Abstract
Lycopene content is an important factor for determining watermelon fruit quality. However, the low DNA polymorphism among cultivated watermelon (Citrullus lanatus) has hindered the ability to establish high quality genetic maps and study the quantitative trait loci (QTL) controlling the lycopene content trait. In this study, we successfully constructed a genetic map of watermelon to determine lycopene content and other horticultural fruit traits using a F2 population developed from a cross between the two lines of watermelon LSW-177 and Cream of Saskatchewan. The genetic map contained 16 linkage groups covering a total length of 2,039.5 cM, which included 37 SSRs (Simple Sequence Repeat) and 107 CAPSs (Cleaved Amplified Polymorphic Sequences), with all of the CAPS markers developed from high-throughput re-sequencing of data from this study. Three CAPS markers (WII04E07-33,WII04E07-37,WII04E07-40) caused the F2 population to perfectly co-segregate for each F2 population plants. We also obtained 12 QTLs for all of the traits measured. Only one QTL (LCYB4.1) was detected with a high value of trait variation (83.50 %) that related to lycopene content and mapped on Chromosome 4 between CAPS markers WII04E07-33 and WII04E07-40, which could nearly account for all of the differences in lycopene content between the two parental strains. In this study, we highlighted 2,458 CAPS loci that were suitable for primer design with a polymorphism of 48.9 %, which is approximately a 12-fold increase from previous studies. The present map and QTLs will facilitate future studies on determining lycopene content related genes and cloning watermelon genes, while also providing for useful markers for breeding for lycopene content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lycopene is one of the most important natural carotenoids found in fruit, which serves many physiological functions in the human body. As a result, lycopene has been a focus of international research in various fields including nutrition, health care products and cosmetics. Mortensen and colleagues reported that lycopene could protect DNA and protein from free radical injury to circumvent health problems caused by free radical scavenging damage (Mortensen et al. 1997). Lycopene can also enhance immunity, prevent cancer and protect the cardiovascular system (Feng et al. 2010). Given these positive effects, it has been suggested that selectively breeding to increase lycopene content levels in fruits could serve as a feasible approach for preventing certain diseases caused by lycopene deficiency.
Watermelon (Citrullus lanatus L.) is thought to be a good source of lycopene. Lycopene content in commercial watermelon is approximately 4.87 mg/100 g of fruit weight (FW). Given its popularity, watermelon is reported to account for approximately 7 % of the world’s fruit and vegetable cultivation (Ren et al. 2014). A variation in lycopene content exists among watermelons of different flesh color (Liu et al. 2009). Amongst all of the tested watermelons, which included watermelons of different flesh colors, the white-fleshed watermelon was considered one of the lowest lycopene content (approximately 0.1 mg/100 g FW). In contrast, crimson colored watermelons possessed the highest content of lycopene (more than 5.78 mg/100 g FW). Canary yellow and orange-yellow fleshed color watermelons contained about 1 mg/100 g FW lycopene content. Overall, lycopene content showed a strong correlation with the red-flesh color of the watermelon.
Initial studies on lycopene content or flesh color in watermelon and some other cucurbit crops (such as melon and cucumber) have focused primarily on the inheritance of traits without using any molecular markers and genetic linkage map analysis. The inheritance and interaction of different flesh colors in watermelon have been previously reported (Poole 1994; Shimotsuma 1963; Navot et al. 1990). For all of the previous studies, the white color was designated as Wf which was reported to be epistatic to yellow flesh (B). Canary yellow (C) was dominant to other colors (c) except white (Wf). In addition, both B and C were dominant to the red flesh color (Y). Given these findings, the genotype of Wf_B and Wf_bb gave rise to white flesh color, while wfwfB_and wfwfbb possessed yellow and red flesh color, respectively. Henderson and colleagues reported the allele i-C (inhibition of C and C). i-C was epistatic to C and generated red flesh even in the presence of C (Henderson et al. 1998). The red color (Y) was a single recessive gene and dominant to the orange (y0) and salmon yellow (y) colors. Gusmini reported that the watermelon line Dixie Lee and Red-N-Sweet gave rise to a darker red color called scarlet red, which was a genetic model distinct from red (Y) (Gusmini and Wehner 2006). Both Dixie Lee and Red-N-Sweet have the same alleles (Scr), which are dominant single genes. Research has also focused on the origin of flesh color inheritance in cucumber and melon. Shen et al. recently reported that the inheritance of cucumber flesh color traits was quantitative in nature and controlled by the major gene plus polygene (Shen et al. 2011). The inheritance of flesh color fit the pattern of two major genes plus polygene. Cuevas et al. confirmed the recessive nature of the cucumber color gene (orange dominant to non-pigmentation) in the XIS-derived progeny, where two recessive genes controlled the orange coloration to β-carotene quantity (QβC) in the mesocarp, whereas one recessive gene controlled QβC in the endocarp (Cuevas et al. 2010). Cayberg indicated that the green (gf) and white (wf) flesh in melon are recessive to orange, where the gf and wf interact epistatically (Clayberg 1992). Bo et al. reported that the gene controlling QβC was linked to seven SSR markers on linkage group 3 and that SSR07706 may have utility as a marker for cucumber germplasm (Bo et al. 2011). A total of eight genes encoding carotenoid biosynthetic pathway enzymes were isolated and characterized by Bang (Bang 2005). Five were expressed in the flesh and other tissues in watermelon of different flesh colors but they did not exhibit obvious differences in gene expression.
Genetic mapping is a powerful tool for detecting genes of interest and their relationship to phenotypic traits. The first genetic map of watermelon was constructed by Navot and colleagues using an inter-specific backcross population derived from C. lanatus and C. colocynthis using isozyme markers distributed in seven linkage groups (Navot et al. 1990). Hashizume and colleagues constructed a comprehensive watermelon linkage map with the genetic background of a cultivated Japanese C. lanatus var. lanatus line (H-7) and a non-sweet wild race from South Africa (SA-1, presumably C. lanatus var. citroides), for which 447 isozymes, randomly amplified polymorphic (RAPD), restriction fragment length polymorphism (RFLP) and inter-simple sequence repeat (ISSR) markers were used, spanning 2,384 cM with an average interval length of 4.3 cM and 11 linkage groups (Hashizume et al. 2003). There have been only two studies that have developed recombinant inbred lines (RILs; Ren et al. 2012; Zhang et al. 2004) and which similarly used an inter-subspecific cross between C. lanatus var. citroides (PI 296341) and elite C. lanatus var. lanatus inbred lines. The map by Ren and colleagues included 698 markers (SSR, InDel and structure variation markers) with an average distance of 0.8 cM between markers on 11 linkage groups (Ren et al. 2012). Sandlin and colleagues used three populations (two F2 populations and one recombinant inbred line) generated from crosses between two elite cultivars, Klondlike Black Seeded × New Hampshire Midget, which is a wild citron with accession and ZWRM50 × PI 244091 (Sandlin et al. 2012). The lengths of the genetic maps were 1,438, 1,514, and 1,144 cM, respectively, for the three populations. The three maps were used to construct a consensus map containing 388 SNP markers. SSR, amplified fragment length polymorphism (AFLP) and single nucleotide polymorphism (SNP) markers were used as the main markers to construct the linkage maps in watermelon according to previous studies (Zhang et al. 2004; Levi et al. 2006). However, only a few studies have reported using CAPS markers as the main markers to construct the linkage map and detect QTLs in watermelon.
