Abstract
White mold is a severe disease of common bean worldwide. Partial resistance occurs in the primary and secondary gene pools. Factors essential for identifying high levels of resistance that is effective throughout the growing season are not known. Our objectives were to determine which factors are most essential for identifying high levels of resistance including: (i) pathogen isolates screened, (ii) inoculations/plant, (iii) evaluation dates, (iv) area under disease progress curve (AUDPC), and (v) the SCAR markers linked with WM2.2, WM6.1, WM7.1, WM7.3, and WM8.3 resistance QTL. Thirty-one genotypes were inoculated one to three times/plant with ARS12D and ND710 isolates in the greenhouse in Idaho, and CO467 and NY133 in Colorado. Evaluations were made from 7 to 35 days post-inoculation. Disease severity and AUDPC increased until 21 days in Colorado and 35 days in Idaho. Correlations were positive among isolates at each evaluation date. The presence or absence of white mold resistance QTL was not indicative of resistance response. ‘Othello’ was susceptible to all isolates with one inoculation, and VC13-5 was resistant to three isolates and intermediate to ND710 after three inoculations per plant; both of these genotypes lacked the five resistance QTL. SE155-9 with WM2.2, WM7.1, and WM8.3 QTL was resistant to CO467 and NY133. However, SE152-6 with the same QTL was resistant and possessed lower AUDPC values to all isolates after three inoculations. Thus, screening with four isolates, three inoculations/plant, and disease evaluations delayed until 21 days or longer helped identify genotypes with high levels of broad-spectrum resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White mold caused by Sclerotinia sclerotiorum (Lib.) de Bary is one of the most destructive diseases of common bean (Phaseolus vulgaris L., both dry and snap or green bean) in cool to warm and wet production regions worldwide causing yield losses up to 100 % (Schwartz and Singh 2013; Singh and Schwartz 2010; Steadman 1983). The fungus attacks more than 400 hosts, mostly dicotyledonous species (Boland and Hall 1994; Bolton et al. 2006; Purdy 1979; Steadman and Boland 2005). In common bean, under favorable weather conditions, the fungus invades stems, branches, leaves, flowers, pods, and seeds irrespective of the plant growth stages. For example, dark-brown water soaked soft lesions are observed on stems, often the most lignified and hardest aerial plant part. These lesions are caused by the presence of enzymes such as polygalacturonase and pectin methyl esterase, and oxalic acid (Lumsden 1976; Maxwell and Lumsden 1970). Also, plant wilting and white mold growth followed by presence of black sclerotia is observed in and on the infected tissue (Purdy 1979; Steadman and Boland 2005). A sclerotium pre-conditioned in soil can divide carpogenically producing apothecia which contain asci with ascospores (sexual reproduction) or germinate directly through mycelia (asexual reproduction) (Merriman 1976; Schwartz and Steadman 1978). Thus, the number of sclerotia in the field affects disease incidence and one sclerotium per 5 kg soil can cause over 40 % of disease severity (Abawi and Grogan 1979; Schwartz and Steadman 1978). The disease is favored by lower temperatures (<25 °C) and high moisture (>60 %), and plants are more susceptible at pre-flowering and flowering stages (Steadman and Boland 2005).
Genetic studies of S. sclerotiorum diversity showed that polymorphism existed among different isolates from Brazil, despite the fact that all isolates were of the same mycelial compatibility group (Meinhardt et al. 2002). Moreover, different levels of aggressiveness have been reported among isolates from Spain (Pascual et al. 2010) and the United States (Jhala et al. 2014; Kull et al. 2004).
White mold disease management involved the use of cultural practices such as rotation with non-host crops, reduced plant population, row orientation to prevailing wind, reduced frequency of irrigation and/or quantity of water applied in each irrigation, reduced fertilizer application, and deep plowing before planting (Blad et al. 1978; Paula Junior et al. 2009). Furthermore, the application of fungicides at initiation of and during flowering minimized the spread and severity of white mold (Mahoney et al. 2014; Tu 1989). Finally, the use of resistant cultivars is crucial for an economical and integrated control of white mold especially under severe disease pressure (Miklas et al. 2013).
Physiological resistance or simply resistance (commonly assessed in the greenhouse) and plant architectural avoidance or simply avoidance traits (mostly expressed in the field) are used for cultivar development (Kolkman and Kelly 2003; Miklas et al. 2001, 2013). Resistance is related with oxalate sensitivity in common bean (Kolkman and Kelly 2000) and scarlet runner bean (P. coccineus L.) (Chipps et al. 2005). Avoidance mechanisms are associated with plant architectural traits such as tall upright growth habit, porous or open canopy, and resistance to lodging, which help reduce the establishment, severity, and spread of the white mold disease (Kolkman and Kelly 2003; Miklas et al. 2001, 2013; Schwartz et al. 1978).
White small-seeded (<25 g 100−1 seed) navy bean ‘ICA Bunsi’ (synonymous with ‘Ex-Rico 23’) was the first cultivar reported to have partial resistance (or avoidance) to white mold in the field within the Middle American gene pool (Miklas et al. 2004; Tu and Beversdorf 1982). Moreover, breeding lines (BL) USPT-WM-1 (Miklas et al. 2006) and USPT-WM-12 (Miklas et al. 2014) and cultivars Eldorado (Kelly et al. 2012) and OAC Rex (Michaels et al. 2006) derived resistance from ICA Bunsi. These genotypes may help reduce or avoid damage during low to moderate white mold pressure in the field (Miklas et al. 2013), however, under more severe disease pressure they are often inadequate to control white mold. Furthermore, in the greenhouse tests, ICA Bunsi and its derived genotypes were susceptible to white mold (Singh et al. 2014b, c). In contrast, Andean genotypes G 122, PC 50, and NY6020-4 have relatively higher and more effective levels of resistance than ICA Bunsi and its derived genotypes both in the greenhouse and field (Balasubramanian et al. 2014; Maxwell et al. 2007; Miklas and Delorme 2003; Miklas et al. 2001; Park et al. 2001). Similarly, Andean A 195, CORN 601, MO 162, and VA 19 have higher levels of resistance in both conditions (Balasubramanian et al. 2014; Schwartz and Singh 2013; Soule et al. 2011).
