Abstract
Fecundity selection has been hypothesized to drive the evolution of female gigantism in the orb-weaving family Nephilidae. Several species of these spiders also exhibit large amounts of variation in size at maturity in one or both sexes. In this article, we attempt to detect correlations of mean and variation in adult size at a phylogenetic scale between the sexes and with latitude. We tested six predictions derived from three broad developmental, ecological, and age structure hypotheses, using independent contrasts and a recent species-level nephilid phylogeny as well as least squares and other conventional statistics: 1. In both sexes, species with larger mean size will have greater variation in size; 2. Males and females will show correlated changes in mean size and of variation in size; 3. In both sexes, mean size will be negatively correlated with the midpoint of the latitudinal range; 4. In both sexes, tropical species will be more variable; 5. In both sexes, more widespread species will be more variable; 6. Variation in male size will be positively correlated with mean female size. In no cases were male and female development correlated, suggesting that in this lineage male and female body size evolve independently. The only significant trend detected was a positive phylogenetic correlation between variation in female size and latitude, the opposite of prediction 4. Power tests showed that in all tests of the ecological hypothesis, sample sizes were more than adequate to detect significant trends, if present. Our results suggest that evolutionary trends in juvenile development among species are too weak to be detectable in such data sets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arthropods present a unique opportunity for the study of variation in adult size because the fitness benefits of large female size at maturity are well documented in diverse groups and because variation in size can be easily determined by measurement of museum specimens. Orb-weaving spiders of the sister genera Nephila and Nephilengys (Nephilidae) are well known for their extreme sexual size dimorphism, apparently a case of female gigantism driven by strong fecundity selection (Coddington 1994; Coddington et al. 1997; Hormiga et al. 2000; Kuntner and Coddington 2009: however, see Vollrath 1980a, 1998; Vollrath and Parker 1992, 1997). Most species exhibit among- and within-population variation in adult size in both species. Varying size at maturity can dramatically affect the fitness of the individual (reviewed in Roff 2002). Variation in developmental trajectories producing differences in size at maturation may be due to one of four factors (which are not mutually exclusive): phenotypic plasticity, non-adaptive loss of canalization under stress, different selective advantages for individuals of different sizes, and local (genetically-based) adaptation. Evidence for all of these has been found in Nephila species studied in depth.
Adaptive plasticity is an important determinant of size at maturity in at least four species: N. pilipes (Robinson and Robinson 1973; Higgins 2002), N. clavipes (Vollrath 1980b; Higgins 1992, 1993, 2000), N. edulis (Uhl and Vollrath 2000) and N. clavata (Miyashita 1986, 1991). Non-adaptive developmental responses to dietary stress have been demonstrated experimentally in N. clavipes juveniles, which both over-eat (Higgins and Rankin 2001) and fail to reach maturity on low diets (Higgins and Goodnight 2010). There is also strong evidence of size-linked reproductive strategies (Christenson et al. 1985) that, in male N. edulis, produce equivalent reproductive success (Schneider and Elgar 2005). The behavioral “compensation” for maturation at different sizes may contribute to the extreme degree of variation in size in males of this species (Schneider and Elgar 2005). Lastly, many species of Nephila are widely distributed and survive in very distinct habitats with different seasonality and prey availability, and there is evidence of heritable differences in juvenile development among populations (Miyashita 1986; Higgins 2000, Higgins and Goodnight in prep).
Variation in phenotype due to plasticity and due to local adaptation have been studied primarily through comparisons within and among populations of a single species; however, an increasing number of studies has involved comparisons of multiple species in one or a few genera. Nevertheless, interspecific studies remain rare and most use closely –related species pairs to test the predicted correlation of increased plasticity with increased habitat range (e.g., Sultan et al. 1998; Sultan 2001; Dudycha 2003; Van Buskirk 2001; Richter-Boix et al. 2006). These studies cannot distinguish phylogenetic from ecological factors without a larger phylogenetic framework (Pigliucci et al. 1999). Fortunately, the recently published phylogenetic hypothesis for most nephilid species (Kuntner et al. 2008) permits us to analyze patterns of variation in adult size within and among species in a phylogenetically structured setting.
Much ecological work in diverse insects and spiders, including Nephila, has shown that a substantial portion of within- and among-population variation in adult size is likely due to diet-dependent differences in development rate interacting with variation in season length (Robinson and Robinson 1973; Toft 1976, 1983; Vollrath 1980b; Miyashita 1986; 1991; Roff 1980, 1983, 2002; Mousseau and Roff 1989; Higgins and Rankin 1996; Higgins 1992, 2000, 2002; Uhl and Vollrath 2000). While females evidently do risk zero fitness in their drive to be as large as possible, males mature at much smaller and relatively “normal” spider sizes, becoming obligate kleptoparasites of large juvenile and mature females following maturation. During early instars, the sexes are indistinguishable in appearance, behavior, and ecology. Within a cohort, therefore, males may be viewed as controls in a natural experiment in which only females pursue strategies to be as large as possible.
