Abstract
Monocrotophos (MCP) is a highly toxic and broad-spectrum pesticide extensively used for agricultural and household purposes. The present study was aimed to evaluate the genotoxicity and alterations in the biochemical and physiological conditions induced by monocrotophos in a non-target organism, an estuarine bivalve, Donax incarnatus. The bivalves were exposed to three sub-lethal concentrations (6.8, 13.7, and 27.45 ppm) of MCP for a period of 72 h. DNA damage was assessed using the comet assay. Oxidative stress was analyzed using catalase, glutathione peroxidase, and superoxide dismutase. Neurotoxicity was evaluated using the acetylcholinesterase assay (AChE) and the physiological condition was assessed using the condition index (CI). A significant concentration-dependent increase of DNA damage was observed as well as a decline in the activities of the antioxidant enzymes. However, a decrease in DNA damage was observed with advancing time. A significant decrease of AChE activity and CI was observed in the bivalves exposed to MCP. Positive correlations were also observed between DNA damage and the antioxidant enzymes whereas negative correlations were observed between AChE and the antioxidant enzymes indicating MCP toxicity mediated by oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are chemical compounds used for the prevention and eradication of a variety of pests persistent in agriculture, forestry and households. In recent times, the use of pesticides on a larger scale has caused severe environmental implications as well as toxic effects in non-target organisms such as humans, livestock, beneficial insects and microorganisms and even aquatic organisms. Extensive agricultural activities and indiscriminate use of pesticides have led to contamination of the aquatic environment by direct application of the pesticides as well as surface runoff and wind-borne drifts that carry these toxic substances to various water sources (Mundhe et al., 2016). As a result, there has been a gradual increase in the concentration of pesticides and their metabolites in water columns and benthic sediments thus causing severe contamination of the aquatic environment (Patetsini et al., 2013). Chronic or acute exposure to pesticides may cause deleterious problems for non-target organisms, leading to altered or disrupted biochemical processes. There is also often a high expenditure of energy for the removal of the toxicants and repair of damaged processes (Calow, 1989).
Organophosphate (OP) compounds that are present in a majority of pesticides act primarily by inhibiting the action of acetylcholinesterase resulting in an excessive buildup of acetylcholine at different nerves and receptors in the body, leading to their overstimulation and causing a wide range of disruptions of metabolic processes. The overstimulation of the nervous system caused by OPs is followed by an intense generation of reactive oxygen species (ROS) thereby causing oxidative damage and can also inhibit antioxidant defenses (Banerjee et al., 1999; Lukaszewicz-Hussain, 2010). ROS are highly reactive molecules, which indiscriminately interact with essential macromolecules such as DNA, protein, and lipids causing disturbances in physiological processes (Cnubben et al., 2001). These compounds exhibit alkylating properties which are also responsible for DNA damage (Cui et al., 2006). Numerous in vitro and in vivo studies are available that report the genotoxicity of OP pesticides in various organisms like bivalves, fishes, birds, and even in human lymphocytes (Bhunya & Jena, 1993; Mahboob et al., 2002; Pavlica et al., 2000; Revankar & Shyama, 2009; Saleha Banu et al., 2001; Sharma et al., 2007).
Monocrotophos (MCP) is a broad-spectrum systemic and contact OP insecticide which is known to be neurotoxic by inhibiting cholinesterase. It is used on a large scale in many parts of the world, particularly in developing countries due to its high toxicity to phytophagous insects and mites and can also be procured at a low cost. However, as with other pesticides, MCP can also affect non-target organisms such as birds, mammals, and even fishes. It can enter the aquatic environment by wastewater discharge and runoffs from agricultural land and severely affect the native fauna. It is a Category 1 chemical (highly toxic) (USEPA, 1985) and is also listed as a marine pollutant by FAO/UNEP (1997). A few studies are available on the genotoxicity and biochemical alterations caused by MCP in non-target organisms such as fishes (Saleha Banu et al., 2001; Agrahari et al., 2007; Ali & Kumar, 2008; D’Costa et al., 2018) and even fewer reports of the same in bivalves (Mundhe et al., 2016; Revankar & Shyama, 2009).
Bivalves are filter feeders, thus able to accumulate toxins in their tissue, making them good bioindicators of pollution. Donax incarnatus (Gmelin, 1791) being one of the most common bivalves found along the coast of Goa, a state in the South of India and is regularly consumed by the local population as seafood. Although MCP is frequently used as an agricultural pesticide in Goa, no reports are available on the toxicity of this pesticide in D. incarnatus. Hence, to verify the safety of the consumption of this bivalve, various biomarkers of genotoxicity as well as oxidative stress, neurotoxicity, and physiological condition are evaluated in this estuarine bivalve D. incarnatus exposed to monocrotophos.
