Abstract
Screening for pancreatic cancer (PC) in high-risk groups aimed to detect early cancers is currently done only in the research setting, and data on psychological outcomes of screening in these populations is scarce. To determine the psychological impact of a national Australian pancreatic screening program, a prospective study was conducted using validated psychological measures: impact of events scale (IES), psychological consequences questionnaire (PCQ) and the cancer worry scale. Measures were administered at baseline, 1-month and at 1-year post-enrolment and correlations with abnormal endoscopic ultrasound (EUS) results were calculated. Over a 6-year period, 102 participants were recruited to the screening program. Thirty-nine patients (38.2%) had an abnormal endoscopic ultrasound, and two patients (2.0%) were diagnosed with PC and two with other malignancies. Those with a personal history of cancer or a positive BRCA2 mutation demonstrated significantly increased worry about developing other types of cancer at baseline (p < 0.01). Irrespective of EUS result, there was a significant decrease of total IES score at 1 year (Z = − 2.0, p = 0.041). In patients with abnormal EUS results, there was a decrease in the total IES score at 1 year (Z = − 2.5, p = 0.011). In participants deemed to be most distressed at baseline based on their negative PCQ score, there was a significant decrease of the total PCQ (Z = − 3.2, p = 0.001), emotional (Z = − 3.0, p = 0.001), social (Z = 3.0, p = 0.001) and physical (Z = − 2.8, p = 0.002) subscale at 1-year post-intervention. This study provides evidence of the long-term psychological benefits of PC screening in high-risk patients. There was no negative impact of screening in the short-term and the positive benefits appeared at 1-year post-intervention irrespective of screening result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Worldwide, pancreatic cancer (PC) is one of the most common lethal malignancies, being the third most common cause of cancer-related death among men and women [1]. In contrast to declining cancer related deaths for colorectal, breast and prostate cancer, it is projected that PC will be the second most common cause of cancer death by 2030 [2]. The median age of diagnosis is 71 years and the 5-year survival rate is only 8% [1, 3]. The poor prognosis is due to the asymptomatic nature of early PC, with approximately 70% of patients presenting with locally advanced of metastatic disease [4].
About 5–10% of PC results from familial aggregation and/or genetic susceptibility, referred to as familial PC and hereditary PC syndromes respectively [5]. Individuals from these families are considered at high risk of developing PC and screening programs target these individuals aiming to detect PC at an early resectable stage.
Familial pancreatic cancer
Families with familial PC are people with two or more first-degree relatives (FDR) with PC in the absence of a known cancer syndrome [6]. Prospective studies have demonstrated an increased risk of PC in asymptomatic family members related to the number of affected family members. In one meta-analysis, having one affected relative increased overall risk by 1.8 [7]. Having two first-degree relatives with PC confers a 6.4 greater risk (lifetime risk 8–12%) and 3 FDRs a 32-times greater risk (lifetime risk 16–32%) [6, 8, 9].
Hereditary syndromes
Hereditary syndromes have a known genetic defect, and the risk of developing PC depends upon the specific genetic abnormality. Hereditary syndromes with increased PC risk include Peutz–Jeghers syndrome (PJS), Hereditary breast and/or ovarian cancer syndrome (HBOC), familial atypical multiple mole melanoma (FAMMM), hereditary pancreatitis and lynch syndrome [10,11,12,13,14,15,16,17,18,19].
Role of surveillance
Ideally, a successful screening program would detect small asymptomatic PC and high-grade dysplastic lesions amenable for curative resection with the aim of reducing pancreas-related mortality. Given the low incidence of PC in the general population (lifetime risk of about 1.3%), it is not recommended nor feasible to undertake a broad screening program for PC [11]. The International Cancer of the Pancreas Screening (CAPS) Consortium was formed in 2012 to help organise global screening for PC in high-risk individuals and recommends surveillance in individuals with a lifetime risk of PC of 5% or more. The rationale of screening asymptomatic high-risk individuals is to diagnose precursor lesions or early PC when still resectable and, hence, potentially curable and thus improve survival. A Japanese study showed a 100% 1-year survival in 79 patients with tumour sizes less than 1 cm undergoing curative resection [20].
A systematic review showed that screening in familial high-risk individuals led to a higher diagnostic rate of pancreatic tumours than in controls (34% vs 7.2%, p < 0.001) [21]. This review showed that PC screening resulted in a significantly higher curative resection rate (60% vs. 25%, p = 0.001) and a significantly longer median survival time (14.5 month vs. 4 months, p < 0.001), although the 3-year survival rate was not significantly longer (20% vs. 15% p = 0.624).
Screening modalities
Endoscopic ultrasound (EUS) and/or magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) are the methods of choice for screening. EUS has been shown to be superior to MRI in these high risk groups, the overall yield for detecting premalignant and malignant lesions using EUS is 20% and using MRI/MRCP is 14% [22]. EUS performs better for small solid lesions and MRI for cystic lesions. EUS has been shown to be a clinically useful modality to diagnose pancreatic adenocarcinoma with a sensitivity of 91%, specificity of 86% and diagnostic accuracy of 89% [23]. EUS fine needle aspiration (FNA) allows cytological sampling of abnormal areas and has an accuracy of 92% for PC [24].
Psychological impact of screening
While the evidence is mounting that PC screening programs detect early lesions and improves at least short-term survival, there is not enough data yet to show that screening improves long term survival and thus screening is performed in a research setting. Given that these patients are undertaking screening while asymptomatic and have had relatives die from PC, there is a real risk that the process itself may cause emotional and psychological distress, as demonstrated by Breitkopf et al., who showed that HRI had a higher perceived risk of PC and high levels of anxiety associated with PC risk during the screening period [25]. The aim of this study is to determine the short- and long-term psychological impact of screening in participants in a prospective PC screening study using standardised validated psychological testing.
Methods
Participants
Eligible participants were enrolled in the Australian PC Screening study for high-risk individuals performed at St Vincent’s Hospital in Sydney, Australia which had started in 2011. The study was approved by St Vincent’s Hospital Ethics Committee (HREC/10/SVH/33). The participants were asymptomatic, did not have a personal history of PC and met criteria for being a high-risk individual (“Appendix”). Participants were referred by Family Cancer Clinics, the Australian Familial PC Registry, medical practitioners or participants had self-referred.
