Abstract
Dry root rot (DRR) caused by Rhizoctonia bataticola [(Taub.) Butler] is an emerging disease of chickpea (Cicer arietinum L.) and a serious constraint to chickpea production in warm and arid regions. To identify the genomic regions conferring resistance to DRR, a total of 182 F9 derived Recombinant Inbred Lines (RILs) were developed from the cross between a susceptible line BG 212 and moderately resistant breeding line ICCV 08305. The parental lines and RILs were screened against Rb 6 isolate of R. bataticola using paper towel method under controlled environment at ICRISAT during 2016 and 2017. The RILs were genotyped with cost-effective SNP genotyping platform, Affymetrix® Axiom®CicerSNP array. As a result, a high-density genetic map with 13,110 SNP markers spanning 1224.11 cM with an average inter marker distance of 0.09 cM was developed. A single minor QTL (‘qDRR-8’) explaining 6.70% PVE with LOD scores 3.34 was identified on CaLG08 for DRR resistance which could be further explored for mining candidate genes and the linked SNP markers could be further validated for application in marker-assisted selection of DRR resistance in chickpea breeding programs.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is a self-pollinated (2n = 2x = 16), cool season food legume, grown over an area of 17.81 million hectares with a production of 17.19 million tonnes and productivity of 965 kg per hectare (FAOSTAT 2018). Chickpea is the second most important food legume crop in the world after dry beans (Phaseolus vulgaris L.) in terms of annual area and production. India is the largest chickpea producing country with a share of 61.4% (11.38 million tonnes) in production and 65.5% (11.89 million hectares) in area (FAOSTAT 2018). Chickpea seeds are highly nutritious, contain 20–22% protein and 60% carbohydrate (Gil et al. 1996), rich in minerals (phosphorous, calcium, magnesium, iron and zinc), fiber, unsaturated fatty acids and β-carotene, which are important in human nutrition (Williams and Singh 1987; McIntosh and Miller 2001; Jukanti et al. 2012). Since the crop is largely grown under rainfed conditions in the post-rainy season, chickpea often experiences terminal stresses (drought, temperature extremes) which limit its yield potential (Khanna-Chopra and Sinha 1987; Gaur et al. 2008, 2019). Chickpea is a low-input crop, grown extensively in the moisture stress environments on residual soil moisture in semi-arid regions. Chickpea production is largely constrained by both biotic and abiotic stresses (Gaur et al. 2007, 2008, 2019). Among the biotic constraints, dry root rot (DRR) is increasingly becoming a major threat to chickpea production under rainfed ecologies worldwide (Sharma et al. 2010, 2016; Ghosh et al. 2013).
DRR of chickpea is caused by soil borne necrotrophic fungus Rhizoctonia bataticola (Taub.) Butler [Synonyms: Macrophomina phaseolina (Tassi) Goid]. The pathogen is a facultative sporophyte, a soil-inhibiting organism which is more prevalent at higher temperatures mainly in dry and warm regions (Sharma and Pande 2013). In R. bataticola, high levels of pathogenic and genetic variation were reported from different regions of the world (Tripathi and Sharma 1983; Trivedi and Gurha 2006; Aghakhani and Dubey 2009). The severity of DRR disease in chickpea rapidly increases when crop is exposed to high day temperature of above 30 °C and dry soil conditions (deficit soil moisture condition i.e. 60% or less) at flowering and podding stages (Gurha et al. 2003; Sharma and Pande 2013). Leaves and stems of the affected plants become straw coloured and lower leaves turn brown. Tap root turns black and devoid of lateral roots.
Incidence of DRR in chickpea was first reported in India (Mitra 1931), followed by Iran (Kaiser et al. 1968), USA (Westerlund et al. 1974) and several countries in Asia and Africa (Nene et al. 1996; Ghosh et al. 2013). Until recently, DRR was not a major concern in the chickpea growing areas. With changing climatic conditions, particularly longer drought spells, DRR could cause yield losses up to 30–40% under rainfed conditions (Sharma et al. 2016). Many economically important crops are predisposed to the infection and colonisation of R. bataticola under hot and dry environmental conditions can cause drastic yield losses on chickpea (Thripathi and Sharma 1983), soybean (Pearson et al. 1984) and sunflower (Nawaz 2007). Some studies reported the variability in yield losses (49–79%) at different stages of crop growth and also reduction in seed size up to 34% (Ahmad and Mohammad 1986). In addition, it was estimated that annual yield loss up to 20% was caused by DRR disease in chickpea (Vishwadhar and Chaudhary 2001; Gupta et al. 2012). Incorporating genetic resistance into the crop has been the most successful and economically efficient way of controlling biotic stresses (Rubiales and Fondevilla 2012). Developing chickpea cultivars with DRR resistance has been challenging due to lack of sources having high levels of resistance in the cultivated chickpea (Pande et al. 2006). However, some studies identified moderate level of resistance in the cultivated species and suggested that the resistance is controlled by a single dominant gene (Rao and Haware 1987; Talekar et al. 2017). In recent years, the availability of genome sequence (Varshney et al. 2013), germplasm sequencing (Thudi et al. 2016a,b; Varshney et al 2019) and ample genomic resources (Roorkiwal et al. 2020) greatly facilitated mapping of several abiotic (Varshney et al. 2014; Paul et al. 2018) and biotic (Sabbavarapu et al. 2013; Garg et al. 2018) stress resistance genes/QTLs in chickpea. The present study was conducted to understand the genetic behaviour of DRR resistance and identify molecular markers linked to genomic regions/QTLs associated with DRR resistance in chickpea.
