Abstract
Humulus lupulus L., is an economically and ecologically important plant species, which has suffered habitat degradation throughout the Hyrcanian forests (Northern Iran). Towards conservation and exotic breeding germplasm assessment, we conducted a survey in the Hyrcanian forests: 15 wild populations (WPs) were located and 54 samples collected. Genetic diversity and population structure were assessed by scoring sequence-related amplified polymorphism markers (SSR, ISSR, and RAPD) and morphological features. Molecular marker analysis showed that RAPDs (232) and ISSRs (77) produced more polymorphic bands compared to SSRs (64) per marker. SSRs exhibited a higher PIC average value (0.64), than RAPDs (0.24) and ISSRs (0.54). Cluster analyses based on the SSR markers to a high degree discriminated WPs based on geographical regions and were more congruent with morphologic traits than the ISSR–RAPD-based clustering. The ΔK parameter of structure analysis showed five clusters. The grouping of the WPs based on the structure analysis was congruent with the SSR clustering to some extent. The results confirmed that SSR markers are effective tools to detect the genetic diversity in hops, but employing higher number of molecular markers (more SSRs), which have a higher polymorphism and prevalence in the genome, or application of NGS SNPs in the identification and genetic relationship of hop indigenous populations, is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common hop, Humulus lupulus L. (Cannabaceae) is a dioecious, herbaceous perennial climbing species mainly cultivated in the Northern Hemisphere primarily for female inflorescences containing glandular trichomes which accumulate secondary compounds, namely, terpenophenolics (bitter acids) and terpenes (essential oils). Hop phytochemicals provide durability, foam stability, and contribute to flavor and aroma of beer (Rohlf 1998; Zanoli et al. 2005; Zanoli and Zavatti 2008; McAdam et al. 2014). Moreover, biomedical and nutritional applications of hop constituents have been reviewed (Kavalier et al. 2011). The first report on phytochemical potential of Iranian hop collection was reported by Mafakheri and Hamidoghli (2015a), who found that these native wild hops have high ability in oxidant scavenging and are rich in polyphenolic compounds comparable with domestic, commercial cultivars. However, destructive anthropological activities such as development of industrial states and agriculture landscape, logging as well as absence of proper management programs in the last decades across the Hyrcanian forests has jeopardized habitats of wild hop populations, especially in Northern Iran. Due to the rich biodiversity of these forests, including relict species in Tertiary period forests, we add to universal conservation goals that cover a vast area between Iran and Azerbaijan (Gholizadeh et al. 2017; Soofi et al. 2018).
Hop breeders have attempted to provide farmers and brewers with the desired improved cultivars: disease and pest resistance (e.g. powdery mildew), high yield, enhanced storage stability and superior aroma and bitter quality (Seefelder et al. 2000a; Ĉerenak et al. 2011; Patzak and Henychová 2018). In this regard, wild hop germplasm and genetic diversity from under-utilized origins, can serve breeders with a crucial new gene pools to properly address crop improvement and sustainability demands (Hampton et al. 2001; Peredo et al. 2010; Xiong et al. 2016).
Molecular markers have been applied to genetic diversity estimation in hops, for example, using random amplification of polymorphic DNA (RAPD), to assess genetic diversity in hops (Abbott and Fedele 1994; Pillay and Kenny 1996; Vejl 1997; ŠUštar-Vozlič and Javornik 1999). RAPD has been used (1) in sex identification of hop (Polley et al. 1997) (2) construction of genetic linkage maps between male and female hops (Seefelder et al. 2000b) and (3) DNA profiling of hop cultivars (Brady et al. 1996) and detection the level of polymorphism in hop cultivars derived from the European and North American germplasm by Murakami (2000) and Patzak et al. (2010). ISSRs as markers were employed in hops to (1) investigate somaclonal variation (Patzak 2003), (2) identify sex-specific markers (Danilova and Karlov 2006), (3) analyze polymorphism among 10 commonly in use commercial cultivars (Patzak 2001) or 26 Russian and European cultivars (Danilova et al. 2003). SSRs due to their high accuracy, multi-allelism which is responsible for high polymorphism, codominant, high frequency, and freedom from laboratory specificity (Nybom 2004; Smykal et al. 2008; Vieira et al. 2016; Kordrostami and Rahimi 2015) have become a favored PCR technique, especially in relation to quantitative and molecular genetic variation in key features in hops (Jakše et al. 2002; Hadonou et al. 2004; Stajner et al. 2005; Bassil et al. 2008). For example, (1) Jakše et al. (2004) assessed 124 wild and cultivated accessions from North America to Europe and Asia (2) Murakami et al. (2006) evaluated genetic variability and closeness of the hop germplasm throughout the Northern hemisphere, including, recently, (3) an Italian germplasm (Mongelli et al. 2015) with SSRs application of multiple marker systems is invaluable.
In order to investigate WPs, we also used vegetative features as another suitable method (e.g. Srečec et al. 2011; Skomra et al. 2013). Morphological features have been applied to such cultivars as English Fuggles, Goldings and Czech Saaz, hops that were once chosen from WPs (Barth et al. 1994; Moir 2000). Therefore, we additionally applied morphological-based diversity assessment as complementary to molecular methods. In summary, we employed three types of molecular markers (SSR. ISSR and RAPD) in combination with morphological features to characterize genetic diversity and population structure of 15 WPs of H. lupulus L. in Northern Iran. The aim of this study was to evaluate genetic diversity at the population level. This study offers invaluable information on the Iranian hop collection for the very first time.
Material and methods
Study area
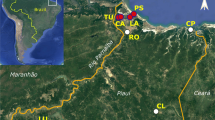
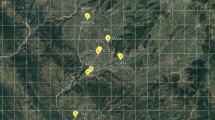
We collected samples in Northern Iran, mainly in Hyrcanian forests, including three provinces, Guilan, Mazandaran, and Golestan, nearly the entire H. lupulus L. distribution range within Iran including various elevations, − 13 to 704 m. Sites were mapped with GPS: 15 WPs were located. Geographical properties (latitude, longitude, and elevation) of all locations were documented (Fig. 1, Table 1). The means, minimum and maximum monthly temperatures, minimum and maximum humid, rainfall, sunny days and evaporation of growing seasons in the five previous years are reported (Supplemental File 1).