Within the secondary gene pool, P. coccineus L. accessions PI 433246, PI 439534, and others possess higher levels of resistance (Gilmore et al. 2002; Schwartz et al. 2006). Furthermore, interspecific BL derived from P. coccineus such as 92BG-7, I9365-25, I9365-31, VCW 54, and VCW 55 (Miklas et al. 1998; Singh et al. 2009a, b, 2014c), and VRW 32 derived from P. costaricensis Freytag & Debouck (Singh et al. 2013) also exhibit partial resistance depending on the fungal isolate used for screening. Breeding lines combining resistance from different gene pools (i.e., pyramided resistance) displayed yet higher levels of resistance (Singh et al. 2014b; Terán and Singh 2009a, 2010).
Resistance to white mold is inherited quantitatively by >25 major and minor effects quantitative trait loci (QTL). Similarly, >35 QTL controlled plant architectural avoidance mechanisms that are distributed in 21 regions across nine of 11 linkage groups (Miklas et al. 2013; Soule et al. 2011). Furthermore, 13 avoidance QTL were co-located with 13 QTL for resistance (Miklas et al. 2013). As discussed below, of the major effect QTL, Soule et al. (2011) reported a sequence characterized amplified region (SCAR) marker SMe1Em5.110 linked with the WM2.2 resistance QTL and WM8.3 QTL linked with SF13R10.410 marker, both present in the Andean common bean BL VA 19. Similarly, the WM6.1 QTL from the Andean snap bean NY6020-4 is linked with SAU.1350 marker (Miklas and Delorme 2003), and WM7.1 QTL present in the Andean common bean germplasm line G 122 is linked with Phs-T gene (Miklas et al. 2001). The marker SF13R10.410 is linked with WM7.3 QTL present in the common bean x P. coccineus interspecific BL I9365-31 (Soule et al. 2011).
Greenhouse screening is used for detection of resistance. However, previously common bean researchers often only used a single pathogen isolate and single inoculation per plant, and evaluated for the white mold disease response after 3–7 days post-inoculation (Hunter et al. 1982; Petzoldt and Dickson 1996). Thus, the resistance response was measured at a single point (often in the vegetative growth stage) during the crop growing period. In contrast, for successful cultivars high levels of resistance would be required throughout the cropping period to reduce pesticide use and production costs. The greenhouse straw test (Petzoldt and Dickson 1996) or its modified cut-stem method (Singh et al. 2014a; Terán et al. 2006) allows use of multiple S. sclerotiorum isolates, multiple inoculations at different points in the vegetative and reproductive growth stages, and repeated evaluations for a prolonged period (e.g., until 35 days post the first inoculation), thus covering both the vegetative and reproductive periods on the same plant (Singh et al. 2014b, c; Terán and Singh 2009b). Although the above screening methodology would be the severest test ever applied in the common bean and related Phaseolus species to the best of our knowledge, it should allow detection of high levels of the pathogen isolate-specific and broad-spectrum resistance that is effective throughout the cropping season. More recent studies, therefore, have used one to two inoculations per plant and evaluations from 8 to 35 days post-inoculation for breeding, germplasm screening, and genetic studies (Pascual et al. 2010; Soule et al. 2011; Terán and Singh 2010; Viteri and Singh 2015).
For the common bean production areas where multiple pathogen isolates of different aggressiveness are sympatric such as the Midwest region of the United States, it is essential to identify genotypes with high levels of broad-spectrum resistance. However, the importance of using more diverse pathogen isolates, multiple inoculations, and evaluations for prolonged periods covering both the vegetative and reproductive growth stages on the same plant in common bean is not known. Thus, our objectives were to determine what factors were more essential for identifying genotypes with high levels of broad-spectrum resistance that is effective throughout the growing season including: (i) number of pathogen isolates screened, (ii) number of inoculations per plant, (iii) the post-inoculation evaluation date, (iv) area under disease progress curve (AUDPC), and (v) the presence or absence of SCAR markers linked with the major effect WM2.2, WM6.1, WM7.1, WM 7.3, and WM8.3 white mold resistance QTL. Also, correlations were determined between mean white mold scores of 31 bean genotypes and four pathogen isolates at each evaluation date.
Materials and methods
Phaseolus bean genotypes
P. coccineus PI 439534 (Schwartz et al. 2006) and 30 common bean genotypes of diverse origins were included in this study (Table 1). The 30 common bean genotypes included ICA Bunsi (Miklas et al. 2004; Tu and Beversdorf 1982); four previously released pinto beans, namely ‘Chase’ (Coyne et al. 1994), ‘UI 320’ (Myers et al. 2001), ‘Othello’ (Burke et al. 1995), and USPT-WM-1 (Miklas et al. 2006); five recently developed interspecific pinto BL (VC13-1, VC13-3, VC13-4, VC13-5, VC13-6) derived from P. vulgaris × P. coccineus interspecific backcross UI 320*2/PI 439534 (Singh et al. 2014c); three previously reported interspecific BL (92BG-7, I9365-31, VCW 54) also derived from crosses between P. vulgaris and P. coccineus (Miklas et al. 1998; Singh et al. 2009b); one interspecific BL (VRW 32) derived from P. costaricensis (Singh et al. 2013); five previously reported white mold resistant Andean genotypes (A 195, G 122, NY6020-4, PC 50, VA 19) (Saladin et al. 2000; Shonnard and Gepts 1994; Singh et al. 2007; Soule et al. 2011); and eleven recently developed BL (SE152-6, SE152-8, SE153-1, SE153-3, SE153-6, SE153-7, SE154-1, SE154-5, SE154-9, SE154-10, SE155-9) with pyramided resistance (i.e., combined from two or more sources) (Singh et al. 2014b). Sources of resistance and multiple-parent crosses from which these latter groups of 11 BL were derived included: SE152 = CORN 501/G 122//A 195/VCW 55, SE153 = USPT-WM-1/CORN 601//USPT-CBB-1/92BG-7, SE154 = VA 19/MO 162//A 195/G 122, and SE155 = A 195/4/NY6020-4/92BG-7///MO 162/I9365-25//ICA Bunsi/G 122 (Singh et al. 2014b).