Materials and methods
Quality assurance and quality control
The procedures of preparation and handling of samples were carried out conforming to the guidelines by APHA, AWWA, WEF (2017). The chemicals used in experimentation were of analytical grade, procured from Himedia (Himedia, India).
Experimental organisms
The clams (Donax incarnatus) were collected from Galgibag, a pristine beach in Goa, India, and transported to the laboratory. The bivalves with an average weight of 1.27 ± 0.3 g and an average length of 1.3 ± 0.5 cm were used for the study. They were transferred to 5L aquaria and acclimatized in seawater from Galgibag for 15 days prior to exposure to MCP. Water in the aquaria was changed daily to prevent fecal contamination and maintained with the following conditions: 25 °C, pH 7.5, salinity 27 ppt, and dissolved oxygen 7.8 mg/L.
Monocrotophos
Anucron-monocrotophos [dimethyl-(E)-1-methyl-2-(methyl carbamoyl) vinyl phosphate] (36%) of commercial grade was procured from Anu Products Ltd., Delhi, India (Batch number: FAP8361). MCP test solutions were prepared by dissolving it directly in water.
Estimation of LC50
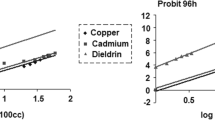
To determine the sub-lethal concentrations to be used for the study, an acute toxicity assay to estimate the 96 h LC50 value for monocrotophos was carried out using standard methods (APHA, AWWA, WEF, 2017). A range-finding test was carried out prior to the definitive test. To estimate the LC50 for definitive testing, the bivalves were divided into 12 groups, comprising 10 specimens each (tests for each group were conducted in triplicates) and exposed to various concentrations of MCP in a semi-static system to estimate mortality for 96 h as follows:
Group | Dose (in ppm) |
|---|---|
1 | 0 (control) |
2 | 10 |
3 | 15 |
4 | 20 |
5 | 25 |
6 | 30 |
7 | 35 |
8 | 40 |
9 | 45 |
10 | 50 |
11 | 55 |
12 | 60 |
During the experiment, the water was changed once daily with a fresh dose of pesticide to the aquarium. Bivalves were considered dead when touching the opened valves did not stimulate their closure and were immediately removed from the tanks. The LC50 values (with 95% confidence limits) were calculated using Probit Analysis Statistical Method (Finney, 1971). Differences in the results obtained were considered to be statistically significant at a p value of < 0.05. The regression equation (Y = a + bX, where Y = probits of mortality; X = log concentrations) was calculated by maximum likelihood using IBM SPSS v23 and the 96 h LC50 was derived from the best-fit line obtained which was found to be 55.49 ppm.
Treatment schedule
To prepare the bivalves for dose response studies, they were first distributed into groups of 10 individuals. Based on the 96 h LC50 value, three sub-lethal concentrations 6.8, 13.7, and 27.45 ppm of monocrotophos (representing 1/8, 1/4, and 1/2 of the LC50 value, respectively) were selected to which the bivalve groups were exposed once in triplicates for a period of 72 h so as to observe any possible short-term effects. A time-dependent response was also studied at three different intervals of exposure to the pesticide, viz., at 24, 48, and 72 h. Another group of clams was exposed to CdCl2 (0.75, 1.5 and 3.0 μg/L), which is a well-known toxicant (Chen et al., 2001; Juhel et al., 2007) so as to validate the biomarkers in the present study.
Comet assay
The comet assay was carried out using the protocol devised by Lee & Steinert (2003). The gills of the clams were extracted and gently nipped in phosphate buffered saline (pH 7.2) to dissociate the cells. This was filtered through a muslin cloth to obtain a clear suspension, devoid of debris. The cell suspension was mixed with low melting agarose (0.5%) and carefully layered over a layer of normal melting agarose (1%) on frosted microscopic slides. After solidification, a third layer was created using 0.5% low melting agarose, and allowed to solidify. The slides were immersed in a cold lysis buffer (pH 10, 4 °C) for 1 h, to allow lysis of cells thereby releasing DNA. The DNA was then allowed to unwind by placing the slides in an alkaline (pH > 10), unwinding buffer for 20 min, following which electrophoresis was performed under alkaline conditions (pH 10) for 20 min and the slides were then placed in a neutralization buffer (pH 7.5) for 5 min. The cells were then stained with ethidium bromide, observed under × 200 magnification of a fluorescence microscope (Olympus BX53, Singapore) and their images were captured. The “comets” were analyzed with the help a software, CASP (Końca et al., 2003), and the percentage of tail DNA was recorded.