Materials and methods
Mapping population
The mapping population used in this study consisted of 182 F9 derived recombinant inbred lines (RILs) obtained from a cross between BG 212 (Desi chickpea line susceptible to DRR) and ICCV 08305 (a Kabuli chickpea line moderately resistant to DRR). RILs were developed by advancing the generations from F2 to F9 following single seed descent (SSD) method. The development of RIL population and evaluation of RILs for DRR resistance and agronomic traits were carried out at the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, India.
Phenotyping of RILs for DRR resistance
A total of 182 RILs along with parental lines were screened against resistance to DRR, R. bataticola (Rb 6 isolate) with three replications for two seasons (during 2016 and 2017) under controlled condition using paper towel technique as described by Pande et al. (2012). The plants were grown in polythene bags in a greenhouse maintained at 25 ± 1 °C for 7 days. The bags were filled up to two-thirds of the volume with sterilized river sand. Seeds were surface-sterilized using 2% sodium hypochlorite for two minutes, rinsed in sterile water for 2–3 min in order to wash off sodium hypochlorite, sown (30 seeds) in plastic bags and allowed to grow for 7 days.
A pure culture of R. bataticola (Rb 6) was mass multiplied on Potato Dextrose Broth (PDB) following the standard procedure (Pande et al. 2012). Roots of test seedlings were dipped in the inoculum for about 30 s. Ten seedlings of each RIL were placed side by side on a blotter paper (size 45 × 25 cm with one-fold; any color; thin) in such a way that only the cotyledons and roots were covered. Uninoculated and inoculated seedlings of susceptible genotype (BG 212) were kept separately with each batch of test seedlings. The folded blotters were kept in a tray and incubated at 35 °C with 12 h of day/night light for eight days in a growth chamber. Seedlings were examined for the extent of DRR severity after eight days. DRR disease severity was recorded visually on 1–9 scale as suggested by Pande et al. (2012) based on the damage caused by the pathogen. In this scale, 1 = Resistant (no infection on roots), 3 = Moderately resistant (very few small lesions on roots), 5 = moderately susceptible (lesions on roots clear but small, new roots free from infection), 7 = Susceptible (lesions on roots many, new roots generally free from lesions) and 9 = Highly susceptible (roots infected and completely discolored).
Statistical analysis for DRR resistance
Genetics of resistance was established through testing of phenotyping frequencies for goodness of fit to postulated ratio using chi-square test. The significance of differences for DRR disease was tested by F-test through analysis of variance (ANOVA) using GenStat (14th Edition), VSN International, Hemel Hempstead, UK (www.GenStat.co.uk).
Evaluation of RILs for agronomic traits
The field experiment for agronomic evaluation was carried out in an Alpha lattice design with 3 replications during post-rainy season of 2015 and 2016 at ICRISAT, Patancheru. Field was solarized for conducting the experiment. Planting was done using cone planter in vertisols. Each RIL was planted in a 4 m row plot with 60 cm distance between rows and 10 cm between plants. All other crop management practices were carried out to ensure good crop establishment and growth. Observations were recorded on days to 50% flowering, plant height (cm), plant biomass, seed yield, 100-seed weight and harvest index (%) in each plot.
Genotyping of RILs
Genomic DNA of the RILs along with two parental genotypes was extracted from fresh young leaves (2 g) collected from 14-day old seedlings following the modified CTAB method as described by Mace et al. (2003). The quality and quantity of DNA was checked on the 0.8% agarose gel. The DNA was normalized to 50 ng per microliter for further genotyping using SNP markers. A cost-effective SNP genotyping platform comprising of 50,590 high quality non-redundant SNPs tiled on to Affymetrix® Axiom®CicerSNP array (Roorkiwal et al. 2018) was used for genotyping the RIL population. All SNP markers with more than 10% missing data and monomorphic among parental lines were excluded before genetic map construction.
Genetic map construction and QTL analysis
A total of 13,110 SNPs were used for genetic map construction using Inclusive Composite Interval Mapping (ICIM) software. Chi-square test was performed (P < 0.05) to test the segregation distortion for each marker. Marker grouping was done with LOD (Logarithm of odds) score of 5 and recombination frequency of 0.40. Distance was calculated using Kosambi’s mapping function and the final linkage map was generated using LinkageMapView package R package (Lisa et al. 2018).
The linkage map data and DRR disease screening data of the RIL population were used for QTL analysis using inclusive composite interval mapping (ICIM) software (Wang et al. 2014). A stepwise regression was performed by ICIM-Add mapping to identify the most significant markers and marker-pair multiplications at 0.001 probability level and scanning step of 1 cM. LOD score threshold was determined by performing 1000 permutations by maintaining the chromosome-wise type I error rate of 0.05. The LOD score peaks were used to estimate the most likely position of a QTL on the linkage map. The amount of variation explained was determined using the coefficient of determination (R2) value and expressed as percent phenotypic variance explained (PVE%).