Inoculum preparation and greenhouse evaluation
Four isolates of S. sclerotiorum, namely ARS12D (collected from an Andean common bean in Tartagal, Argentina in 2012), CO467 (from a Middle American common bean from Lucerne, Colorado in 1996), ND710 (from a Middle American common bean from the Red River Valley of North Dakota), and NY133 (from an Andean snap bean from New York) of varying aggressiveness (Jhala et al. 2014; McCoy et al. 2012; Otto-Hanson et al. 2011; Steadman et al. 2006) were used for inoculation and evaluation of responses to white mold disease. The reasons for using four isolates of different geographical origin and collected from both Andean and Middle American common beans were to determine their role in identifying genotypes with broad-spectrum high levels of resistance and to detect potential cross-over interactions between the bean genotypes and pathogen isolates.
Fresh (48 h old cultures kept at 28 °C) mycelium was multiplied as needed from pre-conditioned sclerotia before each inoculation for each isolate (Schwartz et al. 2006; Singh et al. 2009a). Three plants were sown in a 16.5 × 20.3 cm plastic pot for each genotype and replicate. Response to pathogen isolates ARS12D and ND710 was evaluated in the greenhouse at University of Idaho, Kimberly in 2012. Response to isolates CO467 and NY133 was evaluated in the greenhouse at Colorado State University, Fort Collins in 2013. Plants were inoculated beginning at the fifth internode (approximately 1 month after planting) leaving a 3 cm-long internode intact. A 200 µl eppendorf tip stacked with three plugs of fresh mycelia of each isolate was used for each inoculation per plant. The second and third inoculations on the same plant were made 7 and 14 days after the first inoculation, respectively; however only on the resistant plants with disease scores ≤4, see below. Thus, inoculation of resistant plants continued from about 30–44 days post planting, which covered vegetative and pre-flowering to the end of flowering period. Inoculated plants were kept under high humidity (>80 %) using humidifiers situated under the greenhouse benches and keeping the greenhouse floor wet for at least 1 week after each inoculation. Plants were grown at mean day and night temperatures of 24 and 18 °C, respectively, with 12 h of light. White mold disease severity was evaluated at 7, 14, and 21 days post the first inoculation in Colorado and at 7, 14, 21, 28, and 35 days post-inoculation in Idaho using a 1–9 scale according to Terán et al. (2006). Plants were considered resistant with white mold scores of 1–4, intermediate >4 to <7, and susceptible 7–9. White mold disease severity on 1–9 scale as described above according to Terán et al. (2006) and the area under the disease progress curve (AUDPC, Cooke 2006) were recorded on a single-plant basis. The AUDPC values were calculated using the sum of disease severity progression from 7 to 21 days post-inoculation in Colorado and from 7 to 35 days in Idaho for each pathogen isolate, using the formula described by Cooke (2006) as given below:
where A k = AUDPC value, N indicates the number of assessments in time (i.e., three in the case of Colorado and five in the case of Idaho), y indicates the white mold disease severity at each evaluation date, and t indicates the time in days. Furthermore, the resistance response after three inoculations per plant was verified by visual evaluation at harvest maturity (Singh et al. 2014b, c).
Molecular marker assays
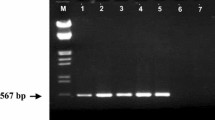
Of the 21 white mold resistance QTL reported by Soule et al. (2011), only the SCAR markers linked with the five QTL derived from the four resistance donor common bean genotypes amplified in this study (Table 2). The SMe1Em5.110 SCAR marker was linked with the WM2.2 resistance QTL and SF13R10.410 was linked with WM8.3 QTL, both from resistant Andean common bean VA 19 (Soule et al. 2011). The marker SAU5.1350 was linked with WM6.1 QTL from resistant Andean snap bean NY6020-4 (Miklas and Delorme 2003). The co-dominant Phs-T phaseolin seed protein gene was associated with WM7.1 QTL from resistant Andean common bean G 122 (Miklas et al. 2001). The SF18R7.410/415 was a co-dominant marker for WM7.3 QTL derived from interspecific BL I9365-31 (Soule et al. 2011). Thus, four resistance QTL (WM2.2, WM6.1, WM7.1, WM8.3) assayed were from Andean common bean and one (WM7.3) was derived from P. coccineus, all of which were known to possess high levels of white mold resistance (Schwartz et al. 2006; Singh et al. 2014b, c). Thus, we assayed the presence (+) or absence (−) of the five markers linked with white mold resistance QTL in the laboratory at University of Idaho, Kimberly Research and Extension Center in 2013. The DNA was extracted from the bulk sample of emerging trifoliolate leaves from all replicates for each genotype using the Dellaporta protocol (Dellaporta et al. 1983). The DNA concentration was adjusted to 10 µg/ml using a BioPhotometer (Eppendorf AG, Hamburg, Germany). The methodology and protocols used for marker assays were those of Miklas and Delorme (2003); Miklas et al. (2001); and Soule et al. (2011). All PCR reactions were carried out in a PTC-100 thermocycler (MJ Research Inc., Waltham, MA). PCR products were run in 1.5 % agarose gel, stained with 1 % of ethidium bromide, and the presence or absence of DNA bands visualized over a UV light box (UVP Products, Upland, CA).