Species of Nephila and Nephilengys inhabit a wide range of environments. Although the majority of species in both genera are tropical, some Nephila species occur in temperate regions (notably N. clavata, found in temperate Asia, and N. edulis from southern Australia and New Zealand). While some species are distributed in a range of habitats over broad latitudinal ranges—N. clavipes is found from the southeastern United States to northeastern Argentina in habitats ranging from lowland rainforest to seasonally dry and temperate woodlands—other species are habitat specialists with moderate (N. senegalensis) or narrow (N. constricta, N. sexpunctata) latitudinal distributions. With the advantage of a phylogenetic hypothesis (Kuntner et al. 2008) and large museum collections for nearly all species of Nephilengys and Nephila, we decided to test multiple predictions concerning the evolution of mean size and variation in size in both males and females. Although Kuntner and Coddington (2009) used the same phylogenetic hypothesis to reconstruct the evolution of female and male size in all nephilid species and test their correlation, that study only used a simple measure of mean size derived from taxonomic descriptions and did not analyze variation in size. The current study thus is novel in considering measurements of individual specimens, analyzing variation in size variation component as a proxy for developmental variation, and in testing precise developmental and ecological hypotheses that attempt to explain the evolution and maintenance of sexual size dimorphism in nephilid spiders. Our predictions are based upon three hypotheses describing intrinsic developmental processes, extrinsic ecological impacts upon development, and the interaction of male and female reproductive strategies.
Developmental hypothesis
The developmental hypothesis proposes that adult size is determined primarily by the number of juvenile instars, and that individuals vary in the number of instars they pass through prior to maturation (Higgins and Rankin 1996; Higgins 2002). This produces both within-sex and between sex predictions.
Female Nephila and Nephilengys are apparently giants because they have added instars—delayed development—relative to males in the same species (Hormiga et al. 2000). Males, however, also vary in the number of juvenile instars with correlated increase in size with delayed maturation. Based on these observations, we hypothesize that the range of instars at which an individual may “opt” to mature increases as additional instars are added to the developmental trajectory, producing a positive correlation between mean size and variation in size across species in both sexes. Log-transformation of the data prior to analysis will eliminate any purely statistical correlation, allowing us to test for the biological correlation.
Between the sexes, all else being equal, genetic correlation between males and females of the same species should produce phenotypic correlation between the sexes (Lande 1980; Slatkin 1984). Moreover, we expect synchronous maturation within a population that should translate into a correlation in size if differences in size are due to changes in the number of instars. In Nephila and Nephilengys, males are obligate kleptoparasites of females. In order to survive males must find a mature or nearly mature female within a few days of maturation. This constraint suggests that if females grow to larger size by adding instars, males should delay maturation to match them, in all likelihood by adding juvenile instars.
-
Prediction 1. Within each sex, mean size will be positively correlated with variation in size.
-
Prediction 2. Between the sexes, male and female mean size and variation in size will be positively correlated.
Ecological hypothesis
It is widely accepted that more broadly distributed species, either in total geographic range or in habitat types utilized, will show greater phenotypic variation due both to plasticity and to local adaptation (e.g., Sultan et al. 1998; Sultan 2001; Dudycha 2003; Van Buskirk 2001; Richter-Boix et al. 2006). Moreover, more tropical locations will generally have longer growing seasons and females will be larger and more variable in size because delaying maturation to a later, larger instar will not carry the strong fitness penalties (end of season events) experienced by females in temperate regions, but other environmental factors may stimulate variation in the instar of maturation (Schoener and Janzen 1968; Higgins 2002). We used Kuntner’s (2005a, b, 2006, 2007) descriptions of latitudinal range and habitat to estimate ecological range for each species (i.e. “moist tropical forests” for N. constricta versus “urban… open bush, savanna… sand forest…spiny forest… and rainforest” for N. inaurata; Kuntner 2005a). These two measures are highly correlated (standard Least Squares analysis, N = 20, R2 = 0.39, F(1, 19) = 12.37, P = 0.002), but we used each in different statistical settings because the discrete character of “habitats” cannot be used in independent contrasts analyses. The absolute value of the midpoint of the latitudinal range is a continuous character that reflects the center of the distribution and determines whether a species is largely tropical or temperate.
-
Prediction 3. In both sexes, mean size will be negatively correlated with the midpoint of the latitudinal range.
-
Prediction 4. In both sexes, variation in size will be negatively correlated with the midpoint of the latitudinal range, with tropical species showing greater variation than temperate species.
-
Prediction 5. In both sexes, variation in size will be positively correlated with latitudinal range and/or number of habitats.
Development interacts with age structure
In populations with longer growing seasons, females will more often achieve their “target” size (Higgins 2000), while the relatively broad season will produce a less age-structured population where both early (smaller) and later (larger) males will locate sexually mature females (such as observed in Papua New Guinea by Higgins 2002). Key to this prediction is the observation that males must locate a female within about 3 days in order to survive (Vollrath 1980b), which would select for delayed maturation in univoltine populations and thus for more uniform size among males in temperate environments (Higgins and Goodnight, submitted).
-
Prediction 6: Variation in male size will be positively correlated with mean female size.
Materials and methods
Specimens
Our data derive from two sources. For most species, we used museum collections assembled for taxonomic revisions of all nephilid genera (for specimen details and museum depositories see Kuntner 2005a, b, 2006, 2007). These revisions included nephilid material available in more than 30 public collections worldwide, and our study used the part of this material available on loan in 2005–2007. We used one species each of Clitaetra and Herennia as outgroups, and the known diversity of the focal group, Nephila + Nephilengys. For two of 15 Nephila species, measurements were available from only one male. Although measurements of single specimens do provide estimates of mean size, SD is unknown, and such taxa were excluded from tests involving SD of male size. For four Nephila species, N. clavipes, N. edulis, N. plumipes, and N. sexpunctata, we used field observations of live specimens (augmented with museum specimens for N. sexpunctata).
