Abstract
Interspecific hybrids were developed between Trifolium alexandrinum cultivar Wardan × Trifolium vesiculosum and T. alexandrinum cultivar BL1 × T. vesiculosum through embryo rescue, as the crosses failed to set seed under natural conditions. Trifolium vesiculosum was used as a donor/male parent in this study as it is reported to possess tolerance to stem rot and high forage yield. Fertilization in crossed florets of the crosses was manifested from the recovery of swollen ovaries (< 7.80%) and confirmed from the presence of one degenerated ovule in most (> 93.00%) of the swollen ovaries. The hybrid embryos at various developmental stages (heart, torpedo and cotyledonary) were rescued at a frequency of 2.56% from Wardan × T. vesiculosum and 6.12% from BL1 × T. vesiculosum. Differentiation occurred only in the cotyledonary stage embryos, resulting in 17 putative interspecific hybrid plantlets. The assessment of plantlet hybridity through SSR markers (for the alleles inherited from the donor parent), micromorphological leaf traits (leaf texture and stomata) and morphological characters (plant height, leaflet length and width) confirmed production of two interspecific hybrids designated as AV1 and BV3 representing both the crosses. AV1 displayed moderate resistance and BV3 was resistant to stem rot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Trifolium (Fabaceae) usually called as clover includes about 300 pasture species (Butkute et al. 2014) of which several are agriculturally important and cultivated as forage crops (Zohary and Heller 1984; Abberton 2007). Egyptian clover or Berseem (Trifolium alexandrinum L.), a diploid species (2n = 16), is a winter annual of tropical and subtropical areas. In India, this multi-cut (four to eight cuts) forage crop is grown on an area of 1.9 m ha (ICAR 2012) and is beneficial for livestock due to its high crude protein (19.28%, dry matter yield 39.2 qt/ha) and palatable green fodder with good digestibility (up to 65%) (Roy et al. 2009, 2015). The crop lacks some important characters, such as profuse basal branching, high growth rate in vegetative stage and resistance to stem rot (Roy et al. 2004), the most devastating fungal disease of forages (Delclos et al. 1997; Öhberg et al. 2008) caused by Sclerotinia trifoliorum Erik. The resistance for this trait may be incorporated from other Trifolium species through interspecific hybridization. The incompatibility barrier between different Trifolium species (Evans 1962) makes hybridization impossible even in closely related species under field conditions (Hendrych 1990; Repkova et al. 2006).
The in vitro embryo rescue technique has been effectively used for developing interspecific hybrids between Trifolium species e.g. T. repens × T. ambiguum (Williams 1978), T. pratense × T. sarosiense (Phillips et al. 1982), T. ambiguum × T. repens (Yamada and Fukuoka 1986), T. repens × T. uniflorum (Pandey et al. 1987), T. repens × T. hybridum (Przywara et al. 1989), T. ambiguum × T. montanum and T. occidentale, T. isthmocarpum × T. repens and T. nigrescens (Ferguson et al. 1990), T. medium × T. pratense (Sawai et al. 1990), T. pratense × T. alpestre (Phillips et al. 1992), T. alexandrinum × T. apertum (Malaviya et al. 2004), T. alexandrinum × T. constantinopolitanum (Roy et al. 2004) and T. pratense × T. medium (Repkova et al. 2006). Interspecific hybridization in Trifolium species through embryo rescue has led to successful transfer of important traits such as clover cyst nematode (Heterodera trifolii) resistance from T. nigrescens into T. repens (Hussain et al. 1997), prolific branching from T. resupinatum into T. alexandrinum (Kaushal et al. 2005) and more number of stems per plant from T. medium into T. pratense leading to release of cultivar Pramedi (Vymyslicky 2014).
In the present study, recombination of economically desirable traits in cultivated T. alexandrinum was attempted through interspecific hybridization with T. vesiculosum following embryo rescue as conventional hybridization did not allow production of fully developed seeds. T. vesiculosum (2n = 16), a diploid annual legume species, was used as donor/male parent in hybridization due to its distinctness for profuse branching (Rampton 1972), lengthy vegetative growth stage (Riffkin et al. 2001), tolerance to stem rot and high forage yield (Taylor et al. 1979; Ovalle et al. 2010). The study describes development of two interspecific hybrids from crosses between T. alexandrinum cultivar Wardan × T. vesiculosum and T. alexandrinum cultivar BL1 × T. vesiculosum with the following highlighting features (i) identification of distinct embryo developmental stages after interspecific hybridization, and recovery of hybrid embryos at high frequency, (ii) confirmation of hybridity through molecular, micromorphological and morphological data, (iii) production of phenotypically normal and fertile interspecific hybrids (AV1, BV3) representing both crosses, and (iv) moderately resistant reaction of AV1 and resistant reaction of BV3 to stem rot.