Data analysis
The experiment was conducted with three treatments in a factorial arrangement. Each of the three treatments were considered a fixed factor. The three treatments were two pathogen isolates (CO476 and NY133 at Colorado State University, and ARS12D and ND710 at University of Idaho), 31 bean genotypes (Table 1), and number of days post-inoculation (7, 14, and 21 days at Colorado State University, and 7, 14, 21, 28, and 35 days at University of Idaho) for evaluation. All possible factorial arrangements of treatments were completely randomized within each of the six blocks, and a separate randomization was used for each block. Thus, unlike the traditional factorial design, no blocking of any kind for any of the three treatments was performed in order to avoid creating micro-climates either due to genotypes, pathogen isolates, and/or the number of days post-inoculation that could affect response of bean genotypes to white mold disease. Therefore, the experiment constitutes a randomized complete block design with six replicates.
For data analysis, replicates were considered a random effect, and genotypes, pathogen isolates, and number of days post-inoculation for evaluation were considered fixed effects. Analysis of variance was used to compare disease scores among treatment levels. Fisher’s least significant difference at P = 0.05 was used to compare treatment means. The range and frequency of resistant plants for each genotype were determined. As noted above, the AUDPC values (using the sum of disease severity progression from 7 to 21 days post-inoculation in Colorado and from 7 to 35 days in Idaho for each pathogen isolate) also were calculated (Cooke 2006). Furthermore, simple correlation coefficients were calculated between the mean white mold scores for 31 bean genotypes and four pathogen isolates for each evaluation date. Data were analyzed using the SAS 9.3 PROC GLM, FREQ, and CORR procedures (SAS 2008).
Results
Mean squares were significant (P ≤ 0.05) for the number of days post-inoculation for evaluation, genotypes, and genotypes × isolates interaction in Colorado and Idaho (Table 3). Pathogen isolates, isolates × number of days post-inoculation for evaluation, and genotypes × number of days post-inoculation for evaluation interactions mean squares were only significant in Idaho. Although the effect of number of inoculations per plant was confounded with the number of days post-inoculation for evaluation, white mold disease scores and the AUDPC values increased from 7 to 21 days post-inoculation in Colorado and from 7 to 35 days in Idaho (Table 4). Similarly, the percentage of resistant plants with white mold disease scores of ≤4 decreased as the number of inoculations per plant increased for each of the four pathogen isolates (Table 5). The isolate ND710 with mean disease score of 5.6 and AUDPC value of 156.6 was more aggressive than ARS12D with white mold score of 5.0 and AUDPC of 139.6 in Idaho. In contrast, significant differences (P > 0.05) were not observed between CO467 and NY133 isolates at any evaluation date in Colorado (Tables 3 and 4). Furthermore, significant positive correlation coefficients (r2 ranged from 0.7 to 0.9; P ≤ 0.01) were observed between the mean white mold scores of 31 bean genotypes and the four pathogen isolates for evaluations made at 7, 14, 21, 28, and 35 days post-inoculation (Table 6). For comparison of results from both greenhouses, however, only data for 21 days post-inoculation will be reported for the 31 bean genotypes for the four S. sclerotiorum isolates. In addition, data for 35 days will be reported for Idaho to assess the effects of evaluations delayed beyond 21 days post-inoculation.
Pinto Othello was susceptible (mean white mold scores 8.2–8.9) to all isolates and had the highest AUDPC values (105.0–116.1) with only one inoculation per plant (Table 7). Also, the Middle American common bean genotypes Chase, UI 320, ICA Bunsi, and USPT-WM-1 were susceptible (7.1–8.9) to ARS12D, ND710, and NY133 after a single inoculation per plant. In contrast, pinto USPT-WM-1 was intermediate to CO467 (5.3) with two inoculations per plant. P. coccineus PI 439534 was intermediate to the four isolates at 21 days after three inoculations per plant. Among BL derived from the secondary gene pool, VCW 54 had significantly lower white mold scores and AUDPC values compared to 92BG-7, I9365-31, and VRW 32 to the four isolates at 21 days post-inoculation (Table 7). The newly developed interspecific pinto VC13 series BL derived from P. coccineus PI 439534 were more resistant than all previously developed interspecific BL, irrespective of the market class, derived from the Phaseolus species of the secondary gene pool. In Idaho, the VC13 series interspecific BL were resistant to ARS12D and intermediate to ND710 at 21 and 35 days post-inoculation. Furthermore, in Colorado, VC13-5 and VC13-6 BL had resistant scores to both isolates at 21 days with three inoculations per plant.
Similarly, all newly developed pyramided BL of the SE152, SE153, SE154, and SE155 series were significantly more resistant than Andean G 122, PC 50, and NY6020-4 in both greenhouses (Table 7). However, only Andean SE152-6 and Middle American pinto BL SE153-7 were resistant and had lower AUDPC values in response to the four isolates at 21 days post-inoculation (Table 7). These two genotypes and pinto interspecific BL VC13-5 had the highest percentage of resistant plants. In contrast, previously developed Middle American common bean genotypes namely ICA Bunsi, Chase, UI 320, and USPT-WM-1 had lower percentages of resistant plants, higher AUDPC values, and broader ranges for white mold disease scores (Table 7).
Evaluations until 35 days in Idaho helped determine if the resistant or intermediate white mold scores persisted longer than previously reported. For example, P. coccineus PI 439534 was intermediate to pathogen isolates ARS12D and ND710 at 21 days, however, it had susceptible scores at 35 days post-inoculation (Table 7). Similarly, Andean G 122, PC 50, and NY6020-4 and interspecific BL VCW 54 derived from P. coccineus were intermediate to ND710 at 21 days post-inoculation, however, they were susceptible and had higher AUDPC values at 35 days (Table 7). In contrast, newly developed BL VC13-1, VC13-5, SE152-6, and SE154-1 were resistant to ARS12D and were close to resistant or intermediate (4.2–6.3), and had lower AUDPC values (99.2–149.1) to the most aggressive isolate ND710 even at 35 days post-inoculation.