Screening protocol
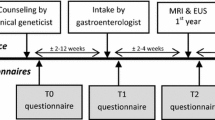
Once their eligibility was confirmed, at enrolment participants were mailed demographic, medical history and psychological questionnaires including the Cancer Worry Scale, Impact of Event and Psychological Consequences Questionnaire. These were returned using reply paid enveloped together with the consent form. Then the participants underwent genetic counselling with a geneticist and/or an accredited genetic counsellor in Family Cancer Clinics across Australia. The topics covered by the familial cancer clinics included verification of family history, assessment of the individual risk for PC, psychosocial issues related to participation and undergoing genetic testing where appropriate.
The screening intervention was an EUS, which was performed by either one of two experienced gastroenterologists with expertise in EUS. Allocation of appointments was purely determined on scheduling availability. All procedures were performed under sedation at St Vincent’s Hospital in Sydney. To determine the short- and long-term psychological impact of screening, two psychological questionnaires, the Impact of Event Scale (IES) and Psychological Consequences Questionnaire (PCQ), were also administered at 1-month post-EUS and 1-year post EUS by mail. Research bloods were also collected from each patient. Patients without any pancreatic abnormalities or non-concerning features on their EUS, were scheduled for annual surveillance. Patients who had abnormal EUS findings were scheduled to have more frequent EUS (either 3 or 6 monthly depending upon level of clinical concern) and additional imaging with an MRI, or surgery if deemed appropriate. The abnormal results were discussed in Multidisciplinary Meeting (MDT).
Measures
Baseline demographics were collected including gender, age, ethnicity and screening indication. The validated measures, which were utilised to assess the psychological impact of screening are described below.
Impact of event scale
This 15-item scale measures anxiety responses in relation to a specific stressor and has well documented psychometric properties [26, 27]. In our study, the particular stressor was concern about being at risk for developing PC. Participants were asked to rate symptoms of anxiety (for example, ‘I had strong waves of feelings about being at risk for PC) on a scale ranging from ‘Not at all’ to ‘Often’. The scale was included because being at risk for PC may constitute a traumatic stressor by some individuals, thereby giving rise to intrusive and avoidant thoughts. The scale consists of the Intrusion subscale (range 0 to 35 scores) and Avoidance subscale (range 0 to 40). A score of 40 or higher on the Impact of Event scale is considered to be strongly predictive of a significant stress response syndrome [28].
Psychological consequences questionnaire
The PCQ is a validated questionnaire that specifically measures the psychological impact of screening. This measure was selected on the basis that it was designed specifically to measure the psychological consequences specific to the cancer screening process [29]. The measure aims to detect the psychological (positive and negative) consequences and any long term-changes, which might be the result of attending screening. This measure looks at the effect on major life dimensions: physical, emotional and social impact. Each subscale is divided into quartiles, where the bottom 25% indicates no or minimal dysfunction. The second, third and fourth quartile indicate mild, moderate and marked disturbance respectively [29].
Cancer worry scale
Cancer worry scale was administered at baseline only to assess level of PC worry and the worry of developing other cancers using the questions “How worried are you that you may develop PC?” and “How worried are you that you may develop another type of cancer?” [30]. Response options ranged from 1 = not at all worried to 5 = extremely worried.
Statistical analyses
Data analysis was performed using IBM SPSS Statistics for Windows (Version 25.0. Armonk, NY).All dichotomous variables were presented as numbers and percentages, and continuous variables as either mean (standard deviation) or median (interquartile range). Statistical differences between patients with a normal and abnormal EUS were calculated using a Fisher’s exact test for discrete variables and a Mann–Whitney U Test for continuous variables. Variables assessed for statistical differences in EUS included age, gender, BRCA2 status, youngest age of familial PC diagnosis, personal cancer history and the number of FDRs and SDRs diagnosed with PC. Sub-analyses on the demographic data were carried out by splitting the data based on the variable of interest and statistical analysis was conducted using the aforementioned approach of Fisher’s exact test and the Mann–Whitney U test. Wilcoxon signed-rank tests were conducted to test for changes of avoidance, intrusion, total IES scores, positive and negative PCQ subscale scores over time. Sub-analyses by EUS, gender and BRCA2 status were conducted by firstly splitting the data using the variable of interest, then carrying out the Wilcoxon signed-rank test on the split data.
A quartile sub-analyses was carried out for the PCQ. The initial data (pre-intervention for negative consequence and at 1-month for positive consequences) was binned into 4 quartiles, and then subsequently, each quartile was analysed separately using a Wilcoxon signed-rank test. For all analyses, a two-tailed p value less than 0.05 was considered significant. A Spearman’s rho was used to calculate correlations between age of participants and the negative as well as positive PCQ scores.
Results
Study cohort demographics
Between 2011 and March 2017, 102 individuals completed pre-screening assessments. Nighty (88.2%) and 86 (84.3%) completed the 30 day and 1-year questionnaires respectively. Eighty-nine (87.3%) participants were Caucasian, 12 Jewish White/Caucasian and one Asian. The major indications for screening were familial PC (n = 79), BRCA 2 mutations with a family history (n = 22) and PJS (n = 1). Seventeen (16.7%) patients self-reported current or previous depression. Twenty patients (19.6%) had a personal history of cancer; 14 breast cancer, two melanoma, one thyroid, prostate, liver and brain. Table 1 shows demographic and medical characteristics for the total cohort and separately for those with a normal and abnormal EUS.
Abnormal EUS results demographics
Thirty-nine participants received abnormal EUS test results, which required more intensive screening. Of those who had abnormal findings, two had confirmed pancreatic malignancy (one pancreatic adenocarcinoma and one pancreatic neuroendocrine tumour). They went onto have successful resection. Two other extra-pancreatic cancers were found as part of the screening program (one liver cancer and one breast cancer). The liver cancer was detected on EUS and the breast cancer on MRI pancreas. The rest of the EUS abnormalities included cysts, branch duct IPMN or diffuse pancreatic abnormalities without a mass that did not meet criteria to proceed to surgery.
Patients who had an abnormal EUS had a median age of 58 and 23 (59%) were female. Twenty-eight HRI were screened due to FPC, and eleven (30.0%) because of BRCA2 mutations with a family history of PC. Ten (25.6%) of patients with an abnormal EUS had a personal history of cancer. Patients with an abnormal EUS had a median age 58.0 (IQR 53.0–64.0 compared to those with a normal EUS; median age 55.0 (49.0–61.0), this was not statistically significant, p = 0.097). The median age of familial PC diagnosis was 50.0 (IQR 44.0–66.0) and 60.0 (IQR 44.0–68.0) for those with a normal and abnormal EUS respectively, p = 0.546.