Sampling the germplasm, and plant material
Fifty-four samples were obtained from 15 WPs (Table 1). Only aspects of female plants with reproductive structures are reported. Gene flow and seed dispersal are under continued study in the WPs, but not reported here. Populations are defined as any group of organisms of a species existing in a specific space and functioning actively as part of biotic community, thus, the hop individuals presented at a particular area were considered as ‘a population’ (Odum and Barrett 1971). For further morphological evaluation (leaves and cones), and DNA extractions (leaves), samples were kept in an ice-box and carried to the laboratory, and stored under − 80 °C until DNA was extracted. A part of harvested cone hops in September 2016 was dried similar to the methods described by Hofmann et al. (2013), then stored in 4 °C. For plant systematic identification, from each population, a voucher specimen was prepared and deposited in Herbarium of Plant Systematic Department of the University of Guilan, Rasht, Iran.
Morphological analysis
Seventeen morphological traits of Iranian hop germplasm were evaluated, 13 traits from cones (width, length, cone size, cone shape, cone intensity of green color, cones fresh and dry weight (100 cones), and bracts (width and length, bract ratio width/length, length apex of bract and length of bracts), one trait each from leaves (size of blade), main shoots (anthocyanin coloration of main shoot), flowering habit and, growth habit for which values of descriptors are defined in a table (Supplemental Table S1). Descriptors are based on UPOV (2006), Rígr and Faberová (2000) and Čeh et al. (2012). Thirty leaves and 30 cones collected per sample were measured by a digital caliber with precision 0.01 mm. “Dwarf” is defined as having average main bine internodes of less than 6 cm.
DNA extraction, SSR analysis
Fresh leaves were used for DNA extraction with a CTAB method (Saghai-Maroof et al. 1984). The quality and quantity of the DNA were evaluated by analysis on a 1% agarose gel. Twenty SSR markers were selected based on Stajner et al. (2005). Fourteen SSR primer-pairs were selected that yielded clear and visible bands within the expected range and without presence of non-specific bands (Supplemental Table S2).
PCR conditions were as follows 10 μl PCR reactions contained 0.4 mM of each primer, 10 μM deoxyribonucleotides, 50 mM KCl, 10 mM TRIS–HCl (pH 8.3), 1.5 mM MgCl2, 0.01% gelatin, 50–100 ng of DNA, and 1 unit of Taq polymerase. The PCR was performed with a profile of 94 °C for 5 min, followed by 35 cycles at 94 °C for 1 min, at 55 °C for 1 min, at 72 °C for 2 min, and finally for 5 min at 72 °C for the final extension. PCR conditions were as described in Panaud et al. (1996). Taq DNA polymerase (1 U) and DNA (20 ng). The PCR was performed with a profile of 95 °C for 2 min, followed by 45 cycles at 94 °C for 1 min, at 42 °C for 1 min, at 72 °C for 2 min, and finally for 5 min at 72 °C for the final extension. Next, a total of 10 μl PCR products were run on 1.5% agarose gels. Products were also examined on sequencing gels, a total of 3 μl PCR products were denatured and run on 6% polyacrylamide denaturing gels, and marker bands were revealed using the silver staining as that described by Panaud et al. (1996).
For ISSR analysis, 14 primers were used (Supplemental Table S3) to amplify each individual. PCR conditions were as described in Morales et al. (2011). In summary, 25 μl PCR reactions contained 40 ng of genomic DNA, 2.5 μl of 1 × PCR buffer, 1.5 mM of MgCl2, 200 μM of each dNTP, 10 μmol of primer, 1 U Taq polymerase and water. The PCR was performed with a profile of 95 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, at 50 °C for 45 s, at 72 °C for 2 min, and finally for 7 min at 72 °C for the final extension. Ten μl PCR products were run on 1.5% agarose gels.
For RAPD analysis, five primers were used (Supplemental Table S3) to amplify the individuals. PCR conditions were as described in Morales et al. (2011). In summary, 10 μl PCR reactions contained PCR buffer (1 ×), MgCl2 (2.5 mM), dNTPmix (0.2 mM), primer (25 ng), Taq DNA polymerase (1 U) and DNA (20 ng). The PCR was performed with a profile of 95 °C for 2 min, followed by 45 cycles at 94 °C for 1 min, at 42 °C for 1 min, at 72 °C for 2 min, and finally for 5 min at 72 °C for the final extension. Ten μl PCR products were analyzed with 1.5% agarose gels.
Data analysis
For each primers combination (SSR, ISSR and RAPD markers), the total number of bands was determined, and only the polymorphic ones were taken into account to estimate the percentage of polymorphic bands (PPB). Polymorphic information content) (PIC), was calculated as reported by Lynch and Walsh (1998). For SSR, PIC was calculated according to Nei (1973): \(PIC = 1 - \sum p_{ij}^{2}\) where pij is the frequency of the jth allele for ith locus across all alleles at a locus.
For RAPD and ISSR, polymorphism information content was calculated according to Anderson et al. (1993): \(PIC = 1 - \mathop \sum \limits_{1}^{k} p_{i}^{2}\) where k is the total number of alleles detected for a given marker locus and Pi is the frequency of the ith allele in the set of genotypes investigated.
Cluster analysis
SSRs bands were scored on the molecular weight of the bands and designated A, B, C, etc. following Gao et al. (2017), Ćurčić et al. (2017) and Yelome et al. (2018). For SSRs Bray–Curtis dissimilarity values were calculated to reveal the relationships among samples based on the scores. The hierarchical clustering software Cluster version 3.03 was used for computing the tree and the calculated tree was visualized using Java TreeView version 1.1.6.4. RAPD and ISSR bands were scored as 1 for their presence or 0 for their absence to generate a matrix. The genetic similarity among genotypes was calculated using simple matching genetic distance (SM = m/n), where ‘m’ is the number of matches and ‘n’ is the total number of variables. Clustering analysis was obtained using the un-weighted pair group method of arithmetical average (UPGMA) algorithm. All of these computations were carried out using NTSYS 2.02 software. To determine how accurately the dendrograms represent the estimates of genetic similarity among the genotypes, a cophenetic matrix was generated for each dendrogram and compared with the corresponding similarity matrix by the Mantel matrix correspondence test (Mantel 1967). The same Mantel statistic was used to compare the similarity matrices as well as the dendrograms produced by the SSR, RAPD and ISSR techniques. All these procedures were performed by appropriate routines in NTSYSpc version 2.0 (Rohlf 1998).