Othello, Chase, UI 320, ICA Bunsi, USPT-WM-1, PI 439534, 92BG-7, and the VC13 series BL lacked all five SCAR markers linked with white mold resistance QTL (Table 8). Interspecific BL I9365-31, VCW 54, and VRW 32 derived from the secondary gene pool possessed the WM7.3 QTL. The pinto SE153 series BL had the WM7.1 QTL. Similar to the Andean parents or checks, all Andean pyramided common bean BL of the SE152, SE154, and SE155 series exhibited the SCAR markers linked with WM2.2, WM7.1, and WM8.3 resistance QTL. The Andean snap bean NY6020-4 amplified for the SCAR markers linked with the WM6.1, WM7.1, and WM8.3 resistance QTL. Nonetheless, the marker linked with the WM6.1 QTL was not observed in Andean pyramided BL SE155-9 even though it had NY6020-4 as a parent (Table 8). Thus, none of the 31 bean genotypes tested in this study had all five white mold resistance QTL.
Discussion
The white mold severity and AUDPC increased as the number of inoculations per plant and number of days post-inoculation for evaluation increased. Thus, significant differences in white mold severity from 7 to 21 days in Colorado and Idaho occurred. Furthermore, white mold severity significantly increased from 21 to 35 days post-inoculation in Idaho. Because common bean plants may be infected multiple times during the vegetative and reproductive growth stages in the field and resistance for the entire growing season is essential for the survival of a successful cultivar, earlier evaluations (e.g., at 7 or 14 days) could lead to misidentification of presumed resistant genotypes. For instance, ICA Bunsi had a resistant response to one inoculation with CO467 at 7 days, however, it was susceptible after three inoculations at 21 days post-inoculation. Therefore, selection of more resistant genotypes should be based on at least three inoculations per plant and evaluation delayed at least until 21 days post-inoculation or longer, thus covering most of the vegetative and reproductive growth stages with verification of the resistance response at maturity (Singh et al. 2014b, c). Terán and Singh (2009a, 2010) identified resistant genotypes at 33 days post-inoculation and Viteri and Singh (2015) determined inheritance of white mold resistance in an Andean common bean BL A 195 based on evaluations at 35 days post-inoculation.
Identification of highly resistant bean genotypes required three inoculations per plant. For example, with only one inoculation pinto Othello was susceptible to each of the four isolates at 7 days. In contrast, Andean common bean BL SE152-6 exhibited resistant or near resistant scores with three inoculations per plant to the four isolates up to 21 days post-inoculation and maintained that score until maturity. As noted above, to the best of our knowledge, this screening methodology of using multiple pathogen isolates of different aggressiveness and inoculations per plant, and delayed evaluations covering both the vegetative and reproductive growth stages is the severest test ever applied in common bean and related Phaseolus species to assess the response against white mold disease. Thus, the above screening methodology should ensure that the white mold resistance is effective throughout the entire cropping period (i.e., covering both the vegetative and reproductive periods). Furthermore, greenhouse space utilization would be optimized when dealing with higher plant populations that are inoculated with multiple isolates on the same plant with susceptible plants discarded, and delayed evaluations of surviving resistant plants. This also should minimize expense, time, and labor used for the subsequent inoculum multiplications, inoculations, and evaluations required for the identification of genotypes with high levels of broad-spectrum resistance that is effective throughout the growing season (Singh et al. 2014b, c; Terán and Singh 2009a, 2010).
Variation occurs in aggressiveness among S. sclerotiorum isolates in multisite greenhouse and field screening nurseries of common bean (Jhala et al. 2014; McCoy et al. 2012; Otto-Hanson et al. 2011; Steadman et al. 2006). The use of multiple greenhouses and inoculations with different S. sclerotiorum isolates in each greenhouse helps identify genotypes with specific as well as broad-spectrum resistance. Furthermore, significant crossover interactions, if any, between common bean genotypes and pathogen isolates could be identified. For example, McCoy et al. (2012), Otto-Hanson et al. (2011); Singh et al. (2014b, c), and Steadman et al. (2006) used multiple isolates to identify white mold resistant common bean genotypes with specific and broad-spectrum resistance. Identification of genotypes with isolate-specific resistance may have merit for areas where only that isolate occurs, assuming that this pathogen information is known and monitored over time. Therefore, if we only used the most aggressive isolate ND710 in Idaho, we would probably select only resistant genotypes SE152-6 and SE154-1. However, pinto BL VC13-5, VC13-6, SE153-1, SE153-6, and SE153-7 with high levels of resistance to other isolates would be discarded. In contrast, if only the less aggressive isolates ARS12D or CO467 were used, the differentiation for genotypes with a broad-spectrum resistance between the VC13 series of interspecific pinto BL would have been difficult.
Although the mean white mold scores for the more aggressive isolate ND710 were often higher than those for ARS12D, there were no significant crossover interactions between genotypes and pathogen isolates in Idaho. This is consistent with the positive correlations for mean white mold disease scores between 31 bean genotypes and the four pathogen isolates at each evaluation date. Therefore, bean genotypes resistant to one isolate would be expected to be resistant to other isolates. Nonetheless, while bean genotypes resistant to ND710 isolate would also be expected to be resistant to ARS12D, the reverse did not always hold true.