Results
Phenotypic variation and frequency distribution for DRR resistance
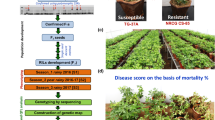
The parental line ICCV 08305 exhibited moderately resistant reaction (disease score between 3.3 to 4.3) and BG 212 showed high susceptibility (an average disease score of above 8) (Table 1; Fig. 1). Analysis of variance for disease severity revealed highly significant differences among the RILs (P < 0.001) in both the screenings and also in pooled analysis of variance (Table 1). A high broad sense heritability of 92.92%, 93.00% and 95.65% was recorded for DRR disease incidence during the first and the second screening and the pooled analysis, respectively (Table 1). The frequency distribution of RILs for DRR disease severity (recorded on 1–9 disease score) depicted a normal distribution (Fig. 2). Based on the disease score, a total of 12 RILs were found resistant, 77 moderately resistant, 65 susceptible and 28 highly susceptible in the first screening (2016). During the second screening (2017), 12 RILs were found resistant, 76 moderately resistant, 77 susceptible and 17 highly susceptible (Table 2). The differential response of parental lines and RILs to DRR is shown in the Fig. 1. Further, the phenotypic data was subjected to chi-square test by combining the first two classes (resistant and moderately resistant) as resistant and the last two classes (susceptible and highly susceptible) as susceptible. As a result, a good fit to the ratio of 1 Resistant: 1 susceptible ratio (2016: χ2 = 0.043; P = 0.83 and 2017: χ2 = 0.099; P = 0.75) was observed in both the years of screening (Table 2). These results indicated a major gene controlling the inheritance of DRR resistance in this cross.
Differential response of parental lines and RILs to dry root rot disease. BG 212 is the susceptible parent (disease score of 8.6) and ICCV 08305 is the moderately resistant (disease score of 4.7) parent. The disease scores of RILs were given as 3, 5, 6, 7 and 9 by following the disease scoring scale as mentioned in Table 1
Genetic map construction
A total of 13,110 SNPs were found highly polymorphic among the parental genotypes of the mapping population. A high-density genetic linkage map was constructed containing 13,110 SNPs distributed across eight linkage groups with a total map length of 1224.11 cM having an average inter-marker distance of 0.09 cM. The map length of linkage groups ranged from 89.93 cM (CaLG08) to 230.77 cM (CaLG04) with an average of 153.01 cM per group. The inter-marker distance ranged from 0.05 cM (CaLG04) to 0.71 cM (CaLG03) with an average marker density of 0.09 cM per group (Table 3; Fig. 3).
QTL mapping of DRR resistance
Genotyping data of mapped 13,110 SNPs was integrated with the DRR screening data and analyzed using ICIM-Add mapping. As a result, a minor QTL “qDRR-8” for DRR resistance explaining 6.70% phenotypic variation (PV) with LOD score of 3.34 was identified on CaLG08. This QTL was flanked by markers Ca8_3970986 and Ca8_3904895. (Table 4; Fig. 4).
Performance of RILs for agronomic traits
The performance of the RILs along with parental lines is presented in Supplementary Table 1. The moderately resistant line, ICCV 08305 recorded early flowering and early maturity as compared to BG 212. The line BG 212 was taller, had higher biomass per plant and gave higher grain yield per plant as compared to ICCV 08305. Whereas, ICCV 08305 had larger seed size and higher harvest index compared to BG 212 in both the seasons. The RILs exhibited wide variation for the traits studied in both the seasons (Supplementary Table 1). Agronomic data of the RILs from field evaluation was compared with the disease score data recorded from growth chamber to identify RILs with superior performance coupled with DRR resistance. Genotypes in each category were averaged to observe the differences between categories for different traits. Results indicated that there was no difference among categories for all the traits studied (Table 5). Relative comparison was made between resistant and susceptible RILs for yield and yield related traits (Supplementary Table 2). As a result, a non-significant difference was observed for all yield related traits (P < 0.001). This implies that disease resistance has no relationship with days to flowering, days to maturity, biomass, seed yield and 100 seed weight.
Promising DRR resistant RILs
Among 182 RILs, 12 lines were found resistant (ICCRIL14-0005, ICCRIL14-0058, ICCRIL14-0105, ICCRIL14-0107, ICCRIL14-0128, ICCRIL14-0138, ICCRIL14-0141, ICCRIL14-0142, ICCRIL14-0144, ICCRIL14-0145, ICCRIL14-0147, ICCRIL14-0152) and 77 as moderately resistant to DRR. Some of these RILs were superior in agronomic performance (Supplementary Table 3). These include, ICCRIL 14-0133 for early flowering and early maturity; ICCRIL 14-0145 for plant height; ICCRIL 14-0119 for plant biomass; ICCRIL 14–0152 for grain yield and harvest index; ICCRIL 14-0154 for 100-seed weight and ICCRIL14-0066 for harvest index during 2015–2016. Whereas during 2016–2017, the RILs with superior performance included ICCRIL14-0105 for early flowering and early maturity; ICCRIL14-0080 for plant height; ICCRIL14-0058 for grain yield and plant biomass; ICCRIL14-0021 for 100-seed weight and ICCRIL14-0018 for harvest index..