Baseline cancer worry scores
Cancer worry scores were assessed at baseline. For all patients, the median baseline cancer worry score for developing PC was 3 (IQR 3–4) and other cancer types it was 3 (IQR 2–4). Patients with a normal EUS at baseline had a median PC worry score of 3 (IQR 3–4), whereas for other cancer types it was 3 (IQR 2–4). Participants who were BRCA2 mutation carriers had a median baseline cancer worry score for developing PC of 3 (IQR 3–4) compared to a median score of 3 (IQR 3–4) for those that did not carry a mutation, p = 0.943. When it came to worry about developing other cancers, BRCA2 mutation carriers had a median score of 4 (IQR 3–4), compared to those who were not carrier (median 3, IQR 2–3). This result was statistically significant, Z = − 3.0, p = 0.003.
Those with a personal history of cancer had a median worry score for developing PC of 3 (IQR 3–4) juxtaposed to their counterparts with no personal history of cancer who had a median worry score of 3 [3, 4], which was not statistically significant, p = 0.385. Interestingly, those with a personal history of cancer had a higher median worry score for developing other cancers of 3.5 (IQR 3–4) compared to their counterparts who had a median score of 3 (IQR 2–3), p = 0.009 (Supplementary Table 1).
Impact of event scale scores
Entire cohort analysis
Pre-intervention to 1-month
The avoidance IES scores did not differ significantly from pre-intervention (Mean = 5.3, SD = 5.9) to 1 month (mean = 4.6, SD = 5.5) (Z = − 0.97, p = 0.328). Similarly, the intrusion IES subscale did not differ significantly from pre-intervention (Mean = 4.4, SD = 4.5) to 1 month for the entire cohort (Mean = 3.8, SD = 4.2) (Z = − 1.3, p = 0.20). Analysis of total IES scores from pre-intervention (Mean = 9.7, SD = 9.8) to 1-Month (Mean = 8.5, SD = 8.9) did not demonstrate a significant decrease (Z = − 1.2, p = 0.22).
Pre-intervention to 1-year
The total IES score decreased significantly at 1 year (Mean = 7.7, SD = 8.6), compared to pre-intervention (Mean = 9.7, SD = 9.8) (Z = − 2.0, p = 0.041), as seen in Table 2. Similarly, the intrusion subscale scores decreased significantly at 1 year (Mean = 3.3, SD = 3.7), compared to pre-intervention (Mean = 4.4, SD = 4.5) (Z = 2.1, p = 0.038). There were no significant changes relating to any of the other subscales.
Sub-analysis by EUS status
Pre-intervention to 1-month
There was no significant difference between pre-intervention and 1-month in avoidance-(Z = − 0.5, p = 0.621), intrusion-(Z = − 0.9, p = 0.368) and total impact scores (Z = − 0.7, p = 0.473) in those with a normal EUS. This was similar in those with an abnormal EUS for the intrusion (Z = − 0.9, p = 0.400), avoidance (Z = − 0.9, p = 0.378) and total impact scores (Z = − 0.8, p = 0.410).
Pre-intervention to 1-year
In those participants with an abnormal EUS, intrusion subscale scores were significantly lower at 1 year (Mean = 2.2, SD = 2.4) compared to pre-intervention (Mean = 4.6, SD = 5.0) (Z = − 2.4, p = 0.013), this is demonstrated in Table 3. This was similar for the avoidance subscale, where IES scores were significantly less at 1 year (Mean = 3.4, SD = 4.4), compared to pre-intervention (Mean = 5.6, SD = 6.3) (Z = − 2.2, p = 0.025) in those with an abnormal EUS. The mean IES total scale score at 1 year (Mean = 5.7, SD = 6.2) was significantly less in those with an abnormal EUS compared to scores at pre-intervention (Mean = 10.3, SD = 10.4) (Z = − 2.5, p = 0.011). There was no significant difference in intrusion (Z = − 0.8, p = 0.442), avoidance (Z = − 0.6, p = 0.582) or total IES score (Z = − 0.7, p = 0.469) between pre-intervention and 1 year in patients within the normal EUS group.
Sub-analysis by gender
Pre-intervention to 1 month
There were no significant decrease in intrusion (Z = − 0.4, p = 0.726), avoidance (Z = − 0.1, p = 0.915) and total IES subscale scores (Z = − 0.2, p = 0.850) for males from pre-intervention to 1 Month. This was similar for females in the intrusion (Z = − 0.9, p = 0.400), avoidance (Z = − 0.9, p = 0.378) and total IES scores (Z = − 0.8, p = 0.410).
Pre-intervention to 1 year
For the female patient cohort there was a reduction in intrusion subscale scores from pre-intervention (Mean = 4.5, SD = 4.5) to 1 year (Mean = 3.3, SD = 3.5) (Z = − 2.2, p = 0.026) as shown in Table 4. There was also a significant reduction in the avoidance subscale from (Mean = 5.6, SD = 6.0) at pre-intervention to 1 year (Mean = 4.1, SD = 5.3) (Z = − 2.1, p = 0.039). Similarly, there was a decrease in the total score from pre-intervention (Mean = 10.1, SD = 9.9) to 1 year (Mean = 7.4, SD = 8.1) (Z = − 2.2, p = 0.030). There was no significant change in intrusion (Z = − 0.4, p = 0.697), avoidance (Z = − 0.6, p = 0.965), or total score (Z = − 0.2, p = 0.882) between pre-intervention to 1 year for the male cohort.
Sub-analysis by personal history of cancer
Pre-intervention to 1 month
There were no significant decreases in intrusion (Z = − 0.6, p = 0.577), avoidance (Z = − 1.3, p = 0.220) and total IES subscale scores (Z = − 1.0, p = 0.349) in those with a personal history of cancer at 1 month compared to pre-intervention. Those without a personal history of cancer also demonstrated no significant decrease in intrusion (Z = − 0.6, p = 0.577), avoidance (Z = − 1.3, p = 0.220) and total IES subscale scores (Z = − 1.0, p = 0.349) at 1 month compared to pre-intervention.
Pre-intervention to 1 year
Participants with a personal history of cancer demonstrated a decrease in their total IES score from pre-intervention (Mean = 12.6, SD = 10.8) to 1-year post EUS (Mean = 6.8, SD = 6.0) (Z = − 2.4, p = 0.012) (Table 5). Similarly, there was a decrease in intrusion subscale scores from pre-intervention (Mean = 5.2, SD = 5.1) to 1-year post EUS (Mean = 2.4, SD = 2.3) (Z = − 2.3, p = 0.022). There was no significant decrease in intrusion (Z = − 1.3, p = 0.194), avoidance (Z = − 0.9, p = 0.378) and total scale IES score (Z = − 1.2, p = 0.254) in those with no history of cancer at 1 year compared to pre-intervention.