Materials and methods
Plant material
Two interspecific crosses between (i) T. alexandrinum cultivar Wardan (female parent) × T. vesiculosum (donor/male parent) and (ii) T. alexandrinum cultivar BL1 (female parent) × T. vesiculosum (donor/male parent) were attempted in the glasshouse facility of School of Agricultural Biotechnology (SAB), Punjab Agricultural University (PAU), Ludhiana during the main cropping season (October to May, 2007–2013) for developing hybrids. Seeds of Wardan and T. vesiculosum were obtained from Indian Grassland and Fodder Research Institute, Jhansi, Uttar Pradesh, India and that of BL1 from Forage and Millets Section, Department of Plant Breeding and Genetics, PAU, Ludhiana. The healthy seeds of Wardan and BL1 were sown directly in earthen pots (8 inch diameter) containing soil (field soil and farmyard manure mixed in 3:1 ratio), whereas the seeds of T. vesiculosum were germinated by scratching gently with a zero number sand paper and placing on a sterile water-soaked filter paper in a disposable petridish (90 mm, Tarsons, India) under dark conditions. The germinated seeds were then transferred to pots containing soil. The sowing dates were staggered at an interval of 2 days till the month of November to lengthen availability of flowers. The pots were watered regularly for proper growth of plantlets. An additional light of 1000 W with sodium lamp (SON-T 1000 W E E40 1SL/4, Phillips, India) was supplied to T. vesiculosum from 5 p.m. to 8.30 a.m. during December–January, to ensure flowering in February, whereas for Wardan and BL1, flowering occurred under natural conditions in the glasshouse during February.
Interspecific hybridization and embryo rescue
The interspecific crosses between T. alexandrinum cultivar Wardan × T. vesiculosum and T. alexandrinum cultivar BL1 × T. vesiculosum were attempted during February. Flowers of T. alexandrinum at 3/4th floret formation stage were selected and florets were emasculated prior to anther dehiscence (Supplementary Fig. 1) as described by Kaushal et al. (2005) using a watchmaker lens (4½ inch power, Suissco, India). The upper and lower whorls of the florets surrounding the whorl of florets to be crossed were plucked before pollination; and subsequently the freshly emasculated florets were pollinated by T. vesiculosum pollen following the method given by Kaushal et al. (2005). After pollination, each flower containing crossed florets was enveloped with a butter paper bag to avoid any cross pollination, tagged with a jewel tag and left for embryo development. The crossed florets were collected daily from 10 to 16 days after pollination (DAP) in a 50 ml beaker (Borosil, India) containing Milli Q water (Direct Ultra TUVF-5, Bio-Age, India), brought to the tissue culture and transformation facility, SAB, PAU and used for rescuing embryos inside a laminar airflow cabinet (Klenzoids, India). A small number of interspecific crosses were left undisturbed (experimental control) for verifying seed set under natural conditions in the glasshouse.
The ovaries from crossed florets were excised in a sterile petridish using a pair of needles under a stereozoom microscope (MSZ, Olympus Opto Stereozoom Microscope, India) and surface-sterilized as per Kaushal et al. (2005). The ovules were microscopically excised from the ovaries and eventually embryos were dissected with a sharp needle and scalpel. The excised embryos were cultured individually on semi-solid embryo rescue medium (ER) [MS (Murashige and Skoog 1962) salts + 0.5 mg/l BAP + 3% (w/v) sucrose + 0.8% (w/v) agar] in test tubes (150 × 25 mm2, Borosil). The cultures were incubated in dark for the first 2 days and then subjected to 16:8 h light:dark conditions of 1.5 Klux/m2/s at 19 ± 2 °C. The plantlets obtained from the rescued embryos were clonally propagated in vitro by culturing axillary bud bearing nodal stem segments (1 cm) on semi-solid shoot induction medium LSP3 (Roy et al. 2004) [L2 basal medium (Phillips and Collins 1984) + 0.0008 mg/l NAA (naphthalene acetic acid) + 0.15 mg/l BAP (6-benzylaminopurine) + 2.5% (w/v) sucrose + 0.8% (w/v) agar)], and incubating under 18:6 h light:dark period at 19 ± 2 °C. The shoot clusters formed on stem segments were split and sub-cultured on LSP3 medium after every 28 days. After sub-culturing, the cultures were kept in dark for first 24 h and thereafter in 16:8 h light:dark conditions at 19 ± 2 °C. Shoot elongation occurred simultaneously on LSP3 medium. The individual elongated shoots were cultured on semi-solid medium RL2 [RL basal medium (Phillips and Collins 1984) + 0.42 mg/l IAA (indole-3-acetic acid) + 2.5% (w/v) sucrose + 0.8% (w/v) agar] for root induction. These cultures were wrapped with black paper to cover the lower portion of test tubes and subsequently incubated under 16:8 h light:dark conditions at 19 ± 2 °C for two weeks. The media constituents utilized in the study were acquired from HiMedia, India. The plantlets were taken out from the culture tubes; roots washed in tap water and then kept for hardening on moist cotton overlaid with freshly squashed Rhizobium nodules harvested from soil-grown Egyptian clover plants. The plantlets were incubated at 20–25 °C under 16 h light for a week. The embryo culture regenerated plantlets of parents were used as experimental control. The parental and putative hybrid plants were transferred to soil in polythene bags containing Egyptian clover root nodules during October.