Results
Morphological features
ANOVA for 17 morphological features assessed among the WPs revealed significant differences among the traits at 1 and 5% probability levels. The highest and lowest CV (coefficient of variation) were observed in leaf size of blade (11.29%) and degree of opening of bracts (0.1%), respectively (Tables 2, 3).
Comparison of means between WPs in Table 4 showed that the populations from Bandar-e Gaz and Aliabad-e-Katul had the highest value for cone width (2.12 and 2.15 cm, respectively) and cone length (2.79 and 2.76 cm, respectively), bract width (0.72 and 0.70 cm, respectively) and bract length (1.06 and 1.00 cm, respectively), cone fresh weight (49.94 and 48.40 gr, respectively) and cone dry weight (10.30 and 10.17 gr, respectively), while the populations from Rostamabad and Totkabon had the lowest values for these traits. The bract size value varied in six populations (Rostamabad, Totkabon, Someh Sara, Saravan, Rahimabad and Lahijan,) exhibited score “small”, while only two populations were “large” (Bandar-e Gaz and Aliabad-e-Katul), the other seven populations had “medium” bract size. The growth type did not vary notably between populations, most of populations were “normal” except for Rostamabad and Totkabon populations which were “dwarf”. Similarly, time of flowering was not markedly variant between populations; although eleven populations showed “late” (September) flowering habit and only two populations were “early” (July) flower (Rostamabad and Totkabon). Conversely, leaf size of blade varied greatly amongst the studied populations from “small” (Rostamabad, Lahijan, Ranekouh, Kochsfhan) to “large” (Bandar-e Gaz, Aliabad-e-Katul). The degree of opening of bracts as another important character exhibited a great variation, which many of populations had “closed” cones (Chalus, Nowshahr, Bandar-e Gaz, Aliabad-e-Katul, Ranekouh and Rahimabad). In 12 populations the “intensity of green color” were medium, and other three ones were “low” (Rostamabad, Totkabon and Someh Sara). The descriptive values for “length of apex” in bract were “long” in populations of Kisom and Kochsfhan, “very short” in Rostamabad and Totkabon, and other population were “short” or “medium”. Populations commonly had “medium” and “large” cone width/length ratio, excluding Lahijan which was “small”.
Correlation analysis indicated strong positive and negative correlations among morphological characteristics (Table 5). Positive correlation between cone shape and cone width, cone length, bract width, bract length, leaf size of blade and width/length ratio was observed. It is interesting to note that a significant negative correlation between cone shape and all the other measured features was observed, except for cone size, width/length ratio, degree of opening of bracts, time of flowering, leaf size of blade and main shoot anthocyanin. In addition, we observed a positive correlation between time of flowering and growth type. There was also a strong positive correlation between cone fresh weight and cone dry weight and the yield related traits such as cone size, cone width, cone length, bract width, bract length, and leaf size of blade and width/length ratio.
Based on the cluster analysis, the WPs were divided into three distinct groups (Fig. 2). In the first group, the populations from Rostamabad and Totkabon were clustered together, while these two populations had the smallest values for cone width and cone length, bract width and bract length, cone fresh weight and cone dry weight. The second cluster, had two sub-clusters. In the first sub-cluster, the WPs from Bandar-e Gaz and Aliabad-e-Katul were grouped, and these two populations had the highest values for cone width, cone length, bract width, bract length, cone fresh weight and cone dry weight. The second sub-cluster had two populations from Kisom and Kochsfhan, and these WPs, in terms of cone width, cone length, bract width, bract length, cone fresh weight and cone dry weight, had the highest value after Bandar-e Gaz and Ali-abad. The third cluster consisted of the rest of the populations (Table 4), which had middle values of the descriptors and quantitative yield associated characteristics.
Molecular analysis
SSR analysis
A total of 64 score-able SSR markers were produced using fourteen molecular markers with an average of 4.57 bands per marker (Table 6). The polymorphism percentage (P %) was 100% for each pair of SSR primers. The polymorphism information content value ranged from 0.50 (Hl-AGA1) to 0.97 (Hl-GT24) with a mean of 0.64. The total number of bands from SSRs was 64. In this study, average expected (He) and observed (Ho) heterozygosis estimated 66% and 55%, respectively (Table 7). Cluster analysis based on SSR data using Bray–Curtis dissimilarity analysis placed WPs in five groups. The first and second groups each contained 1 WP from Bandar-e Gaz (P15) and Rahimabad (P7), respectively, that were out-grouped (Fig. 3). The populations from Mazandaran province: Shasavar, Ramsar and Noshahr (P5, P9 and P12, respectively) and 1 WP, Ranekouh (P8) from Guilan, clustered together as third group. The fourth group encompassed the highest number of WPs (7) from Guilan province including Rostamabad, Totkabon, Someh Sara, Saravan, Lahijan, Kisom, Kochsfhan (P1, P2, P3, P4, P6, P10 and P11, respectively). The last cluster had two WPs 1 from Mazandaran (P13: Chalus) and Golestan (P14: Aliabad-e-Katul). The similarity coefficient among the WPs were between 44 and 92%.
ISSR and RAPD Analysis
A total of 232 score-able markers were produced by fourteen polymorphic ISSRs with an average of 16.6 bands per marker, while RAPDs had less bands (70), the mean number of bands per marker was 14 (Table 6). The PPB for ISSRs and RAPDs were 71.6% and 91.9%, respectively, which in this index RAPDs were notably more informative than ISSRs. For ISSR primer combination, the PIC value ranged from 0.26 to 0.35 with a mean of 0.30. In RAPDs, the PIC value varied from 0.20 to 0.30 with an average of 0.24. Cluster analysis through the UPGMA method with simple matching distances placed WPs in 4 groups in which the first group (1) had 4 WPs, 75% belong to Guilan area (Totkabon (2), Someh Sara (3) and Kochsfahan (11) and 25% to Golestan region (Bandar-e Gaz (15), and WPs of this group had similarity coefficient between 0.36 and 0.68 which consisted of WPs of 4 parts of the two areas. The second group (2) included 3 WPs, P6 and P7 from Guilan (Rahimabad and Lahijan) and P9 from Mazandaran (Ramsar), respectively, which the similarity coefficient was between 0.40 and 0.71. The third group (3), as the biggest group, included 5 WPs which the WPs from Guilan (Rostamabad (1) and Saravan(4), Mazandaran (Nowshahr (12) and Chalus (13) and Golestan (Aliabad-e-Katul (14) were clustered together, and in the fourth group (4) WPs from Guilan (Ranekouh (8) and Kisom (10)) and Mazandaran (Shahsavar (5) were clustered together; the similarity coefficient was 0.41 and 0.74. Therefore, in ISSR-RAPD dendrograms, WPs, two by two, had less similarity and most of WPs grouped together with similarity coefficient between 0.41 and 0.65 (Fig. 4).
