Abstract
Bending-type lodging is one of the most important factors affecting the yield and grain quality of rice. This study identified quantitative trait loci (QTLs) for physical strength of the upper culms, and evaluated QTL effects on lodging resistance. In 2010 and 2011, QTLs for breaking strength, length, and diameter of the top three internodes were identified by analyzing chromosomal segment substitution lines (CSSLs) developed from ‘Koshihikari’ and ‘Kasalath’. The QTL analysis indicated that ‘Kasalath’ had two types of QTLs: one to strengthen specific internodes and one to simultaneously improve the physical strengths of plural internodes or the top three internodes. A QTL for breaking strengths of the top three internodes (bsuc11) was detected on chromosome 11 in both years. This QTL did not overlap with that for internode length. To evaluate the effects of bsuc11 on lodging resistance, this study selected three CSSLs with bsuc11 and analyzed the breaking strengths of the top three internodes after heading and the pushing resistance of the lower part. Internodes of ‘Koshihikari’ showed decreased breaking strengths after grain filling, while those of CSSLs with bsuc11 did not show this decrease in breaking strength. The pushing resistance of the lower part at the fully ripe stage was the same in ‘Koshihikari’ and CSSLs with bsuc11. These results suggested that bsuc11 could be a target to improve the physical strength of the upper culms to resist bending-type lodging, and that the physical strengths of upper and lower parts are controlled by different genetic factors in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bending-type lodging is the main type of rice lodging, which is one of the most important problems in rice production as it leads to low yields and poor grain quality (Kono 1995). Several Japanese rice cultivars, e.g. ‘Koshihikari’, are prone to bending-type lodging because of their heavy panicle weights, which make them vulnerable to environmental stresses from rain and wind at the fully ripe stage (Kashiwagi et al. 2007). This lodging-type is associated with heavier panicles, weaker upper culms, and higher plant stature, and reducing plant height has been the main target for breeding lodging-resistant varieties. When breeding for higher yield or greater biomass, an additional improvement in the physical strength of culms is one of the best traits for resistance to bending-type lodging. In fact, the New Plant Type (NPT) developed by the International Rice Research Institute showed thick and sturdy stems (Peng et al. 1999a), and a few second-generation NPTs produced high yields as a result of accumulating large amounts of aboveground biomass (Peng et al. 2008). The next breeding objective for lodging resistance is to target the physical strength of the plant, rather than plant height.
In previous studies, several techniques have been used to evaluate the physical strength of the plant body. Bending stress and breaking strength were measured in specific parts of cereal plants. Kokubo et al. (1989) measured maximum bending stress of the fourth internode in barley. Similarly, stiffness and the bending moment at breaking were measured for rice culms (Kashiwagi et al. 2008; Ookawa et al. 2010). Other ways to evaluate the physical strength of plant body include measuring resistance to artificial lodging by pushing the plant body. Pushing resistance has been used to evaluate lodging resistance in rice and maize (Fouéré et al. 1995; Kashiwagi and Ishimaru 2004). In their study on stem and root lodging resistance, Berry et al. (2003) used field-based measurement techniques to evaluate resistance to rotational displacement. Physical strength measurement is a useful way to evaluate lodging resistance in crops, and is required for breeding to reduce lodging damage.
The physical strength of the plant body has also been evaluated by analyzing morphological traits and chemical components. Among the morphological traits of rice and wheat, diameter and weight of stems were significantly correlated with the physical strength of the plant (Atkins 1938; Zuber et al. 1999; Kashiwagi et al. 2008). Culm wall thickness also was strongly related to lodging score and culm stiffness (Tripathi et al. 2003; Kashiwagi et al. 2008). Structural carbohydrates including cellulose and lignin are known to contribute to physical strength in plants (Kokubo et al. 1991; Jones et al. 2001; Li et al. 2003). In rice, it was reported that high re-accumulation of non-structural carbohydrates in culms at the fully ripe stage was a character of cultivars with high lodging resistance (Sato 1957; Takaya and Miyasaka 1983; Yang et al. 2001). The amount of minerals in culms is also important for lodging resistance, especially silicon, which increases culm rigidity (Idris et al. 1975). Thus, the physical strength of the plant body comprises various traits. Understanding the genetic controls of these traits is important in breeding for lodging resistance.