Among the Andean common beans, A 195 and VA 19 were the most resistant genotypes to four isolates, supporting previous results (Soule et al. 2011; Terán and Singh 2009a, 2010). A 195 and VA 19 were derived from ‘Red Kloud’, and both possessed the WM2.2, WM7.1, and WM8.3 white mold resistance QTL (Soule et al. 2011; this study). Thus, it would be important to determine the white mold response and confirm if Red Kloud carries these (and other) QTL expressed in the greenhouse and field in future studies. Also, G 122 and PC 50 carried the same WM2.2, WM7.1, and WM8.3 QTL, however they had higher mean white mold disease scores compared to A 195 and VA 19. Thus, A 195 and VA 19 may possess additional genes/QTL or different resistance allele(s) that would be important to identify and tag. Alternatively, G 122 and PC 50 may be variable or are a mixture of resistant and susceptible genotypes and need to be selected for a uniform resistance response against each pathogen isolate of interest for future genetic and breeding studies.
The WM7.3 resistance QTL from interspecific BL I9365-31 was observed in interspecific BL VCW 54 and VRW 32. It would be useful to determine if WM7.3 QTL from I9365-31 derived from P. coccineus is present in P. coccineus G 35172 (Singh et al. 2009b) that was used in VCW 54, and P. costaricensis G 40604 (Singh et al. 2013) that was used in VRW 32 as white mold resistant donor parents. The VC13 series of interspecific pinto BL derived from P. coccineus PI 439534 did not possess the WM7.3 and other four resistance QTL. Therefore, white mold resistance in the VC13 series interspecific BL would be expected to be different from those of I9365-31, VCW 54, VRW 32, and the three Andean common bean resistance donor parents, namely G 122, NY6020-4, and VA 19.
Among pinto bean genotypes, the VC13 series interspecific BL derived from UI 320*2/PI 439534, had higher levels of resistance compared to UI 320, Chase, and USPT-WM-1. These new BL also had significantly higher levels of resistance than most of the previously developed interspecific BL (92BG-7, I9365-31, VCW 54, VRW 32). Because UI 320 was susceptible to white mold, the P. coccineus PI 439534 contributed higher levels of white mold resistance to the VC13 series pinto BL. Schwartz et al. (2006) identified a dominant resistance gene in UI 320/PI 439534, which needs to be tagged and mapped for marker-assisted selection and its presence verified in the VC13 series BL and PI 439534. Furthermore, concerted efforts should be made to identify contrasting and complementary sources of white mold resistance in other Phaseolus genotypes of the secondary gene pool species, introgressed, and/or pyramided into common bean genotypes.
Breeding line SE152-6 with pyramided white mold resistance QTL WM2.2, WM7.1, and WM8.3 was significantly more resistant than A 195 which also possesses the same three QTL. Similarly, SE155-9 was more resistant than VA 19 in Colorado; and both SE152-6 and SE155-9 had higher percentage of resistant plants compared to their other parents in both greenhouses. Thus, pyramiding white mold resistance from diverse germplasm sources was a sound strategy to maximize selection gains and breed for higher levels of broad-spectrum white mold resistance (Singh et al. 2014b; Terán and Singh 2009a, 2010). Because none of the pyramided BL or other genotypes tested in this study had all five white mold resistance QTL, further pyramiding efforts would be justified. Breeding lines such as 92BG-7, NY6020-4, VCW 54, VC13-3 or VC13-6, SE152-6, and SE153-3 or SE153-7 could be combined into a multiple-parent cross using gamete (Singh 1994; Terán and Singh 2009a) and/or recurrent (Terán and Singh 2010) selections, and systematically screened using both direct disease screening with isolates of different aggressiveness and marker-assisted selection to develop BL with yet higher levels of broad-spectrum resistance possessing all five (and other) white mold resistance QTL.
The range and percentage of resistant plants, in addition to mean white mold disease scores, were used for development of the SE (Singh et al. 2014b) and VC (Singh et al. 2014c) series of BL. Consequently, some of these newly developed BL had 100 % of plants resistant to some isolates (e.g., VC13-1 and VC13-5 in response to ARS12D) with comparatively low mean white mold disease scores and AUDPC values. Thus, selection gains may be considerably enhanced by combining the range of white mold disease scores, percentage of resistant plants, AUDPC values, and mean disease scores in the selection process.
In summary, identification of bean genotypes with high levels of broad-spectrum resistance required use of multiple pathogen isolates of diverse origin, three inoculations per plant, and evaluations delayed until 21–35 days post-inoculation. Furthermore, the resistance response was verified at maturity, and the range, percentage of resistant plants, and mean white mold disease scores together were used in the selection process from the early segregating generations (Singh et al. 2014b, c). Thus, highly resistant interspecific pinto BL (i.e., the VC13 series) from UI 320*2/PI 439534 and 11 BL with pyramided resistance (the SE series) from three multiple-parent crosses were developed. Their white mold resistance was significantly higher than that of previously developed Middle American pinto BL and cultivars, Andean common beans, and interspecific BL derived from the secondary gene pool Phaseolus species. Effectiveness of these newly developed BL to combat white mold disease with and without fungicides and other control strategies should be determined. Also, their high levels of resistance should be transferred into cultivars of different market classes using the severest screening methodology applied in the greenhouse in this study to combat white mold disease in common bean worldwide.