The advent of SNP technology has provided a powerful tool to create genetics maps (Henry 2008; Kole and Abbott 2008). However, this was not previously possible for watermelon due to the lack of sequence and marker availability. SNP and CAPS markers have been routinely used in agricultural breeding programs, plants and animal variation studies, genome mapping and association mapping studies (Kole and Abbott 2008). CAPS markers were proven to be useful for increasing marker resolution in cucurbita crops to identify genes related to plant diseases, but little attention has focused on flesh color markers for watermelon (Femandez-Silva et al. 2008; Ling et al. 2009; Morales et al. 2005; Tezuka et al. 2009; Zhang et al. 2011). Only one CAPS marker (Phe226) has been identified thus far (Bang et al. 2010), which could perfectly co-segregate the flesh color phenotype to distinguish red and canary yellow flesh color.
In this study, we aimed to use CAPS markers to create a genetic map of watermelon using a F2 population generated from LSW-177 and COS as well as to detect QTLs associated with lycopene content, flesh color and other important horticulture traits, which may serve as a meaningful step toward studying lycopene content and flesh color related genes of the watermelon genome.
Materials and methods
Plant materials
A total of 234 F2 individuals were developed from a cross between LSW-177 (the female parent with a red flesh color) and Cream of Saskatchewan (short for ‘COS’, the male parent with a pale yellow flesh color). The material seeds were offered by Angela R. Davis from the U.S. Department of Agriculture, Agricultural Research Service, South Central Agricultural Research Laboratory. LSW-177 is a midseason monoecious watermelon line, producing an elongated fruit with red flesh color, dark green rind and dark green stripes. The Brix content is 7 % in the center and 5.5 % at the edge (Davis et al. 2008). COS fruit is also a monoecious watermelon line which has a spherical fruit shape, green rind and dark green stripes, and an especially pale yellow flesh color. The Brix content is 10 % in the center and 8.6 % at the edge. F1, F2, and backcross (BC1P1 and BC1P2) populations were generated in a greenhouse.
Six generations (P1, P2, F1, F2, BC1P1 and BC1P2) of watermelon were grown in the greenhouse in XiangFang Experiment Agricultural Station of Northeast Agricultural University, Harbin (44°04′N, E125°42′), China, during the summer of 2013. Two parent and F1 plants were grown in triplicate with 15 plants each. A total of 234 F2 generation plants were planted in the green house as part of the experiment design. For analysis, all fruits were harvested at full maturity between 35 to 40 days after pollination and a single fruit per plant was harvested. Harvested fruits were cut longitudinally and photographed to evaluate the following traits: (1) Flesh Color: visual observation was used to estimate flesh color and scored as 3 for red, 2 for canary yellow and 1 for pale yellow, (2) Fruit length and width: measured using a ruler and results were expressed in centimeters and (3) Brix: a hand refractometer was used to measure Brix content in juice squeezed from the central and edge sections of each fruit, with three samples measured per fruit. Flesh samples were taken from the central tissue and four other points around the edge, which were stored at −80 °C for high-performance liquid chromatography (HPLC) analysis. Leaf tissues were collected and stored individually at −20 °C for genotype analysis.
Lycopene analysis
Fruit was sectioned into slices, which was then cut into smaller pieces. The lycopene extraction method was performed according to Yuan and colleagues (Yuan et al. 2012). Frozen samples were ground to a fine juice with a homogenizer (Bio-Gen Series PRO200, United States) in centrifuge tubes. Five grams of watermelon juice was weighed and added to 5 ml of absolute ethanol as a dehydration treatment and run four times through vacuum filtration. Lycopene was extracted three times using 15 ml of methyl alcohol until the eluant became colorless. Watermelon residues were washed with 2 % dichloromethane-petroleum ether as soon as the tissue became white. The lycopene solution was concentrated using rotary evaporators and samples were dissolved with 50 ml of moving phase combination of methyl alcohol: acetonitrile: dichloromethane (10: 25: 15 V/V). For HPLC analysis, the samples were passed through a 0.22 µm nylon filter and kept at −40 °C in darkness for no more than 24 h before HPLC analysis. The HPLC instrument (Waters) with Waters PDA detector 2535 and Agilent LCZORBAXSB-C18 column (4.6 × 250 mm, 5 µ) was used for HPLC analysis as described by Yuan and colleagues (Yuan et al. 2012). A lycopene authentic standard (Sigma) was purchased and dissolved using dichloromethane to get the solution to a concentration of 5 μg/mL. Lycopene was identified by the retention time, UV–V spectra (at 472 nm) and injection spikes of lycopene standards (Sigma, USA), which were quantified by utilizing curves of authentic standards and BREEZE software (Waters). Lycopene content was determined as µg/g flesh weight.
DNA preparation
DNA was extracted from young leaf tissues of fifteen plants from each of the parental lines and F1 generation. DNA of each F2 generation plant was extracted individually using a modified CTAB (Hexadecyl Trimethyl Ammonium Bromide) method as previously reported (Luan et al. 2008).
Marker analysis and genetic map construction
SSR markers
A total of 449 pairs of watermelon SSR markers (including 23 pairs of core watermelon SSR marker) and 556 pairs of melon SSR markers were used for marker selection. All of the SSR markers were derived from published literature (Danin-Poleg et al. 2001; Fazio et al. 2002; Silberstein et al. 2003; Yi et al. 2003; Gonzalo et al. 2005; Joobeur et al. 2006; Zalapa et al. 2007; Fernandez-Silva et al. 2008; Zhang et al. 2011). The PCR mixture for SSR amplification contained: 20 ng plant genomic DNA, 8–10 pmol primers, 0.25 mM dNTPs, 10 × Taq buffer and 1 unit of Taq polymerase in a total volume of 10 µl. PCR was performed by preheating samples for 5 min at 94 °C followed by 35 cycles of 60 s at 94 °C, 60 s at 50 °C, and 90 s at 72 °C, finishing with post-heating for 5 min at 72 °C. SSR amplification products were run on 6 % denaturing polyacrylamide gel electrophoresis and detected by silver staining.