Measurements
We chose the SD of leg I tibia-patella length (TPL) in mature males and females as our measure of developmental variation. These spiders cease molting at maturity, and TPL measures a rigid part of the exoskeleton and is therefore a consistent measure of size at maturity. Large-bodied Nephila and Nephilengys specimens were measured to the nearest 0.1 mm with Helios™ needle-nose dial calipers, and the small-bodied Clitaetra and Herennia were measured to the nearest 0.01 mm using a Leica stereomicroscope micrometer eyepiece. Damaged specimens lacking identifiable legs were excluded from the analyses. No laboratory-reared specimens were knowingly included in these data. Museum specimens of N. sexpunctata, N. plumipes and N. edulis are rare, and we supplemented or replaced measurement of museum specimens with field measurement of live animals (N. sexpunctata: Hilton Japyassu, pers. comm.; N. plumipes and N. edulis: Jutta Schneider and Mark Elgar, pers. comm.). For N. clavipes, we used observations of live animals from Mexico and Panama (Higgins 2000; described below). Measurement imprecision of live females in the field is ± 2% (Higgins 1993), and is likely lower for laboratory measurements of museum specimens. We recognize that the field data for the rare species N sexpunctata may not represent the full range of sizes achieved by this species, but this could also be argued for species that are rare in museum specimens. The summary data for each species are presented in Table 1.
Within-species
The field data from N. clavipes provided us with the opportunity to test for differences in amount of variation among populations within a species, and to test predictions 1 and 2, which we expect to hold within as well as among species. We had data from Gigante Peninsula, Barro Colorado National Monument, Panama (1985–1986), and six locations in Mexico (1988–1991): coastal Veracruz (3 locations), a mid-altitude temperate forest, a mid-altitude desert, and a Pacific-coast seasonally dry scrub forest (Higgins 2000). Samples from each site ranged from a low of 9 males (the desert site) to a high of 202 females (the Pacific coast site). These analyses are distinct from the Higgins (2000) analysis of the same data testing the hypothesis that male and female size varied over time within a cohort (season) within each population. Those analyses did not compare males with females, nor test for patterns in variation in size among populations, as predicted in by the Development Hypothesis. The latitudinal range and number of populations in this data set are insufficient to allow testing of the ecological hypothesis within N. clavipes.
Statistical analysis
We log-transformed TPL in the among-species data set prior to any statistical analyses to normalize the data and to eliminate purely statistical correlation between mean and SD. We calculated mean and SD of log-transformed TPL for each sex in each species (JMP V.6) and imported these values into Mesquite (v.2.6; Maddison and Maddison 2009) as continuous variables. The among-population N. clavipes data were normally distributed for each sex and required no transformation.
Within N. clavipes
We used a parametric Bartlett’s test (JMP V.6; Snedecor and Cochran 1989) on males and females separately to detect significant differences in SD among populations. Having no phylogenetic hypothesis for the relationships among these populations, we used standard least squares analysis to test for correlations between mean and SD within and between males and females across all populations.
Among species
We used a parametric Bartlett’s test (JMP V.6; Snedecor and Cochran 1989) on males and females separately to detect significant differences in SD among species. Because sample size varied by two orders of magnitude among species, we also employed a bootstrap version of the same test on the residuals remaining after a one-way analysis of variance of mean TPL with species as a factor. The residuals have the same mean for all species, but preserve the variation within species. We verified the validity of this manipulation by confirming that a parametric Bartlett’s test run on the residual data gave results identical to those obtained with the original data.
In the bootstrap analysis, we randomized the data among species to eliminate differences in SD but otherwise preserved data structure (e.g. sample sizes) and resampled the data for each sex 10,500 times with replacement. Thus, a Bartlett’s test on these data presents what the test statistic would equal if no true differences existed. Because of small sample sizes, and sampling with replacement, in some cases the same individual of a species could have been assigned to all of its slots. In that case the variance for that species would be zero, and the Bartlett’s statistic (χ2) would be infinity. For the female data set, 82 (0.78% of the bootstrap samples) had a χ2 = infinity and for the male data set, 40 (0.38% of the bootstrap samples) had a χ2 = infinity. These cases were not included in the analyses. For each sex, we compared the actual test statistic to the ranked bootstrap values. Since these analyses showed that SD varied significantly among species, we calculated Welch’s unequal-variances F-statistic to determine whether mean size varied among species.
We tested for phylogenetic corrected correlation of mean and SD across species within each sex using PDAP for Mesquite v. 2.6 (Midford et al. 2007), mapping each onto the phylogeny of Kuntner et al. (2008) under squared change parsimony (Figs. 1 and 2; Maddison and Maddison 2009). There are 21 species involved in these analyses, and the phylogenetic hypothesis was constructed using morphological and behavioral traits (Kuntner et al. 2008). Thus, we have no genetic measure of branch length such as would be provided in molecular phylogenies. We therefore tested 11 different branch length transformations/assumptions (Table 2). Of these, five passed the PDAP diagnostic test, meaning that there was no correlation between character value and branch length (Garland et al. 1999; Garland and Ives 2000; indicated by asterisks in Table 2). In one case (model #8), the midpoint of the geographic range was nearly significantly correlated with branch length (P = 0.054) but this is due to the branch with two narrowly distributed species (N. turneri and N. komaci) being an outlier, and we decided to include this model in subsequent analyses.
Mean (left tree) and SD (right tree) of log-transformed tibia-patella length of adult female nephilid spiders mapped on the phylogeny of Kuntner et al. (2008)
Mean (left tree) and SD (right tree) of log-transformed tibia-patella length of adult male nephilid spiders mapped on the phylogeny of Kuntner et al. (2008). Species for which no standard deviations are reported as unknown (?)