Certain genetic factors, including quantitative trait loci (QTLs) for lodging resistance have been studied in several crops, e.g. rice, wheat, barley, soybean, and field pea (Lee et al. 1996; Keller et al. 1999; Spaner et al. 1999; Tar’an et al. 2003; Kashiwagi and Ishimaru 2004). The improvement of lodging resistance requires effective targets for breeding, because lodging resistance is related to complex factors including morphological traits, chemical contents, and physiological characteristics. Mulder (1954) proposed that lodging results from a disproportionate balance between the weight of upper parts and the sturdiness of the basal parts. Genetic approaches to improve these factors have been reported in rice. For example, the semidwarf gene, sd-1, the famous gene for the “Green revolution”, reduced plant height through the mutation of a gibberellin-biosynthesis gene (Khush 1999; Sasaki et al. 2002), and reduced the moment of upper parts. In other cereal crops, genetic factors related to gibberellin, e.g. Rht-B1 and Rht-D1 in wheat (Peng et al. 1999b) and Dwf2, gai, and gal in barley (Börner et al. 1999; Ivandic et al. 1999) contributed to dwarfing. Genetic factors related to another plant hormone, brassinosteroid, also reduced plant height in rice (Yamamuro et al. 2000; Mori et al. 2002; Hong et al. 2003). As an example of the sturdiness of basal parts, a QTL for the pushing resistance of the lower part, prl5, physically strengthened the lower part of the plant by delaying leaf senescence and non-structural carbohydrate accumulation in culms at the fully ripe stage (Kashiwagi and Ishimaru 2004; Kashiwagi et al. 2006). Culm strength has also been a genetic target for lodging resistance. Ookawa et al. (2010) identified STRONG CULM2 (SCM2), which increased the section modulus of all internodes and the bending moment at breaking of internode IV. A QTL for stem diameter, sdm8, thickened the diameters of internodes II and III, and increased culm stiffness (Kashiwagi et al. 2008).
In addition to reducing the moment of upper parts, genetic improvement strategies to increase lodging resistance can focus on increasing the physical strengths of internodes at mid-to-low levels or the lower parts of the plant. Greater physical strength of the plant parts at mid-to-low levels contributes to reducing lodging damage in rice. On the other hand, the upper culms, including internodes I to III, are thinner and weaker than the lower culms, e.g. the basal intenode, and are easily bent by environmental stresses and panicle weight at the fully ripe stage. Identification of the genetic factors affecting the physical strength of upper culms, therefore, would provide a new approach for improving bending-type lodging resistance. In this study, QTL for the physical strength of internodes I to III was identified using chromosomal segment substitution lines (CSSLs). This QTL was evaluated the change in the physical strength of internodes over time after heading, and the effects of the physical strength of the lower part of the plant on lodging.
Materials and methods
Plant materials
For QTL analysis, this study used 39 CSSLs of rice with chromosomal segments of ‘Kasalath’ in the genetic background of ‘Koshihikari’, developed by the Rice Genome Resource Center in Tsukuba, these substituted chromosomal segments covered most of the genome other than small regions at the distal end of the short arm on chromosome 8 and at distal end of the long arm on chromosome 12 (Ebitani et al. 2005). The seeds of these CSSLs and their parents were sown in a greenhouse in late April of 2010 and 2011. Seedlings were transplanted into paddy fields in Utsunomiya, Japan (latitude 36°N), in late May with a single plant per hill spaced at 30 × 30 cm, and were grown under natural conditions with 10 plants per line. The CSSLs and their parents were arrayed in a row, respectively, on one experimental plot in each year. In 2011 and 2012, seeds of three CSSLs with bsuc11 and ‘Koshihikari’ were sown in late April, and these seedlings were transplanted on 22 and 16 May, respectively, at a spacing of 15 cm between hills and 30 cm between rows. Each plot of CSSL or ‘Koshihikari’ consisted of five rows with 20 hills per row, and the plot position for each line or variety in the paddy field was different in each of the 2 years. N, P2O5, and K2O fertilizers were applied as basal dressing at 6 g m−2, respectively, before transplanting in 2010–2012.