Psychological consequences scores
Entire cohort analysis
The total negative PCQ (NPCQ) score (Z = − 0.2, p = 0.987), and emotional (Z = − 0.2, p = 0.809), physical (Z = − 0.4, p = 0.664) and social (Z = − 0.5, p = 0.609) subset scores did not differ significantly at 1 month compared to pre-intervention. The total NPCQ score (Z = − 0.6, p = 0.550), emotional (Z = − 0.9, p = 0.353), physical (Z = − 0.5, p = 0.617) and social (Z = − 0.4, p = 0.713) subset scores at 1 year compared to pre-intervention did not differ significantly (Supplementary Table 2). The total Positive PCQ (PPCQ) score (Z = − 0.4, p = 0.637 as well as emotional (Z = − 1.2, p = 0.243), physical (Z = − 0.5, p = 0.647) and social (Z = − 0.4, p = 0.687) subset scores did not differ significantly at 1 year compared to 1 month (Supplementary Table 3).
Sub-analysis by gender
There was no significant difference between pre-intervention to 1-month for the negative emotional (Z = − 0.3, p = 0.779), physical (Z = − 0.2, p = 0.938), social (Z = − 0.6, p = 0.592) subscales scores, and total NPCQ score (Z = − 0.4, p = 0.719) for males. This was similar for females, where there was no significant change in emotional (Z = − 0.4, p = 0.674), physical (Z = − 0.7, p = 0.536), social (Z = − 0.2, p = 0.845) and total NPCQ score (Z = − 0.1, p = 0.896) from pre-intervention to 1 month post screening EUS. Analysis of males NPCQ at 1 year compared to preintervention demonstrated no significant difference in the emotional (Z = − 0.6, p = 0.552), physical (Z = − 0.9, p = 0.516), social (Z = − 1.2, p = 0.273) and total NPCQ scores (Z = − 1.0, p = 0.349). This was also demonstrated in the female population, where there was no significant change at 1 year compared to pre-intervention in the emotional (Z = − 1.5, p = 0.146), physical (Z = − 1.0, p = 0.349), social (Z = − 1.3, p = 0.204) and total NPCQ score (Z = − 1.3, p = 0.190) (Supplementary Table 4–5).
Similarly, there was no difference between 1-month and 1 year for total (Z = − 0.5 p = 0.659) emotional (Z = − 0.3 p = 0.800), physical (Z = − 0.9, p = 0.389) and social (Z = − 0.5, p = 0.672) positive PCQ subscales in the males. In females, there was no significant change in total PPCQ score at 1 year compared to 1 month (Z = − 0.3, p = 0.764), emotional (Z = − 1.1, p = 0.267), physical (Z = − 1.2, p = 0.226) and social (Z = − 0.7, p = 0.487) subscales (Supplementary Table 6).
Sub-analysis by BRCA2 status
There was no significant difference in emotional (Z = − 0.9, p = 0.359), physical (Z = − 0.3, p = 0.770), social (Z = − 0.2, p = 0.874) and total NPCQ (Z = − 0.4, p = 0.686) scores at 1 month compared to pre-intervention in those with a negative BRCA2 genetic screen. This was similar for 1 year compared to pre-intervention, where there was no significant difference in the emotional (Z = − 0.9, p = 0.385), physical (Z = − 0.8, p = 0.412), social (Z = − 0.6, p = 0.575) and total NPCQ scores (Z = − 0.6, p = 0.527).
In those with a positive BRCA2 genetic screen, there was no significant difference in emotional (Z = − 1.0, p = 0.346), physical (Z = − 0.4, p = 0.875), social (Z = − 1.7, p = 0.133) and total NPCQ scores (Z = − 0.8, p = 0.425) at 1 month compared to pre-intervention. This was similar at 1 year compared to pre-intervention, where there was no significant change in emotional (Z = − 0.4, p = 0.773), physical (Z = − 0.4, p = 0.750), social (Z = − 0.3, p = 0.844) and total NPCQ scores (Z = − 0.2, p = 0.867). In those with a negative BRCA2 genetic screen, there was no significant change in emotional (Z = − 1.2, p = 0.240), physical (Z = − 0.4, p = 0.673), social (Z = − 0.1, p = 0.890) and total PPCQ scores (Z = − 0.6, p = 0.555) at 1 year compared to 1 month. This was similar for those with a positive BRCA2 genetic screen, where there was no significant change in emotional (Z = − 0.4, p = 0.750), physical (Z = − 0.4, p = 0.734), social (Z = − 0.6, p = 0.625), and total PPCQ score (Z = − 0.1, p = 0.924) at 1 year compared to 1 month (Supplementary Table 7–9).
Sub-analysis by EUS status
There was no significant difference in emotional (Z = − 1.1, p = 0.284), physical (Z = − 0.5, p = 0.652), social (Z = − 0.4, p = 0.717) and total NPCQ score (Z = − 0.4, p = 0.715) at 1 month compared to pre-intervention in those with a normal EUS. This was similar for those with an abnormal EUS with there being no significant change in emotional (Z = − 0.6, p = 0.556), physical (Z = − 0.1, p = 0.994), social (Z = − 0.3, p = 0.758) and total NPCQ score (Z = − 0.3, p = 0.756) at 1 month compared to pre-intervention. Furthermore, at 1 year compared to pre-intervention, there was no significant different in emotional (Z = − 0.9, p = 0.367), physical (Z = − 0.5, p = 0.666), social (Z = − 0.1, p = 0.899) and total NPCQ score (Z = − 0.7, p = 0.491) in those with a normal EUS. In those with an abnormal EUS, at 1 year there was no significant difference in emotional (Z = − 0.4, p = 0.702), physical (Z = − 0.2, p = 0.858, social (Z = − 0.3, p = 0.775) and total NPCQ scores (Z = − 0.3, p = 0.774) compared to pre-intervention (Supplementary Table 10–11).
There was no significant difference in emotional (Z = − 1.6, p = 0.110), physical (Z = − 0.4, p = 0.720), social (Z = − 1.0, p = 0.318) and total PPCQ score (Z = − 0.6, p = 0.546) at 1 year compared to 1 month in those with a normal EUS. This was similar for those with an abnormal EUS, where no significant difference was detected in the emotional (Z = − 0.2, p = 0.817), physical (Z = − 0.2, p = 0.859), social (Z = − 0.6, p = 0.560) and total PPCQ scores (Z = 0, p = 0.976) at 1 year compared to 1 month (Supplementary Table 12).