We used Felsenstein’s (1985) independent contrasts analyses as implemented in PDAP (Garland and Ives 2000) to test the two developmental and ecological hypotheses for both males and females (predictions 1–5), and the combined hypothesis predicting correlation of male variation with female mean size (prediction 6). Because of the small number of species, and because there are no power tests available within the PDAP framework, we also ran standard least squares analyses to test these predictions. To present a more intuitive statement of the power of each test, we converted the least significant value of the slope (LSV) to the least detectable correlation between the two variables (r) using the following equation: r = [LSV * SD (x)]/SD (y).
Results
We start our test of the three hypotheses by determining whether there are differences in the amount of variation among populations of N. clavipes and among species of Nephilengys and Nephila.
Within N. clavipes, females of all populations have the same SD (Bartlett’s test F(6, 548) = 1.48, P = 0.18) despite significant differences in mean size among populations (ANOVA F(6, 548) = 74.52, P < 0.001) as has been reported before (Higgins 1992, 2000). The spiders in the coastal Veracruz population of Los Tuxtlas (sampled in 1986) were the largest, and the spiders in the mid-altitude desert, Tehuacan, Puebla (sampled 1989–1990), were the smallest. A posteriori comparisons show that this significance is not due to a single out-lying population.
Males from these seven sampling locations show significant among-site differences in the amount of variation in size (Bartlett’s test F(6, 435) = 2.58, P = 0.0169). Importantly, this was not due to the sample from the bivoltine population of Gigante (which data we combined across generations for these tests): the most variable males were from Los Tuxtlas and the least variable from Tehuacan and Chamela. A non-parametric Welch ANOVA, appropriate due to the heteroscedasticity of the data, showed significant among-site differences in mean size of males (F(6, 435) = 16.75, P < 0.001). Mild heteroscedasticity such as found in these data does not generally have a great effect on parametric ANOVA. Therefore, we ran a parametric ANOVA to do a posteriori tests for patterns of male size among sites. This analysis showed that the Pacific coast site of Chamela and the Veracruz coast site of Nanciyaga tended to have large males, the desert site of Tehuacan had small males, and the remaining sites had similarly-sized males.
Among species, we detected significant differences in variation in both males and females using the parametric Bartlett’s test (males: Bartlett’s χ2 = 166.2, df = 8, P < 0.0001; females: Bartlett’s χ2 = 261.0, df = 10, P < 0.0001). The bootstrap version of this test was repeated 5,000 times with replacement for each sex. The largest bootstrap χ2 values observed (males: 28.6, females 88.8) were an order of magnitude less than the observed χ2 value in both sexes confirming the results of the parametric analysis, that the probability of observing differences this great or greater when there was no true difference is less than P < 0.0001.
Developmental hypothesis
The females of Nephila and Nephilengys are famous for their large size relative to males of the same species, which is due to the addition of juvenile instars during female development. Increased range of instars prior to sexual maturation should produce increased variation in adult size. In addition, developmental changes in male and female size should be correlated over evolutionary time (Lande 1980; Slatkin 1984).
Within each sex, mean size will be positively correlated with variation in size
Within N. clavipes, this hypothesis can only be tested for males because females showed no differences in SD of size across these populations. In males, mean size was not significantly correlated with SD. Moreover, the (non-significant) relationship between mean size and SD across these sampling sites was negative, the opposite that would be found if variation within a population was a simple function of mean size.
Among species, female mean size was significantly correlated with SD in female size under two branch length models, both with the outgroups excluded (#5: R2 = 0.18, df = 17, P = 0.035; #8: R2 = 0.20, df = 17, P = 0.028). However, removal of N. constricta, which is an outlier due to extreme variation, eliminated the significance (#5: R2 = 0.081, df = 16, p = 0.13; #8: R2 = 0.072, df = 16, P = 0.14). Moreover, the correlation detected between mean and SD is in the opposite direction from that predicted by our hypothesis: SD declined with increasing mean female size in these analyses. Least squares analysis showed no detectable correlation with or without N. constricta (with: R2 = 0, slope = −0.003, F(1, 19) = 0.048, P = 0.95; without: R2 = 0, slope = −0.007, F(1, 18) = 0.047, P = 0.83; outgroups excluded). The least detectable correlation with these data is greater than 1, indicating that these data provide a weak test of this prediction, probably because the differences in mean size greatly exceed the differences in SD among species.
Male SD did not co-vary with male mean size in any of the branch length models (all P ≥ 0.13). Least squares analysis with outgroups excluded showed a detectable but non-significant correlation (R2 = 0.14, P = 0.13) with a positive slope (slope = 0.13, F(1, 16) = 2.54). The power test showed that this test also was weak (correlation of less than 1 undetectable), again probably because mean size varied much more than SD of size among species.
Between the sexes, male and female mean size and variation in size will be correlated
Within N. clavipes, this prediction can only be tested for mean size, as females show no among-population differences in SD of size (above). Linear regression of mean male size on mean female size showed a non-significant positive relationship (slope = 0.19; F(1, 6) = 0.77, P = 0.42).
Among species, the mean sizes of males and females were not phylogenetically correlated under any of the models (all P ≥ 0.064). Least squares analysis showed a positive but non-significant correlation between male and female mean size (slope = 0.21, R2 = 0.07, F(1,18) = 1.30, P = 0.26). The smallest detectable correlation was 0.8, likely due to the greater variation of mean size in females compared to males.
The SD of size in males and SD of size in females were not phylogenetically correlated under any of the tree models (all P ≥ 0.15). Least squares analysis showed a positive but non-significant correlation between variation in male size and variation in female size (slope = 0.19, R2 = 0.01, F(1,16) = 0.14, P = 0.07). This test was weak, and only a perfect correlation (r = 1) between males and females could be reliably detected with the available data.