Molecular characterization
Genomic DNA from six tender leaves of clonally propagated plantlets of putative hybrids and parents was extracted according to cetyl trimethylammonium bromide method (Saghai-Maroof et al. 1984). The characterization for hybridity was carried out through simple sequence repeat (SSR) analysis using eight white clover specific SSR markers (Table 1) selected on the basis of PIC values (Kölliker et al. 2001). The PCR mixture (20 µl total volume) comprised 5X PCR buffer (final concentration per PCR = 1X), 25 mM MgCl2 (1.5 mM), 1 mM dNTPs (0.2 mM), 5 µM of each primer (0.25 µM), 40 ng/µl template DNA (120 ng) and 5 units Taq DNA polymerase (1 µl) [Promega, USA]. The mixtures were placed in a thermocycler (Eppendorf, Germany) programmed for an initial denaturation at 94 °C for 4 min, followed by 35 cycles of 94 °C for 1 min, annealing (Table 1) for 1 min and 72 °C for 1 min; the final extension at 72 °C was held for 7 min. The amplified products were size-separated through electrophoresis in 2.5% (w/v) agarose gel by running the gel at 5 V/cm for 3 h, and visualized under gel documentation system (Avegene, USA).
Morphological characterization
Morphological data were recorded on the clones of 60 day-old interspecific hybrid plants AV1, BV3 and their parents for comparing plant height, leaflet width, stem diameter, leaflet length, stipule length and number of primary branches. The pollen viability of parents and hybrid plants were determined at 50% flowering stage by macerating the mature anthers in 2% acetocarmine (HiMedia) and counting the number of round, stained pollen grains vs. shrivelled pollen grains in at least 200 grains at 200X magnification under compound light microscope (CH20i, Olympus). The number of seeds formed was recorded on 240 day-old mature plants of parents and hybrids. Data were statistically analyzed using Microsoft Excel software.
Scanning electron microscopy
The micromorphological traits (leaf texture, trichomes and stomata) on abaxial leaf surface of parents (T. alexandrinum cultivar Wardan, T. vesiculosum, T. alexandrinum cultivar BL1) and two interspecific hybrids (AV1 and BV3) were examined through scanning electron microscopy (SEM). The pre-processing steps involved thorough washing of the abaxial and adaxial leaf surfaces with sterilized triple distilled water to remove the dust particles adhering on the surfaces. The washed leaves were placed on an absorbent tissue paper to remove excess water. The leaves were not dab dried to avoid any injury or erosion of the delicate trichomes and the outer leaf surface. Small leaf squares (approximately 10 mm2) were cut from the internerval region at the place of maximum leaf blade width. These cuttings were placed in the primary fixative i.e. 2.5% glutaraldehyde (Bozzola and Russell 1999) for 24 h at 4 °C to chemically fix the tissue by extensive protein interlinking through the aldehyde group. The primary fixative was later drained and the tissue was washed with sodium cacodylate buffer (pH 7.2–7.4). It was then post-fixed in the secondary fixative, osmium tetraoxide (OsO4) to further fix the lipids in the tissue sample. The tissue was again rinsed with sodium cacodylate buffer to wash of the excess OsO4. Further, the tissue was dehydrated by treatment with graded ascending ethanol series (from 30 to 100%). The dehydrated samples were dried in a critical point drier (Quorum Polaron E3000, UK) and placed on aluminium stub using double sided carbon sticky tape. The stubbed samples were then sputter coated with 10–20 nm gold layer in an ion sputter coater (Hitachi E-1010, Japan). The images were obtained using a scanning electron microscope (Hitachi S-3400 N, Japan) @ 10–15 kV in back scattered electron imaging mode at different magnifications. The micromorphological leaf traits were viewed at 500X magnification on standard leaf area of 0.25 mm2. The leaf texture was examined for the occurrence of epicuticular wax on the leaf surface and structural wax plates in the epistomatal chambers; trichome features i.e. length and density of trichomes were observed; stomatal density was counted and its aperture parameters i.e. pore length (length of the long axis of the area bounded by outer stomatal ledges), pore width (length of the short axis between the edges of outer ledges) and guard cell width were measured. Data on stomatal aperture parameters were statistically analyzed by Microsoft Excel software.
Screening for reaction to stem rot
The parents and interspecific hybrids (AV1, BV3) were screened against S. trifoliorum for reaction to stem rot under glasshouse conditions. S. trifoliorum strain was isolated from the infected field-grown Berseem plants showing typical symptoms of stem rot. The stem cuttings (4–5 mm) were surface sterilized with 0.1% (w/v) mercuric chloride for 1 min followed by 4–5 washings with sterile water aseptically. The cuttings were inoculated on potato dextrose agar slants and incubated at 20 ± 1 °C for 7 days to obtain mycelial growth. The cultures were purified by hyphal tip method and raised on sterile whole gram in 250 ml Erlenmeyer flask (Borosil) at 20 ± 1 °C for 40 days with manual shaking at an interval of 7 days. The inoculum dosage for screening was standardized on 30 day-old T. alexandrinum cultivars Wardan and BL1 i.e. female parents (20 plants each) by applying inoculum at concentrations ranging from 0.5 to 5 g with an increment of 0.5 g at the collar region. These plants were highly susceptible to stem rot at an inoculum concentration of 2 g (approximately 10,000 spores/g) and this dosage was used for screening. The inoculated plants/clones were covered with polythene bags to maintain proper humidity for growth of the fungus in the glasshouse. The scoring for disease reaction was carried out 14 days after inoculation on the basis of scale given by Malaviya et al. (1999) i.e. 0–10% mortality as resistant; 10–20% mortality as moderately resistant; 20–30% mortality as susceptible and > 30% mortality as highly susceptible.