Physical strength measurements
The breaking strengths of internodes I, II, and III, (first, second, and third internode just below the neck node of the panicle, respectively) were measured a digital force gauge (SX-5, Aikoh Engineering, Osaka, Japan) on a manual test stand. The central part of the internode was immediately measured at a distance of 6-cm between two supporting points after sampling. Breaking strength was defined as the maximum value at broken internodes. The measurement used three culms with the average culm length per plant, and six plants for each line were analyzed. The pushing resistance of the lower part was measured at 6 weeks after heading with a prostrate tester (Daiki Rika Kogyou Co., Tokyo, Japan), according to the method reported by Kashiwagi and Ishimaru (2004). For pushing resistance of the lower part, the upper part of the plant was removed at a height of 40 cm. The prostrate tester was placed vertically next to the stem at a height of 20 cm above the soil surface, and was pushed until the plants had inclined to an angle of 45° to the vertical. Pushing resistance of the lower part per one tiller (PRL/TN) was calculated using the following equation, PRL/TN = pushing resistance of the lower part/tiller no. per plant. Pushing resistances were determined using 10 plants in each line.
Morphological traits of upper culms
The length and diameter of internodes I, II, and III were measured at 6 weeks after heading. Internode diameter was measured at the major axis at the central part of each internode. As in the case of breaking strength measurement, lengths and diameters of internodes were measured for three culms per plant, and six plants in each line.
QTL analysis
QTLs for breaking strength, length, and diameter of internodes I, II, and III were determined by comparing the phenotypes and genotypes of 130 restriction fragment length polymorphism markers (Ebitani et al. 2005). QTL detection was based on the t test of difference between each CSSL and Koshihikari. According to the method of Madoka et al. (2008), the threshold for detection of QTLs was a probability level of 0.05. The effect of a QTL was evaluated as positive (plus) or negative (minus), compared with the ‘Koshihikari’ phenotype.
Statistical analyses
Statistical analyses were conducted with Microsoft Excel 2008 for Mac. Mean and standard error was calculated from six or ten plants. For the comparison between each CSSL and Koshihikari, means were separated using the t test at a probability of P = 0.05 only when the F-test showed significance at 0.05 probability levels.
Results
Breaking strength, length, and diameter of upper culms in CSSLs
The breaking strength of internode I, II, and III in ‘Koshihikari’ was 1.02 ± 0.06, 2.14 ± 0.16, and 3.53 ± 0.28 N, respectively, in 2010 (Fig. 1; Table 1). Compared with their corresponding values in ‘Koshihikari’, the breaking strength of internodes I, II, and III showed a maximum increase of 104, 90, and 94 %, respectively, in the CSSLs. The proportion of CSSLs with a significantly higher breaking strength than that of ‘Koshihikari’ was 85, 59, and 77 % for internodes I, II, and III, respectively. A few CSSLs showed significantly decreased breaking strengths; three CSSL lines showed decreased breaking strengths of internode II, and one showed decreased breaking strength of internode III. In 2011, the breaking strength of ‘Koshihikari’ internodes I, II, and III was 1.06 ± 0.04, 1.87 ± 0.14, and 3.34 ± 0.18 N, respectively. The maximum breaking strength of internodes I, II, and III in CSSLs was 68, 65, and 141 % higher, respectively, than its corresponding value in ‘Koshihikari’. Compared with those of ‘Koshihikari’, 56, 74, and 69 % of CSSLs had significantly higher breaking strengths for internodes I, II, and III, respectively. The donor parent ‘Kasalath’ showed 1.3-, 1.6-, and 1.7-times higher breaking strength of internodes I, II, and III, respectively, in 2010, and 1.2-, 2.2-, and 2.2-times higher breaking strength of internodes I, II, and III, respectively, in 2011, compared with the corresponding values in ‘Koshihikari’ (Table 1).