Ecological hypothesis
We predicted that the more tropical species would have larger mean size, and the more broadly distributed species, either in total geographic range or in habitat types utilized, would show greater size variation, due either to plasticity, local genetic adaptation, or both.
In both sexes, mean size will be negatively correlated with the midpoint of the latitudinal range
Under no branch-length model was mean female size correlated with the midpoint of the latitudinal range (all P ≥ 0.33). The least squares test for a correlation of female size with the midpoint of the distribution likewise revealed no correlation of mean female size with latitude (R2 = 0.04, F(1, 19) = 0.69, P = 0.41, slope = −0.008). In contrast to the developmental hypotheses, this test is quite powerful: the least detectable correlation with these data is 0.049.
Similarly, mean male size was not correlated with the midpoint of the latitudinal range of the species. Within a phylogenetic framework, Felsenstein correlations were non-significant across all trees (all P ≥ 0.39). The least squares analysis was likewise non-significant (R2 = 0.05, F(1, 18) = 1.02, P = 0.33, slope = 0.009). These data could have detected a correlation of 0.09.
In both sexes, variation in size will be negatively correlated with the midpoint of the latitudinal range, with tropical species showing greater variation than temperate species
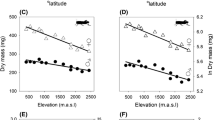
The variation in female size (SD) was positively associated with the midpoint of the range under all but one branch-length model (#4, 5, 7 and 8 R2 ≥ 0.12, df = 17 or 19, P = 0.07; tree #3 R2 = 0.016, df = 19, P = 0.29). Because N. constricta was an outlier in these analyses (Fig. 3), as it is a very narrowly distributed tropical specialist with extraordinarily high variation in adult female size (Kuntner 2005a), we ran these tests on branch-length models #4, 5, 7 and 8 without N. constricta. With its removal, all branch-length models showed a significant positive evolutionary correlation between SD in female size and the latitudinal midpoint of the distribution: more temperate species are more variable (Table 3). This result only holds in a phylogenetic context: the least squares test for a correlation of female size with the midpoint of the distribution revealed no correlation of variation in size with the midpoint of the distribution (R2 = 0.06, F(1, 19) = 1.22, P = 0.28, slope = −0.002). The test is powerful, as a correlation of 0.03 would be detectable with these data. Removing N. constricta from the least squares analysis of SD did not change the results (R2 = 0.00, F(1, 19) = 0.02, P = 0.89, slope = 0.0002).
Unlike in females, there was no correlation of the variation in male size with the midpoint of the geographic range in any analysis. Within a phylogenetic framework, Felsenstein correlations were non-significant across all trees (all P ≥ 0.21). Male N. constricta is not an outlier, and we did not drop this taxon from these analyses. The least squares analysis was likewise non-significant (R2 = 0.04, P = 0.42, F(1, 16) = 0.70, slope = 0.003). The least detectable correlation is 0.09.
In both sexes, variation in size will be positively correlated with latitudinal range and/or number of habitats
In neither sex was there a detectable phylogenetic correlation of variation in size with latitudinal range under any tree model (females: all P ≥ 0.19; males: all P ≥ 0.19). Since latitudinal range was used in these analyses as a proxy measure of the range of habitats, which has been shown to be correlated with developmental plasticity in a range of organisms, we used the actual number of habitats from which specimens have been collected for each species in the standard least squares analyses (Kuntner 2005a). As noted in the methods, these two measures of habitat range are highly positively correlated. The least squares analyses showed no correlation of variation in size in either sex with the number of habitats (females: R2 = 0.00, P = 0.82, F(1, 19) = 0.054, slope = −0.003; males: R2 = 0.02, P = 0.58, F(1, 16) = 0.32, slope = 0.01). The power test shows that this is a strong test for females (least detectible r = 0.03) but less strong for males (least detectible r = 0.10).
Development interacts with age structure
We predicted that in species inhabiting less seasonal environments, females would be larger and there would be relaxed selection for delayed male maturation, so males would mature at a wider range of sizes.
Variation in male size will be positively correlated with mean female size
There was no detectable phylogenetic correlation between mean female size and variation in male size at maturity under any of the tree models used (all R2 < 0.08, P ≥ 0.15). These results held in the least squares analysis (R2 = 0.02 P = 0.61, F(1, 17) = 0.27, slope = 0.03). This is not a powerful test of the prediction, with the least detectable r = 1. Again, this appears related to the difference in amount of variation among species in the two sexes and the two measures: there are greater differences among species in mean female size than in SD of male size.
Discussion
We initiated this study to test three hypotheses, not mutually exclusive, describing the evolution of mean size and variation in size in two genera of Nephilidae spiders with female gigantism. Developmentally, variation in size within a species was expected to covary with mean adult size because, in a species that has increased mean size through additional juvenile stages, an individual growing under poor conditions may retain the ability to “opt out” at a smaller, earlier instar. Moreover, genetic correlation between males and females (Lande 1980; Slatkin 1984) leads to the prediction that male and female development will covary. Ecologically, classic patterns of variation among species with different geographic ranges have supported various adaptational explanations of arthropod development (e.g. Schoener and Janzen 1968). In an arthropod restricted to a single generation per year, species in tropical habitats should be larger and less variable than those in temperate habitats due to the increased duration of the growing season (Higgins 2000). Species inhabiting a wide range of habitats should exhibit greater variation, through local adaptation and because juvenile development interacts with differences in food availability and season length (e.g., Sultan et al. 1998; Sultan 2001; Dudycha 2003; Van Buskirk 2001; Richter-Boix et al. 2006). Lastly, we expected an interaction between male and female development in different ecological circumstances. Because feeding males are obligate kleptoparasites (stealing from females because after maturity they can no longer spin sticky silk; Vollrath 1980b; Christenson and Goist 1979) and only larger juvenile females will tolerate male kleptoparasitism, males are under selection to delay maturation (Higgins and Goodnight submitted). This selection should be relaxed in tropical sites, where populations are less age structured (e.g. N. pilipes, see Higgins 2002). Because we also expect greater mean female size in tropical populations, we predicted a positive correlation of female size with variation in male size.