Discussion
Development of DRR resistant varieties is a priority in chickpea breeding programs as this is an emerging disease of chickpea in warm and arid regions and its occurrence is increasing due to changing climatic conditions (Sharma et al. 2016). Efforts have been made to identify sources of DRR resistance from germplasm and breeding lines/cultivars using either or both of these screening techniques by various researchers (Nene et al. 1981; Reddy et al. 1990; Baker and Ahmed 1991; Bekele et al. 1992; Jayanti Bhatt 1993; Gupta 1995; Oad et al. 1995; Gurha et al. 2003; Pande et al. 2004, 2006; Ashraf et al. 2005; Gupta and Babbar 2006; Shareef et al. 2009; Gupta et al. 2012). However high level of DRR resistance has not been identified so far (Sharma et al. 2016). Nevertheless, using partial resistance available in germplasm collections, breeders have developed several chickpea cultivars with moderate levels of DRR resistance (Muehlbauer et al. 1998a, 1998b, 2004; Muehlbauer and Kaiser 2002; Malhotra et al. 2003; Rubio et al. 2003, 2004; Vandenberg et al. 2003a, b; Warkentin et al. 2005). ICRISAT has developed several breeding lines, such as ICCV 05530, ICCV 08305, ICCV 05529, ICCV 05532, ICCV 07117 and ICCV 07112 with moderate level of resistance to DRR (Sharma Mamta, Legumes Pathology, ICRISAT, unpublished results). Among these, ICCV 08305 was used as male parent for construction of mapping population used in the present study. Analysis of variance for disease severity revealed highly significant differences among the RILs (P < 0.001) in both the screening and also in pooled analysis of variance. The heritability (h2) estimates were found high for DRR resistance indicating less influence of environmental variability (Table 2). This suggests that selection would be effective for DRR resistance. In this study, a total of 12 RILs showed resistant and 76 moderately resistant reactions to DRR under controlled environment condition in both screenings. The identified resistant and moderately resistant sources to DRR need to be reconfirmed under artificial epiphytotic conditions in sick pots/plots with the existing variability in R. bataticola isolates. Previously, several germplasm and breeding lines were evaluated using these two screening techniques by various researchers. Pande et al. (2006) identified resistant sources (ICC 1710 and ICC 2242) for DRR among 211 mini core accessions. Jayalakshmi et al. (2008) reported four genotypes (GCP- 101, GBM-2, GBM-6 and ICCV-10) as tolerant to DRR. Iftikhar and Ilyas (2000) found ICCV 97112 as resistant to DRR among 108 chickpea lines screened. Resistant sources to DRR were also reported from other studies in chickpea (Gangwar et al. 2002; Prajapati et al. 2003; Mishra et al. 2005; Pande et al. 2006; Gupta et al. 2012 and Khan et al. 2013). The identified resistant sources can be utilized in breeding programs to develop DRR resistant cultivars.
Understanding the genetics of resistance is the first step for the successful disease resistance breeding programme. The parental line ICCV 08305 maintained its resistance against Rhizoctonia (Rb6) with disease score of 3.3 to 4.3 and recorded as moderately resistant. The disease score of ≥ 8 of susceptible parent BG 212 indicated that this genotype was susceptible to DRR (Table 2). Interestingly, the frequency distribution of the DRR disease scores based on mean values was normal and the DRR disease scores extended beyond the parents suggested transgressive segregation (Fig. 2). Here, some of the RILs showed transgressive segregation for DRR resistance (higher level of DRR resistance than that in DRR resistant parent) suggesting that the DRR susceptible parent also contributed some alleles for DRR resistance. Thus, there is possibility of increasing DRR resistance by accumulating favorable alleles from diverse sources of DRR resistance. The transgressive segregation also suggests the resistance to be quantitative in nature. The distribution of DRR resistance scores in RILs suggests quantitative inheritance of DRR resistance. However, some earlier studies reported monogenic inheritance of DRR resistance (Rao and Haware 1987; Talekar et al. 2017). This could be due to different approaches in the classification of resistant and susceptible groups or differences in the resistance level of parental genotypes used in the previous studies. In the present study also, a good fit to 1 Resistant: 1 Susceptible ratio for DRR resistance in RILs was observed when score up to 5 was considered resistant and above 5 as susceptible (Table 3). More studies are needed to understand and confirm the genetic nature of DRR resistance.