In both 2010 and 2011, the lengths of internodes II and III in ‘Kasalath’ were significantly longer than those in ‘Koshihikari’ (Table 1). For each internode, the mean length in the CSSLs was similar to that of the corresponding internode in ‘Koshihikari’. For the CSSLs with the longest internodes, the internode lengths were at least 1.2-times longer than those of ‘Koshihikari’. In the CSSLs with the shortest length of internode III, the length of internode III was 0.6 times or less than that of ‘Koshihikara’. Compared with those of ‘Koshihikari’, the diameters of internodes of ‘Kasalath’ were significantly greater (Table 1). Comparing mean values, the diameters of internodes I, II, and III were similar between ‘Koshihikari’ and the CSSLs. In 2010 and 2011, the CSSLs with maximum internode diameters showed 1.2-, 1.2 to 1.7-, and 1.3-times greater diameters of internodes I, II, and III, respectively, compared with those of the corresponding internodes of ‘Koshihikari’. The smallest internode diameters in the CSSLs were 0.8- to 0.9-times those of the corresponding internodes of ‘Koshihikari’.
Correlations among upper culm traits
For breaking strengths, there were significant positive correlations among internodes (P < 0.05, Table 2). The breaking strength of internode I was very strongly correlated with that of internode III (P < 0.001). Breaking strength was positively correlated with the length and diameter of internode I (P < 0.001). Internode II showed a weak positive correlation between breaking strength and diameter (P < 0.05). Breaking strength of internode III negatively correlated with its length, but positively correlated with its diameter (P < 0.001). Only internode I showed a significant correlation between length and diameter (P < 0.001). The length of internode II was positively correlated with those of internodes I and III (P < 0.01), and there were positive correlations among the diameters of the top three internodes (P < 0.001).
QTL analysis
This study evaluated nine traits: breaking strengths, lengths, and diameters of the top three internodes. For the nine traits, this study detected 65 and 85 QTLs in 2010 and 2011, respectively (Table 3, 4). In 2010, nine and eight QTLs for breaking strength of internodes I and III, respectively, had positive effects from the ‘Kasalath’ allele (Table 3). With respect to the QTLs for breaking strength of internode II, eight alleles from ‘Kasalath’ showed positive effects, and two alleles on chromosomes 1 and 4 decreased the breaking strength. ‘Kasalath’ alleles for increased breaking strength of the top three internodes were detected on chromosomes 7, 10, 11, and 12, while those for increased strength of specific internodes were detected on chromosomes 1, 2, and 3. In 2011, seven, eight, and eight QTLs for breaking strength of internodes I, II, and III, respectively, showed positive effects from the ‘Kasalath’ allele (Table 4). Three ‘Kasalath’ alleles on chromosomes 3, 4, and 5 contributed a decrease in the breaking strength of internode I. On chromosomes 1, 7, and 11, there were regions associated with increased breaking strength of the top three internodes. QTLs on chromosomes 5, 9, and 10 contributed to strength of specific internodes.
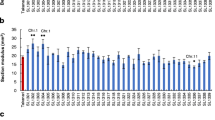
Comparing the QTLs detected in the 2 years, 21 corresponding QTLs were found for breaking strengths, lengths, and diameters of the top three internodes (Fig. 2). On chromosome 11 (marker C535–G257), there was the only chromosomal region (bsuc11) associated with a simultaneous increase in the breaking strengths of the top three internodes. QTLs for breaking strength of specific internodes were detected on chromosomes 2 and 9. Two chromosomal regions on chromosomes 10 and 12 improved breaking strengths of internode II and III, and of internode I and II, respectively. A QTL for elongation of internode I was detected on chromosome 10, and three QTLs for elongation of internode II were detected on chromosomes 1, 2, and 12. A chromosomal region associated with simultaneous shortening of internodes I and III was found on chromosome 3. Three QTLs on chromosomes 7, 9, and 11 were related to an increase in the diameter of internode II.