Sub-analysis by quartile (negative psychological consequences)
All patients were divided into 4-quartiles based on their pre-intervention NPCQ score. Those with the lowest score were in the 1st quartile, whereas those with the highest score were in the 4th quartile. In quartile 4 participants, there was a significant reduction in total NPCQ score from pre-intervention (Mean = 13.5, SD = 4.8) compared to 1 month (Mean = 7.3, SD = 5.5) (Z = − 2.4, p = 0.013), and 1 year (Mean = 4.4, SD = 4.9) (Z = − 3.2 p = 0.001). At 1 month, a reduction in the emotional subscale from pre-intervention (Mean = 7.0, SD = 2.4) to 1 month (Mean = 3.7, SD = 2.7) was noted (Z = − 2.8, p = 0.002). At 1 year the reduction in scores within the quartile 4 group was reflected across all 3 subscales. The emotional subscale reduced from pre-intervention (Mean = 7.0, SD = 2.4) to 1 year (Mean = 2.7, SD = 2.8) (Z = − 3.0, p = 0.001). Similarly, the physical subscale reduced from pre-intervention (Mean = 3.0, SD = 1.8) to 1 year (Mean = 1.0, SD = 1.8) (Z = − 2.8, p = 0.002). The social subscale too reduced from pre-intervention (Mean = 3.6, SD = 2.3) to 1 year (Mean = 0.7, SD = 1.0) (Z = 3.0, p = 0.001). These results are depicted in Table 6.
In those participants that were deemed to be quartile 1, there was an increase in their total NPCQ score at pre-intervention (Mean = 0.0, SD = 0.0) to 1 month (Mean = 1.6, SD = 3.4) (Z = − 2.9, p = 0.001), and pre-intervention to 1 year (Mean = 1.4, SD = 2.9) (Z = 2.7, p = 0.004). This change was reflected in the increase in the emotional subscale from pre-intervention (Mean = 0, SD = 0) to 1 month (Mean = 1.1, SD = 2.3) (Z = − 3.0, p = 0.001) to 1 year (Mean = 0.9, SD = 1.9) (Z = − 2.7, p = 0.004).
Sub-analysis by quartile (positive psychological consequences)
All patients were divided into 4-quartiles based on their 1-month PPCQ score (Table 7). Those with the lowest score were in the 1st quartile. Patients in quartile 1 demonstrated an increase in the emotional subscale from 1 month (Mean = 2.1, SD = 1.7) to 1 year (Mean = 6.3, SD = 5.4) (Z = − 2.0, p = 0.040). In patients within the 4th quartile, total PPCQ score was significantly reduced from 1-month (Mean = 27.8, SD = 1.8) to 1-year (Mean = 23.7, SD = 7.2) (Z = − 2.4, p = 0.016). Similarly, the physical subscale score in quartile 4 patients reduced from 1 month (Mean = 8.4, SD = 0.9) to 1 year (Mean = 6.0, SD = 3.4) (Z = − 2.8, p = 0.004).
Correlation analysis
There was a significant negative correlation between age of participants and total NPCQ score at 1 month (correlation coefficient = − 0.247, p = 0.041). Similarly, there was also a significant negative correlation between age of participants and emotional score at 1 month (correlation coefficient = − 0.257, p = 0.033) as well as social score (correlation coefficient = − 0.343, p = 0.004).
Discussion
In this prospective analysis of questionnaires designed to assess the psychological impact of a PC screening program in high-risk individuals, the screening program did not show any negative short- or long-term psychological effects on participants, irrespective of the EUS result. Furthermore, at 1-year post-EUS, analysis of the cohort demonstrated a decrease in the total IES score, with those with an abnormal EUS reporting less avoidant and intrusive behaviour, highlighting a positive impact of the screening program. The lack of negative psychological impact of the screening program on quality of life was demonstrated by nil significant increase in the NPCQ total score from pre intervention to 1 month and 1 year. Furthermore, there was no increase in any of the NPCQ subscales that measure the effect of screening on an individual’s functioning on emotional, social and physical life domains, in either the normal or the abnormal EUS group. In addition to these results, implementation of this screening program in those who were deemed to have the greatest level of distress as determined by those who scored in the top 25% of NPCQ total score, was significantly effective in reducing their overall level of distress at both 1 month and 1-year post intervention.
Although not a direct aim of this psychological study, two participants (2.0%) were found to have pancreatic malignancies on EUS, which is similar to the findings published by the Italian Association for the Study of the Pancreas (AISP) Registry [31]. Two other malignancies were found as part of the screening program, one primary liver cancer and one early breast cancer detected on MRI of the pancreas, which brings the malignancy detection rate of the screening program to 3.9%, which is consistent with the results of other screening programs. A recent meta-analysis of PC screening programs showed a pooled prevalence of 3.3% of lesions considered a successful target of surveillance [32]. Their subgroup analysis showed a lesion detection rate of 3.0% in FPC, 4.0% in hereditary pancreatitis, 5.0% in familial melanoma, 6.3% in HBOC, and 12.2% in Peutz–Jeghers syndrome. Thirty-four percent of our participants had some pancreatic abnormalities (mainly cystic), which is similar to the pooled prevalence of cystic lesions of 33.6% found in studies with a high-quality score as described by Signoretti et al. [32].
This study shows that those with a personal history of cancer reported higher worry scores in regard to developing cancers other than PC. This underscores the findings of Douma et al., however it is in contrast to the findings of Konings et al, who demonstrated that a personal history of cancer was not associated with high cancer worries [33, 34]. Furthermore, the current study identified that individuals with a positive BRCA2 genetic mutation also demonstrated higher median worry scores in relation to developing other cancers compared to those without a mutation.
There are only a small number of studies that have investigated the psychological effect of screening for PC in high-risk individuals. Franke et al. assessed communication with the physician, reassurance, nervous anticipation and specific perceived disadvantages and were able to establish that in those at increased risk of PC, regular screening had minimal psychological impact [35]. This study was limited, however, with only a reported 50% of individuals at risk regularly participating in the proposed screening program, and only 67% having completed the questionnaire compared to 84.3% in the current study. Other studies have shown that cancer worries and psychological stress appear acceptable to patients participating in pancreatic screening programs, and that there is significant increase in risk perceptions, cancer worries and/or general distress in patients with FPC compared to those with a BRCA2 mutation and controls [35, 36]. Konings et al. were able to show that from pre-intervention to 3-years post-surveillance initiation, participants reported decreased anxiety in relation to their next screening EUS, and that respondents’ cancer worries decreased significantly over time. Both anxiety and depression scores remained stable or low over the 3-year period, and having a family history of PC under the age of 50 was a predictor of cancer worries experienced after 2 years of surveillance [33, 37]. A recent systematic review [38] that also included our preliminary results presented in abstract form [39], encompassing six cohort studies and one cross-sectional study came to the conclusion that, although screening may not always be reassuring, in high-risk individuals screening does result in positive psychological outcomes.