Structure analysis
STRUCTURE yielded K and ΔK and the two-dimensional graph is presented in Fig. 5, showing a two-way graph for determining the optimal K. The best K, which is the peak of the curve, was 5. Supplemental Table S4 shows the placement of each genotype in each cluster. S10, G2, G10, G13, G14, G16, G18 G46 and G47 WPs were clustered in group 1; G4, G16, G20, G21 G28 and G36 WPs were clustered in group 2; G3, G24, G26, G29, G31, G32, G37, G39 and G54 WPs were clustered in the 3rd group; G7, G9, G22, G25 and G30 WPs were clustered in the 4th group and G42, G44, G48, G50, G51, G52 WPs were clustered in the last group. The bar plot diagram for the population structure is shown in Fig. 6. Of the 4 WPs in cluster 1, 50% belonged to Guilan and 50% belonged to Mazandaran. In the cluster 2, 20% of the WPs belonged to Mazandaran and 80% belonged to Guilan Province. In cluster 3, 11% of the WPs belonged to Golestan Province, 22% of them belonged to Mazandaran Province and 67% belonged to Guilan Province. In cluster 4, all the WPs belonged to Guilan Province. Interestingly, in the last cluster, 33% of the WPs were from Mazandaran Province and 67% were from Golestan Province.
Discussion
Identification of genetic diversity as well as natural population relationships and structure are necessary steps toward rational exploitation a new genetic reservoir and pave the way to conserve a germplasm properly, therefore, we attempted to reveal the possible genetic diversity in H. lupulus L. wild germplasm in Northern Iran for the first time. We detected the structure of WPs through two well-proven approaches, including morphological and molecular analysis which frequently have been employed for hops (McAdam 2013; Mongelli et al. 2015; Turchetto et al. 2016). In this study, a large degree of variation among populations were observed for most yield components. Therefore, due to the high genetic diversity for different agronomic traits among native hop populations, especially in terms of yield and yield components, potential to breed cultivars for flower yield, growth characteristics and other yield characteristics exists.
In the process of examining plant genetic diversity, evaluating the correlation of traits is critical perquisite for selection of superior WPs (Ali et al. 2003). Therefore, simple correlation was used to obtain information about the relationship between traits. Strong correlation between cone length, cone width, bract width, bract length, leaf size of blade and width/ length ratio was observed. Due to morphological complexity, breeders may employ our various measures in selecting source plants (Yimram et al. 2009). Our results showed a wide range diversity in the vegetative traits of Iranian hops. Similarly Mongelli et al. (2015) observed a significant variation in evaluated morphological features in an Italian hop germplasm.
Clustering patterns of populations based on morphological characters inclined to be consistent with geographical origins. This finding was in accordance with Hartings et al. (2008) observations on maize, and also Liu et al. (2016) on Ulmus lamellose. Moreover, the dispersion pattern of a species may impact genetic diversity, because typically plants with less dispersion have less genetic variability (Hamrick and Godt 1996). Even though hop populations in Northern Iran are in decline, geographic distribution ranges from east to west of Hyrcanian forests, which predisposes morphological and genetic diversity. Most of the wild hop populations are late flowering, unlike the commercial varieties, suggesting lack of domestication in the WPs. Russian native hops, especially in the main hop growing areas, are early flowering: in order to cope with the constraint cold climate (less than 100 days to vegetation phase), domestic hops have undergone breeding selection for such commercial agronomics (information from official booklet of Research and Technological Institute of Hop Production, Chuvashia, Russian Federation). Another example which as Skomra et al. (2013) reported, domestic hops have shorter branches in comparison with other wild hop collections in Europe. Short branches are correlated to early maturation, however, the short branch pattern has been observed in some late-maturing lines. On the contrary, our inquiries from local people (where WPs were sited) and literature search in the available early documentation of hop in Northern Iran, gave no indication of neither common use of hop nor its cultivation. Understanding the scope of genetic variation and genetic structure of the gene pool is a criterion for managing and efficient use of germplasm resources. Population structure analysis using the STRUCTURE software allows the population to be segmented into subgroups with different structures. The subgroups are genetically distinct from each other, thus admixed WPs can be identified (Dadras et al. 2014). New genetic technologies, especially DNA sequencing, has led to the development of new methods for measuring the similarity and genetic variation of plant species and populations (Xu et al. 2010). The results of molecular cluster and structure analysis showed that the genetic diversity of collected WPs does not follow their geographic distribution origins: no correlation between the collection site and the genetic distance between the native populations; and the WPs collected from an area were not necessarily similar and had a great genetic distance. Solouki et al. (2008) reported similar results on Matricaria chamomilla, in which genetic variation in masses were not matched to geographical distribution. Similarly, resultant of experiment conducted on Jatropha curcas by Vasquez-Mayorga et al. (2017) to assess the genetic diversity in its Costa Rican germplam revealed that accessions were grouped separately from their sample sites. Other studies reported lack of uniformity between the genetic origin and the collection localities in their observations (Ambrosi et al. 2010; Maghuly et al. 2015).
RAPD-ISSR markers have been applied for detecting genetic variability in H. lupulus; for example, ŠUštar-Vozlič and Javornik (1999) investigated 65 hop cultivars by RAPD, and observed the polymorphism of 38.6%. Patzak (2001), also used RAPD, ISSR, STS and AFLP to analysis diversity in ten hop varieties and compared the outcomes, in which RAPD and ISSR polymorphism were 32.6% and 42.3% respectively. In the both studies above, the polymorphism was significantly lower in comparison to our Iranian wild hop collection using the similar markers; RAPD (71.6%) and ISSR (91.9%).