Positions of QTLs for breaking strengths, lengths, diameters of top three internodes on the rice genetic map. Data indicate corresponding QTLs between 2010 and 2011. RFLP markers were defined by Ebitani et al. (2005)
Effects of bsuc11 on lodging resistance characteristics
In 2011 and 2012, this study analyzed three CSSLs containing bsuc11; CSSL233, 234, and 235, and compared the breaking strengths of their top three internodes with those of ‘Koshihikari’ after heading (Fig. 3). Internode I in ‘Koshihikari’ showed an increase in breaking strength from heading to 4 weeks after heading (WAH) in 2011, and the breaking strengths of internode II and III increased until 2 WAH. After 6 WAH, the top three internodes of ‘Koshihikari’ showed a decrease in breaking strength. CSSLs with bsuc11 showed increased breaking strengths of internode I at 6 WAH; their breaking strengths were 1.61- to 1.78- times higher than that of internode I of ‘Koshihikari’ at corresponding time points (P < 0.01). In CSSLs with bsuc11, internode II showed significantly higher breaking strengths at 4 and 6 WAH than those of internode II of ‘Koshihikari’ at corresponding time points (P < 0.05, 1.19–1.46 times higher at 4 WAH, and 1.58–1.67 times higher at 6 WAH). For internode III, CSSLs with bsuc11 showed higher breaking strengths at heading and 6 WAH, compared with those of ‘Koshihikari’ at corresponding time points (P < 0.05, 1.39–1.49 times higher at heading, 1.49–1.65 times higher at 6 WAH). In 2012, ‘Koshihikari’ showed increased breaking strength of only internode I until 2 WAH, and then the breaking strength of the top three internodes decreased after 2 WAH. At heading and 6 WAH, CSSLs with bsuc11 showed 1.42–1.69 times higher and 1.21–1.44 times higher breaking strength of internode I, compared with those of ‘Koshihikari’ at corresponding time points. The breaking strength of internode II gradually decreased after heading in CSSLs with bsuc11, but these lines showed higher breaking strengths than those of ‘Koshihikari’ from 4 WAH to 6 WAH (1.20–1.26 times higher and 1.22–1.46 times higher, respectively). Internode III showed the same breaking strength in CSSLs with bsuc11 and ‘Koshihikari’ from heading until 4 WAH, but the internode breaking strengths in these CSSLs were 1.10–1.31 times higher than that of internode III of ‘Koshihikari’ at 6 WAH.
Changes in breaking strengths of top three internodes in ‘Koshihikari’ (black line) and CSSLs with bsuc11 (gray lines) after heading. Data are means of six plants in each line. Vertical bars indicate standard errors. Double asterisk and single asterisk indicate significant differences at 0.01 and 0.05 probability levels, respectively, between ‘Koshihikari’ and three CSSLs with bsuc11
The pushing resistance of the lower part (PRL) in ‘Koshihikari’ was 7.15 ± 0.57 kPa cm−2, and the PRL per one tiller (PRL/TN) was 0.56 ± 0.06 kPa cm−2 in 2011 (Table 5). Two CSSLs (CSSL234 and 235) showed significant decreases in PRL compared with that of ‘Koshihikari’ (P < 0.05), while the values for PRL/TN were the same in ‘Koshihikari’ and CSSLs with bsuc11. For ‘Koshihikari’ in 2011, the values of PRL and PRL/TN were 6.10 ± 0.31 and 0.53 ± 0.03 kPa cm−2, respectively. Only CSSL233 showed a significantly lower PRL than that of ‘Koshihikari’ (P < 0.05), but three CSSLs showed similar PRL/TN values to that of ‘Koshihikari’.
