos A, Barra R (2005) Reproductive, physiological and biochemical responses in juvenile female rainbow trout (Oncorhynchus mykiss) exposed to sediment from pulp and paper mill industrial discharge areas. Environ Toxicol Chem 24(8):1935–1943
Pankhurst NW, Porter MJR (2003) Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish Physiol Biochem 28:385–389
Person-Le Ruyet J, Mahé K, Le Bayon N, Le Delliu H (2004) Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture 237:269–280
Pickering AD (1986) Changes in blood cells composition of the brown trout, Salmo trutta L., during spawning season. J Fish Biol 29:335–347
Pickering AD, Christie P (1981) Changes in the concentrations of plasma cortisol and thyroxine during sexual maturation of the hatchery-reared brown trout, Salmo trutta L. Gen Comp Endocrinol 44:487–496
Pierson PM, Lamers A, Flik G, Mayer-Gostan N (2004) The stress axis, stanniocalcin, and ion balance in rainbow trout. Gen Comp Endocrinol 137:263–271
Pololi-Anagnostou L, Schade S, Anagnostou A (1981) The effect of estrogens on erythroid stem cells in polycythemic mice. Mol Cell Endocrinol 24(1):73–84
Pulsford AL, Lemaire-Gony S, Tomlinson M, Collingwood N, Glynn PJ (1994) Effects of acute stress on the immune system of the dab, Limanda limanda. Comp Biochem Physiol 109C:129–139
Rodak BF (1992) Routine laboratory evaluation of erythrocytes. In: Lotspeich-Steininger CA, Stiene-Martin EA, Koepke JA (eds) Clinical haematology: principles, procedures, correlations. Lippincott, USA, pp 107–125
Rouff R, Rensing L (2004) Temperature effects on circadian clocks. J Therm Biol 29:445–456
Saha NL, Usami T, Suzuki Y (2004) In vitro effects of steroid hormones on IgM-secreting cells and IgM secretion in common carp (Cyprinus carpio). Fish Shellfish Immunol 17:149–158
Silveira-Coffigny R, Prieto-Trujillo A, Ascencio-Valle F (2004) Effects of different stressors in haematological variables in cultured Oreochromis aureus S. Comp Biochem Physiol 139(C):245–250
Slater CH, Schreck CB (1993) Testosterone alters the immune response of Chinook Salmon, Oncorhynchus tshawytscha. Gen Comp Endocrinol 89:291–298
Slater CH, Fitzpatrick MS, Schreck CB (1995) Characterization of an androgen receptor in salmonid lymphocytes: possible link to androgen–induced immunosuppression. Gen Comp Endocrinol 100:218–225
Sumpter JP (1997) Environmental control of fish reproduction: a different perspective. Fish Physiol Biochem 17:25–31
Takafumi Y, Watanuki H, Sakai M (2001) Effects of estradiol, progesterone and testosterone on the function of carp, Cyprinus carpio, phagocytes in vitro. Comp Biochem Physiol 129:49–55
Valenzuela AE, Silva VM, Klempau AE (2006a) Qualitative and quantitative effects of constant light photoperiod on rainbow trout (Oncorhynchus mykiss) peripheral blood erythrocytes. Aquaculture 251(2–4):596–602
Valenzuela AE, Silva VM, Klempau AE (2006b) Effects of constant light on haematological parameters of cultured rainbow trout (Oncorhynchus mykiss) in the southern hemisphere. Fish Physiol Biochem 32:113–120
Vanecek J (1998) Cellular mechanisms of melatonin action. Physiol Rev 78(3):687–711
Wedemeyer GA, Barton BA, McLeay DJ (1990) Stress and acclimation. In: Schreck CB, Moyle PB (eds) Methods for fish biology. Bethesda, MD, American Fisheries Society, pp 451–489
Wiik R, Andersen K, Uglenes I, Egidius E (1989) Cortisol-induced increased in susceptibility of Atlantic salmon together with effects on the blood cell pattern. Aquaculture 83:201–215
Wira C, Fahey JV, Abrahams VM, Rossoll RM (2003) Influence of stage of the reproductive cycle and estradiol on thymus cell antigen presentation. J Steroid Biochem Mol Biol 84:79–87
Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA (2003) Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci 72:2183–2198
Wojtaszek J, Dziewulska-Szwajkowska D, Lozinska-Gabska M, Adamowicz A, Dzugaj A (2002) Hematological effects of high dose of cortisol on the carp (Cyprinus carpio L.): Cortisol effect on the carp blood. Gen Comp Endocrinol 125:176–183
Yellon SM, Fagoaga OR, Nehlsen-Cannarella SL (1999) Influence of photoperiod on immune cell functions in the male Siberian hamster. Am J Physiol 276:R97–R102
Zapata G, Varas A, Torroba M (1992) Seasonal variations in the immune system of lower vertebrates. Immunol Today 13(4):142–147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valenzuela, A.E., Silva, V.M. & Klempau, A.E. Some changes in the haematological parameters of rainbow trout (Oncorhynchus mykiss) exposed to three artificial photoperiod regimes. Fish Physiol Biochem 33, 35–48 (2007). https://doi.org/10.1007/s10695-006-9115-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-006-9115-4