SSR markers indicated high diversity in hop populations: our results were the same or even higher than to level of variation that Bassil et al. (2008) reported in European hop accessions, but the indices that they reported in American wild hops were higher than our Iranian WPs. For our WPs, the polymorphism level revealed by SSRs was higher in comparison to RAPDs and ISSRs, which indicates that SSRs are highly informative and useful markers for hops. Clustering patterns for SSR diversity was observed to successfully separate WPs based on their geographical origins, particularly for Guilan and Mazandaran WPs where mostly clustered together. Suitability of SSRs over other markers also reported in several cases in other plants (Goulão and Oliveira 2001; Palombi and Damiano 2002; Belaj et al. 2003).
STRUCTURE analysis produced 5 groups which they grouped similar to the SSRs distance-based clustering. Commonly, the genetic structure of a germplasm depends on factors (geographical distribution, dispersal paradigm, population size and gene flow) which vary by historical and biological background (Loveless and Hamrick 1984; Lynch and Walsh 1998; Nybom 2004). Ecological circumstances are generators of selective pressures which impose changes at population level that eventually causes distinction between them. Consequently, a reliable prediction of genetic diversity in a plant germplasm cannot be achieved without contemplating the environmental components (Ohsawa et al. 2008; Wang et al. 2018). Ecological factors varied in the locations of WPs, in the respect of not only yearly precipitation, temperature, and light intensity, but the soil features (physically i.e. clay, silt, sand, moss percentage, and chemically, i.e., macro and micro element, pH, EC) (data not shown, available upon request). Based on our field survey, hops in Iran seem fairly adaptable to the wide range of soil properties.
For our WP locations, the climatological elements had varied significantly, as a case in point, yearly average temperature and precipitation in Rostamabad were 16.2 °C and 950 mm, respectively, while in Aliabad-e-Katul, average values were 17.9 °C and 445 mm, respectively. Further, elevation in our survey varied from − 13 (Someh Sara) to 714 (Rahimabad). Detectable changes in plant diversity (i.e. richness and phonotypical variation) often occur in elevations above 700 m (Bonanomi et al. 2016; Xu et al. 2017), and it's generally accepted that with increasing elevation the height of plants shrink (Boscutti et al. 2018). We observed the ‘dwarf’ growth habit in areas with the highest elevations (714 and 520 m), and speculate that elevation might have a key role. Two WPs in the highest elevation showed the lowest quantity of stature and floral components, perhaps, conditioned by the negative impacts that increasing in elevation has on dry matter, volume or height (Worrell 1987). Soil components in the collection sites were highly variable; for example, pH and nitrogen, both of which potentially affect plant recruitment, were 4.8 and 0.48%, respectively in Lahijan whereas in Rostamabad they were 6.8 and 0.93%, respectively (data not shown, available upon request). Molecular analyses did not distinguish populations according to their geographical region suggesting gene flows between populations (Martins et al. 2006; Liu et al. 2013; Yang et al. 2016; Vasquez-Mayorga et al. 2017). Although vegetative traits are prone to be interpreted prejudicially (Ravi et al. 2003; Baránek et al. 2006; Yook et al. 2014; Zhang et al. 2018) assessing plant morphological variation as a basic procedure still allows discrimination of plant populations in a convenient and cost-saving way. Tackling erosion of genetic diversity is the core of saving endangered plant species (Avise and Hamrick 1996). Our results can be used in conservation programs to slow down or halt declining H. lupulus populations in the Hyrcanian forests in a most effective way. First and foremost, detecting the frequency of populations and diversity of germplasm now, allows tracking of deterioration taking place in each locality over time, recognize the populations that are in danger the most and require the protection urgently. Secondly, by being aware of the wide range of climatic and ecologic factors where hop populations remain can help recover those populations, even reintroduce hop to the sites that once had hops, and this population can be propagated efficiently ex situ under in vitro condition Mafakheri and Hamidoghli (2015b), and provides the suitable propagation material to repopulate the hop populations.
Conclusions
A large spectrum of diversity was demonstrated in the Iranian hop germplasm, which offers a unique opportunity to access a new reservoir of suitable alleles that breeders can utilize to cope with novel challenges in developing new cultivars. Evaluating a germplasm through relying on morphological features and using different molecular methods (SSR, ISSR, and RAPD) can generate a reliable information on such previously unexplored germplasm, but it is recommended, first, to use more efficient molecular markers (more SSRs and SNPs), which have a high polymorphism and exist abundantly in the genome, in the identification and genetic relationship of hop indigenous populations. Also, we are profiling hop secondary metabolites as the third approach to differentiate between populations.
References
Abbott MS, Fedele MJ (1994) A DNA-based varietal identification procesure for hop leaf tissue. J Inst Brew 100:283–285. https://doi.org/10.1002/j.2050-0416.1994.tb00825.x
Ali N, Javidfar F, Elmira JY, Mirza M (2003) Relationship among yield components and selection criteria for yield improvement in winter rapeseed (Brassica napus L.). Pak J Bot 35:167–174
Ambrosi DG et al (2010) DNA markers and FCSS analyses shed light on the genetic diversity and reproductive strategy of Jatropha curcas L. Diversity 2(5):810–836. https://doi.org/10.3390/d2050810
Anderson JT, Wilson SM, Datar KV, Swanson MS (1993) NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol 13:2730–2741. https://doi.org/10.1128/MCB.13.5.2730
Avise JC, Hamrick JL (1996) Conservation genetics: case histories from nature, vol 575.17 CON.