Molecular markers linked to DRR resistance would be very much useful in identification of resistant genotypes in the early generations and improving precision and efficiency of breeding programs aimed at improving DRR resistance. The advent of next generation sequencing (NGS) technologies has enabled the development of sequence-based markers and subsequently high-density genetic maps for fine mapping of trait of interest. Single Nucleotide Polymorphisms represent the most abundant DNA sequence variation in the genome and are well chronicled for use in high-resolution genetic mapping. In the present study, we screened the parental lines and the RIL population with a total of 50,590 SNP probes on the Axiom®CicerSNP array. Of these, a total of 13,110 SNPs found highly polymorphic between the parental genotypes of the mapping population. Based on the genotyping data of RILs, a high-density genetic linkage map was constructed comprising of 13,110 SNPs distributed on to eight groups spanning a total of 1224.11 cM with an average inter-marker distance of 0.09 cM (Table 4; Fig. 3). The resolution of the high-density map developed in the present study is higher than the chickpea genetic maps constructed using the axiom array (Barmukh et al. 2020; Soren et al. 2020) and GBS approach (Gaur et al. 2015; Verma et al. 2015; Deokar et al. 2019; Sab et al. 2020). Overall, the high-density genetic map has a sufficient number of markers to capture the majority of the recombination events in the population which will increase precision in QTL mapping and subsequent identification of candidate genes. In this study, a minor QTL, ‘qDRR-8’ explained 6.70% phenotypic variation (PV) with LOD scores 3.34 was identified on CaLG08 for DRR resistance flanked by markers Ca8_3970986 and Ca8_3904895 (Table 5; Fig. 4). In an earlier study, Talekar et al. (2017) conducted bulk seggregant analysis in 129 F2:3 progeny derived from the cross L550 × PG06102, found monogenic inheritance of DRR resistance. In addition, they identified four SSR markers (ICCM0299, TR29, CaM111 and ICCM0120b) differentiated the resistant and susceptible bulks. On linkage analysis found that two markers (ICCM0299 and ICCM0120b) were co-segregating with resistance to DRR. Previous studies mapped these SSR markers on different linkage groups of the chickpea genetic map. For instance, the marker TR29 was mapped on LG01, 05 & 07, and marker CaM111 on LG01 & 07. Similarly, the marker ICCM0120b was mapped on LG05 of chickpea genetic map (https://cegresources.icrisat.org/cmap/). These findings indicate that the genomic regions conferring resistance to DRR could be distributed on to more than one linkage group in the chickpea genome indicating polygenic nature. Therefore, further studies are needed to confirm these results and conclusively establish the genetic inheritance of DRR resistance. In this study, the RILs exhibited a wide variation for agronomic traits studied in both the seasons. A relative comparison made between resistant and susceptible RILs revealed a non-significant difference for yield related traits (Supplementary Table 2). This indicates that disease resistance has no relationship with these traits. In this study, agronomically superior lines with high level of DRR resistance (e.g., ICCRIL14-0058 with disease score 3) (Supplementary Table 3) were identified which could be used as donor parent in chickpea breeding programs for improving DRR resistance.
The present study revealed that DRR resistance in chickpea was found to be polygenic in nature. The RILs studied had high phenotypic variation with high heritability for DRR disease resistance suggesting that selection will be effective for this trait. A minor QTL for DRR resistance was identified on linkage group 8. The identified genomic region could be further explored for mining candidate genes and the linked SNP markers could be validated and used for development of cost affective marker systems for application in marker assisted breeding programs. Also, the transgressive segregants with high level of DRR resistance identified in the present study could be used as resistance sources in chickpea breeding programs for enhancing DRR resistance.
References
Aghakhani M, Dubey SC (2009) Determination of genetic diversity among Indian isolates of Rhizoctonia bataticola causing dry root rot of chickpea. Antonie Van Leeuwenhoek 96:607–619
Ahmad Q, Mohammad A (1986) Losses in yield due to Rhizoctonia root rot of chickpea in Bihar. Indian Phytopath 39:590–592
Ashraf MS, Khan TA, Hasan S (2005) Reaction of chickpea varieties to Macrophomina phaseolina and their effect on peroxidase activity. Pak J Bot 37:761–767
Barmukh R, Roorkiwal M, Jaba J, Chitikineni A, Mishra SP, Sagurthi SR, Munghate R, Sharma HC, Varshney RK (2020) Development of a dense genetic map and QTL analysis for pod borer Helicoverpa armigera (Hübner) resistance component traits in chickpea (Cicer arietinum L.). Plant Genome 14(1):e20071. https://doi.org/10.1002/tpg2.20071