Preparation of homogenate for enzymatic tests
Whole soft tissues of clams were gently homogenized in phosphate buffer (pH 7.2) using a Potter–Elvehjem homogenizer and centrifuged at 10,000×g for 20 min at 4 °C. The supernatant was collected and used for the subsequent enzymatic tests.
Catalase assay
The activity of catalase (CAT) was estimated following the protocol by Sinha (1972). The assay mixture consisted of H2O2, phosphate buffer (0.01 M, pH 7.0), and deionized water, and the supernatant was added to initiate the reaction. The reaction was arrested by adding dichromate / acetic acid reagent to the mixture at intervals of 15, 30, 45, and 60 s. The mixture was subsequently heated in a boiling water bath until a stable green color developed which was read at 610 nm. The activity was expressed as μmoles of H2O2 decomposed /min/mg protein.
Glutathione peroxidase assay
Glutathione peroxidase (GPx) activity was determined as per Paglia and Valentine (1967). The reaction mixture consisted of EDTA (2 mM), sodium azide (0.1 mM), reduced glutathione (4 mM), H2O2, sodium phosphate buffer (0.4 M, pH 7.0), distilled water, and supernatant. The reaction was terminated with trichloroacetic acid (10%), centrifuged, and the supernatant was added to phosphate buffer and DTNB reagent. The optical density was measured at 412 nm and the activity of GPx was expressed in terms of µg of glutathione utilized/min/mg protein.
Superoxide dismutase assay
The activity of superoxide dismutase (SOD) activity was assessed by the method of Das et al. (2000). Briefly, phosphate buffer (50 mM, pH 7.4), L-methionine (20 mM), Triton X-100, hydroxylamine hydrochloride (10 mM), and EDTA (50 mM) was added to the sample supernatant in microfuge tubes and incubated at 30 °C for 5 min. Riboflavin (50 M) was then added to the mixture, and the tubes were exposed to 200-W fluorescent lamps for a period of 10 min following which Greiss reagent was then added. The optical density of the mixture was measured at 543 nm. The activity was calculated as the amount of SOD which could inhibit 50% of nitrite formation.
Acetylcholinesterase assay
The activity of acetylcholinesterase (AChE) was measured using the protocol outlined by Ellman et al. (1961) with modifications by Galloway et al. (2002). The sample supernatant was incubated with DTNB (270 μM, pH 7.4) at 25 °C for 5 min. The activity of the enzyme was initiated by the addition of acetylthiocholine iodide (3 mM), and the optical density was measured at 412 nm. The activity of AChE was expressed as nmol thiocholine/min/mg protein.
Protein estimation
The protein content of the supernatant was measured using the standard protocol developed by Lowry et al. (1951).
Condition index
The condition index (CI) of D. incarnatus was following the protocol of Filgueira et al. (2013). Briefly, soft tissue was carefully separated from the shells and both were dried in an oven at 65 °C. CI was calculated as the ratio of dry soft tissue weight and dry shell weight.
Statistical analysis
Data were analyzed using IBM SPSS v23. Data were tested for normality and homogeneity, and parametric or non-parametric tests were used wherever applicable. ANOVA or Kruskall–Wallis test was applied to test the effect (dose and time) of monocrotophos concentration on the biomarkers. Dunnet’s test was performed post hoc to compare the differences with the control within the same treatment group. Correlations (Pearson’s) were tested for all the parameters to check for significant associations. The significance level was considered at p < 0.05.
Results
The biomarkers were validated using CdCl2 as a known toxicant. All the concentrations of CdCl2 induced significant toxic effects such as increased % tail DNA, CAT, GPx, and SOD, as well as decreased AChE and CI in the clams (Supplementary data Table S1).
The gill cells of D. incarnatus showed a significant concentration-dependent increase in the percentage tail DNA at all the time intervals (Fig. 1). The highest concentration of monocrotophos induced the highest DNA damage (% tail DNA) at 24 h (40.16 ± 3.08, p < 0.001). However, the percentage tail DNA was found to decrease significantly as the time of exposure advanced for all the concentrations.