This study shows that in those high-risk individuals who reported the greatest level of psychological distress as indicated by the NPCQ score at baseline, implementation of this screening program had significant positive effects short term (1 month) as well as long term (1 year). This supports the findings of Hart et al. who demonstrated, through using the IES to measure psychological distress, that those individuals with the greater baseline distress showed a significant decrease in cancer-related intrusive thoughts over time [40]. They showed age was an important factor in determining psychological response to screening. Our study identified a significant negative correlation between increasing age and total NPCQ score and emotional and social subscale scores at 1-month post-intervention. This demonstrates that older participants have lower psychological distress post-screening.
The results of this study show that there was a significant decrease in total IES score, as well as both avoidance and intrusion subsets at 1 year compared to baseline in those with an abnormal EUS. To the authors’ knowledge this is the first time this phenomenon has been documented in the literature. Konings et al. were able to identify no significant difference in cancer worry scores in post-detection of a pancreatic cystic lesion on EUS [33]. The results of this study take this further, by demonstrating that there is a positive psychological effect of lesion detection on EUS during screening as demonstrated by the significant decrease in IES at 1 year compared to pre-intervention.
The results of this study, although assessing PC, can be compared to previous studies that have assessed the effect of screening programs for other types of cancers. A recent review assessing the psychological burden associated with lung cancer screening demonstrated that although there may be a short-term adverse psychological burden associated with screening, particularly in those participants who receive a false positive result, these adverse outcomes diminish over time [41]. This is similar in HBOC syndrome, where screening is not reported to create anxiety in participants if the result is normal; however significant anxiety in the short-term has been reported in those requiring further investigation [42, 43]. Furthermore, in the case of prostate cancer, 96.8% of screening participants with no history of prostate cancer were reassured, while a large Norwegian study assessing the psychological impact of colorectal cancer screening demonstrated no significant psychological distress associated with participating in the screening program or receiving a positive result [44, 45]. Although the general consensus favours a positive psychological outcome associated with cancer screening programs, individuals at high risk of developing multiple tumours have been identified as a target subgroup demonstrating increased distress and lower quality of life [46].
In regards to the screening methodology, a recent study has highlighted that in those deemed to be at highest risk of developing PC, EUS is favoured compared to MRI from a personal and cost-analysis perspective [47, 48]. EUS is not without its risks, and although it is an invasive procedure, Harinck et al. and Franke et al, showed that in 88% and 93% of participants respectively, the advantages of participating in an endoscopic screening outweighed the disadvantages, while Breitkopf et al. highlighted a high level of receptivity to screening in high-risk individuals [25, 35, 49]. This is further supported by the findings of Canto et al, who showed that in those high- risk patients in whom PC is detected, the survival rate at 3 years is 85% [50]. Recently, a meta-analysis assessing the diagnostic yield of PC screening programs showed that 135 high risk patients needing to be screened to identify one patient with a high-risk lesion [51]. The results of our study highlight an increased frequency of PC high-risk lesions detected (1 lesion in 51 patients screened) and supports current international screening implementation.
To overcome some of the limitations imposed by methods used by previous studies we used a measurement tool (PCQ) designed specifically to measure the psychological consequences of screening and to determine the long-term effect of screening. Although this study provides evidence for a positive psychological outcome in those undergoing screening for PC, the size of the cohort included for assessment is a recognised limitation. Only 10% of PC cases are deemed to have a hereditary origin, and therefore this figure in itself provides a basis for a limited cohort size [52]. This limitation is not novel, with previous non-controlled studies on pancreatic screening in high-risk individuals enrolling similar if not smaller cohorts for analysis [38, 53, 54].The authors acknowledge the absence of a control group in this study as a limitation; however the absence of a control group in previous studies is well documented limitation of PC screening in high-risk individuals [38, 53, 54]. Interobserver reliability has been previously identified as a limitation of screening utilising EUS; however through the involvement of a multidisciplinary team in the review of the enrolled participants this limitation was deemed to be negligible by the study authors and was not directly assessed by this study [55].
Conclusion
This is the first study to investigate the impact of PC screening on the quality of life of the participants assessing the emotional, social and physical wellbeing. In addition, this study builds on the findings of Konings et al, who found that those individuals in whom an abnormality was detected during PC screening, there was no increase in psychological distress [33]. Furthermore, it is the first of its kind to demonstrate that in high-risk patients participating in a pancreatic screening program, those with an abnormal EUS show positive long-term psychological outcomes, demonstrated by decreased total and subgroup IES scores at 1 year. In addition to this, those with a previous cancer history demonstrated decreased total and subgroup IES scores at 1 year. For the rest of the cohort, screening did not cause any psychological harm. This study shows that in those participants with the greatest psychological distress at baseline as determined by the NPCQ score, screening was deemed to be the most efficacious producing significantly positive results in emotional, social and physical domain at 1 year post-intervention.
The implementation and continuation of a screening program in high-risk individuals is further justified by the findings of Canto et al., who reported that 90% of tumours identified during routine PC surveillance were resectable, and an increased 3-year survival rate (85% vs. 25%) for individuals with resectable disease, compared to those without surveillance [50].