Baker MA, Ahmed F (1991) Additional sources of resistance of will and root rot of chickpea in Bangladesh. Int Chickpea Newsl 25:28–29
Bekele H, Paul YS, Hailu T (1992) Evaluation of chickpea lines for resistance to root rot and wilt in Northwestern Ethiopia. Int Chickpea Newsl 27:18–19
Deokar A, Sagi M, Tar’an, B, (2019) Genome-wide SNP discovery for development of high-density genetic map and QTL mapping of ascochyta blight resistance in chickpea (Cicer arietinum L.). Theor Appl Genet 132:1861–1872. https://doi.org/10.1007/s00122-019-03322-3
FAOSTAT (2018) http://faostat.fao.org/site. Accessed Oct 2020
Garg T, Mallikarjuna BP, Thudi M, Samineni S, Singh S, Sandhu JS, Kaur L, Singh I, Sirari A, Basandrai AK, Basandrai D, Varshney RK, Gaur PM (2018) Identification of QTLs for resistance to Fusarium wilt and Ascochyta blight in a recombinant inbred population of chickpea (Cicer arietinum L.). Euphytica 214:45
Gangwar RK, Prajapati RK, Srivastava SSL, Kumar K (2002) Resistance in chickpea germplasms against the dry root rot. Ann Plant Protect Sci 10:393–394
Gaur PM, Gowda CLL, Knights EJ, Warkentin TD, Acikgoz N, Yadav SS, Kumar J (2007) Chapter 19: breeding achievements. In: Yadav SS, Redden B, Chen W, Sharma B (eds) Chickpea breeding and management. CABI, London, pp 391–416
Gaur PM, Krishnamurthy L, Kashiwagi J (2008) Improving drought-avoidance root traits in chickpea (Cicer arietinum L.): current status of research at ICRISAT. Plant Prod Sci 11:3–11
Gaur PM, Samineni S, Thudi M, Tripathi S, Sajja SB, Jayalakshmi V, Mannur DM, Vijayakumar AG, Ganga Rao NVPR, Ojiewo C, Fikre A, Kimurto P, Kileo RO, Girma N, Chaturvedi SK, Varshney RK, Dixit GP (2019) Integrated breeding approaches for improving drought and heat adaptation in chickpea (Cicer arietinum L.). Plant Breed 138:389–400
Gaur R, Jeena G, Shah N, Gupta S, Pradhan S, Tyagi AK, Jain M, Chattopadhyay D, Bhatia S (2015) High density linkage mapping of genomic and transcriptomic SNPs for synteny analysis and anchoring the genome sequence of chickpea. Sci Rep 5:13387. https://doi.org/10.1038/srep13387
Ghosh R, Sharma M, Telangre R, Pande S (2013) Occurrence and distribution of chickpea diseases in central and southern parts of India. Am J Plant Sci 4:940–944
Gil J, Nadal S, Luna D, Moreno M, De Haro A (1996) Variability of some physicochemical characters in Desi and Kabuli chickpea types. J Sci Food Agric 71:179–184
Gupta O (1995) Identification of chickpea genotypes with dual resistance against wilt and root rots. Inter Chickpea Pigeonpea Newsl 2:27–28
Gupta O, Babbar A (2006) Identification of desi and kabuli chickpea genotypes for multiple disease resistance against soil borne diseases. Indian J Pulses Res 19:129–130
Gupta O, Rathi M, Mishra M (2012) Screening for resistance against Rhizoctonia bataticola causing dry root rot in chickpea. J Food Legumes 25:139–141
Gurha SN, Singh G, Sharma YR (2003) Diseases of chickpea and their management. In: Ali M, Kumar S, Singh NB (eds) Chickpea research in India. Army Printing Press, Lucknow, pp 195–227
Iftikhar K, Ilyas MB (2000) Screening of chickpea germplasm against dry root rot disease (Macrophomina phaseolina) in pots/glass house. Pak J Phytopathol 12:66–70
Jayalakshmi SK, Usharani S, Benagi VI, Mannur DM (2008) Sources of resistance to dry root rot of chickpea caused by Rhizoctonia bataticola. Agri Sci Digest 28:147–148
Jayanti Bhatt J (1993) Reaction of chickpea cultivars to R. bataticola (Taub.) Butler. Indian J Pulss Res 6:118–119
Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Br J Nutr 108:S11–S26
Kaiser WJ, Danesh D, Okhovat M, Mossahebi H (1968) Diseases of pulse crops (edible legumes) in Iran. Plant Dis Rep 52:687–691
Khan RA, Bhat TA, Kumar K (2013) Screening of chickpea (Cicer arietinum L.) germplasm lines against dry root rot caused by Rhizoctonia bataticola (taub.) Butler. Asian J Pharm Clin Res 6:211–212
Khanna-Chopra R, Sinha SK (1987) Chickpea: physiological aspects of growth and yield. In: Saxena MC, Singh KB (eds) The chickpea. CAB International, Wallingford, pp 163–189
Lisa AO, Robert WR, Steven GB, Cory RB (2018) Linkage map view-rendering high-resolution linkage and QTL maps. J Bioinform 34:306–307
Mace ES, Buhariwalla HK, Crouch JH (2003) A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Mol Biol Rep 21:459–460
Malhotra RS, Baum M, Udupa SM, Bayaa B, Kabbabe S, Khalaf G (2003) Ascochyta blight resistance in chickpea: present status and future prospects. In: Proceedings of international chickpea conference, Raipur, India, pp 20–22
McIntosh M, Millar C (2001) A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr Rev 59:52–55
Mishra AN, Prasad SN, Ram RM (2005) Search for source of resistance of dry root rot in chickpea. Ann Plant Prot Sci 13:465–529