A significant increase in the activity of catalase was observed in the soft tissues of the bivalves at all the time intervals of monocrotophos exposure (Fig. 2). Catalase activity in the lowest concentration group was non-significant compared to the control at 24 h of exposure. Catalase activity was found to be the highest at 72-h exposure in the highest concentration group (49.47 ± 5.52, p < 0.001).
The activity of glutathione peroxidase was observed to be significantly increased in the soft tissues of the bivalves exposed to the medium and high concentrations of monocrotophos (Fig. 3). However, the activity of GPx was found to be non-significant in the bivalves exposed to the lowest concentration of monocrotophos at all the time intervals. Significant variation was observed across all the concentrations and time intervals (F = 12.83, p < 0.001).
Superoxide dismutase activity was also found to be significantly increased in the tissues of the bivalves in all the pesticide-exposed groups (Fig. 4) (F = 26,279.92, p < 0.001). Further, SOD activity was found to be elevated in a time-dependent manner for each pesticide concentration (F = 153.19, p < 0.001).
The analysis of AChE activity in the soft tissues of bivalves exposed to monocrotophos showed a significant decrease in its activity (F = 1452.20, p < 0.001). A constant decline of activity was observed as the time progressed for all the concentrations (F = 114.58, p < 0.001). Further, the activity of AChE remained significantly low at all time intervals in all the doses (Fig. 5).
A significant dose-dependent and time-dependent decrease in the condition index of the bivalves as compared to the control (Fig. 6) was observed. As the dose of the pesticide and time of exposure increased, the ratio of dry tissue weight to dry shell weight decreased (concentration F = 309.21, p < 0.001, and time F = 9.33, p < 0.001). Condition index was found to be the lowest at the highest concentration of monocrotophos at 72 h of exposure.
The two-way ANOVA carried out with time and concentration as the independent variables and the tested parameters as dependent variables are represented in Table 1. Significant variance was observed within the dependent variables with increasing concentration or advancing time. The variance within the dependent variables by the interaction of both concentration and time was also found to be significant except for CAT activity which was found to be non-significant.
Based on the correlation matrix (Table 2), a significant positive correlation was observed between % tail DNA and SOD (p < 0.01) as well as between % tail DNA and CAT (p < 0.05) and GPx (p < 0.05). A significant negative correlation was observed between % tail DNA and AChE activity (p < 0.05). Significant correlations were also observed between CAT and SOD (p < 0.05), CAT and GPx (p < 0.05), as well as between SOD and GPx (p < 0.001).
Discussion
The excessive and unregulated use of OP compounds as pesticides has often led to severe toxic effects on non-target organisms which include alterations in their DNA, biochemistry, and physiology.
DNA damage
Results of the present study indicate that monocrotophos induced significant DNA damage in the gill cells of the bivalves. This finding is on par with the studies of Mundhe et al. (2016) in which they observed significant DNA damage in mussels (Lamellidens marginalis) exposed to monocrotophos for 7 days. The present results also find similarity with that of Revankar and Shyama (2009) in which they observed a high level of DNA damage in the clam Meretrix ovum exposed to monocrotophos. Various genetic studies are available that help provide a better understanding of the mechanisms that cause this DNA damage. Saleha Banu et al. (2001) reported that the phosphate moiety of monocrotophos might cause phosphorylation of DNA leading to strand breaks. OPs can cause alkylation of DNA bases thereby altering DNA (Bhinder & Chaudhry, 2013). Additionally, the phosphorous group in the OP may be a substrate for nucleophilic attack which could cause DNA phosphorylation and subsequent damage (Das et al., 2007; Rahman et al., 2002). The results of the two-way ANOVA indicate that both concentration and time influence the formation of DNA strand breaks. Further, a significant decrease in the % tail DNA was observed as the time of exposure increased.