Results
Interspecific hybridization and embryo rescue
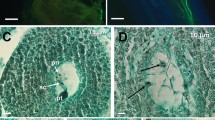
Two interspecific crosses between T. alexandrinum cultivar Wardan × T. vesiculosum and T. alexandrinum cultivar BL1 × T. vesiculosum were attempted for developing hybrids that failed to set seed under natural conditions (Table 2), suggesting the presence of post-fertilization barrier(s). Subsequently, attempts were made for the development of interspecific hybrids through embryo rescue from several hundred crossed florets of the two interspecific crosses (Table 2). The petals of more than 60% florets withered 24 h after pollination. The in vitro recovery of 32 and 88 swollen ovaries from crossed florets of Wardan × T. vesiculosum and BL1 × T. vesiculosum, respectively pointed towards cross-fertilization (Table 2). The examination of swollen ovaries revealed degeneration of one of the two ovules (Fig. 1a) in most of the ovaries (seven from Wardan × T. vesiculosum and eighteen from BL1 × T. vesiculosum) excised 11 DAP, whereas all the ovaries excised at 12, 13, 14 and 15 DAP contained only one ovule implying degeneration of the unfertilized ovule, confirming cross-fertilization to have taken place. The maximum number of swollen ovaries were collected 12 DAP for embryo rescue in both crosses.
Embryo developmental stages after interspecific hybridization and production of plantlets. a Degeneration of one of the two ovules (indicated with arrow). b Embryo rescued at heart stage. c Embryo rescued at torpedo stage. d Embryo rescued at cotyledonary stage e plantlet formation. f Clonal propagation. g Hardening of interspecific hybrid plantlets. h Interspecific hybrid plants in soil
The heart, torpedo and cotyledonary stage embryos (Fig. 1b–d) were rescued from Wardan × T. vesiculosum, whereas the heart stage missed out from the rescued embryos of BL1 × T. vesiculosum. The number of rescued embryos from both the crosses at different developmental stages is shown in Table 2. The maximum number of embryos were rescued at cotyledonary stage 12 DAP. The delayed collection of florets i.e. at 15 DAP from Wardan × T. vesiculosum and 16 DAP from BL1 × T. vesiculosum led to degeneration of fertilized ovule as well, and hence no swollen ovaries could be collected. The cotyledonary stage embryos differentiated normally in both the crosses (Table 2) and the plumules emerged within 6 days leading to plantlet formation in 28 days of culture initiation (Fig. 1e), whereas the embryos at heart and torpedo stages were non-responsive in vitro. A total of 17 putative interspecific hybrid plantlets were produced from both the crosses (Table 2). The plantlets were clonally propagated from axillary bud bearing nodal stem segments leading to shoot initiation after 10 days and multiple shoot formation within 28 days (Fig. 1f); and root induction (16.67%) on individual shoots after 45 days of culturing. Subsequently, a representative plantlet from each clonal population was characterized for verification of hybridity.
Molecular characterization of putative hybrids
Genomic DNA representing 17 putative interspecific Trifolium hybrids (AV1 to AV4 from Wardan × T. vesiculosum and BV1 to BV13 from BL1 × T. vesiculosum) and their parents was analyzed for assessment of hybridity using eight SSR markers (Table 1). Two amplicons of 121 and 147 bp corresponding to the donor/male parent were detected in four interspecific hybrids AV1, BV3, BV4 and BV12. The 121 bp amplicon was observed by TRSSRA02B08 marker in AV1, BV3, BV4 and BV12 (Fig. 2a, b), likewise 147 bp fragment was amplified by TRSSRA02C02 marker in AV1, BV3, BV4 and BV12 (Fig. 2c, d) indicating hybridity. The markers designated as TRSSRA02A05, TRSSRA01H11 and TRSSRAXX31 detected polymorphism in putative AV hybrids (Supplementary Fig. 2A); polymorphism was also detected in putative BV hybrids by TRSSRA02A05 and TRSSRAXX31 (Supplementary Fig. 2B) without confirming hybridity. The generation of 17 amplicons in putative AV hybrids was observed from five polymorphic SSR markers (TRSSRA02A05, TRSSRA02B08, TRSSRA02C02, TRSSRA01H11 and TRSSRAXX31) with an average of 3.4 loci per marker. In putative BV hybrids, 10 amplicons were generated from four polymorphic SSR markers (TRSSRA02A05, TRSSRA02B08, TRSSRA02C02 and TRSSRAXX31) with an average of 2.5 loci per marker. The markers TRSSRA02A05 and TRSSRA01H11 amplified a maximum number of five loci each, and the marker TRSSRA02C02 amplified three loci in putative AV hybrids; in putative BV hybrids, maximum number of loci (three each) was amplified by the markers TRSSRA02B08 and TRSSRAXX31 (Table 1). The four interspecific hybrids designated as AV1, BV3, BV4 and BV12 were transferred for in vitro hardening (Fig. 1g); subsequently nine AV1 clones and twelve BV3 clones survived in soil (Fig. 1h).