Mitra M (1931) Report of the imperial mycologist. Sci Rep Agric Res Inst 1929–1930:58–71
Muehlbauer FJ, Kaiser WJ (2002) Registration of ‘Evans’ chickpea. Crop Sci 42:301
Muehlbauer FJ, Kaiser WJ, Kusmenoglu I (1998a) Registration of ‘Dwelley’ chickpea. Crop Sci 38:283
Muehlbauer FJ, Kaiser WJ, Kusmenoglu I (1998b) Registration of ‘Sanford’ chickpea. Crop Sci 38:282
Muehlbauer FJ, Temple SR, Chen W (2004) Registration of ‘Sierra’ chickpea. Crop Sci 44:1864
Nawaz KS (2007) Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopathology 5:111–118
Nene YL, Haware MP, Reddy MV (1981) Resistance screening techniques in chickpea disease. Inform. Bull. No. 10. ICRISAT, Patancheru, Andhra Pradesh, India
Nene YL, Shelia VK, Sharma SB (1996) A world list of chickpea and pigeonpea pathogens. 5th ed. Hyderabad: International Crops Research Institute for Semi-Arid Tropics; p 27
Oad SM, Khuhro GA, Oad GL, Junejo RA, Chandio LB (1995) Release of two new chickpea varieties in Pakistan-Dokri Gram89 (DG89) and Dokri Gram 92 (DG92). Int Chickpea Pigeonpea Newsl 2:16–17
Pande S, Kishore GK, Rao JN (2004) Evaluation of chickpea lines for resistance to dry root rot caused by Rhizoctonia bataticola. Int Chickpea Pigeonpea Newsl 11:37–38
Pande S, Kishore GK, Upadhyaya HD, Rao JN (2006) Identification of sources of multiple disease resistance in mini-core collection of chickpea. Plant Dis 90:1214–1218
Pande S, Sharma M, Avuthu N, Telangre R (2012) High throughput phenotyping of chickpea diseases: stepwise identification of host plant resistance. Inf Bull No 92. Technical Report. Patancheru: International Crops Research Institute for Semi-Arid Tropics
Paul PJ, Samineni S, Thudi M, Sajja SB, Rathore A, Das RR, Khan AW, Chaturvedi SK, Lavanya GR, Varshney RK, Gaur PM (2018) Molecular mapping of QTLs for heat tolerance in chickpea. Int J Mol Sci 19:2166
Pearson CAS, Schwenk FW, Crowe FJ (1984) Colonization of soybean roots by Macrophomina phaseolina. Plant Dis 68:1086–1088
Prajapati RK, Gangwar RK, Srivastava SSL (2003) Resistant sources of chickpea against dry root rot. Farm Sci J 12:86
Rao A, Haware (1987) Inheritance of Dry Root Rot (Rhizoctonia bataticola) Resistance in Chickpea (Cicer arietinum L.). Plant Breed 98:349–352
Reddy MV, Raju TN, Nene YL (1990) Evaluation of chickpea lines in the all India coordinated varietal trails for wilt and dry root rot resistance. Int Chickpea Newsl 22–23
Roorkiwal M, Jain A, Kale SM, Doddamani D, Chitikineni A, Thudi M, Varshney RK (2018) Development and evaluation of high-density Axiom® CicerSNP Array for high-resolution genetic mapping and breeding applications in chickpea. Plant Biotechnol J 16:890–901
Roorkiwal M, Bharadwaj C, Barmukh R, Girish PD, Thudi M, Gaur PM, Chaturvedi SK, Fikre A, Hamwieh A, Kumar S, Sachdeva S, Ojiewo CO, Taran B, Wordofa NG, Singh NP, Siddique KHM, Varshney RK (2020) Integrating genomics for chickpea improvement: achievements and opportunities. Theor Appl Genet 133:1703–1720
Rubiales R, Fondevilla S (2012) Future prospects for ascochyta blight resistance breeding in cool season food legumes. Front Plant Sci 3:27
Rubio J, Moreno MT, Martinez C, Gil J (2003) Registration of CA2969, an ascochyta blight resistant and double-podded chickpea germplasm. Crop Sci 43:1567–1568
Rubio J, Martinez C, Gil J, Moreno MT (2004) Registration of ascochyta blight and fusarium wilt resistant CA2954 kabuli chickpea germplasm. Crop Sci 44:1881–1882
Sab S, Lokesha R, Mannur DM, Somasekhar, Jadhav K, Mallikarjuna BP, C L, Yeri S, Valluri V, Bajaj P, Chitikineni A, Vemula A, Rathore A, Varshney RK, Shankergoud I, Thudi M (2020) Genome-wide SNP discovery and mapping QTLs for seed iron and zinc concentrations in chickpea (Cicer arietinum L). Front Nutr 7:559120.
Sabbavarapu M, Sharma M, Chamarthi SK, Swapna N, Rathore A, Thudi M, Gaur PM, Pande S, Singh S, Kaur L, Varshney RK (2013) Molecular mapping of QTLs for resistance to fusarium wilt (race 1) and Ascochyta blight in chickpea (Cicer arietinum L.). Euphytica 193:121–133
Shareef AL, Tayed A, Mustafa AS (2009) Wilt/root-rots screening for resistance and effect of temperature on growth of the causal fungi. M.Sc (Agri) Thesis, Sudan Univ Sci Tech 115 pp
Sharma M, Ghosh R, Pande S (2016) Dry root rot [Rhizoctonia bataticola (Taub.) Butler] an emerging disease of chickpea—where do we stand? Arch Phytopath Plant Prot 48:1–16
Sharma M, Mangla UN, Krishnamurthy L, Vadez V, Pande S (2010) Drought and dry root rot of chickpea. In 5th International Food Legumes Research Conference (IFLRC V) Antalya, Turkey, p 263
Sharma M, Pande S (2013) Unravelling effects of temperature and soil moisture stress response on development of dry root rot [Rhizoctonia bataticola (Taub.)] Butler in Chickpea. Am J Plant Sci 4:584–589