Antioxidant enzymes
Antioxidant enzymes convert reactive oxygen species into stable non-toxic molecules, thus rendering them harmless and therefore constitute the most important defense mechanism against oxidative stress-induced cell damage. For instance, the dismutation of the highly reactive superoxide radical (O2-) into hydrogen peroxide (H2O2) and oxygen (O2) is catalyzed by SOD. The H2O2 is then further converted into water (H2O) and oxygen (O2) by CAT. Glutathione peroxidase (GPx) also catalyzes the reduction of H2O2 to water by glutathione. The activity of the SOD increased significantly in response to the dose of monocrotophos and time of exposure (as well as the interaction between both) to the pesticide. This could be attributed to the increased oxidative stress in bivalves induced by monocrotophos. Our present observations are in agreement with the findings of Mundhe and Pandit (2014) in which they observed a significant increase of SOD activity in bivalves exposed to monocrotophos. In another similar study, SOD was found to increase significantly in the clam Corbicula fluminea exposed to organophosphate flame retardants in sediment (Li et al., 2018). The significant increase of CAT activity observed in the present study in a dose- and time-dependent manner is on par with the findings of Bianco et al. (2013) who observed a significant increase of CAT activity in the gastropod Chilina gibbosa exposed to the organophosphate pesticide azinphos-methyl. These findings are also similar to that of Mundhe and Pandit (2014) who reported a time dependent increase of CAT activity in bivalves exposed to monocrotophos. Our results also find similarities with that of (Al-Fanharawi et al., 2019) wherein they reported significant increases of both SOD and CAT in the soft tissues of mussel Unio tigridis exposed to 22 mg/L and 35 mg/L of the organophosphate pesticide chlorpyrifos for a period of 21 days. The activity of GPx was also found to increase significantly with increased dose and time as a result of oxidative stress induced by monocrotophos. This is in accordance with the studies of Yu and Ai-li (2011) in which they observed a significant increase in the activity of GPx in the soft tissues of mussels (Mytilus edulis) exposed to various concentrations of an organophosphate pesticide methamidophos. Thus, it is evident by the increase of the activities of antioxidant enzymes that monocrotophos induces oxidative stress in D. incarnatus.
Additionally, although the activity of antioxidant enzymes increased over a period of 72 h, there was a concurrent decrease of DNA damage. This may be due to the DNA repair systems in the gill cells and the increased activity of first line of defense of the antioxidant enzymes (CAT, GPx, and SOD) to combat the ROS generated by the pesticide and thereby decreasing the chances of ROS interacting with DNA. Thus, the increase in antioxidant enzymes can be correlated to the decrease in DNA damage over a period of 3 days.
Acetylcholinesterase
The bivalves exposed to monocrotophos in the present study appeared to be lethargic and showed impaired movement. This may be due to inhibition of acetylcholinesterase induced by monocrotophos. Acetylcholinesterase is found in neuromuscular junctions, and is responsible for the termination of synaptic transmissions, by the breakdown of the neurotransmitter acetylcholine into acetate and choline. A significant decrease in AChE activity was observed in the MCP exposed bivalves both in a time- and concentration-dependent manner. Further, the interaction of both concentration and time influenced the inhibition of AChE which was inferred from the two-way ANOVA. A similar observation was made by (Cooper & Bidwell, 2006) who witnessed a considerable decrease of cholinesterase activity in the Asian clam (Corbicula fluminea) exposed to an organophosphate pesticide chlorpyrifos. The results of the present study also find similarity with the reports of Moncaleano-Niño et al. (2018) in which they observed a decline in cholinesterase activity in oysters (Saccostrea sp.) exposed to an organophosphate pesticide chlorpyrifos. It can therefore be concluded that organophosphate pesticides are potent inhibitors of acetylcholinesterase activity and can potentially exert neurotoxic effects in exposed non-target organisms such as bivalves.
Condition index
Condition index is an important parameter to measure the overall health of an organism. The use of dry tissue weight measurements eliminates the weight fluctuations caused by changes in water content of the whole tissue. In the present study, there was a significant decrease in the condition index values due to physiological stress in the bivalves exposed to monocrotophos. Low condition index values of the bivalves may be due to an increase in energy expenditure to avoid/detoxify the pesticide (Lucas & Beninger, 1985). This is in agreement with the studies of Kim et al. (2004) wherein they reported a decrease in the shell length and body weight of the Manilla clam (Ruditapes phillippinarum) exposed to an organophosphate pesticide chlorpyrifos. Another reason for decreased CI ratio could be due to a mechanism of survival adaption in which feeding is reduced and the bivalves’ shells close as a result of pollutant exposure.
Correlation analysis
In the Pearson’s correlation analysis (Table 2), DNA damage in the form of % tail DNA was found to be significantly positively correlated with the antioxidant enzymes CAT, SOD, and GPx. This correlation indicates that DNA damage in the bivalves occurred due to oxidative stress as a consequence of MCP exposure. The DNA damage observed may thus be due to the generation of ROS which can directly interact with macromolecules like DNA and induce genotoxicity by causing single and double stranded breaks (Mundhe et al., 2016). These free radicals may also affect DNA indirectly through lipid peroxidation and could cause DNA strand breaks. Therefore, oxidative stress may be caused due to the production of ROS that surpasses the initial rate of detoxification and repair by the organism’s antioxidant enzymes (Ojha et al., 2013; Yaduvanshi et al., 2010). A significant negative correlation was observed between AChE activity and the antioxidant enzymes which could indicate that the inhibition of AChE activity may be linked to oxidative stress. A significant negative correlation was observed between % Tail DNA and CI. The decrease of CI may be attributed to altered DNA function which in turn may cause abnormal protein synthesis and function which is vital for normal physiological processes of the organism.