Molecular characterization of putative interspecific Trifolium hybrids by SSR markers. a Detection of 121 bp amplicon by TRSSRA02B08 in AV1 corresponding to the donor/male parent; M standard 100 bp DNA ladder (Gene DireX); P1 T. alexandrinum cultivar Wardan; P2 T. vesiculosum (donor/male parent); AV1 to AV4 putative interspecific hybrids. b Detection of 121 bp amplicon by TRSSRA02B08 in BV3, BV4 and BV12 matching with the donor/male parent; M standard 100 bp DNA ladder (Gene DireX); P1 T. alexandrinum cultivar BL1; P2 T. vesiculosum (donor/male parent). c Amplification of 147 bp fragment by TRSSRA02C02 in AV1 similar to the donor/male parent. d Identification of 147 bp amplicon by TRSSRA02C02 in BV3, BV4 and BV12 comparable to the donor/male parent
Morphological characterization of interspecific hybrids
The AV1 interspecific hybrid transgressed significantly for plant height, as compared to both the parents; likewise its leaflet width and stem diameter increased significantly from the female parent (Table 3), indicating hybridity. The leaflet length was transformed in the direction of female parent (Table 3). The difference in stipule length was observed to be statistically non-significant from either parent. The BV3 interspecific hybrid had numerically more primary branch number and leaflet width than the female parent; plant height, leaflet length were found to be intermediate between the parents, and stipule length was significantly less as compared to both the parents (Table 3), suggesting hybridity. The pollen viability of AV1 was similar to the female parent and significantly lower than the male parent, whereas pollen viability of BV3 was significantly lower than both the parents (Table 3). The seed formation in both the hybrids was significantly lower than the parents (Table 3) due to less number of flowers per inflorescence and presence of empty flowers in inflorescence of hybrid plants. The hybrid seed was shrivelled and showed negligible germination even under in vitro conditions (Supplementary Table 1) and plantlets were necrotic.
Micromorphological characterization of interspecific hybrids
The micromorphological traits on abaxial leaf surface of parents and the two interspecific hybrids were examined through SEM. The leaf texture analysis of AV1 and its parents revealed that the foliage of female parent was glaucous and smooth with uniform distribution of epicuticular wax on the whole leaf surface (Fig. 3a) and that of male parent was non-glaucous and rough (Fig. 3b), the foliage of AV1 was intermediate with inclination towards the male parent due to the presence of trichomes (Fig. 3c) confirming its hybridity. The parents of BV3 hybrid demonstrated rough leaf surface (Fig. 3d, b), whereas the hybrid had smooth surface (Fig. 3e).
Scanning electron micrographs of micromorphological traits (leaf texture, trichomes and stomata) showing comparisons among parents and interspecific hybrids. a T. alexandrinum cultivar Wardan (female parent). Bar represents 20 μm. b T. vesiculosum (male parent). Bar represents 10 μm. c AV1 hybrid. Bar represents 20 μm. d T. alexandrinum cultivar BL1 (female parent). Bar represents 10 μm. e BV3 hybrid. Bar represents 50 μm
The stomatal density was lower in AV1 as compared to the parents (Table 4). The comparisons made for stomatal aperture parameters revealed that the hybrid possessed intermediate stomatal pore length and guard cell width between the parents, while its stomatal pore width was significantly smaller (Fig. 3c) as compared to both the parents (Fig. 3a, b, Table 4). The micromorphological traits i.e. stomatal pore length and guard cell width confirmed AV1 hybridity. The stomatal density in BV3 was intermediate between the two parents (Table 4). The comparison of stomatal aperture parameters showed that the hybrid had significantly larger stomatal pore length, guard cell width (Fig. 3e) than both the parents (Fig. 3d, b), and significantly larger stomatal pore width as compared to female parent (Fig. 3e, Table 4), which along with intermediate stomatal density established its hybridity. The closer view of micromorphological leaf traits of parents and the two interspecific hybrids are shown in Supplementary Fig. 3A–E.
Reaction to stem rot
The AV1, BV3 interspecific hybrids and their parents were screened against S. trifoliorum for reaction to stem rot. The female parent of AV1 was highly susceptible (Fig. 4a) exhibiting 100% mortality, AV1 was moderately resistant (Fig. 4b) displaying 11.1% mortality and the male parent was resistant to the disease (Fig. 4c) without mortality (Table 5). The female parent of BV3 was also highly susceptible to the disease with 80% mortality, whereas the hybrid was resistant with 8.3% mortality. The decrease in disease incidence on the hybrids as compared to their female parents pointed towards recombination of stem rot resistant trait from the male parent. The hybrid clones were successfully grown to maturity in the glasshouse.
Discussion
This study demonstrated development of two interspecific hybrids between Trifolium species following embryo rescue by overcoming post-fertilization barrier(s) leading to recombination of stem rot resistance in cultivated T. alexandrinum. The highlighting feature was confirmation of hybridity independently through molecular, micromorphological, morphological analyses and also by their combination. The combined use of molecular and micromorphological markers for the assessment of hybridity has been found to be highly consistent (Bruschi et al. 2000).