These tests are only as reliable as the phylogenetic framework used for the analyses. The phylogeny used in these analyses is based upon morphological and behavioral traits (Kuntner et al. 2008), as the accumulation of genetic data is only just underway (Kuntner pers. comm.). No genetic measures of branch length are available, and therefore, we ran all analyses on five different models of branch length transformation (see also Kuntner and Coddington 2009). Results across the different models were consistent and are combined for the remainder of this discussion. Because of the small number of species, we also ran least-squares analyses for all predictions, which allowed us to construct power tests. In all analyses, only one correlation—female SD versus the latitudinal midpoint of the range—was significant, and that was only significant in the phylogenetically-informed tests. The power tests performed as part of the least-squares analyses are therefore important for the interpretation of our results.
Variation in adult size is an evolutionarily labile character in both sexes, as is size itself, particularly in male spiders of highly dimorphic species (Kuntner and Coddington 2009). We do not believe that the significant differences in SD among species are due to sampling error: Both parametric and boot-strapped Bartlett’s tests show significant among-species differences in the amount of variation in adult size, and the boot-strapped version of the test would not be significant if variation in sample size among species was the cause. With documented variation among species in adult size, we could test for evolutionary patterns predicted by diverse hypotheses.
The predictions based upon the developmental hypothesis were all rejected in this analysis. In both sexes, mean and variation in size (prediction 1) are evolving independently. A similar result stemmed from a phylogenetic analysis of variation in size at maturation among Arabidopsis species (Pigliucci et al. 1999; Pollard et al. 2001). That male development is evolving independently from female development in this group (prediction 2) is seen both within N. clavipes and across species. Among the nephilid spiders, males and females are clearly under very different selective regimes with regard to optimal body size (Lande 1980; Slatkin 1984; Roff 2002; Kuntner and Coddington 2009). Female arthropods can increase fecundity via increased body size (e.g., Darwin 1871; Head 1995; Nylin and Gotthard 1998), and fecundity selection appears to be driving female gigantism in this group: in N. pilipes, one additional instar increases fecundity over 150% (Higgins 2002). Kuntner and Coddington (2009) showed that across species, female Nephila have gradually increased from large to enormous while males did not show any evolutionary trend. Rather, male size repeatedly increased and decreased on fairly small phylogenetic scales. The selective forces maintaining small male size despite strong selection on large females are poorly understood (reviewed in Foellmer and Moya-Laraño 2007), but are likely related to the balance of natural versus sexual selection (Kuntner and Coddington 2009; Higgins and Goodnight submitted). Compared with previous studies (e.g. Higgins 2002; Kuntner and Coddington 2009) we attempted to find more precise factors, related to spiders’ ecology, geography and development, that were hypothesized to correlate with the evolution of size on both genders. The pervasive pattern of nonsignificant correlations is challenging to explain.
The tests of the predictions from the developmental hypothesis and the predicted correlation of developmental strategies with female age structure are uniformly weak. Multiple uncontrolled sources of variation could affect these statistics: non-random sampling, variable sample sizes, within and between-year variation in climate (sun, temperature, rainfall), ecological succession, land-use trends over decades, geography, food resource variation, and no doubt other unidentified factors. Moreover, the different magnitude of size and variation in size between giant females and dwarf males may obscure any actual correlation between the sexes. The weakness of the tests of the developmental hypotheses indicate that these uncontrolled sources of variation may be an important part of these results.
Schoener and Janzen (1968) were among the first to discuss latitudinal patterns of arthropod size based on broad comparisons of temperate and tropical insect communities. As expected, those data show that the mean size of insects and the variation in insect size are both greater in tropical habitats. Likewise, Higgins (2002) found that mean female size in eight species of Nephila was larger in tropical regions. We expected to find this same ecological pattern in the current study. However, all but one prediction from the ecological hypothesis were rejected for both males and females: in neither sex did size vary with latitude. Moreover, these negative results appear robust. The results were consistent across all branch-length models and between the PDAP and least squares analyses, and the power tests indicate that very slight correlations would be detectible with our data. There are two major differences between this study of nephilid size and the prior study of Higgins (2002). First, the prior analysis used field data from individual populations, and each species was represented by only a single population. Second, the prior analysis did not include all species of nephilid spiders. It is possible that the 2002 analysis reflects primarily an among-populations signal (missing in the analysis of N. clavipes reported here due to the narrow geographic range of populations) that was misinterpreted as an among-species difference. Perhaps mean body size primarily evolves locally rather than at the species level, so that species means will not be correlated with midpoints of distribution. The failure to detect the Schoener/Janzen pattern in body size among these species may also be due to our data being primarily tropical/subtropical and relatively sparse both phylogenetically and in terms of sample size.