Conclusion
Exposure of Donax incarnatus to monocrotophotos induced a substantial amount of damage within the organism. The organophosphate monocrotophos induced the generation of ROS in the bivalves which in turn have deleterious effects on various macromolecules including nucleic acids. Significant DNA damage and an increase in the activities of anti-oxidant enzymes like catalase, glutathione peroxidase, and superoxide dismutase were observed as a result of monocrotophos exposure, thus implying that the bivalves experienced substantial oxidative stress. The pesticide also caused significant inhibition of acetylcholinesterase and decreased condition index. Comet assay, biomarkers of oxidative stress, acetylcholinesterase activity, and condition index can be reliably used to assess the toxicity of monocrotophos in the environment. Donax incarnatus can be considered as a suitable bio-indicator for assessment of pesticide pollution in the estuarine environment using appropriate markers.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrahari, S., Pandey, K. C., & Gopal, K. (2007). Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pesticide Biochemistry and Physiology, 88(3), 268–272. https://doi.org/10.1016/j.pestbp.2007.01.001
Al-Fanharawi, A. A., Rabee, A. M., & Al-Mamoori, A. M. J. (2019). Multi-biomarker responses after exposure to organophosphates chlorpyrifos in the freshwater mussels Unio tigridis and snails Viviparous benglensis. Human and Ecological Risk Assessment: An International Journal, 25(5), 1137–1156. https://doi.org/10.1080/10807039.2018.1460800
Ali, D., & Kumar, S. (2008). Long-term genotoxic effect of monocrotophos in different tissues of freshwater fish Channa punctatus (Bloch) using alkaline single cell gel electrophoresis. The Science of the Total Environment, 405(1–3), 345–350. https://doi.org/10.1016/j.scitotenv.2008.05.037
AWWA, Apha, & WPCF . (2017). Standard methods for the examination of water and wastewater. (23rd ed.). American Publication of Health Association.
Banerjee, B. D., Seth, V., Bhattacharya, A., Pasha, S. T., & Chakraborty, A. K. (1999). Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicology Letters, 107(1–3), 33–47. https://doi.org/10.1016/s0378-4274(99)00029-6
Bhinder, P., & Chaudhry, A. (2013). Mutagenicity assessment of organophosphates using polymerase chain reaction-restriction fragment length polymorphism assay. Toxicology International, 20(3), 254–260. https://doi.org/10.4103/0971-6580.121678
Bhunya, S. P., & Jena, G. B. (1993). Studies on the genotoxicity of monocrotophos, an organophosphate insecticide, in the chick in vivo test system. Mutation Research/Environmental Mutagenesis and Related Subjects, 292(3), 231–239. https://doi.org/10.1016/0165-1161(93)90026-V
Bianco, K., Yusseppone, M. S., Otero, S., Luquet, C., Ríos de Molina, M. D. C., & Kristoff, G. (2013). Cholinesterases and neurotoxicity as highly sensitive biomarkers for an organophosphate insecticide in a freshwater gastropod (Chilina gibbosa) with low sensitivity carboxylesterases. Aquatic Toxicology (Amsterdam, Netherlands), 144–145, 26–35. https://doi.org/10.1016/j.aquatox.2013.09.025
Calow, P. (1989). Proximate and ultimate responses to stress in biological systems. Biological Journal of the Linnean Society, 37(1–2), 173–181. https://doi.org/10.1111/j.1095-8312.1989.tb02101.x
Chen, C. M., Yu, S. C., & Liu, M. C. (2001). Use of Japanese medaka (Oryzias latipes) and Tilapia (Oreochromis mossambicus) in toxicity tests on different industrial effluents in Taiwan. Archives of Environmental Contamination and Toxicology, 40(3), 363–370. https://doi.org/10.1007/s002440010184
Cnubben, N. H., Rietjens, I. M., Wortelboer, H., van Zanden, J., & van Bladeren, P. J. (2001). The interplay of glutathione-related processes in antioxidant defense. Environmental Toxicology and Pharmacology, 10(4), 141–152. https://doi.org/10.1016/s1382-6689(01)00077-1