CAPS markers
CAPS markers were developed by the high-throughput re-sequencing method. Two of the parental watermelon strains (LSW-177 and COS) were re-sequenced by using the IlluminaHiSeq 2000 high-throughput sequencing platform and 10G of data was the output from each strain, which covered more than 20× the watermelon genome. A referenced watermelon genome was used to assemble the original data by BWA software (Trust Sanger Institute, Hong Kong, China). SNP mining of the assembled data was performed by SAMTOOLS software (Trust Sanger Institute, Hong Kong, China), extracting 1,000 bp sequence before and after the SNP locus by self-compiled script on PERL. CAPS locus mining was detected by SNP2CAPS software (Inst Plant Genet & Crop Plant Res, Gatersleben, Germany) using sequences contained within the SNP locus. Results from the bioinformatics analysis identified 272345 SNPs within the locus between both parental strains and 142097 CAPS locus by using SNP2CAPS software. We identified 2458 CAPS loci that were appropriate for PCR primer design by using 11 restriction endonucleases (EcoRI, BsaHI, HindIII, MboII, PstI, ScaI, BamHI, MluI, AluI, DraI and PvuI) (Thermo Scientific, Massachusetts, United States). A total of 15–25 CAPS loci were evenly distributed on each chromosome and were chosen for PCR primer design and PCR products were digested with restriction endonuclease to verify the authenticity of the CAPS markers. The PCR reaction mixture was the same as for the SSR markers and touchdown PCR was performed by preheating samples for 7 min at 94 °C, followed by 30 cycles of 60 s at 94 °C, 20 s at 60 °C with step-wise decrease of 0.5 °C for each cycle and 60 s at 72 °C, as well as 10 cycles of 20 s at 94 °C, 20 s at 45 °C and 60 s at 72 °C followed by post-heating for 7 min at 72 °C. The reaction mixture for the enzyme digestion contained: 5 µl PCR production, 9 µl ddH2O, 0.3 µl restriction enzyme (10 U/µL), which were then incubated at 37 °C for 1–16 h. Enzyme-digested products were examined by 1 % agarose gel electrophoresis.
Map construction and QTL analysis
A total of 148 scored markers (40 SSRs and 108 CAPSs) and 234 F2 plants were used to construct linkage maps using the IciMapping V3.3 software (Institute of Crop Science Chinese Academy of Agricultural Sciences, Beijing, China). Markers were grouped at a minimum LOD score of 3.0 and a maximum threshold rfs value of 0.35. IciMapping V3.3 software was used for the Kosambi mapping function to translate recombination frequency into map distance. The order of the markers in each linkage group was determined by the method of maximum likelihood. The software package Map Chart 2.1 (Plant Research International, Wageningen, Netherlands) was used to graphically represent the linkage groups in the map. QTL analyses were performed using Icimapping V3.3 software. QTLs and their significance was calculated using interval mapping (IM), multiple QTL model (MQM) and permutation analysis. The QTL threshold base in the permutation analysis (1,000 permutation at p = 0.05) ranged between 2.0 and 2.3 for the various traits. A LOD score of 3.0 was used for detection of QTLs.
Statistical analysis
SPSS 19.0 (SPSS Inc, United States) was used to perform statistical analysis such as mean, standard deviation, trait distribution and pairwise correlation analysis.
Results
Trait variations
Phenotypic values and ranges of traits were measured in the parental strains, F1 generation and F2 population (Table 1). Simple linear correlations for traits were measured and the strongest positive correlations were observed between flesh color and lycopene content (Table 2).
Phenotypic segregation for flesh color
Visual analysis of the flesh color was classified into three categories. LSW-177 and COS had a red and pale yellow flesh color, respectively. The F1 generation gave rise to canary yellow flesh color near to that of the COS line, suggesting a dominant effect of the pale yellow parental line. For the F2 population, 234 plants included red, pale yellow and canary yellow. For all of the F2 generation, 55 plants had red flesh color, 129 plants had canary yellow flesh color and 50 plants had pale yellow flesh color. Canary yellow and pale yellow were classified as one non-red group. As a result, the flesh color for the six generations was classified into two color categories by visual observation, which included the red and non-red groups. Statistical analysis of the F2 color segregation indicated a significant fit for 3:1 ratio (non-red group: red group; p < 0.05). Results from the Chi square goodness-of-fit test of the segregation ratios in the F2 and backcross populations are shown in Table 3. Irregular color patterns in the flesh were also identified, consisting of mixed colors of yellow and red swirled together in separate sectors or in mixed patterns in the heart and placental tissues of the fruit. Mixed colors were classified as canary yellow and pale yellow if the flesh color was more than 50 % canary yellow and pale yellow in cross sectional areas, respectively. The flesh color of the BC1P1 population showed five colors including red, pale yellow, canary yellow and two kinds of mixed colors. For the 41 plants of BC1P1 population, 20 plants exhibited red flesh color, while the other 21 plants were non-red flesh color (canary yellow, pale yellow and two kinds of mixed color), perfectly fitting the ratio of 1:1. The BC1P2 population showed just two flesh colors, canary yellow and pale yellow. The ratio of red color group: non-red color group was 0:40 plants, which fit the ratio of 0:1. These results indicated that a single recessive gene determines the red versus non-red color.
Lycopene content analysis in six generations
HPLC analysis of the lycopene content in the ripe fruit showed that the LSW-177 strain was rich in lycopene, containing an average of 43.56 ± 0.31 µg/g, which was much more than 0.23 ± 0.32 and 0.56 ± 0.24 µg/g measured for the COS and F1 generation, respectively, two strains that nearly lack lycopene content. According to the HPLC analysis of COS and F1 generations, we detected a new pigment with a high content. We speculated that the new pigment was neoxanthin and lutein or the mixture of both of these pigments as reported by Bang and colleagues (Bang et al. 2010). A histogram displaying lycopene content distribution in the F2 population was shown in Fig. 1. For the F2 generation, the lycopene content showed a segregation distortion in the group, ranging from 0.28 to 69.57 µg/g with an average of 12.49 ± 1.19 µg/g. The segregation of the F2 population significantly deviated from the normal distribution and consistently performed in such a way that a single gene genetic model of inheritance for this trait was observed. For the BC1P1 population the lycopene content showed a range from 0.67 to 64.19 µg/g. For the BC1P2 population the lycopene content showed a range from 0.48 to 1.57 µg/g. When visually comparing flesh color and lycopene content, we observed that the flesh color was red when the lycopene content was more than 20.19 µg/g. On the other hand, canary yellow and pale yellow plants showed the same lycopene content levels throughout the six generations.