The cross-fertilization in both the interspecific crosses was manifested from withering of petals, swelling of ovaries and degeneration of unfertilized ovules. In Trifolium species, the withering of petals in pollinated florets along with swelling of ovaries is a positive indicator of cross-fertilization (Evans 1962; Roy et al. 2004; Kaushal et al. 2005). Degeneration of unfertilized ovule in a pollinated ovary is also a sign of cross-fertilization (Evans 1962; Roy et al. 2004). In the present study, hybrid embryos were rescued at heart, torpedo and cotyledonary stages; only the cotyledonary stage embryos responded, whereas in other reports, heart (Malaviya et al. 2004; Roy et al. 2004; Kaushal et al. 2005) and early torpedo (Repkova et al. 2006) stages of the hybrid embryos were found responsive. The frequency of obtaining hybrid embryos was 2.56% from Wardan × T. vesiculosum and 6.12% from BL1 × T. vesiculosum, as compared to 13.92% (Malaviya et al. 2004), 5.39% (Roy et al. 2004), 4.68% (Kaushal et al. 2005) and 2.5% (Repkova et al. 2006) in other interspecific crosses between Trifolium species.
The distinctness of leaf surface in hybrids from their parents was obvious, suggesting hybridity. The rough or smooth leaf texture is due to trichomes that are good indicators of hybridization as in the hybrids these do not remain intact as compared to the parents (Hardin 1979). The reliability of micromorphological leaf traits for prediction of hybridity was exhibited in mentha (Saric-Kundalic et al. 2009) and Quercus (Fortini et al. 2015). The non-glaucous foliage of hybrids was evident from absence of epicuticular leaf wax; non-glaucousness of foliage has been correlated with enhanced photosynthetic rate due to decreased light reflectance from leaf surface signifying better adjustment to low light intensity under winter conditions (Reicosky and Hanover 1978). The leaves of BV3 displayed increased stomatal density (1.5-fold), stomatal pore width as compared to female parent, and increased stomatal pore length, guard cell width as compared to both parents. The direct correlation of higher stomatal density with water use efficiency has been reported in common bean (Martinez et al. 2007), wheat (Yang et al. 2007) and Leymus chinensis (Xu and Zhou 2008), pointing that BV3 could have improved capacity to grow under water deficit conditions. Leaves of rice having large sized stomata were reported to have enhanced photosynthetic efficiency (Chandra and Das 2000) and improved grain yield in hybrids (Sarwar et al. 2013) due to tight coupling of stomatal density and size with conductance leading to increased CO2 diffusion and photosynthesis of plants (Maherali et al. 2002; Parkhurst 1994; Zhang et al. 2006; Ohsuni et al. 2007), indicative of enhanced photosynthetic ability in BV3.
The status of putative interspecific Trifolium hybrids was analysed using SSR markers derived from white clover (Trifolium repens L.) (Kölliker et al. 2001). The plantlets of both interspecific crosses inherited SSR alleles from male parent T. vesiculosum confirming hybridity. The alleles specific to female parent were undetected in F1 hybrids, this may be due to genome reorganization by recombination that affects the position of an SSR marker and its binding site. If the marker binding site is affected, PCR amplification by the marker does not occur representing a null allele situation (Weber and May 1989). The detection of only male specific alleles in F1 interspecific hybrid derived from cross between wild and cultivated Helianthus annuus using SSR marker was taken as an evidence of successful hybridization, although allele corresponding to female parent remained undetected (Terzic et al. 2006). Similarly, the detection of distinct male parent specific alleles in F1 hybrids from crosses between Vigna radiata and V. mungo with three SSR markers was used to verify hybridity (Abbas et al. 2015). Visualization of only male parent specific alleles in F1 plants of intraspecific Cynodon transvaalensis cross using SSR markers was used as an indicator of hybridization (Tan et al. 2014). There are reports on interspecific hybridization between Trifolium species (Malaviya et al. 2004; Roy et al. 2004; Kaushal et al. 2005); however evidence on hybrid nature of plants through molecular analysis was not presented.
The morphological traits such as leaflet length and width of two interspecific hybrids were intermediate between their parents. The intermediary nature of such traits in interspecific Trifolium hybrids has been demonstrated to be a sign of hybridity (Roy et al. 2004; Kaushal et al. 2005). The hybridity in AV1 and BV3 was corroborated through integrated molecular, leaf micromorphological and morphological data (Table 6). The screening of AV1 and BV3 hybrids for reaction to S. trifoliorum pointed towards resistance of the hybrids to stem rot. The successful interspecific hybridization between Trifolium species broadens the genetic base of the recipient and may be of interest to plant breeders for developing T. alexandrinum lines carrying desirable alleles for protection against disease and better adaptation to environmental changes.