Future studies should focus on the enrolment of a larger cohort for analysis as this will improve the accuracy and reliability of the results, and potentially validate the results of this study. It is intended that the results of this study will form the basis of a future study investigating the psychological impact 5-years post-enrolment. Australian screening program has expanded to the Austin Hospital, Melbourne, in 2014, and it is intended that this program will continue to expand to all Australian states to improve access to screening. This will improve barriers to screening due to travelling requirement and the generalisability of the results and potentially eliminate any selection bias attributable to a single screening location. In conclusion, this study provides evidence for the beneficial long-term psychological efficacy of PC screening in high-risk patients and justifies the continuation of the current study.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2016. CA Cancer J Clin 68(1):7–30
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11):2913–2921
Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S et al (2013) SEER cancer statistics review, 1975–2010. National Cancer Institute, Bethesda, p 12
Gilbert JW, Wolpin B, Clancy T, Wang J, Mamon H, Shinagare AB et al (2017) Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol 28(9):2067–2076
Shi C, Hruban RH, Klein AP (2009) Familial pancreatic cancer. Arch Pathol Lab Med 133(3):365–374
Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJA et al (2004) Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 64(7):2634–2638
Permuth-Wey J, Egan KM (2009) Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 8(2):109–117
Grover S, Syngal S (2010) Hereditary pancreatic cancer. Gastroenterology 139(4):1076
Tersmette AC, Petersen GM, Offerhaus GJA, Falatko FC, Brune KA, Goggins M et al (2001) Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res 7(3):738–744
Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV et al (2000) Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119(6):1447–1453
Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley J-W, Kamel I et al (2013) International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 62(3):339–347
Van Asperen C, Brohet R, Meijers-Heijboer E, Hoogerbrugge N, Verhoef S, Vasen H et al (2005) Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet 42(9):711–719
Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N et al (2010) PALB2 mutations in European familial pancreatic cancer families. Clin Genet 78(5):490–494
Takai E, Yachida S, Shimizu K, Furuse J, Kubo E, Ohmoto A et al (2016) Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 7(45):74227–74235
Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthai E et al (2011) German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 10(2):323–330
Southey MC, Goldgar DE, Winqvist R, Pylkas K, Couch F, Tischkowitz M et al (2016) PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 53(12):800–811
Lynch HT, Fusaro RM, Lynch JF, Brand R (2008) Pancreatic cancer and the FAMMM syndrome. Fam Cancer 7(1):103–112
Rebours V, Boutron-Ruault M-C, Schnee M, Férec C, Maire F, Hammel P et al (2008) Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol 103(1):111–119
Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM et al (2009) Risk of pancreatic cancer in families with Lynch syndrome. J Am Med Assoc 302(16):1790–1795
Ariyama J, Suyama M, Ogawa K, Ikari T (1986) Screening of pancreatic neoplasms and the diagnostic rate of small pancreatic neoplasms. Nihon Rinsho Jpn J Clin Med 44(8):1729–1734
Lu C, Xu C-F, Wan X-Y, Zhu H-T, Yu C-H, Li Y-M (2015) Screening for pancreatic cancer in familial high-risk individuals: a systematic review. World J Gastroenterol 21(28):8678
Capurso G, Signoretti M, Valente R, Arnelo U, Lohr M, Poley JW et al (2015) Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc 7(9):833–842
Toft J, Hadden WJ, Laurence JM, Lam V, Yuen L, Janssen A et al (2017) Imaging modalities in the diagnosis of pancreatic adenocarcinoma: a systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol 92:17–23
Raut C, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA, Vauthey JN, Lee JE, Pisters PW, Evans DB (2003) Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg 7:118–126
Breitkopf CR, Sinicrope PS, Rabe KG, Brockman TA, Patten CA, McWilliams RR et al (2012) Factors influencing receptivity to future screening options for pancreatic cancer in those with and without pancreatic cancer family history. Hered Cancer Clin Pract 10(1):8
Horowitz M, Wilner N, Alvarez W (1979) Impact of event scale: a measure of subjective stress. Psychosom Med 41(3):209–218
Zilberg NJ, Weiss DS, Horowitz MJ (1982) Impact of event scale: a cross-validation study and some empirical evidence supporting a conceptual model of stress response syndromes. J Consult Clin Psychol 50(3):407
Cella DF, Mahon SM, Donovan MI (1990) Cancer recurrence as a traumatic event. Behav Med 16(1):15–22
Cockburn J, De Luise T, Hurley S, Clover K (1992) Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. Soc Sci Med 34(10):1129–1134
Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A (1991) Psychological side effects of breast cancer screening. Health Psychol 10(4):259
Paiella S, Capurso G, Cavestro GM, Butturini G, Pezzilli R, Salvia R et al (2019) Results of first-round of surveillance in individuals at high-risk of pancreatic cancer from the AISP (Italian Association for the Study of the Pancreas) registry. Am J Gastroenterol 114(4):665–670
Signoretti M, Bruno MJ, Zerboni G, Poley J-W, Delle Fave G, Capurso G (2018) Results of surveillance in individuals at high-risk of pancreatic cancer: a systematic review and meta-analysis. United Eur Gastroenterol J 6(4):489–499
Konings IC, Harinck F, Kuenen MA, Sidharta GN, Kieffer JM, Aalfs CM et al (2017) Factors associated with cancer worries in individuals participating in annual pancreatic cancer surveillance. Fam Cancer 16(1):143–151
Douma KF, Aaronson NK, Vasen HF, Gerritsma MA, Gundy CM, Janssen EP et al (2010) Psychological distress and use of psychosocial support in familial adenomatous polyposis. Psycho-Oncology 19(3):289–298
Franke FS, Matthäi E, Slater EP, Schicker C, Kruse J, Bartsch DK (2018) German National Case Collection for familial pancreatic Cancer (FaPaCa)-acceptance and psychological aspects of a pancreatic cancer screening program. Hered Cancer Clin Pract 16(1):17
Maheu C, Vodermaier A, Rothenmund H, Gallinger S, Ardiles P, Semotiuk K et al (2010) Pancreatic cancer risk counselling and screening: impact on perceived risk and psychological functioning. Fam Cancer 9(4):617–624
Konings IC, Sidharta GN, Harinck F, Aalfs CM, Poley JW, Kieffer JM et al (2016) Repeated participation in pancreatic cancer surveillance by high-risk individuals imposes low psychological burden. Psycho-Oncology 25(8):971–978
Cazacu IM, Chavez AAL, Saftoiu A, Bhutani MS (2019) Psychological impact of pancreatic cancer screening by EUS or magnetic resonance imaging in high-risk individuals: a systematic review. Endosc Ultrasound 8(1):17
Mckay S, Gunasingam N, Meiser B, Williams DB, Stoita A (2017) Pancreatic cancer screening in high risk individuals does not have negative psychological impact in the short or long term. Gastroenterology 152(5):S277