Baránek M, Raddová J, Pidra M (2006) Comparative analysis of genetic diversity in Prunus L. as revealed by RAPD and SSR markers. Sci Hortic 108:253–259. https://doi.org/10.1016/j.scienta.2006.01.023
Barth HJ, Schmidt C, Klinke C (1994) The hop atlas: the history and geography of the cultivated plant, vol 383. Joh. Barth & Sohn, Nuremberg
Bassil NV, Gilmore B, Oliphant JM, Hummer KE, Henning JA (2008) Genic SSRs for European and North American hop (Humulus lupulus L.). Genet Resour Crop Evol 55:959–969. https://doi.org/10.1007/s10722-007-9303-9
Belaj A, Satovic Z, Cipriani G, Baldoni L, Testolin R, Rallo L, Trujillo I (2003) Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effectiveness in establishing genetic relationships in olive. Theor Appl Genet 107:736–744. https://doi.org/10.1007/s00122-003-1301-5
Bonanomi G, Stinca A, Chirico GB, Ciaschetti G, Saracino A, Incerti G (2016) Cushion plant morphology controls biogenic capability and facilitation effects of Silene acaulis along an elevation gradient. Funct Ecol 30:1216–1226. https://doi.org/10.1111/1365-2435.12596
Boscutti F, Casolo V, Beraldo P, Braidot E, Zancani M, Rixen Ch (2018) Shrub growth and plant diversity along an elevation gradient: evidence of indirect effects of climate on alpine ecosystems. PLoS ONE 13(4):e0196653. https://doi.org/10.1371/journal.pone.0196653
Brady JL, Scott NS, Thomas MR (1996) DNA typing of hops (Humulus lupulus) through application of RAPD and microsatellite marker sequences converted to sequence tagged sites (STS). Euphytica 91:277–284. https://doi.org/10.1007/BF00033088
Čeh B, Naglič B, Luskar MO (2012) Hop (Humulus lupulus L.) cones mass and lenght at cv. Savinjski golding. Hmelj Bilt 19:12–16
Ĉerenak A, Pavlovic M, Luskar MO, Košir I (2011) Characterisation of slovenian hop (Humulus Lupulus L.) varieties by analysis of essential oil. Hmelj Bilt Hop Bull 18:27–32
Ćurčić Ž, Taški-Ajduković K, Nagl N (2017) Relationship between hybrid performance and genetic variation in self-fertile and self-sterile sugar beet pollinators as estimated by SSR markers. Euphytica 213:108. https://doi.org/10.1007/s10681-017-1897-1
Dadras AR, Sabouri H, Nejad GM, Sabouri A, Shoai-Deylami M (2014) Association analysis, genetic diversity and structure analysis of tobacco based on AFLP markers. Mol Biol Rep 41:3317–3329. https://doi.org/10.1007/s11033-014-3194-6
Danilova TV, Karlov GI (2006) Application of inter simple sequence repeat (ISSR) polymorphism for detection of sex-specific molecular markers in hop (Humulus lupulus L.). Euphytica 151:15–21. https://doi.org/10.1007/s10681-005-9020-4
Danilova TV, Danilov SS, Karlov GI (2003) Assessment of genetic polymorphism in hop (Humulus lupulus L.) cultivars by ISSR–PCR analysis. Rus J Genet 39:1252–1257. https://doi.org/10.1023/B:RUGE.0000004140.26775.db
Gao J, Li N, Xuan Z, Yang W (2017) Genetic diversity among “Qamgur” varieties in China revealed by SSR markers. Euphytica 213:204. https://doi.org/10.1007/s10681-017-1988-z
Gholizadeh H, Saeidi Mehrvarz S, Naqinezhad A, Jafari M (2017) Peucedanumhyrcanicum (Apiaceae), a new species of Peucedanum s. lato from Northern Iran. Ann Bot Fenn. https://doi.org/10.5735/085.054.0612
Goulão L, Oliveira CM (2001) Molecular characterisation of cultivars of apple (Malus × domestica Borkh.) using microsatellite (SSR and ISSR) markers. Euphytica 122:81–89. https://doi.org/10.1023/A:1012691814643
Hadonou AM, Walden R, Darby P (2004) Isolation and characterization of polymorphic microsatellites for assessment of genetic variation of hops (Humulus lupulus L.). Mol Ecol Res 4:280–282. https://doi.org/10.1111/j.1471-8286.2004.00641.x
Hampton R, Small E, Haunold A (2001) Habitat and variability of Humulus lupulus var. lupuloides in upper Midwestern North America: a critical source of American hop Germplasm. J Torrey Bot Soc 128:35–46. https://doi.org/10.2307/3088658
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond Ser B Biol Sci 351:1291–1298. https://doi.org/10.1098/rstb.1996.0112
Hartings H, Berardo N, Mazzinelli G, Valoti P, Verderio A, Motto M (2008) Assessment of genetic diversity and relationships among maize (Zeamays L.) Italian landraces by morphological traits and AFLP profiling. Theor Appl Genet 117:831. https://doi.org/10.1007/s00122-008-0823-2
Hofmann R, Weber S, Rettberg N, Thörner S, Garbe L, Folz R (2013) Optimization of the hop kilning process to improve energy efficiency and recover hop oils. Brew Sci 66:23–30
Jakše J, Bandelj D, Javornik B (2002) Eleven new microsatellites for hop (Humulus lupulus L.). J Mol Ecol Notes 2:544–546. https://doi.org/10.1046/j.1471-8286.2002.00309.x
Jakše J, Satovic Z, Javornik B (2004) Microsatellite variability among wild and cultivated hops (Humulus lupulus L.). Genome 47:889–899. https://doi.org/10.1139/g04-054
Kavalier AR, Litt A, Ma C, Pitra NJ, Coles MC, Kennelly EJ, Matthews PD (2011) Phytochemical and morphological characterization of hop (Humulus lupulus L.) cones over five developmental stages using high performance liquid chromatography coupled to time-of-flight mass spectrometry, ultrahigh performance liquid chromatography photodiode array detection, and light microscopy techniques. J Agric Food Chem 59:4783–4793. https://doi.org/10.1021/jf1049084
Kordrostami M, Rahimi M (2015) Molecular markers in plants: concepts and applications. Genet 3rd Millenn 13:4024–4031
Liu Y, Wei W, Ma K, Li J, Liang Y, Darmency H (2013) Consequences of gene flow between oilseed rape (Brassica napus) and its relatives. Plant Sci 211:42–51. https://doi.org/10.1016/j.plantsci.2013.07.002
Liu L et al (2016) Genetic diversity of Ulmuslamellosa by morphological traits and sequence-related amplified polymorphism (SRAP) markers. Biochem Syst Ecol 66:272–280. https://doi.org/10.1016/j.bse.2016.04.017
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95. https://doi.org/10.1146/annurev.es.15.110184.000433