References
Abbas G, Hameed A, Rizwan M, Ahsan M, Asghar MJ, Iqbal N (2015) Genetic confirmation of mungbean (Vigna radiata) and mashbean (Vigna mungo) interspecific recombinants using molecular markers. Front Plant Sci 6:1107
Abberton MT (2007) Interspecific hybridization in genus Trifolium. Plant Breed 126:337–342
Bozzola JJ, Russell LD (1999) Electron microscopy. Jones and Bartlett Publishers, Sudbury
Bruschi P, Vendramin GG, Bussotti F, Grossoni P (2000) Morphological and molecular differentiation between Quercus petraea (Matt.) Liebl. and Quercus pubescens Willd. (Fagaceae) in Northern and Central Italy. Ann Bot 85:325–333
Butkute B, Lemeziene B, Dabkeviciene G, Jakstas V, Vilcinskas E, Janulis V (2014) Source of variation of isoflavone concentrations in perennial clover species. Pharmacogn Mag 10:181–188
Chandra K, Das AK (2000) Correlation and interaction of physiological parameters in rice under rainfed transplanted condition. J Crop Res Assam Agric Univ 19:251–254
Delclos B, Mousset-D´eclas C, Raynal G (1997) A simple method for the evaluation of red clover (Trifolium pratense L.) resistance to Sclerotinia trifoliorum. Euphytica 93:173–179
Evans AM (1962) Species hybridization in Trifolium. II. Investigating the prefertilization barriers to compatibility. Euphytica 11:256–262
Ferguson NH, Rupert EA, Evans PT (1990) Interspecific Trifolium hybrids produced by embryo and ovule culture. Crop Sci 30:1145–1149
Fortini P, Antonecchia G, Marzio PD, Maiuro L, Viscosi V (2015) Role of micromorphological leaf traits and molecular data in taxonomy of three sympatric white oak species and their hybrids (Quercus L.). Plant Biosystems 149:546–558
Hardin JW (1979) Patterns of variation in foliar trichomes of eastern North American Quercus. Am J Bot 66:576–585
Hendrych R (1990) The third series of complement on Trifolium—monograph by Zohary and Heller (Plantae hybridae). Preslia Praha 62:43–60
Hussain SW, Williams WM, Mercer CF, White DWR (1997) Transfer of clover cyst nematode resistance from Trifolium nigrescens Viv. to T. repens L. by interspecific hybridization. Theor Appl Genet 95:1274–1281
ICAR (2012) Forage crops and grasses. Handbook of agriculture, 6th edn. Kenya Institute of Organic Farming, Nairobi
Kaushal P, Malaviya DR, Roy AK, Kumar B, Tiwari A (2005) Trifolium alexandrinum × T. resupinatum -interspecific hybrids developed through embryo rescue. Plant Cell Tissue Organ Cult 83:137–144
Kölliker R, Jones ES, Drayton MC, Dupal MP, Forster JW (2001) Development and characterisation of simple sequence repeat (SSR) markers for white clover (Trifolium repens L.). Theor Appl Genet 102:416–424
Maherali H, Reid CD, Polley HW, Johnson HB, Jachson RB (2002) Stomatal acclimation over a subambient to elevated CO2 gradient in a C3/C4 grassland. Plant Cell Environ 25:557–566
Malaviya DR, Bhaskar RB, Roy AK, Kaushal P (1999) Screening of berseem genotypes for resistance to root rot and stem rot diseases under pot culture condition. Crop Improv 26:232–235
Malaviya DR, Roy AK, Kaushal P, Kumar B, Tiwari A (2004) Development and characterization of interspecific hybrids of Trifolium alexandrinum × T. apertum using embryo rescue. Plant Breed 123:536–542
Martinez JP, Silva H, Ledent JF, Pinto M (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26:30–38
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Öhberg H, Ruth P, Bang U (2008) Differential responses of red clover cultivars to Sclerotinia trifoliorum under diverse natural climatic conditions. Plant Pathol 57:459–466
Ohsuni A, Kanemura T, Homma K, Horie T, Shiraiwa T (2007) Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Prod Sci 10:322–328
Ovalle CM, Del Pozo A, Fernandez F, Chavarria J, Arredondo S (2010) Arrowleaf clover (Trifolium vesiculosum Savi): a new of annual legumes for high rainfall areas of the mediterranean climate zone of Chile. Chil J Agric Res 70:170–177
Pandey KK, Grant JE, Williams EG (1987) Interspecific hybridization between Trifolium. Aust J Bot 35:171–182
Parkhurst DF (1994) Diffusion of CO2 and other gases inside leaves. New Phytol 126:449–479
Phillips GC, Collins GB (1984) Red clover and other forage legumes. In: Sharp WR, Evans DA, Ammirato PV, Yamada Y (eds) Hand book of plant cell culture, vol 2. Macmillan Publishing Co., New York, pp 169–210
Phillips GC, Collins GB, Taylor NL (1982) Interspecific hybridization of red clover (Trifolium pratense L.) with T. sarosiense Hazsl. using in vitro embryo rescue. Theor Appl Genet 62:17–24
Phillips GC, Grosser JW, Berger S, Taylor NL, Collins GB (1992) Interspecific hybridization between red clover and Trifolium alpestre using in vitro embryo rescue. Crop Sci 32:1113–1115
Przywara L, White DWR, Sanders PM, Maher D (1989) Interspecific hybridization of Trifolium repens with T. hybridum using ovule and embryo culture. Ann Bot 64:613–624
Rampton HH (1972) Seed production of arrowleaf clover in Western Oregon. Circular of Information 635, Agricultural Experiment Station, Oregon State University, Corvallis, pp 1–4