Abbreviations
- AUDPC:
-
Area under the disease progress curve
- BL:
-
Breeding line or lines
- QTL:
-
Quantitative trait locus or loci
- SCAR:
-
Sequence characterized amplified region
References
Abawi GS, Grogan RG (1979) Epidemiology of diseases caused by Sclerotinia species. Phytopathology 69:899–904
Balasubramanian PM, Conner RL, McLaren DL, Chatterton S, Hou A (2014) Partial resistance to white mould in dry bean. Can J Plant Sci 94:683–691
Blad BL, Steadman JR, Weiss A (1978) Canopy structure and irrigation influence white mold disease and microclimate of dry edible beans. Phytopathology 68:1431–1437
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol 16:93–108
Bolton MD, Thomma BP, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16
Burke DW, Silbernagel MJ, Kraft JM, Koehler H (1995) Registration of Othello pinto bean. Crop Sci 35:943
Chipps TJ, Gilmore B, Myers JR, Stotz HU (2005) Relationship between oxalate, oxalate oxidase activity, oxalate sensitivity and white mold susceptibility in Phaseolus coccineus. Phytopathology 95:292–299
Cooke BM (2006) Disease assessment and yield loss. In: Cooke BM, Jones DG, Kaye B (eds) The epidemiology of plant diseases, 2nd edn. Springer, Dordrecht, pp 43–74
Coyne DP, Nuland DS, Lindgren DT, Steadman JR (1994) ‘Chase’ pinto dry bean. HortScience 29:44–45
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: Version II. Plant Mol Biol Rep 1:19–21
Gilmore B, Myers JR, Kean D (2002) Completion of testing of Phaseolus coccineus plant introductions (PIs) for white mold, Sclerotinia sclerotiorum, resistance. Annu Rep Bean Improv Coop 45:64–65
Hunter JE, Dickson MH, Boettger MA, Cigna JA (1982) Evaluation of plant introductions of Phaseolus spp. for resistance to white mold. Plant Dis 66:320–322
Jhala R, Higgins R, Eskridge K, Steadman JR (2014) Characterized isolates of Sclerotinia sclerotiorum can facilitate identification and verification of resistance to white mold in dry and snap beans. Annu Rep Bean Improv Coop 57:57–58
Kelly JD, Mkwaila W, Varner GV, Cichy KA, Wright EM (2012) Registration of ‘Eldorado’ pinto bean. J Plant Regul 6:233–237
Kolkman JM, Kelly JD (2000) An indirect test using oxalate to determine physiological resistance to white mold in common bean. Crop Sci 40:281–285
Kolkman JM, Kelly JD (2003) QTL conferring resistance and avoidance to white mold in common bean. Crop Sci 43:539–548
Kull LS, Pedersen WL, Palmquist D, Hartman GL (2004) Mycelial compatibility groupings and virulence of Sclerotinia sclerotiorum. Plant Dis 88:325–332
Lumsden RD (1976) Pectolytic enzymes of Sclerotinia sclerotiorum and their localization in infected bean. Can J Bot 54:2630–2641
Mahoney KJ, McCreary CM, Gillard CL (2014) Response of dry bean white mould [Sclerotinia sclerotiorum (Lib.) de Bary, causal organism] to fungicides. Can J Plant Sci 94:905–910
Maxwell DP, Lumsden RD (1970) Oxalic acid production by Sclerotinia sclerotiorum in infected bean and in culture. Phytopathology 60:1395–1398
Maxwell JJ, Brick MA, Byrne PF, Schwartz HF, Shan X, Ogg JB, Hensen RA (2007) Quantitative trait loci linked to white mold resistance in common bean. Crop Sci 47:2285–2294
McCoy S, Higgins B, Steadman JR (2012) Use of multisite screening to identify and verify partial resistance to white mold in common bean in 2011. Annu Rep Bean Improv Coop 55:153–154
Meinhardt LW, Wulff NA, Bellato CM, Tsai SM (2002) Telomere and microsatellite primers reveal diversity among Sclerotinia sclerotiorum isolates from Brazil. Fitopatol Bras 21:211–215
Merriman PR (1976) Survival of sclerotia of Sclerotinia sclerotiorum in soil. Soil Biol Biochem 8:385–389
Michaels TE, Smith TH, Larsen J, Beattie AD, Pauls KP (2006) OAX Rex common bean. Can J Plant Sci 86:733–736
Miklas PN, Delorme R (2003) Identification of QTL conditioning resistance to white mold in snap bean. J Am Soc Hortic Sci 128:564–570
Miklas PN, Grafton KF, Kelly JD, Schwartz HF, Steadman JR (1998) Registration of four white mold resistant dry bean germplasm lines: I9365-3, I9365-5, I9365-31, and 92BG-7. Crop Sci 38:1728
Miklas PN, Johnson WC, Delorme R, Gepts P (2001) QTL conditioning physiological resistance and avoidance to white mold in dry bean. Crop Sci 41:309–315
Miklas PN, Hauf DC, Henson RA, Grafton KF (2004) Inheritance of ICA Bunsi-derived resistance to white mold in a navy x pinto bean cross. Crop Sci 44:1584–1588
Miklas PN, Grafton KF, Hauf D, Kelly JD (2006) Registration of partial white mold resistant pinto bean germplasm line USPT-WM-1. Crop Sci 46:2339
Miklas PN, Porter LD, Kelly JD, Myers JR (2013) Characterization of white mold disease avoidance in common bean. Eur J Plant Pathol 135:525–543
Miklas PN, Kelly JD, Steadman JD, McCoy S (2014) Registration of pinto bean germplasm line USPT-WM-12 with partial white mold resistance. J Plant Reg 8:183–186
Myers JR, Stewart-Williams KD, Hayes RE, Kolar JJ, Singh SP (2001) Registration of ‘UI 320’ pinto dry bean. Crop Sci 41:1642–1643
Otto-Hanson L, Steadman JR, Higgins R, Eskridge KM (2011) Variation in Sclerotinia sclerotiorum bean isolates from multisite resistance screening locations. Plant Dis 95:1370–1377
Park SO, Coyne DP, Steadman JR, Skroch PW (2001) Mapping of QTL for resistance to white mold disease in common bean. Crop Sci 41:1253–1262
Pascual A, Campa A, Pérez-Vega E, Giraldez R, Miklas PN, Ferreira JJ (2010) Screening common bean for resistance to four Sclerotinia sclerotiorum isolates collected in northern Spain. Plant Dis 94:885–890