The only ecological pattern detected by the current analysis is increasing variation in female size with increasing latitude. The interaction of female developmental responses to food availability with the end of the season in higher-latitude, more seasonal habitats may explain this correlation. In more seasonal locations, well-fed, rapidly-growing individuals can pass through many instars and mature at a large size before the season ends, while poorly-fed, slowly growing individuals pass through few instars and mature at a small size, making the “best of a bad lot” (Dawkins 1980; e.g., Nylin et al. 1996; Miyashita 1986, 1991; Schneider 1997; Higgins 2000). Females of tropical species will more often achieve their target (maximum or optimal) body size because there is no “end of season” event curtailing development regardless of foraging success and thus these species show less variation (Higgins and Rankin 1996). Males do not show this pattern because all males mature at about half the number of instars compared to females, long before the end of the season even in high-latitude populations: males can achieve their “target” size even under conditions of low food availability. This explanation, however, fails to account for the variation seen among females in the tropical African species N. constricta or males of the Australian N. edulis. Both are far more variable than other species at similar latitudes: clearly, other factors are at work in determining individual size in these species.
The comparison of body sizes among species of arthropods with known phylogenetic relationships ought to provide a strong framework for testing for ecological and developmental patterns in the evolution of juvenile developmental strategies. On the other hand, replicated, strictly controlled studies of the life cycles of multiple species throughout globally distributed environments would be difficult and expensive to obtain. Our data instead exemplify easily and cheaply available comparative data: body size and habitat/seasonality as estimated from museum specimens and their localities. Our predictions were nearly entirely rejected by our analyses, and the tests for ecological patterns in female size were particularly strong. It may be that while these relatively simple ecological hypotheses for the evolution of size and variation in size can explain differences among populations within species and among small groups of closely related species, they are inadequate to explain the evolution of size and variation on a larger evolutionary scale. The alternative explanation, that the phylogeny we built our analyses upon is utterly wrong, is unlikely, because other evolutionary analyses have used the same topology getting significant and expected results (Kuntner et al. 2009; Kuntner and Coddington 2009). Our results at least suggest that evolutionary trends in developmental plasticity among species with distinct ecologies are relatively weak, too weak to be detectable in such data sets. Because experimentally-based and field-based comparative data would be so difficult to get, this study should be replicated in other lineages with similar sexual size dimorphism and ecological variability to test whether these negative results are general.
References
Christenson TE, Goist KC (1979) Costs and benefits of male-male competition in the orb-weaving spider, Nephila clavipes. Behav Ecol Sociobio 5:87–92
Christenson TE, Brown SG, Wenzel PA, Hill EM, Goist KC (1985) Mating behavior of the golden-orb-weaving spider, Nephila clavipes: I. Female receptivity and male courtship. J Comp Psych 99:160–166
Coddington JA (1994) The roles of homology and convergence in studies of adaptation. In: Eggleton P, Vane-Wright RI (eds) Phylogenetics and ecology. Academic Press, London, pp 53–78
Coddington JA, Hormiga G, Scharff N (1997) Giant female or dwarf male spiders? Nature 385:687–688
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Dawkins R (1980) Good strategy or evolutionarily stable strategy? In: Barlow GW, Silverberg J (eds) Sociobiology: beyond nature/nurture?. Westview Press, Boulder, pp 331–367
Dudycha JL (2003) A multi-environment comparison of senescence between sister species of Daphnia. Oecologia 135:555–563
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Foellmer MW, Moya-Laraño J (2007) Sexual size dimorphism in spiders: patterns and processes. In: Fairbairn DJ, Blanckenhorn WU, Szekely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 71–81
Garland T, Ives AR (2000) Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–364
Garland T Jr, Midford PE, Ives AR (1999) An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral states. Am Zool 39:374–388
Head G (1995) Selection on fecundity and variation in the degree of sexual size dimorphism among spider species (Class Araneae). Evolution 49:776–781
Higgins LE (1992) Developmental plasticity and fecundity in the orb-weaving spider Nephila-clavipes. J Arachnol 20:94–106
Higgins L (1993) Constraints and plasticity in the development of juvenile Nephila clavipes in Mexico. J Arachnol 21:107–119
Higgins L (2000) The interaction of season length and development time alters size at maturity. Oecologia 122:51–59
Higgins L (2002) Female gigantism in a New Guinea population of the spider Nephila maculata. Oikos 99:377–385
Higgins L, Goodnight C (2010) Nephila clavipes females have accelerating dietary requirements. J Arachnol 38:150–152
Higgins LE, Rankin MA (1996) Different pathways in arthropod postembryonic development. Evolution 50:573–582
Higgins LE, Rankin MA (2001) Mortality risk of rapid growth in the spider Nephila clavipes. Func Ecol 15:24–28
Hormiga G, Scharff N, Coddington JA (2000) The phylogenetic basis of sexual size dimorphism in orb-weaving spiders (Araneae, Orbiculariae). Sys Bio 49:435–462
Kuntner M (2005a) Systematics and evolution of nephilid spiders (Araneae, Nephilidae new rank). George Washington University, Washington, DC
Kuntner M (2005b) A revision of Herennia (Araneae, Nephilidae, Nephilinae), the Australasian “coin spiders”. Invert Syst 19:391–436