Segregation and genetic analysis of other horticultural traits
Fruit length (FL) and fruit width (FW)
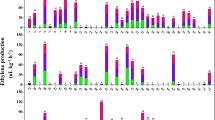
The two parental strains were different from each other in terms of fruit length and width, with the LSW-177 fruits averaging 19.00 ± 0.31 cm in length and 12.33 ± 0.52 cm in width, while the COS fruits averaging 16.40 ± 0.59 cm in length and 17.65 ± 0.25 cm in width (Fig. 2). For the F1 generation, measured data revealed 18.80 ± 0.44 and 16.45 ± 0.44 cm in fruit length and width respectively, which was close to the high end of the scale for the parental strains. Among the F2 generation, fruit lengths ranged from 8.1 to 32 cm, and fruit widths ranged from 7.7 to 26.1 cm, exhibiting a continuous distribution, which suggested that the large size fruit traits exhibit some dominance if there is a non-additive component.
Brix
Brix content of both the central and edge of the fruit was measured within the six generations. COS was rich in Brix content, averaging 9.84 ± 0.43 and 7.76 ± 0.43 % in both the central and edge regions, respectively. These levels are more than that measured from the LSW-177 (7.70 ± 0.30 and 6.30 ± 0.30 % for central and edge regions, respectively) parental strain. The F1 generation showed a 9.00 ± 0.31 and 7.05 ± 0.16 % in the central and edge regions, respectively, for the two traits. For the F2 generation both of the traits performed a continuous distribution, a characteristic of quantitative character, indicating that the Brix content was controlled by polygenes.
Re-sequencing data
A total of 2458 CAPS loci were detected and selected for primer design using 11 restriction endonucleases. A total of 227 pairs of CAPS primers were designed using Primer Premier 5 software (Premier Biosoft International, Palo Alto, United States). Among the primers for CAPS markers, PCR amplification showed that 200 pairs could produce the proper PCR products (88.1 %). In order to verify the PCR products, enzymatic digestion reactions were performed. Out of the 227 pairs of CAPS primers, 110 pairs produced the correct bands and polymorphisms between the parental strains, with a rate of 48.5 %. All of the polymorphic CAPS markers were found to be co-dominate in the LSW-177, COS and F1 generation. The fragment lengths of the PCR products ranged from 966 to 312 bp, while the enzyme-digested products ranged from 856 to 158 bp. The 110 pairs of CAPS markers covered 11 chromosomes in watermelon. According to the re-sequencing data, each CAPS marker has its location in the watermelon genome and distributed in the 11 chromosomes so the CAPS markers could be used as the anchor markers in the linkage map construction. We also identified some CAPS loci from the re-sequencing data that were grouped as chromosome 0, which means that the CAPS loci did not fit within the 11 chromosomes analyzed within watermelon but existed in the watermelon genome. Some primers were designed with the chromosome 0 sequence data and some designed primers identified polymorphisms between the LSW-177 and COS. We grouped these markers into watermelon chromosomes by the method of linkage map construction.
Genetic map
Based on the parental strains, diverse polymorphic markers from a total of 148 polymorphic markers (40 SSRs, 108 CAPSs) were used to construct a genetic map using the F2 population. Twenty-three pairs of core SSR markers reported by Zhang and colleagues were used for primer selection (Zhang et al. 2011). Among them, 13 pair of primers showed polymorphisms between the two parental strains. Finally, nine pair of core primers were arranged into eight groups. The resulting order of markers and map distances between the markers are shown graphically as linkage maps in Fig. 3. Using IciMapping V3.3, 144 polymorphic markers were produced by 37 SSR and 107 CAPS were mapped on the watermelon linkage map with four markers being removed. All of the CAPS markers identified in this study have not been previously reported. The LSW-177 by COS linkage map covered 2,039.5 cM and is divided into 11 major and 5 minor linkage groups with an average of 14.16 cM between markers. According to the CAPS markers, the 5 minor linkage groups were merged into 11 major linkage groups. The order of the CAPS markers was compared within the physical map and most of the distributed markers were the same as the physical map.
A linkage map of watermelon chromosomes constructed based on a F2 population of a cross between LSW-177 and COS parental strains. The names of the markers are shown on the right of the chromosomes while the intervals of the two markers are shown on the left. The boxes in the right of the chromosomes indicate the most likely positions of QTLs identified for different fruit traits. LCYB lycopene content, FL fruit length, FW fruit width, BCC Brix content central, BCE Brix content edge, FC flesh color
Identification of QTLs for flesh color
The watermelon linkage map was used for the QTL analysis to identify markers associated with flesh color and lycopene content as well as flesh color related traits. Three newly developed CAPS markers (WII04E07-33, WII04E07-37 and WII04E07-40) associated with flesh color were detected on chromosome 4. Chi square test of the segregation ratios between red, pale yellow and canary yellow flesh color using watermelon CAPS markers WII04E07-33, WII04E07-37 and WII04E07-40 in the F2 population was performed and the results were listed in Table 4. The QTL related with lycopene content and was detected on the basis of the three CAPS markers. Four QTLs associated with flesh color, which included one major and three minor QTLs that were detected in four linkage groups (chromosome 3, 4, 6 and 11). The four QTLs could explain the phenotypic variation, which ranged from 34.68 to 2.56 % as well as the combined effects of flesh color, which was 43.68 % of the total variation. For all QTLs, FC3.1 showed a reduced additive effect for flesh color in watermelon. A major QTL (FC4.1) related with flesh color was detected on chromosome 4, with a LOD (Limit of Detection) score of 31.77 and total phenotypic variation of 34.68 %. The QTL FC4.1 was located between the CAPS marker WII04E07-37 and WII04E07-40 and just 0.2 cM away from the CAPS marker WII04E07-40. The other minor QTLs associated with flesh color (FC6.1 and FC11.1) were detected on chromosome 6 and 11 with LOD scores of 3.87 and 2.57, respectively, with both of them showing an increased additive effect.
Identification of QTLs for lycopene content
Only one major QTL (LCYB4.1) was identified for lycopene content on chromosome 4, which accounted for 83.5 % of the total variation and LOD score of 92.3 as well as additive effect of 19.79. The QTL LCYB4.1 was located between the CAPS markers WII04E07-33 and WII04E07-40, which was just 0.35 and 1.5 cM, respectively, away from the CAPS markers for FC4.1. These results support that LCYB4.1 is a major QTL that significantly increased lycopene content in this population with a strong additive effect. The significant QTLs for fruit traits were identified by interval mapping analysis (Table 5).
Identification of QTLs for other horticultural traits
Interval mapping analysis identified a total of 12 significant QTLs for the four traits: Brix (central and edge region), fruit length and fruit width. The number of QTLs ranged from 1 to 3 per trait. The most significant QTLs were for fruit width with a LOD score of >7.