Discussion
This study identified one QTL, bsuc11, for breaking strengths of the top three internodes at the fully ripe stage over the 2 years, which did not relate to the internodes length (Fig. 2). The donor parent ‘Kasalath’ had greater diameters and breaking strengths of the top three internodes, but these internodes were long (Table 2). These properties in ‘Kasalath’ were inherited by the CSSLs in the genetic background of ‘Koshihikari’, and the CSSLs with maximum breaking strengths of top three internodes showed greater strengths than those of ‘Kasalath’. Thus, ‘Kasalath’ would be a valuable genetic resource for physical strength of the upper culms, at least in the Koshihikari background. From the results of QTL analysis, ‘Kasalath’ had QTLs to strengthen specific internodes and to simultaneously improve the physical strengths of plural internodes or the top three internodes (Tables 3, 4). To improve lodging resistance, it is important that culm strength is genetically controlled by two types of QTL. The most useful QTLs for breeding will be those that improve the physical strengths of plural internodes. bsuc11 physically strengthened the top three internodes, and did not overlap with QTLs for internode elongation (Fig. 2). Therefore, bsuc11 would efficiently improve resistance to bending in upper culms and could be a useful genetic target to reduce bending-type lodging.
Culm diameter is one of the traits that contributes to physical strength, and thus to lodging resistance (Atkins 1938; Zuber et al. 1999; Kashiwagi et al. 2008). In this study, internode diameter was correlated with breaking strength (Table 2), and QTLs for diameter partly overlapped with those for breaking strength of each internode (Tables 3, 4). These results indicate that some QTLs for internode diameter would contribute to significant correlations between diameters and breaking strengths of internodes, but all QTLs to increase in culm diameter do not necessarily improve lodging resistance. In a previous study, a QTL for stem diameter in ‘Kasalath’, sdm8, increased the culm wall thickness and physical strength of internodes in ‘Nipponbare’ (Kashiwagi et al. 2008). In this study, bsuc11 overlapped with a QTL to increase the diameter of internode II in the QTL analyses in both years (Fig. 2). Therefore, the improvement in the breaking strengths of the top three internodes by bsuc11 might be partly related to morphological changes in culm diameter and culm wall thickness.
In terms of properties associated with higher lodging resistance, bsuc11 prevented the decrease in the breaking strengths of top three internodes after grain filling, rather than increasing the strengths of internodes from heading (Fig. 3). Takaya and Miyasaka (1983) reported that breaking strengths of culms decreased after heading. The top three internodes of ‘Koshihikari’ showed decreases in breaking strength after 2 WAH. However, lines with bsuc11 did not show this decrease in internode strength after 2 WAH, and therefore showed greater physical strength than that of ‘Koshihikari’ at the fully ripe stage (Fig. 3). These results indicated that the decrease in culm strength after heading contributes to bending-type lodging; thus, preventing this decrease in strength would be a good target to improve lodging resistance. This study evaluated the effects of bsuc11 on the physical strength of the lower part of the plant. This study found that bsuc11 increased the physical strength of the upper culms but did not improve the pushing resistance of the lower part (Table 5). This result implies that the physical strengths of upper and lower parts of rice plants are controlled by different genetic factors, and the improvement of both strengths could develop cultivars with greater lodging resistance.
A previous study on the genetic factors associated with lodging resistance identified some QTLs and genes in rice. Ookawa et al. (2010) revealed that SCM2 on chromosome 6, which was identical to ABERRANT PANICLE ORGANIZATION (APO1), enhanced culm strength at 20 days after heading and increased the diameters of the minor and major axes of all internodes. From this result, SCM2 could be expected to increase the maximum strength of culms after heading. This effect differs from the improvement in culm strength associated with bsuc11, because the CSSLs with bsuc11 had similar internode breaking strengths to those of ‘Koshihikari’, when ‘Koshihikari’ showed maximum culm strength (Fig. 3). Therefore, there are two approaches to improve culm strength; to increase the maximum strength of the culm, or to prevent the decrease in culm strength after heading. The QTL for lodging resistance in a typhoon on chromosome 5, lrt5, increased young’s modulus of internode I and II, but the stem diameter was the same as that of the control (Ishimaru et al. 2008). This QTL physically strengthened the upper culms but did not increase the PRL, an indicator of the physical strength of the lower parts. This study found that ‘Koshihikari’ and the CSSLs with bsuc11 had the same PRL at the fully ripe stage (Table 5), suggesting that bsuc11 would be similar to lrt5 in terms of its effect on lodging resistance. lrt5 delayed senescence in the upper leaves and resulted in high starch content in the upper culms. Non-structural carbohydrate (NSC) re-accumulation is an indicator of breaking-type lodging resistance in rice (Yagi 1983). Other components of culms, e.g., cellulose, lignin, and silicon, also contribute to the physical strength of the plant body (Kokubo et al. 1991; Jones et al. 2001; Li et al. 2003; Idris et al. 1975). Because bsuc11 did not affect the diameters of all internodes, the greater culm strength associated with bsuc11 might result from differences in culm components.