Reicosky DA, Hanover JW (1978) Physiological effects of surface waxes I. Light reflectance for glaucous and nonglaucous Picea pungens. Plant Physiol 62:101–104
Repkova J, Jungmannova B, Jakesova H (2006) Identification of barriers to interspecific crosses in the genus Trifolium. Euphytica 151:39–48
Riffkin PA, Evans P, Wright A (2001) Extending pasture quality later into the season. In: Rowe B, Donaghy D, Mendham N (eds) Science and technology: delivering results for agriculture? Proceedings of 10th Aust Agronomy Conference on, Hobart, Tasmania. http://www.regional.org.au/au/asa/2001/p/9/riffkin.htm
Roy AK, Malaviya DR, Kaushal P, Kumar B, Tiwari A (2004) Interspecific hybridization of Trifolium alexandrinum with T. constantinopolitanum using embryo rescue. Plant Cell Rep 22:705–710
Roy AK, Malaviya DR, Kaushal P, Chandra A, Singh UP (2009) Descriptors for tropical forage legume—Egyptian clover/Berseem Trifolium alexandrinum L. IGFRI, Jhansi
Roy DC, Ray M, Tudu NK, Kundu CK (2015) Impact of phosphate solubilizing bacteria and phosphorous application on forage yield and quality of berseem in West Bengal. IJAEB 8:315–321
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer length polymorphisms in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Saric-Kundalic B, Filalova S, Dobes CD, Olzant S, Tekelova D, Grancai D, Reznicek G, Saukel J (2009) Multivariate numerical taxonomy of Mentha, hybrids, varieties and cultivars. Sci Pharm 77:851–876. https://doi.org/10.3797/scipharm.0905-10
Sarwar AKMG, Karim MA, Rana SMAM (2013) Influence of stomatal characteristics on yield and yield attributes of rice. J Bangladesh Agric Univ 11:47–52
Sawai A, Ueda S, Gau M, Uchiyama K (1990) Interspecific hybrids of Trifolium medium L. × 4× T. pratense L. obtained through embryo culture. J Jpn Soc Grassl Sci 35:267–272
Tan C, Wu Y, Taliaferro CM, Bell GE, Martin DL, Smith MW (2014) Development and characterization of genomic SSR markers in Cynodon transvaalensis Burtt-Davy. Mol Genet Genom. https://doi.org/10.1007/s00438-014-0829-1
Taylor AO, Hughes KA, Hunt BJ (1979) Annual cool-season legumes for forage I. A survey of lines for yield and disease resistance at Kaitaia and Palmerston North. N Z J Exp Agric 7:141–147
Terzic S, Atlagic J, Pankovic D (2006) Characterization of F1 interspecific hybrids between wild Helianthus annuus L. populations and cultivated sunflower. Genetika 38:159–168
Vymyslicky T (2014) Breeding of minor fodder crops for sustainable agriculture. Ratar Povrt 51:1–6
Weber JL, May PE (1989) Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet 44:388–396
Williams E (1978) Hybrid between Trifolium repens and T. ambiguum obtained with the aid of embryo rescue. N Z J Bot 16:499–506
Xu Z, Zhou G (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59:3317–3325
Yamada T, Fukuoka H (1986) Production of interspecific hybrids between Trifolium ambiguum M. Bieb. and T. repens L. by ovule culture. Jpn J Breed 36:233–239
Yang L, Han M, Zhou G, Li J (2007) The changes of water-use efficiency and stoma density of Leymus chinensis along Northeast China transect. Acta Ecol Sin 27:16–24
Zhang YP, Wang ZM, Wu YC, Zhang X (2006) Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Ecol Sin 32:70–75
Zohary M, Heller D (1984) The genus Trifolium. The Israel Academy of Sciences and Humanities, Jerusalem
Acknowledgements
The financial support from Department of Biotechnology, New Delhi (DBT Reference No. BT/PR8158/AGR/02/382/2006) on ‘Development of interspecific hybrids using embryo rescue in Trifolium’ in the network project on “Biotechnological approach towards forage crop improvement” is thankfully acknowledged.
Author information
Authors and Affiliations
Contributions
AK attempted Trifolium alexandrinum cultivar Wardan x T. vesiculosum crosses, rescued embryos, recorded data on morphological traits of AV1 interspecific hybrid and drafted the manuscript. KPK attempted T. alexandrinum cultivar BL1 x T. vesiculosum crosses, rescued embryos, carried out SSR analysis of putative AV and BV interspecific hybrids and recorded data on morphological traits of BV3 interspecific hybrid. AK conducted SEM analysis. UR conducted preliminary screening of interspecific hybrids for disease reaction to stem rot. JGK captured embryo developmental stages, recorded pollen viability and seed formation of parents and interspecific hybrids under compound light microscope. RS clonally propagated putative hybrid plantlets and parents. DM provided seed material of Wardan, T. vesiculosum and imparted training on embryo culture. RK analyzed and interpreted data on morphological traits of interspecific hybrids. JSS planned, coordinated the experiments, interpreted results and drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, A., Kaur, K.P., Kalia, A. et al. Generation of interspecific hybrids between Trifolium vesiculosum and T. alexandrinum using embryo rescue. Euphytica 213, 253 (2017). https://doi.org/10.1007/s10681-017-2042-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-2042-x