Brix
Three minor QTLs were found to be significant for Brix content by IciMapping V3.3. The three QTLs were distributed over two linkages (chromosome 2 and 8). Of the three QTLs, two were for the edge and one was for the central region of the Brix content. The three QTLs were for minor loci and no major QTL was detected. The two QTLs for the edge region of the Brix content (BCE2.1 and BCE8.1) were located on chromosome 2 and 8, with LOD scores of 2.69 and 2.70, respectively, and individual effects of 6.14 and 5.27 % trait variation, respectively. The combined effect was 23.8 % of the total phenotypic variance. An additional QTL for the central region of the Brix content (BCC8.1) was found with a LOD score of 3.26 on chromosome 8, near the locus controlling the length and width of the fruit. The interval between BCC8.1 and the CAPS marker WIII08E08-8 was 2.3 cM.
Fruit length and width
For fruit length and width, three QTLs were detected in two linkage groups (chromosome 8 and 11) with individual effects of 11.33, 5.16, 7.31 % trait variations, respectively, and a combined effect accounting for 23.8 % of trait variation. For all of the QTLs, FL8.1contributed to reduced fruit length and other QTLs (FL11.1and FL11.2) showed increased effect to fruit length. FL11.1 was 0.4 cM from the CAPS marker, WII11E05-59. For fruit width, only one QTL was detected (FW8.1) with a LOD score of 7.33, which and accounted for 13.45 % phenotypic variation. This QTL was 2.1 cM away from the SSR marker WMU06151.FW8.1 and FL8.1, which were located in the same region, with both QTLs showing a reduced effect on fruit length and width.
Discussion
The inheritance of lycopene content and flesh color
An interesting phenomenon found in this study is that all of the plants in the high-lycopene content group showed a red flesh color, while the low-lycopene content group showed either canary yellow and pale yellow flesh color. Previous reports suggested that a single LCYB gene was the determinant of canary yellow or red flesh color (Bang et al. 2007), although studies by Henderson and colleagues reported the presence of a two-gene model for flesh color inheritance (Henderson et al. 1998). The results obtained herein agree in principle with the single recessive inheritance model. HPLC analysis indicated that the segregation of lycopene content in the F2 population significantly deviated from the normal distribution, consistent with a single gene genetic model of inheritance for this trait. This is different from that of tomato, which also exhibited a high lycopene content, for which other studies showed that the lycopene content followed an additive genetic model of inheritance (Chen et al. 1999; Kinkade and Foolad 2013). Thus, the inheritance of lycopene content in watermelon appears to be different from tomato.
The color segregation of the F2 progeny in this study adequately fits a 3:1 ratio in flesh color between the non-red and red groups as well as the high-lycopene and low-lycopene content groups, indicating the action of one major gene for red flesh color and high-lycopene content. Similar results were also observed by Bang and colleagues for the gene (C) controlling canary yellow flesh color, which was dominant to the red flesh color gene (Y) (Bang et al. 2010). These results suggest that one recessive gene could control red-flesh color and high-lycopene content traits.
The lycopene content data from canary yellow and pale yellow showed that both of the flesh colors have low-lycopene content but no obvious differences could be observed between these flesh colors in lycopene content. We hypothesize that there are other gene(s) for the canary yellow and pale yellow flesh color in watermelon. This may be supported by the report from Bang and colleagues, which showed that a single recessive gene py operating in the presence of a dominant allele resulted in the pale yellow phenotype (Bang et al. 2010).
CAPS markers for linkage map construction
Our linkage map based on the cross between LSW-177 and COS was created with 144 markers. Among them 108 CAPS markers were developed, with the SSR and CAPS markers being co-dominant. Important horticultural traits, such as Brix content, flesh color, lycopene content, fruit length and fruit width, could be analyzed in this linkage map. Thus the present map may be a useful framework for mapping horticultural traits in watermelon and for studies on the synteny of various cucurbita crops.
Linkage map construction in watermelon has been difficult due to the low DNA polymorphism among cultivated watermelon. In a previous study, only 3.9 % of the amplified DNA bands were different from the parents of commercial hybrid cultivars (Li et al. 2008). We also used melon SSR markers to screen for selective primers but the results also suggested a comparatively low polymorphism (1.6 %). Thus, identifying new markers through linkage map construction has been a major priority for research in the watermelon field. In our study, 2458 CAPS loci were detected to be suitable for primer design using 11 restriction endonucleases, with a polymorphism rate of 48.9 %, which is a more than 12 fold increase when compared to the low polymorphism using SSR selection. The results presented in this study may therefore provide a promising tool to derive high quality watermelon linkage maps.
According to our re-sequencing data, each CAPS marker had its own location distributed in the 11 watermelon chromosomes. As a result, we could distinguish linkages from chromosome 1 to 11, which would correspond to the watermelon chromosomes 1 to 11. The order of the CAPS markers in the linkage map was mostly the same as the distribution of the sequence data. Five minor linkage groups were combined with the major linkages according to the location of the CAPS markers. Gaps between the minor linkages could be merged using markers developed in this study. Finally, both the core watermelon SSR and CAPS markers were used as anchor points, and both markers were distributed to the same chromosome. Some CAPS markers designed from chromosome 0 were also arranged into different chromosomes in the linkage map. It should be noted that there were gaps of more than 30 cM between the markers and some linkage groups. However, this issue can be resolved if more CAPS markers were developed in the gap area to enrich the linkage map.
Three CAPS markers associated with red and non-red flesh color
In our study, three CAPS markers (WII04E07-33, WII04E07-37 and WII04E07-40) were developed, which could perfectly co-segregate with the red and non-red groups. The three CAPS were on chromosome 4 in the watermelon genome, according to the high-throughput re-sequencing data. Through bioinformatics analysis and using high-throughput re-sequencing data we constructed a physical map of watermelon. The position of the three CAPS markers was obtained from the physical map and QTLs for lycopene content and flesh color were detected in the intervals of the three markers. The three newly developed CAPS markers were different from the CAPS marker reported by Bang and colleagues, where one CAPS marker (Phe226) was developed to co-segregate with the canary yellow and red flesh color phenotype (Bang et al. 2007). This marker was found by comparing the partial cDNA sequence of the LCYB in canary yellow to the red watermelon and subsequently isolated using degenerate PCR primers and RACE (Bang et al. 2007). Based on re-sequencing data from the COS and LSW-177 strains, we were able to align the full-length LCYB cDNA in the region between the two CAPS markers (WII04E07-33 and WII04E07-40) on chromosome 4. These results indicated that the intervals between the three CAPS markers could have genes or loci that could distinguish between red versus non-red flesh color that are near the coding region of LCYB and co-segregate with genes controlling flesh color and lycopene content.