This study showed that bsuc11 prevented the decrease in strengths of the upper culms after heading. The improved culm strength includes the thick culm diameter or culm wall and the changes in culm components. Genetic information about culm strength will provide a new approach in breeding for lodging resistance. To clarify the function of bsuc11, further research is required to reveal the morphological characters and components of culms, and to identify the gene locus. These would be key findings to improving the lodging resistance of rice.
References
Atkins IM (1938) Relation of certain plant characters to strength of straw and lodging in winter wheat. J Agric Res 56:99–120
Berry PM, Spink J, Sterling M, Pickett AA (2003) Methods for rapidly measuring the lodging resistance of wheat cultivars. J Agron Crop Sci 189:390–401
Börner A, Korzun V, Malyshev S, Ivandic V, Graner A (1999) Molecular mapping of two dwarfing genes differing in their GA response on chromosome 2H of barley. Theor Appl Genet 99:670–675
Ebitani T, Takeuchi Y, Nonoue Y, Yamamoto T, Takeuchi K, Yano M (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segment of indica rice cultivar “Kasalath” in a genetic background of japonica elite cultivar “Koshihikari”. Breed Sci 55:65–73
Fouéré A, Pellerin S, Duparque A (1995) A portable electronic device for evaluating root lodging resistance in maize. Agron J 87:1020–1024
Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15:2900–2910
Idris MD, Hossain MM, Choudhury FA (1975) The effect of silicon on lodging of rice in presence of added nitrogen. Plant Soil 43:691–695
Ishimaru K, Togawa E, Ookawa T, Kashiwagi T, Madoka Y, Hirotsu N (2008) New target for rice lodging resistance and its effect in a typhoon. Planta 227:601–609
Ivandic V, Malyshev S, Korzun V, Graner A, Börner A (1999) Comparative mapping of a gibberellic acid-insensitive dwarfing gene (Dwf2) on chromosome 4HS in barley. Theor Appl Genet 98:728–731
Jones L, Ennos AR, Turner SR (2001) Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J 26:205–216
Kashiwagi T, Ishimaru K (2004) Identification and functional analysis of a locus for improvement of lodging resistance in rice. Plant Physiol 134:676–683
Kashiwagi T, Madoka Y, Hirotsu N, Ishimaru K (2006) Locus prl5 improves lodging resistance of rice by delaying senescence and increasing carbohydrate reaccumulation. Plant Physiol Biochem 44:152–157
Kashiwagi T, Hirotsu N, Madoka Y, Ookawa T, Ishimaru K (2007) Improvement of resistance to bending-type lodging in rice. Jpn J Crop Sci 76:1–9
Kashiwagi T, Togawa E, Hirotsu N, Ishimaru K (2008) Improvement of lodging resistance with QTLs for stem diameter in rice (Oryza sativa L.). Theor Appl Genet 117:749–757
Keller M, Karutz Ch, Schmid JE, Stamp P, Winzeler M, Keller B, Messmer MM (1999) Quantitative trait loci for lodging resistance in a segregating wheat × spelt population. Theor Appl Genet 98:1171–1182
Khush GS (1999) Green revolution: preparing for the 21st century. Genome 42:646–655
Kokubo A, Kuraishi S, Sakurai N (1989) Culm strength of barley: correlation among maximum bending stress, cell wall dimensions, and cellulose content. Plant Physiol 91:876–882
Kokubo A, Sakurai N, Kuraishi S, Takeda K (1991) Culm brittleness of barley (Hordeum vulgare L.) mutants is caused by smaller number of cellulose molecules in cell wall. Plant Physiol 97:509–514
Kono M (1995) Physiological aspects of lodging. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H (eds) Science of the rice plant, vol. 2: physiology, vol 2. Food and Agriculture Policy Research Center, Tokyo, pp 971–982
Lee SH, Bailey MA, Mian MAR, Shipe ER, Ashley DA, Parrott WA, Hussey RS, Boerma HR (1996) Identification of quantitative loci for plant height, lodging, and maturity in a soybean population segregating for growth habit. Theor Appl Genet 92:516–523
Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X, Chen M, Li J (2003) BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15:2020–2031
Madoka Y, Kashiwagi T, Hirotsu N, Ishimaru K (2008) Indian rice “Kasalath” contains genes that improve traits of Japanese premium rice “Koshihikari”. Theor Appl Genet 116:603–612
Mori M, Nomura T, Ooka H, Ishizaka M, Yokota T, Sugimoto K, Okabe K, Kajiwara H, Satoh K, Yamamoto K, Hirochika H, Kikuchi S (2002) Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol 130:1152–1161
Mulder EG (1954) Effect of mineral nutrition on lodging of cereals. Plant Soil 5:246–306
Ookawa T, Hobo T, Yano M, Murata K, Ando T, Miura H, Asano K, Ochiai Y, Ikeda M, Nishitani R, Ebitani T, Ozaki H, Angeles ER, Hirasawa T, Matsuoka M (2010) New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat Commun 1:132
Peng S, Cassman K, Virmani SS, Sheehy J, Khush GS (1999a) Yield potential trends of tropical rice since the release of IR8 and the challenge of increasing rice yield potential. Crop Sci 39:1552–1559
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999b) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Peng S, Khush GS, Virk P, Tang Q, Zou Y (2008) Progress in ideotype breeding to increase rice yield potential. Field Crop Res 108:32–38
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) A mutant gibberellin-synthesis gene in rice. Nature 416:701–702
Sato K (1957) Studies on the starch contained in the tissues of rice plant. (IV) Starch content in the culm related to lodging. Jpn J Crop Sci 26:19
Spaner D, Rossnagel BG, Legge WG, Scoles GJ, Eckstein PE, Penner GA, Tinker NA, Briggs KG, Falk DE, Afele JC, Hayes PM, Mather DE (1999) Verification of a quantitative trait locus affecting agronomic traits in two-row barley. Crop Sci 39:248–252
Takaya T, Miyasaka A (1983) Prevention of lodging of rice plants under direct sowing culture on well-drained paddy field. II Transition of the characters related to lodging resistance after the heading. Jpn J Crop Sci 52:7–14
Tar’an B, Warkentin T, Somers DJ, Miranda D, Vandenberg A, Blade S, Woods S, Bing D, Xue A, DeKoeyer D, Penner G (2003) Quantitative trait loci for lodging resistance, plant height and partial resistance to mycosphaerella blight in field pea (Pisum sativum L.). Theor Appl Genet 107:1482–1491
Tripathi SC, Sayre KD, Kaul JN, Narang RS (2003) Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: effects of genotypes, N levels and ethephon. Field Crops Res 84:271–290
Yagi T (1983) Studies on breeding for culm stiffness in rice. 1. Varietal differences in culm stiffness and its related traits. Jpn J Breed 33:411–422
Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12:1591–1605
Yang J, Zhang J, Wang Z, Zhu Q (2001) Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J Exp Bot 52:2169–2179
Zuber U, Winzeler H, Messmer MM, Keller M, Keller B, Schmid JE, Stamp P (1999) Morphological traits associated with lodging resistance of spring wheat (Triticum aestivum L.). J Agron Crop Sci 182:17–24
Acknowledgments
This work was supported by Grant-in-Aid for Young Scientists (B, 23780014). I thank Ms. M. Oota and S. Yashiro, Utsunomiya University, for their help in QTL analyses, and Mr. J. Munakata and K. Satou, Utsunomiya University, for help in measurement of lodging resistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashiwagi, T. Identification of quantitative trait loci for resistance to bending-type lodging in rice (Oryza sativa L.). Euphytica 198, 353–367 (2014). https://doi.org/10.1007/s10681-014-1111-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1111-7