Cooper, N. L., & Bidwell, J. R. (2006). Cholinesterase inhibition and impacts on behavior of the Asian clam, Corbicula fluminea, after exposure to an organophosphate insecticide. Aquatic Toxicology (Amsterdam, Netherlands), 76(3–4), 258–267. https://doi.org/10.1016/j.aquatox.2005.09.012
Cui, Y., Guo, J., Xu, B., & Chen, Z. (2006). Potential of chlorpyrifos and cypermethrin forming DNA adducts. Mutation Research, 604(1–2), 36–41. https://doi.org/10.1016/j.mrgentox.2005.12.003
D’Costa, A. H., Shyama, S. K., Praveen Kumar, M. K., & Fernandes, T. M. (2018). Induction of DNA damage in the peripheral blood of zebrafish (Danio rerio) by an agricultural organophosphate pesticide, monocrotophos. International Aquatic Research, 10(3), 243–251. https://doi.org/10.1007/s40071-018-0201-x
Das, K., Samanta, L., & Chainy, G. (2000). A Modified Spectrophotometric Assay of Superoxide Dismutase Using Nitrite Formation by Superoxide Radicals. Indian Journal of Biochemistry & Biophysics, 37(3), 201-204
Das, P. P., Shaik, A. P., & Jamil, K. (2007). Genotoxicity induced by pesticide mixtures: in-vitro studies on human peripheral blood lymphocytes. Toxicology and Industrial Health, 23(8), 449–458. https://doi.org/10.1177/0748233708089040
Ellman, G. L., Courtney, K. D., Andres, V. J., & Feather-Stone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88–95. https://doi.org/10.1016/0006-2952(61)90145-9
FAO/UNEP. (1997). Monocrotophos. PIC Decision Guidance Document.
Filgueira, R., Comeau, L. A., Landry, T., Grant, J., Guyondet, T., & Mallet, A. (2013). Bivalve condition index as an indicator of aquaculture intensity: A meta-analysis. Ecological Indicators, 25, 215–229. https://doi.org/10.1016/j.ecolind.2012.10.001
Finney, D. J. (1971). Probit Analysis, 3rd ed., Cambridge University Press, 32 E. 57th St., New York, Ny 10022, 1971. xv + 333 pp. 14.5 × 22 cm. Price $18.50. Journal of Pharmaceutical Sciences, 60(9), 1432. https://doi.org/10.1002/jps.2600600940
Galloway, T. S., Millward, N., Browne, M. A., & Depledge, M. H. (2002). Rapid assessment of organophosphorous/carbamate exposure in the bivalve mollusc Mytilus edulis using combined esterase activities as biomarkers. Aquatic Toxicology, 61(3), 169–180. https://doi.org/10.1016/S0166-445X(02)00051-6
Gmelin J.F. (1791). Caroli a Linnaei systema naturae per regna tria naturae. Ed. 13, G.E. Beer, Vermes Tome. Lipsiae 1(6), 3021-3910.
Juhel, G., O’Halloran, J., Culloty, S. C., & O’riordan, R. M., Davenport, J., O’Brien, N. M., James, K. F., Furey, A., & Allis, O. (2007). In vivo exposure to microcystins induces DNA damage in the haemocytes of the zebra mussel, Dreissena polymorpha, as measured with the comet assay. Environmental and Molecular Mutagenesis, 48(1), 22–29. https://doi.org/10.1002/em.20271
Kim, W.-S., Yoon, S.-J., & Yang, D.-B. (2004). Effects of chlorpyrifos on the endogenous rhythm of the Manila clam, Ruditapes philippinarum (Bivalvia: Veneridae). Marine Pollution Bulletin, 48(1–2), 182–187. https://doi.org/10.1016/j.marpolbul.2003.09.005
Końca, K., Lankoff, A., Banasik, A., Lisowska, H., Kuszewski, T., Góźdź, S., Koza, Z., & Wojcik, A. (2003). A cross-platform public domain PC image-analysis program for the comet assay. Mutation Research, 534(1–2), 15–20. https://doi.org/10.1016/s1383-5718(02)00251-6
Lee, R. F., & Steinert, S. (2003). Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutation Research, 544(1), 43–64. https://doi.org/10.1016/s1383-5742(03)00017-6
Li, D., Wang, P., Wang, C., Fan, X., Wang, X., & Hu, B. (2018). Combined toxicity of organophosphate flame retardants and cadmium to Corbicula fluminea in aquatic sediments. Environmental Pollution, 243, 645–653. https://doi.org/10.1016/j.envpol.2018.08.076