Two mixed flesh colors were identified in the F2 generation of our study. These mixed colors were also reported in other studies (Bang et al. 2010; Navot et al. 1990) and thought to be caused by environmental conditions rather than genetics and developmental abnormalities. Gusmini and Wehner presumed that flesh color arose by many genes, with flesh color from different parts of the mature watermelon being controlled by different genes. It was thought that the mixed color could be due to genetic recombination or the expression of a gene regulating flesh color. We used our three new CAPS markers to genotype all of the plants in the F2 generation including the mixed color plants. The results of our study showed that all of the mixed color watermelons resulted in heterozygous bands in the F1 generation. Other studies have suggested that there should be other genes, gene epistasis effects or environmental conditions that could underlie the mixed color phenotype (Bang et al. 2010; Navot et al. 1990).
QTL analysis for lycopene content and flesh color
Breeding for increased lycopene content in watermelon fruit is an important focus for horticultural quality, consumer acceptance and human health. Fruit color and lycopene content are key traits to be analyzed in watermelon QTL mapping studies. In our study, lycopene content QTL was identified as one major QTL on chromosome 4. This QTL was detected by CAPS marker WII04E07-40 and WII04E07-33 and was found only 0.35 cM from the CAPS marker WII04E07-33. The LOD score (92.32) and trait variation (83.50 %) indicated that the QTL was a major QTL for lycopene content with a large additive effect to increase lycopene content.
Prior to this study, little research was focused on lycopene content QTL analysis in watermelon and the carotenoid biosynthetic pathway of watermelon was extrapolated from the carotenoid biosynthesis pathways in plants (Isaacson 2002; Isaacson et al. 2004). Grassi and colleagues reported that the developmental origins of watermelon were similar to tomato; however, they still suggested complex and different regulatory systems for carotinoid biosynthesis pathways between these two fruits (Grassi et al. 2013). Two lycopene cyclases, lycopene β-cyclase (LCYB) and lycopene ε-cyclase (LCYE) could convert lycopene-β or α-carotene with ring structures (Ronen et al. 2000). As a result, a likely candidate gene for color determination between red and other flesh color in watermelon could be the gene immediately downstream of the lycopene synthesis pathway (Bang 2005). In the COS strain and the F1 generation, we detected a new pigment by HPLC analysis as reported by Bang and colleagues (Bang et al. 2010). We speculated that this new pigment could be neoxanthin or lutein. According to the re-sequencing data, the sequence between the two CAPS markers WII04E07-33 and WII04E07-40 detected the lycopene content QTL LCYB4 from BLAST analysis. Blast analysis results demonstrated that lycopene β-cyclase (Lcyb) mRNA, LCYB-red allele and complete cDNAs were detected in the same region between the two CAPS markers. In melon, seven genes from the carotenoid biosynthetic pathway have been previously mapped (Cuevas et al. 2008, 2010), and five were re-mapped to similar locations with different markers, as reported by Harel-Beja and colleagues (Harel-Beja et al. 2010). Identifying the gene and functional polymorphism underlying this position may further shed light on the carotenoid biosynthetic process.
One major and three minor QTLs for flesh color were mapped in our study on chromosome 3, 4, 6 and 11, respectively. The major QTL for flesh color (LOD = 31.77) with trait variation of 34.68 % was located on chromosome 4, which mapped to the same region as the lycopene content QTL. Co-localization of the major QTL for flesh color and lycopene content is a reflection of a single locus determining the role of lycopene content and flesh color in watermelon fruits. Unlike lycopene content, we detected four QTLs related to flesh color in watermelon, which indicated that there were many genes in the carotenoid biosynthetic pathway that control pigment content and flesh color. Previous studies in cucurbit crops suggested that no single gene controlled flesh color. Harel-Beja and colleagues reported 11 QTLs for orange flesh color and carotenoid content in melon using a RIL population derived from two subspecies of Cucumis melo L.: PI 414723 (subspecies agrestis) and ‘Dulce’ (subspecies meld) (Harel-Beja et al. 2010).
Hashizume and colleagues mapped the red flesh color locus in watermelon using a comprehensive linkage map on chromosome 2 and 8, which was not detected in the cross between LSW-177 and COS F2 population (Hashizume et al. 2003). The QTL related with red flesh color was identified using a cross between red and yellow flesh genotypes. QTL at chromosome 2 and 8 were associated with the presence and absence of flesh carotenoids, while the LSW-177 and COS F2 population was established from a cross of red and pale yellow flesh color. Some studies showed that genetic manipulation of the quantity of β-carotene in cucumber and melon fruit (endocarp and mesocarp) was controlled by at least two recessive genes (Clayberg 1992; Cuevas et al. 2010). It was suggested that the LG6 in melon QTL may contain a gene that stimulates the carotenoid metabolic pathway in general, leading to the formation of β-carotene (Monforte et al. 2004; Cuevas et al. 2008). These additional QTLs suggest an accumulation of carotenoid regulators at this locus, which may lead to increased carotenoid levels in cucurbit crops. The fact that QTLs for both flesh and lycopene were mapped to the same region on chromosome 4 suggests that chromosome 4 may contain a gene that stimulates the lycopene metabolic pathway and lycopene in general, which may be controlled by the same (or closely linked) gene. A potential limitation of this study is that our study only detected lycopene content QTL in watermelon but did not reveal other pigment QTLs from the carotenoid biosynthetic pathway. However, measuring the content of other pigments in watermelon flesh could alleviate this limitation. Although preliminary, our study clearly identified the location of the lycopene content gene in the watermelon genome.
QTL analysis for other horticulture traits
Brix is a common measurement for predicting fruit sugar content and was shown to strongly correlate with sugar accumulation (Zhang et al. 2006). The three QTLs we detected were for minor QTLs accounting for just 6.87, 5.14 and 5.27 % trait variations, respectively. According to the segregation observed in the F2 population, it is possible that there was a major gene controlling Brix content. On chromosome 8 we detected two QTLs associated with Brix content, which was similar to results from another study (Sandlin et al. 2012). Our study presents two QTLs that may be the main loci controlling gene expression of Brix content, based on previous studies (Guo et al. 2006). Harel-Beja and colleagues detected six QTLs for sugar content in melon that interacted in an additive manner to account for nearly all of the differences in sugar content between the two parental strains (Harel-Beja et al. 2010).
Fruit size has been well studied in many plants but seldom in watermelon. Fruit size or fruit shape index is influenced by fruit length and fruit width. To our knowledge fruit length and width can be diverse as well as ultimately affect fruit weight and size. Perin and colleagues detected seven QTLs for fruit shape (Perin et al. 2002), and seven QTLs in melon (Monforte et al. 2004). In our studies, we detected three QTLs related to fruit length and one QTL related to fruit width. The fruit width associated QTL (FW8.1) was a major QTL on chromosome 8. FL11.1 and FL11.2 were located on chromosome 11 of the same chromosome as previously reported for the fruit length QTL in watermelon (Sandlin et al. 2012). FW8.1 and FL8.1 were mapped to the same region and similar to previous reports (Perin et al. 2002; Sandlin et al. 2012) suggesting that some regions or genes may control both traits.