Kuntner M (2006) Phylogenetic systematics of the Gondwanan nephilid spider lineage Clitaetrinae (Araneae, Nephilidae). Zool Scr 35:19–62
Kuntner M (2007) A monograph of Nephilengys, the pantropical ‘‘hermit spiders’’ (Araneae, Nephilidae, Nephilinae). Syst Entomol 32:95–135
Kuntner M, Coddington JA (2009) Discovery of the largest orbweaving spider species: the evolution of gigantism in Nephila. PLoS ONE 4(10):7516. doi:10.1371/journal.pone.0007516
Kuntner M, Coddington JA, Hormiga G (2008) Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24:147–217
Kuntner M, Coddington JA, Schneider JM (2009) Intersexual arms race? Genital coevolution in nephilid spiders (Araneae, Nephilidae). Evolution 63:1451–1463
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–307
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 2.6. http://mesquiteproject.org
Midford PE, Garland Jr T, Maddison WP (2007) PDAP: PDTREE Package of Mesquite, Version 1.11. http://mesquiteproject.org/pdap_mesquite/
Miyashita T (1986) Growth, egg-production, and population-density of the spider, Nephila clavata in relation to food conditions in the field. Res Pop Ecol 28:135–149
Miyashita T (1991) Direct evidence of food limitation for growth rate and body size in the spider Nephila clavata. Acta Arachnol 40:17–21
Mousseau TA, Roff DA (1989) Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43:1483–1496
Nylin S, Gotthard K (1998) Plasticity in the life-history traits. Ann Rev Entom 43:63–83
Nylin S, Gotthard K, Wiklud C (1996) Reaction norms for age and size at maturity in Lasiommata butterflies: predictions and tests. Evolution 50:1351–1358
Pigliucci M, Cammell K, Schmitt J (1999) Evolution of phenotypic plasticity a comparative approach in the phylogenetic neighbourhood of Arabidopsis thaliana. J Evol Biol 12:779–791
Pollard H, Cruzan M, Pigliucci M (2001) Comparative studies of reaction norms in Arabidopsis. I. Evolution of response to daylength. Evol Ecol Res 3:129–155
Richter-Boix A, Llorente GA, Montori A (2006) A comparative analysis of the adaptive developmental plasticity hypothesis in six mediterranean anuran species along a pond permanency gradient. Evol Ecol Res 8:1139–1154
Robinson MH, Robinson B (1973) Ecology and behavior of the giant wood spider Nephila maculata (Fabricius) in New Guinea. Smithson Contrib Zool 149:1–73
Roff DA (1980) Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia 45:202–208
Roff DA (1983) Phenological adaptation in a seasonal environment: a theoretical perspective. In: Brown VK, Hodek I (eds) Diapause and life cycle strategies in insects. Springer, New York, pp 253–270
Roff DA (2002) Life history evolution. Sinauer Associates, Massachusetts
Schneider JM (1997) Timing of maturation and the mating system of the spider, Stegodyphus lineatus (Eresidae): how important is body size? Biol J Linn Soc 60:517–525
Schneider JM, Elgar MA (2005) The combined effects of pre- and post-insemination sexual selection on extreme variation in male body size. Evol Ecol 19:419–433
Schoener TW, Janzen DH (1968) Notes on environmental determinants of tropical versus temperate insect size patterns. Am Nat 102:207–224
Slatkin M (1984) Ecological causes of sexual dimorphism. Evolution 38:622–630
Snedecor GW, Cochran WG (1989) Statistical methods. Blackwell Publishing, Oxford
Sultan SE (2001) Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology 82:328–343
Sultan SE, Wilczek AM, Hann SD, Brosi BJ (1998) Contrasting ecological breadth of co-occurring annual Polygonum species. J Ecol 86:363–383
Toft S (1976) Life-histories of spiders in a Danish beech wood. Nat Jutlandica 19:5–40
Toft S (1983) Life cycles of Meta segmentata (Clerck 1757) and Meta mengei (Blackwall 1869) in western Europe (Arachnida: Araneae: Tetragnathidae). Verhandlungendes Naturwissenschaftlichen Vereins in Hamburg 26:265–276
Uhl G, Vollrath F (2000) Extreme body size variability in the golden silk spider (Nephila edulis) does not extend to genitalia. J Zool 251:7–14
Van Buskirk J (2001) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160:87–102
Vollrath F (1980a) Why are some spider males small? A discussion on Nephila clavipes. In: Gruber J (ed) Verhandlungen. 8. Internationaler Arachnologen—Kongress abgehalten ander Universitat fur Bodenkultur Wien. H. Egermann, Vienna, pp 165–169
Vollrath F (1980b) Male body size and fitness in the web-building spider Nephila clavipes. Z Tierpsychol 53:61–78
Vollrath F (1998) Dwarf males. TREE 13:159–163
Vollrath F, Parker GA (1992) Sexual dimorphism and distorted sex ratios in spiders. Nature 360:156–159
Vollrath F, Parker GA (1997) Reply to ‘Giant female or dwarf male spiders?’. Nature 385:688
Acknowledgements
We are grateful to many people for help in the preparation of this manuscript, especially numerous patient curators. Financial support during the collection of data and preparation of the manuscript came from the US National Science Foundation with grants to LEH (INT-1235778) and JC (PEET grant DEB-9712353 and EAR-0228699), from the Slovenian Research Agency to MK and JC (ARRS grants Z1-7082-0618, BI-US/06-07-026) and the European Commission’s 6th FP to MK (MIRG-CT-2005 036536). From the Smithsonian NMNH, D. DeRoche helped with the specimen management and K. Darrow prepared all the figures. Specimen measurements and data entry were done in part by M. Gregorič (Ljubljana) and A. Gallant Bernstein, J. Madden, R. Webber, B. Mulcahey and S. Wanamaker at UVM. Additional data were graciously contributed by J. Schneider, M. Elgar, and H. Japyassu. T. Garland and P. Midford helped us navigate the intricacies of PDAP within Mesquite. Conversations with K. Pickett also aided in our understanding of the statistics employed in these analyses. Comments from several anonymous reviewers were very helpful in preparation of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higgins, L., Coddington, J., Goodnight, C. et al. Testing ecological and developmental hypotheses of mean and variation in adult size in nephilid orb-weaving spiders. Evol Ecol 25, 1289–1306 (2011). https://doi.org/10.1007/s10682-011-9475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9475-9