Paula Junior TJ, Vieira RF, Rocha PR, Bernardes A, Costa EL, Carneiro JES, Riberiro do Vale FX, Zambolim L (2009) White mold intensity on common bean in response to plant density, irrigation frequency, grass mulching, Trichoderma spp., and fungicide. Summa Phytopathol 35:44–48
Petzoldt R, Dickson H (1996) Straw test for resistance to white mold in beans. Annu Rep Bean Improv Coop 39:142–143
Purdy LH (1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology 69:875–880
Saladin F, Arnaud-Santana E, Nin JC, Godoy-Lutz G, Beaver JS, Coyne DP, Steadman JR (2000) Registration of ‘PC-50’ red mottled bean. Crop Sci 40:858
Schwartz HF, Singh SP (2013) Breeding common bean for resistance to white mold: a review. Crop Sci 53:1832–1844
SAS Institute (2008) SAS/STAT User’s Guide. SAS Institute Inc, Cary
Schwartz HF, Steadman JR (1978) Factors affecting sclerotium populations of and apothecium production by Sclerotinia sclerotiorum. Phytopathology 68:383–388
Schwartz HF, Steadman JR, Coyne DP (1978) Influence of Phaseolus vulgaris blossoming characteristics and canopy structure upon reaction to Sclerotinia sclerotiorum. Phytopathology 68:465–470
Schwartz HF, Otto K, Terán H, Lema M, Singh SP (2006) Inheritance of white mold resistance in Phaseolus vulgaris × P. coccineus crosses. Plant Dis 90:1167–1170
Shonnard GC, Gepts P (1994) Genetics of heat tolerance during reproductive development in common bean. Crop Sci 34:1168–1175
Singh SP (1994) Gamete selection for simultaneous improvement of multiple traits in common bean. Crop Sci 34:352–355
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to diseases: a review. Crop Sci 50:2199–2223
Singh SP, Terán H, Lema M, Schwartz HF, Miklas PN (2007) Registration of white mold resistant dry bean germplasm line A 195. J Plant Reg 1:62–63
Singh SP, Terán H, Schwartz HF, Otto K, Lema M (2009a) Introgressing white mold resistance from Phaseolus species of the secondary gene pool into common bean. Crop Sci 49:1629–1637
Singh SP, Terán H, Schwartz HF, Otto K, Lema M (2009b) White-mold resistant interspecific common bean germplasm lines VCW 54 and VCW 55. J Plant Reg 3:191–197
Singh SP, Terán H, Schwartz HF, Otto K, Debouck DG, Roca W, Lema M (2013) White mold-resistant, interspecific common bean breeding line VRW 32 derived from Phaseolus costaricensis. J Plant Reg 7:95–99
Singh SP, Schwartz HF, Steadman JR (2014a) A new scale for white mold disease rating for the common bean cut-stem method of inoculation in the greenhouse. Annu Rep Bean Improv Coop 57:231–232
Singh SP, Schwartz HF, Terán H, Viteri DM, Otto K (2014b) Pyramiding white mold resistance between and within common bean gene pools. Can J Plant Sci 94:947–953
Singh SP, Schwartz HF, Viteri DM, Terán H, Otto K (2014c) Introgressing white mold resistance from Phaseolus coccineus PI 439534 into common pinto bean. Crop Sci 54:1026–1032
Soule M, Porter L, Medina J, Santana G, Blair M, Miklas PN (2011) Comparative QTL map for white mold resistance in common bean, and characterization of partial resistance in dry bean lines VA19 and I9365-31. Crop Sci 51:123–139
Steadman JR (1983) White mold: a serious yield-limiting disease of bean. Plant Dis 67:346–350
Steadman JR, Boland G (2005) White mold. In: Schwartz HF, Steadman JR, Hall R, Forster RL (eds) Compendium of bean diseases, 2nd edn. Am Phytopathol Soc, St. Paul, pp 44–46
Steadman JR, Otto-Hanson LK, Breathnach J, Kurowski C, Mainz R, Kelly J, Griffiths P, Myers J, Miklas PN, Schwartz H, Singh S, Oppelar A (2006) Identification of partial resistance to Sclerotinia sclerotiorum in common bean at multiple locations in 2005. Annu Rep Bean Improv Coop 49:223–224
Terán H, Singh SP (2009a) Gamete selection for improving physiological resistance to white mold in common bean. Euphytica 167:271–280
Terán H, Singh SP (2009b) Efficacy of three greenhouse screening methods for the identification of physiological resistance to white mold in dry bean. Can J Plant Sci 89:755–762
Terán H, Singh SP (2010) Recurrent selection for physiological resistance to white mould in dry bean. Plant Breed 129:327–333
Terán H, Lema M, Schwartz HF, Duncan R, Gilberson R, Singh SP (2006) Modified Petzoldt and Dickson scale for white mold rating of common bean. Annu Rep Bean Improv Coop 49:115–116
Tu JC (1989) Management of white mold of white beans in Ontario. Plant Dis 73:281–285
Tu JC, Beversdorf WD (1982) Tolerance to white mold (Sclerotinia sclerotiorum (Lib.) de Bary) in Ex Rico 23, a cultivar of white bean (Phaseolus vulgaris L.). Can J Plant Sci 62:65–69
Viteri DM, Singh SP (2015) Inheritance of white mold resistance in an Andean common bean A 195 and its relationship with Andean G 122. Crop Sci 55:44–49
Acknowledgments
This research was partially supported by the USDA-ARS National Sclerotinia Initiative Grants No. 58-5442-7-228 and 058-5442-8-235 “Gamete Selection for Simultaneously Pyramiding and Introgressing White Mold Resistance from Phaseolus Species into Pinto Bean” in 2009 and 2010. The authors also gratefully acknowledge complementary support from the Idaho and Colorado Agricultural Experiment Stations, and thank Dr. Mark Brick for reviewing the manuscript and his valuable comments, and Carlos Centeno for assisting in the greenhouse and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viteri, D.M., Otto, K., Terán, H. et al. Use of four Sclerotinia sclerotiorum isolates of different aggressiveness, three inoculations per plant, and delayed multiple evaluations to select common beans with high levels of white mold resistance. Euphytica 204, 457–472 (2015). https://doi.org/10.1007/s10681-015-1366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1366-7