Soren KR, Madugula P, Kumar N, Barmukh R, Sengar MS, Bharadwaj C, Sharma PC, Singh S, Bhandari A, Singh J, Sanwal SK, Pal M, RSP P, Mann A, Sagurthi SR, Ps S, Siddique KHM, Singh NP, Roorkiwal M, Varshney RK (2020) Genetic dissection and identification of candidate genes for salinity tolerance using Axiom®CicerSNP array in chickpea. Int J Mol Sci. 21(14):5058. https://doi.org/10.3390/ijms21145058
Talekar SC, Lohithaswa HC, Viswanatha KP (2017) Identification of resistant sources and DNA markers linked to genomic region conferring dry root rot resistance in chickpea (Cicer arietinum L.). Pl Breed 136:161–166
Tripathi NN, Sharma BK (1983) Incidence of chickpea dry root rot (Rhizoctonia bataticola) in Southern Haryana. Int Chickpea Newsl 8:22–23
Trivedi S, Gurha SN (2006) Status of some soil borne pathogens infecting chickpea in Bundelkh and region of Uttar Pradesh. Indian J Pulses Res 19:88–90
Thudi M, Chitikineni A, Liu X, He W, Roorkiwal M, Yang W, Jian J, Doddamani D, Gaur PM, Rathore A, Samineni S, Saxena RK, Xu D, Singh NP, Chaturvedi SK, Zhang G, Wang J, Datta SK, Xu X, Varshney RK (2016a) Recent breeding programs enhanced genetic diversity in both desi and kabuli varieties of chickpea (Cicer arietinum L.). Sci Rep 6:38636
Thudi M, Khan AW, Kumar V, Gaur PM, Katta AVSK, Garg V, Roorkiwal M, Samineni S, Varshney RK (2016b) Whole genome re-sequencing reveals genome wide variations among parental lines of mapping populations in chickpea (Cicer arietinum). BMC Plant Biol 16(Suppl1):10
Vandenberg A, Warkentin T, Slinkard A (2003a) CDC Anna desi chickpea. Can J Plant Sci 83:797–798
Vandenberg A, Warkentin T, Slinkard A (2003b) CDC Nika desi chickpea. Can J Plant Sci 83:799–800
Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar’an B, Millan T, Zhang X, Ramsay LD, Iwata A, Wang Y, Nelson W, Farmer AD, Gaur PM, Soderlund C, Penmetsa RV, Xu C, Bharti AK, He W, Winter P, Zhao S, Hane JK, Carrasquilla-Garcia N, Condie JA, Upadhyaya HD, Luo MC, Thudi M, Gowda CLL, Singh NP, Lichtenzveig J, Gali KK, Rubio J, Nadarajan N, Dolezel J, Bansal KC, Xu X, Edwards D, Zhang G, Kahl G, Gil J, Singh KB, Datta SK, Jackson SA, Wang J, Cook DR (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nature Biotech 31:240–246
Varshney RK, Thudi M, Nayak SN, Gaur PM, Kashiwagi J, Krishnamurthy L, Jaganathan D, Koppolu J, Bohra A, Tripathi S, Rathore A, Jukanti AK, Jayalakshmi V, Vemula A, Singh S, Yasin M, Sheshshayee MS, Viswanatha KP (2014) Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor Appl Genet 127:445–462
Varshney RK, Thudi M, Roorkiwal M, He W, Upadhyaya HD, Yang W, Bajaj P, Cubry P, Rathore A, Jian J, Doddamani D, Khan AW, Garg V, Chitikineni A, Xu D, Gaur PM, Singh NP, Chaturvedi SK, Nadigatla GVPR, Krishnamurthy L, Dixit GP, Fikre A, Kimurto PK, Sreeman SM, Bharadwaj C, Tripathi S, Wang J, Lee S-H, Edwards D, Polavarapu KKB, Penmetsa RV, Crossa J, Nguyen HT, Siddique KHM, Colmer TD, Sutton T, von Wettberg E, Vigouroux Y, Xu X, Liu X (2019) Resequencing of 429 chickpea accessions from 45 countries provides insights into genome diversity, domestication and agronomic traits. Nat Genet 51:857–864
Verma S, Gupta S, Bandhiwal N, Kumar T, Bharadwaj C, Bhatia S (2015) High-density linkage map construction and mapping of seed trait QTLs in chickpea (Cicer arietinum L.) using Genotyping-by-Sequencing (GBS). Sci Rep. 5:17512. https://doi.org/10.1038/srep17512
Vishwadhar CSG (2001) Disease resistance in pulse crop-current status and future approaches. In: Nagarajan S, Singh DP (eds) The role of resistance in intensive agriculture. Kalyani, New Delhi, pp 144–157
Wang J, Shi L, Zhu L, Li X, Liu D (2014) Genetic analysis and molecular mapping of leaf rust resistance genes in the wheat line 5R618. Czech J Genet Plant Breed 50:262–267
Warkentin TD, Banniza S, Vandenberg A (2005) CDC Frontier kabuli chickpea. Can J Plant Sci 85:909–910
Westerlund FV, Cambell RN, Kimble KA (1974) Fungal root rots and wilt of chickpea in California. Phytopathology 664:432–436
Williams PC, Singh U (1987) The Chickpea-Nutritional quality and the evaluation of quality. In: Saxena MC, Singh KB (eds) The Chickpea. CAB International, Wallingford, pp 329–356
Acknowledgements
This work was undertaken as part of the CGIAR Research Program on Grain Legumes and KWDP-II (Sujala-III) project funded by the Government of Karnataka state of India.
Author information
Authors and Affiliations
Contributions
PMG conceived the idea, SrS developed the mapping population, AK conducted the experiment and wrote the initial draft of the MS, MS supervised disease screening, MT and BPM helped in data analysis and interpretation of results. KPV, RKV and PMG reviewed and edited the MS. All authors read the manuscript and agreed with its content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karadi, A., Samineni, S., Sajja, S. et al. Molecular mapping of dry root rot resistance genes in chickpea (Cicer arietinum L.). Euphytica 217, 123 (2021). https://doi.org/10.1007/s10681-021-02854-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02854-4