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits, vol 1. Sinauer, Sunderland
Mafakheri M, Hamidoghli Y (2015) Effect of different extraction solvents on phenolic compounds and antioxidant capacity of hop flowers (Humulus lupulus L.). In: International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 1–6. https://doi.org/10.17660/ActaHortic.2019.1236.1
Mafakheri M, Hamidoghli Y (2015) Micropropagation of hop (Humuluslupulus L.) via shoot tip and node culture In: IV international humulus symposium 1236, pp 31–36
Maghuly F, Jankowicz-Cieslak J, Pabinger S, Till BJ, Laimer M (2015) Geographic origin is not supported by the genetic variability found in a large living collection of Jatropha curcas with accessions from three continents. Biotechnol J 10:536–551. https://doi.org/10.1002/biot.201400196
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Martins SR, Vences FJ, Sáenz de Miera LE, Barroso MR, Carnide V (2006) RAPD analysis of genetic diversity among and within Portuguese landraces of common white bean (Phaseolus vulgaris L.). Sci Hort 108:133–142. https://doi.org/10.1016/j.scienta.2006.01.031
McAdam EL (2013) Molecular and quantitative genetic analyses of hop (Humulus lupulus L.). PhD dissertation, University of Tasmania, Tasmania, Newzealand
McAdam EL, Vaillancourt RE, Koutoulis A, Whittock SP (2014) Quantitative genetic parameters for yield, plant growth and cone chemical traits in hop (Humuluslupulus L.). BMC Genet 15:22. https://doi.org/10.1186/1471-2156-15-22
Moir M (2000) Hops—A millennium review. J Am Soc Brew Chem 58:131–146. https://doi.org/10.1094/ASBCJ-58-0131
Mongelli A, Rodolfi M, Ganino T, Marieschi M, Dall’Asta C, Bruni R (2015) Italian hop germplasm: characterization of wild Humulus lupulus L. genotypes from Northern Italy by means of phytochemical, morphological traits and multivariate data analysis. Ind Crops Prod 70:16–27. https://doi.org/10.1016/j.indcrop.2015.02.036
Morales RGF, Resende JTV, Faria MV, Andrade MC, Resende LV, Delatorre CA, Silva PRd (2011) Genetic similarity among strawberry cultivars assessed by RAPD and ISSR markers. Sci Agric 68:665–670. https://doi.org/10.1590/S0103-90162011000600010
Murakami A (2000) Comparison of sequence of rbcL and non-coding regions of chloroplast DNA and ITS2 region of rDNA in Genus Humulus. Breed Sci 50:155–160. https://doi.org/10.1270/jsbbs.50.155
Murakami A, Darby P, Javornik B, Pais MS, Seigner E, Lutz A, Svoboda P (2006) Microsatellite DNA analysis of wild hops, Humulus lupulus L. Genet Res Crop Evol 53:1553–1562. https://doi.org/10.1007/s10722-005-7765-1
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci 70:3321. https://doi.org/10.1073/pnas.70.12.3321
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155. https://doi.org/10.1111/j.1365-294X.2004.02141.x
Odum EP, Barrett GW (1971) Fundamentals of ecology, vol 3. Saunders, Philadelphia
Ohsawa T, Saito Y, Sawada H, Ide Y (2008) Impact of altitude and topography on the genetic diversity of Quercus serrata populations in the Chichibu Mountains, central Japan. Flora 203:187–196. https://doi.org/10.1016/j.flora.2007.02.007
Palombi M, Damiano C (2002) Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep 20:1061–1066. https://doi.org/10.1007/s00299-001-0430-z
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607. https://doi.org/10.1007/BF02172406
Patzak J (2001) Comparison of RAPD, STS, ISSR and AFLP molecular methods used for assessment of genetic diversity in hop (Humulus lupulus L.). Euphytica 121:9–18. https://doi.org/10.1023/A:1012099123877
Patzak J (2003) Assessment of somaclonal variability in hop (Humulus lupulus L.) in vitro meristem cultures and clones by molecular methods. Euphytica 131:343–350
Patzak J, Henychová A (2018) Evaluation of genetic variability within actual hop (Humulus lupulus L.) cultivars by an enlarged set of molecular markers. Czech J Genet Plant Breed 54:86–91. https://doi.org/10.17221/175/2016-CJGPB
Patzak J, Nesvadba V, Krofta K, Henychova A, Marzoev AI, Richards K (2010) Evaluation of genetic variability of wild hops (Humulus lupulus L.) in Canada and the Caucasus region by chemical and molecular methods. Genome 53:545–557. https://doi.org/10.1139/g10-024
Peredo EL, Ángeles Revilla M, Reed BM, Javornik B, Cires E, Prieto JAF, Arroyo-García R (2010) The influence of European and American wild germplasm in hop (Humulus lupulus L.) cultivars. Genet Res Crop Evol 57:575–586. https://doi.org/10.1007/s10722-009-9495-2
Pillay M, Kenny ST (1996) Random amplified polymorphic DNA (RAPD) markers in hop, Humulus lupulus: level of genetic variability and segregation in F1 progeny. Theor Appl Genet 92:334–339. https://doi.org/10.1007/BF00223676
Polley A, Ganal MW, Seigner E (1997) Identification of sex in hop (Humulus lupulus) using molecular markers. Genome 40:357–361. https://doi.org/10.1139/g97-048
Ravi M, Geethanjali S, Sameeyafarheen F, Maheswaran M (2003) Molecular marker based genetic diversity analysis in rice (Oryza sativa L.) using RAPD and SSR markers. Euphytica 133:243–252. https://doi.org/10.1023/A:1025513111279
Rígr A, Faberová I (2000) Descriptor list—genus Humulus L. CHI-Žatec, 3–18
Rohlf FJ (1998) NTSYSpc numerical taxonomy and multivariate analysis system version 2.0 user guide. Applied Biostatistics Inc, Setauket
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci 81:8014–8018. https://doi.org/10.1073/pnas.81.24.8014
Seefelder S, Ehrmaier H, Schweizer G, Seigner E (2000a) Genetic diversity and phylogenetic relationships among accessions of hop, Humulus lupulus, as determined by amplified fragment length polymorphism fingerprinting compared with pedigree data. Plant Breed 119:257–263. https://doi.org/10.1046/j.1439-0523.2000.00500.x