Hart SL, Torbit LA, Crangle CJ, Esplen MJ, Holter S, Semotiuk K et al (2012) Moderators of cancer-related distress and worry after a pancreatic cancer genetic counseling and screening intervention. Psycho-Oncology. 21(12):1324–1330
Wu GX, Raz DJ, Brown L, Sun V (2016) Psychological burden associated with lung cancer screening: a systematic review. Clin Lung Cancer 17(5):315–324
Brett J, Bankhead C, Henderson B, Watson E, Austoker J (2005) The psychological impact of mammographic screening. A systematic review. Psychooncology 14(11):917–938
Brain KE, Lifford KJ, Fraser L, Rosenthal AN, Rogers MT, Lancastle D et al (2012) Psychological outcomes of familial ovarian cancer screening: no evidence of long-term harm. Gynecol Oncol 127(3):556–563
Cantor SB, Volk RJ, Cass AR, Gilani J, Spann SJ (2002) Psychological benefits of prostate cancer screening: the role of reassurance. Health Expect 5(2):104–113
Kirkøen B, Berstad P, Botteri E, Åvitsland TL, Ossum AM, De Lange T et al (2016) Do no harm: no psychological harm from colorectal cancer screening. Br J Cancer 114(5):497
Gopie JP, Vasen HF, Tibben A (2012) Surveillance for hereditary cancer: does the benefit outweigh the psychological burden?—a systematic review. Crit Rev Oncol/Hematol 83(3):329–340
Corral JE, Das A, Bruno MJ, Wallace MB (2019) Cost-effectiveness of pancreatic cancer surveillance in high-risk individuals: an economic analysis. Pancreas 48(4):526–536
Lewis ZK, Frost CJ, Venne VL (2009) Pancreatic cancer surveillance among high-risk populations: knowledge and intent. J Genet Counsel 18(3):229–238
Harinck F, Nagtegaal T, Kluijt I, Aalfs C, Smets E, Poley J-W et al (2011) Feasibility of a pancreatic cancer surveillance program from a psychological point of view. Genet Med 13(12):1015–1024
Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A et al (2018) Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 155(3):740–751
Corral JE, Mareth KF, Riegert-Johnson DL, Das A, Wallace MB (2019) Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic cancer: a meta-analysis of cohort studies. Clin Gastroenterol Hepatol 17(1):41–53
Hruban RH, Canto M, Goggins M, Schulick R, Klein AP (2010) Update on familial pancreatic cancer. Adv Surg 44:293
Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R et al (2012) Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 16(4):771–783
Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z et al (2012) Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142(4):796–804
Topazian M, Enders F, Kimmey M, Brand R, Chak A, Clain J et al (2007) Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc 66(1):62–67
Acknowledgements
We would like to thank the participants for their involvement and the medical practitioners who referred their patients to the screening program. We would like to acknowledge Prof Kathy Tucker, Prof Alan Spiegelman and Dr Lesley Andrews for their expert advice on genetic counselling and testing and Dr Marcia Canto for her contribution to the initial screening program development. A special thanks is given to Prof Andrew Biankin, Prof Anthony Gill, Ms Amber Jones and Australian Pancreatic Cancer Genome Initiative network for their support and ongoing collaboration: http://pancreaticcancer.net.au/about-collaborators. We also acknowledge Ms Skye Mackay, who was the trial coordinator until 2017 and Ms Tanya Dwarte who is the current trial coordinator. Bettina Meiser is supported through a Senior Research Fellowship Level B from the National Health and Medical Research Council.
Funding
The Clinical Research Coordinator positions were supported by PanCare Foundation.
Author information
Authors and Affiliations
Contributions
Dr O’Neill was involved in writing the manuscript and data analysis. Professor Bettina Meiser was involved in designing the psychological questionnaire and reviewing the manuscript. Dr David Williams contributed to the research design, provided revisions to ethics documents, performed screening and revised the final manuscript. Dr Sam Emmanuel was involved in statistical analysis. Dr Alina Stoita developed the screening program; prepared documents for ethics submission, assisted in participant recruitment, performed screening and provided revisions to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Robert O’Neill, Sam Emmanuel, David Williams and Alina Stoita declare they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 [5]. Informed consent was obtained from all patients for being included in the study. Bettina Meiser had a remunerated consultant role with the company Astrazeneca with respect to an unrelated project. No animal studies were carried out by the authors for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Inclusion criteria
Appendix: Inclusion criteria
Australian Pancreatic Cancer Screening Program
Inclusion criteria
High risk group 1: familial pancreatic cancer
-
a.
Aged 50–80 years (or 10 years younger than the youngest relative with PC, AND
-
b.
Member of a family with 2 or more blood relatives with PC on the same side of the family. If only 2 family members are affected, both must be an FDR of the individual being screened. If there are ≥ 3 affected family members, at least one must be an FDR of the individual being screened.
High risk group 2: Peutz–Jeghers syndrome
-
a.
Age > 30 years old and < 80 years old, AND
-
b.
Clinical diagnosis of Peutz-Jeghers Syndrome or carrier of a germline STK11 pathogenic variant.
High risk group 3: BRCA2 pathogenic variant carriers
-
a.
Age > 40 years old and < 80 years old (or 10 years younger than the youngest relative with PC) AND
-
b.
Patient is a carrier of a BRCA2 pathogenic variant AND
-
c.
There is ≥ 1 pancreatic cancer in the family (FDR or SDR, confirmed or likely carrier of the pathogenic variant)
High risk group 4: hereditary pancreatitis
-
a.
Age > 40 years old and < 80 years old (or 10 years younger than the youngest relative with PC) AND
-
b.
Previous diagnosis of Hereditary Pancreatitis or known carrier of a PRSS1 or SPINK1 pathogenic variant.
High risk group 5: PALB2 gene carrier*
-
a.
Age > 50 years old and < 80 years old (or 10 years younger than the youngest relative with PC) AND
-
b.
Patient is a carrier of a PALB2 pathogenic variant AND
-
c.
There is ≥ 1 pancreatic cancer in the family (FDR or SDR, confirmed or likely carrier of the pathogenic variant)
High risk group 6: lynch syndrome mutation carrier /hereditary non polyposis colorectal cancer mutation carrier (MLH1, PMS2, MSH6, MSH2 mutation)*
-
a.
Age > 50 years old and < 80 years old (or 10 years younger than the youngest relative with PC) AND
-
b.
Patient is a Lynch syndrome mutation carrier AND
-
c.
There is a ≥1 FDR with pancreatic cancer
High risk group 7: familial atypical multiple melanoma moles (FAMMM) syndrome (CDKN2A/p16 mutation carrier)*
-
a.
Age > 50 years old and < 80 years old (or 10 years younger than the youngest relative with PC) AND
-
b.
Patient is a carrier of p16/ CDKN2A pathogenic variant
*Groups 5, 6, 7 were added in 2018 and are not included in the current paper
Rights and permissions
About this article
Cite this article
O’Neill, R.S., Meiser, B., Emmanuel, S. et al. Long-term positive psychological outcomes in an Australian pancreatic cancer screening program. Familial Cancer 19, 23–35 (2020). https://doi.org/10.1007/s10689-019-00147-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-019-00147-3