In this study, Brix content, fruit length and fruit width were analyzed by taking advantage of the linkage map. All of the QTLs for the four traits were detected and had a continuous distribution, suggesting the quantitative character quantitative trait of the F2 population. It is possible that polygenic control by a few genes determines the four traits in the cross between LSW-177 and COS population.
In the plant genome, many QTLs exist in clumps, and this phenomenon also appeared in our study. For chromosome 8, 3 QTLs (FL8.1, FW8.1 and BCC8.1) associated with fruit length, fruit width and Brix content (central region) were detected in the same region (between markers WMU06151 and WIII08E08-8). Further correlation analysis demonstrated that the three traits had a high positive correlation. For the three traits (Brix content, fruit length and fruit width) in our early SSR primer selection, all of the detected QTLs had relatively low LOD values (2.74 to 7.33) due to the exploitation of low CAPS marker numbers and limited SSR polymorphism between the two parental strains. Lambel and colleagues successfully used the selective genotype analysis as an efficient method to locate genes for Fusarium oxysporum race1 resistance in watermelon (Lambel et al. 2014). Given our findings, developing more CAPS markers or the selective genotype analysis reported by Lambel and colleagues can be applied to future studies.
References
Bang H (2005) Environmental and genetic strategies to improve carotenoids and quality in watermelon. Dissertation for Ph D Degree at Texas A & M university U.S.A
Bang H, Kim S, Leskovar D, King S (2007) Development of a codominant CAPS marker for allelic selection between canary yellow and red watermelon based on SNP in lycopene β-cyclase (LCYB) gene. Mol Breed 20:63–72
Bang H, Davis AR, Kim S, Leskovar DI, King SR (2010) Flesh color inheritance and gene interactions among canary yellow, pale yellow, and red watermelon. J Am Soc Hortic Sci 135:362–368
Bo K, Song H, Shen J, Qian C, Staub JE, Simon PW, Lou Q, Chen J (2011) Inheritance and mapping of the ore gene controlling the quantity of β-carotene in cucumber (Cucumis sativus L.) endocarp. Mol Breed 30:335–344
Chen FQ, Foolad MR, Hyman J, St. Clair DA, Beelaman RB (1999) Mapping of QTLs for lycopene and other fruit traits in a Lycopersicon esculentum × L. pimpinellifolium cross and comparison of QTLs across tomato species. Mol Breed 5:283–299
Clayberg CD (1992) Interaction and linkage test of flesh color genes in Cucumis melo L. Cucurbit Genet Coop Rep 15:53
Cuevas HE, Staub JE, Simon PW, Zalapa JE, McCreight JD (2008) Mapping of genetic loci that regulate quantity of beta-carotene in fruit of US Western Shipping melon (Cucumis melo L.). Theor Appl Genet 117:1345–1359
Cuevas HE, Song H, Staub JE, Simon PW (2010) Inheritance of beta-carotene-associated flesh color in cucumber (Cucumis sativus L.) fruit. Euphytica 171:301–311
Danin-Poleg Y, Reis N, Tzuri G (2001) Development and characterization of microsatellite in Cucumis. Theor Appl Genet 102:61–72
Davis AR, Collins JK, Perkins Veazie PM, Levi A (2008) LSW-177 and LSW-194: red-fleshed watermelon lines with low-total soluble solids. HortScience 43:538–539
Fazio G, Staub JE, Chung SM (2002) Development and characterization of PCR markers in cucumber. HortScience 127:545–557
Femandez-Silva I, Eduardo I, Blanca J, Esteras C, Pico B, Nuez F, Arus P, Garcia-Mas J, Monforte AJ (2008) Bin mapping of genomic and EST-derived SSRs in melon (Cucumis melo L.). Theor Appl Genet 118:139–150
Feng D, Ling WH, Duan RD (2010) Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-k B in macrophages. Inflamm Res 59:115–121
Fernandez-Silva I, Eduardo I, Blanca J, Esteras C, Pico B, Nuez F, Arus P, Garcia-Mas J, Monforte AJ (2008) Bin mapping of genomic and EST-derived SSRs in melon (Cucumis melo L.). Theor Appl Genet 118:139–150
Gonzalo MJ, Oliver M, Garcia-Mas J, Monfort A, Dolcet-Sanjuan R, Katzir N, Arus P, Monforte AJ (2005) Simple-sequence repeat markers used in merging linkage maps of melon (Cucumis melo L.). Theor Appl Genet 110:802–811
Grassi S, Piro G, Lee JM, Zheng Y, Fei ZJ, Dalessandro G, Giovannoni JJ, Lenucci MS (2013) Comparative genomics reveals candidate carotenoid pathway regulators of ripening watermelon fruit. BMC Genomics 14:781
Guo SG, Xu Y, Zhang HY, Gong GY (2006) QTL analysis of soluble solids content in watermelon under different environments. Molecular Plant Breeding 4:393–398
Gusmini G, Wehner TC (2006) Qualitative inheritance of rind pattern and flesh color in watermelon. J Hered 97:177–185
Harel-Beja R, Tzuri G, Portnoy V, Lotan-Pompan M, Lev S, Cohen S, Dai N, Yeselson L, Meir A, Libhaber SE, Avisar E, Melame T, van Koert P, Verbakel H, Hofstede R, Volpin H, Oliver M, Fougedoire A, Stalh C, Fauve J, Copes B, Fei Z, Giovannoni J, Ori N, Lewinsohn E, Sherman A, Burger J, Tadmor Y, Schaffer AA, Katzir N (2010) A genetic map of melon highly enriched with fruit quality QTLs and EST markers, including sugar and carotenoid metabolism genes. Theor Appl Genet 121:511–533
Hashizume T, Shimamoto I, Hirai M (2003) Construction of a linkage map and QTL analysis of horticultural traits for watermelon [Citrullus lanatus (THUNB.) MATSUM & NAKAI] using RAPD, RFLP and ISSR markers. Theor Appl Genet 106:779–785
Henderson W, Scott G, Wehner T (1998) Interaction of flesh color genes in watermelon. J Hered 89:50–53
Henry RJ (2008) Plant genotyping II: SNP technology. CABI, Wallingford
Isaacson T (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14:333–342
Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136:4246–4255
Joobeur T, Gusmini G, Zhang X, Levi A, Xu Y, Wehner TC, Oliver M, Dean AR (2006) Construction of a watermelon BAC library and identification of SSRs anchored to melon or Arabidopsis genomes. Theor Appl Genet 2:1553–1562