Seefelder S, Ehrmaier H, Schweizer G, Seigner E (2000) Male and female genetic linkage map of hops, Humulus lupulus. Plant Breed 119:249–255. https://doi.org/10.1046/j.1439-0523.2000.00469.x
Skomra U, Bocianowski J, Agacka-Mołdoch M (2013) Agro-morphological differentiation between European hop (Humulus lupulus L.) cultivars in relation to their origin. J Food Agric Environ 11:1123–1128. https://doi.org/10.1234/4.2013.4812
Smykal P, Horacek J, Dostalova R, Hybl M (2008) Variety discrimination in pea (Pisum sativum L.) by molecular, biochemical and morphological markers. J Appl Genet 49:155–166. https://doi.org/10.1007/bf03195609
Solouki M, Mehdikhani H, Zeinali H, Emamjomeh AA (2008) Study of genetic diversity in Chamomile (Matricaria chamomilla) based on morphological traits and molecular markers. Sci Hortic 117:281–287. https://doi.org/10.1016/j.scienta.2008.03.029
Soofi M et al (2018) Livestock grazing in protected areas and its effects on large mammals in the Hyrcanian forest. Iran Biol Conserv 217:377–382. https://doi.org/10.1016/j.biocon.2017.11.020
Srečec S, Zechner-Krpan V, Marag S, Špoljarić I, Kvaternjak I, Mršić G (2011) Morphogenesis, volume and number of hop (Humulus lupulus L.) glandular trichomes, and their influence on alpha-acid accumulation in fresh bracts of hop cones. Acta Bot Croat 70:1–8. https://doi.org/10.2478/v10184-010-0017-2
Stajner N, Jakse J, Kozjak P, Javornik B (2005) The isolation and characterisation of microsatellites in hop (Humulus lupulus L.). Plant Sci 168:213–221. https://doi.org/10.1016/j.plantsci.2004.07.031
ŠUštar-Vozlič J, Javornik B (1999) Genetic relationships in cultivars of hop, Humulus lupulus L., determined by RAPD analysis. Plant Breed 118:175–181. https://doi.org/10.1046/j.1439-0523.1999.118002175.x
Turchetto C, Segatto AL, Mader G, Rodrigues DM, Bonatto SL, Freitas LB (2016) High levels of genetic diversity and population structure in an endemic and rare species: implications for conservation. AoB Plants. https://doi.org/10.1093/aobpla/plw002
UPOV (2006) International Union for the Protection of New Varieties of Plants: hop Guidelines for the conduct of tests for distinctness, uniformity and stability. https://www.upov.int/portal/index.html.en
Vasquez-Mayorga M et al (2017) Molecular characterization and genetic diversity of Jatrophacurcas L. in Costa Rica. Peer J 5:e2931. https://doi.org/10.7717/peerj.2931
Vejl P (1997) Identification of genotypes in hop (Humulus lupulus L.) by rapd analysis using program gel manager for windows. Plant Prod 43:325–331
Vieira MLC, Santini L, Diniz AL, Munhoz CdF (2016) Microsatellite markers: what they mean and why they are so useful. Genet Mol Biol 39:312–328. https://doi.org/10.1590/1678-4685-GMB-2016-0027
Wang L, Bai P, Yuan X, Chen H, Wang S, Chen X, Cheng X (2018) Genetic diversity assessment of a set of introduced mung bean accessions (Vigna radiata L.). Crop J 6:207–213. https://doi.org/10.1016/j.cj.2017.08.004
Worrell R (1987) Geographical variation in Sitka spruce productivity and its dependence on environmental factors. PhD dissertation, University of Edinburgh, Edinburgh, UK
Xiong H et al (2016) Genetic diversity and population structure of cowpea (Vignaunguiculata L. Walp). PLoS ONE 11:e0160941. https://doi.org/10.1371/journal.pone.0160941
Xu MY, Aragon AD, Mascarenas MR, Torrez-Martinez N, Edwards JS (2010) Dual primer emulsion PCR for next-generation DNA sequencing. Biotechniques 48:409–412. https://doi.org/10.2144/000113423
Xu M, Ma L, Jia Y, Liu M (2017) Integrating the effects of latitude and altitude on the spatial differentiation of plant community diversity in a mountainous ecosystem in China. PLoS ONE 12:e0174231. https://doi.org/10.1371/journal.pone.0174231
Yang J, Gao Z, Sun W, Zhang C (2016) High regional genetic differentiation of an endangered relict plant Craigia yunnanensis and implications for its conservation. Plant Divers 38:221–226. https://doi.org/10.1016/j.pld.2016.07.002
Yelome OI, Audenaert K, Landschoot S, Dansi A, Vanhove W, Silue D, Van Damme P, Haesaert G (2018) Analysis of population structure and genetic diversity reveals gene flow and geographic patterns in cultivated rice (O. sativa and O. glaberrima) in West Africa. Euphytica 214:215. https://doi.org/10.1007/s10681-018-2285-1
Yimram T, Somta P, Srinives P (2009) Genetic variation in cultivated mungbean germplasm and its implication in breeding for high yield. Field Crops Res 112:260–266. https://doi.org/10.1016/j.fcr.2009.03.013
Yook MJ et al (2014) Assessment of genetic diversity of Korean Miscanthus using morphological traits and SSR markers. Biomass Bioenergy 66:81–92. https://doi.org/10.1016/j.biombioe.2014.01.025
Zanoli P, Rivasi M, Zavatti M, Brusiani F, Baraldi M (2005) New insight in the neuropharmacological activity of Humulus lupulus L. J Ethnopharmacol 102:102–106. https://doi.org/10.1016/j.jep.2005.05.040
Zanoli P, Zavatti M (2008) Pharmacognostic and pharmacological profile of Humulus lupulus L. J Ethnopharmacol 116:383–396. https://doi.org/10.1016/j.jep.2008.01.011
Zhang H et al (2018) Genetic variation and diversity in 199 Melilotus accessions based on a combination of 5 DNA sequences. PLoS ONE 13:e0194172–e0194172. https://doi.org/10.1371/journal.pone.0194172
Acknowledgements
We are grateful to Dr. Hamid Gholizadeh for contributing to the collection of plant materials.
Author information
Authors and Affiliations
Contributions
MM and PM conceived and designed research. MM and MK conducted experiments. MK, MM, MR, and PM analyzed data. MK, PM and MM wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mafakheri, M., Kordrostami, M., Rahimi, M. et al. Evaluating genetic diversity and structure of a wild hop (Humulus lupulus L.) germplasm using morphological and molecular characteristics. Euphytica 216, 58 (2020). https://doi.org/10.1007/s10681-020-02592-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02592-z