Kinkade MP, Foolad MR (2013) Validation and fine mapping of lyc12.1, a QTL for increased tomato fruit lycopene content. Theor Appl Genet 126:2163–2175
Kole C, Abbott AG (2008) Principles and practices of plant genomics, vol 1. Genome mapping Science Publishers, Enfield
Lambel S, Lanini B, Vivoda E, Fauve J, Wechter WP, Harris-Shultz KR, Massey L, Levi A (2014) A major QTL associated with Fusarium oxysporum race 1 resistance identified in genetic populations. Theor Appl Genet 127:2105–2115
Levi A, Thomas CE, Trebitsh T, Salman A, King J, Karalius J, Newman M, Reddy OUK, Xu Y, Zhang X (2006) An extended linkage map for watermelon based on SRAP, AFLP, SSR, ISSR, and RAPD markers. J Am Soc Hortic Sci 131:393–402
Li N, Zhang HY, Chen NL, Wang YJ, Xu Y, Gong GY, Guo SG (2008) Comparison of versatility and polymorphism of SSRs in the cucurbitaceae. Acta Agriculture Boreali-Sinica 23:1109–1114
Ling KS, Harris KR, Meyer JD, Levi A, Guner N, Wehner TC, Bendahmane A, Havey MJ (2009) Non-synonymous single nucleotide polymorphisms in the watermelon eIF4E gene are closely associated with resistance to Zucchini yellow mosaic virus. Theor Appl Genet 120:191–200
Liu QH, Wang HL, Zhou ZC (2009) Comparative study on lycopene content of different watermelon. North Hortic 8:44–46
Luan FS, Delannay I, Staub JE (2008) Chinese melon (Cucumis melo L.) diversity analyses provide strategies for germplasm curation, genetic improvement, and evidentiary support of domestication patterns. Euphytica 164:445–461
Monforte AJ, Oliver M, Gonzalo MJ, Alvarez JM, Dolcet-Sanjuan R, Arus P (2004) Identification of quantitative trait loci involved in fruit quality traits in melon (Cucumis melo L.). Theor Appl Genet 108:750–758
Morales M, Orjeda G, Nieto C, van Leeuwen H, Monfort A, Charpentier M, Caboche M, Arus P, Puigdomenech P, Aranda MA, Dogimont C, Bendahmane A, Garcia-Mas J (2005) A physical map covering the nsv locus that confers resistance to Melon necrotic spot virus in melon (Cucumis melo L.). Theor Appl Genet 111:914–922
Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA (1997) Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett 418:91–97
Navot N, Sarfatti M, Zamir D (1990) Linkage relationships of genes affecting bitterness and flesh color in watermelon. J Hered 81:162–165
Perin C, Hagen LS, Giovinazzo N, Besombes D, Dogimont C, Pitrat M (2002) Genetic control of fruit shape acts prior to anthesis in melon (Cucumis melo L.). Mol Genet Genom 266:933–941
Poole CF (1994) Genetics of cultivated cucurbits. J Hered 35:122–128
Ren Y, Zhao H, Kou QH, Jiang J, Guo SG, Zhang HY, Hou WJ, Zou XH, Sun HH, Gong GY, Levi A, Xu Y (2012) A High Resolution Genetic Map Anchoring Scaffolds of the Sequenced Watermelon Genome. PLoS ONE 7:e29453
Ren Y, McGregor C, Zhang Y, Gong GY, Zhang HY, Guo SG, Sun HH, Cai WT, Zhang J, Xu Y (2014) An integrated genetic map based on four mapping populations and quantitative trait loci associated with economically important traits in watermelon (Citrullus lanatus). BMC Plant Biol 14:33
Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Betaandold-goldcolor mutations in tomato. Proc Natl Acad Sci USA 97:11102–11107
Sandlin K, Prothro J, Heesacker A, Khalilian N, Okashah R, Xiang W, Bachlava E, Caldwell DG, Taylor CA, Seymour DK, White V, Chan E, Tolla G, White C, Safran D, Graham E, Knapp S, McGregor C (2012) Comparative mapping in watermelon [Citrullus lanatus (Thunb.) Matsum. et Nakai]. Theor Appl Genet 125:1603–1618
Shen D, Fang ZY, Li XX, Li QH, Cheng JQ, Song JP, Wang HP, Qiu Y (2011) Inheritance of fruit flesh color in Cucumis sativus L. J Plant Genet Resour 12:216–222
Shimotsuma M (1963) Cytogenetical studies in the genus Citrullus. VI. Inheritance of several characters in watermelons. Jpn J Breed 13:235–240
Silberstein L, Kovalski I, Brotman Y, Perin C, Dogimont C, Pitrat M, Klingler J, Thompson G, Portnoy V, Katzir N, Perl-Treves R (2003) Linkage map of Cucumis melo including phenotypic traits and sequence-characterized genes. Genome 46:761–773
Tezuka T, Waki K, Yashiro K, Kuzuya M, Ishikawa T, Takatsu Y, Miyagi M (2009) Construction of a linkage map and identification of DNA markers linked to Fom-1, a gene conferring resistance to Fusarium oxysporum f.sp. melonis race 2 in melon. Euphytica 168:177–188
Yi K, Xu XL, Lu XY, Xu Y, Xiao LT, Wang YJ, Kang G (2003) Construction of molecular genetic map of watermelon by SSR and ISSR technology. J Hunan Agric Univ (Nat Sci) 29:333–347
Yuan LP, Lu XQ, Liu WG, Zhao JS, Lu JS, Yan ZH, He N, Guan LY, Zhu HJ (2012) Measurement of lycopene contents in watermelon. China Cucurbits Veg 25:1–4
Zalapa JE, Staub JE, McCreight JD, Chung SM, Cuevas H (2007) Detection of QTL for yield-related traits using recombinant inbred lines derived from exotic and elite US Western Shipping melon germplasm. Theor Appl Genet 114:1185–1201
Zhang RB, Xu Y, Yi K, Zhang HY, Liu LG, Gong GY, Levi A (2004) A genetic linkage map for watermelon derived from recombinant inbred lines. J Am Soc Hortic Sci 129:237–243
Zhang F, Gong G, Wang Q, He H, Xu Y (2006) Analysis of watermelon quality structure. J Fruit Sci 23:266–269
Zhang H, Wang H, Guo S, Ren Y, Gong G, Weng Y, Xu Y (2011) Identification and validation of a core set of microsatellite markers for genetic diversity analysis in watermelon, Citrullus lanatus Thunb. Matsum. and Nakai. Euphytica 186:329–342
Acknowledgments
This research was supported by the National Nature Science Foundation of China (31272186). This work was also supported by the China Agriculture Research System (CARS-26-02).
Conflict of interest
The authors declare that there are no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Gao, P., Wang, X. et al. Mapping of quantitative trait loci for lycopene content and fruit traits in Citrullus lanatus . Euphytica 202, 411–426 (2015). https://doi.org/10.1007/s10681-014-1308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1308-9