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193(1), 265–275
Lucas, A., & Beninger, P. G. (1985). The use of physiological condition indices in marine bivalve aquaculture. Aquaculture, 44(3), 187–200
Lukaszewicz-Hussain, A. (2010). Role of oxidative stress in organophosphate insecticide toxicity – short review. Pesticide Biochemistry and Physiology, 98(2), 145–150. https://doi.org/10.1016/j.pestbp.2010.07.006
Mahboob, M., Rahman, M. F., Danadevi, K., Banu, B. S., & Grover, P. (2002). Detection of DNA damage in mouse peripheral blood leukocytes by the comet assay after oral administration of monocrotophos. Drug and Chemical Toxicology, 25(1), 65–74. https://doi.org/10.1081/dct-100108472
Moncaleano-Niño, A. M., Luna-Acosta, A., Gómez-Cubillos, M. C., Villamil, L., & Ahrens, M. J. (2018). Cholinesterase activity in the cup oyster Saccostrea sp. exposed to chlorpyrifos, imidacloprid, cadmium and copper. Ecotoxicology and Environmental Safety, 151, 242–254. https://doi.org/10.1016/j.ecoenv.2017.12.057
Mundhe, A. Y., & Pandit, S. V. (2014). Assessment of toxicity of monocrotophos in freshwater bivalve, Lamellidens marginalis. Using Different Markers. Toxicology International, 21(1), 51–56. https://doi.org/10.4103/0971-6580.128793
Mundhe, A. Y., Bhilwade, H., & Pandit, S. V. (2016). Genotoxicity and oxidative stress as biomarkers in fresh water mussel, Lamellidens marginalis (Lam.) exposed to monocrotophos. Indian Journal of Experimental Biology, 54(12), 822–828.
Ojha, A., Yaduvanshi, S. K., Pant, S. C., Lomash, V., & Srivastava, N. (2013). Evaluation of DNA damage and cytotoxicity induced by three commonly used organophosphate pesticides individually and in mixture, in rat tissues. Environmental Toxicology, 28(10), 543–552. https://doi.org/10.1002/tox.20748
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine, 70(1), 158—169. http://europepmc.org/abstract/MED/6066618
Patetsini, E., Dimitriadis, V. K., & Kaloyianni, M. (2013). Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquatic Toxicology, 126, 338–345. https://doi.org/10.1016/j.aquatox.2012.09.009
Pavlica, M., Klobučar, G. I. V, Vetma, N., Erben, R., & Papeš, D. (2000). Detection of micronuclei in haemocytes of zebra mussel and great ramshorn snail exposed to pentachlorophenol. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 465(1), 145–150. https://doi.org/10.1016/S1383-5718(99)00222-3
Rahman, M. F., Mahboob, M., Danadevi, K., Saleha Banu, B., & Grover, P. (2002). Assessment of genotoxic effects of chloropyriphos and acephate by the comet assay in mice leucocytes. Mutation Research, 516(1–2), 139–147. https://doi.org/10.1016/s1383-5718(02)00033-5
Revankar, P. R., & Shyama, S. K. (2009). Genotoxic effects of monocrotophos, an organophosphorous pesticide, on an estuarine bivalve, Meretrix ovum. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 47(7), 1618–1623. https://doi.org/10.1016/j.fct.2009.04.010
Saleha Banu, B., Danadevi, K., Rahman, M. F., Ahuja, Y. R., & Kaiser, J. (2001). Genotoxic effect of monocrotophos to sentinel species using comet assay. Food and Chemical Toxicology, 39(4), 361–366. https://doi.org/10.1016/S0278-6915(00)00141-1
Sharma, S., Nagpure, N. S., Kumar, R., Pandey, S., Srivastava, S. K., Singh, P. J., & Mathur, P. K. (2007). Studies on the genotoxicity of endosulfan in different tissues of fresh water fish Mystus vittatus using the comet assay. Archives of Environmental Contamination and Toxicology, 53(4), 617–623. https://doi.org/10.1007/s00244-006-0228-7
Sinha, A. K. (1972). Colorimetric assay of catalase. Analytical Biochemistry, 47(2), 389–394. https://doi.org/10.1016/0003-2697(72)90132-7
USEPA. (1985). Pesticide fact sheet No 72: Monocrotophos. USEPA, Washington D.C.
Yaduvanshi, S. K., Ojha, A., Pant, S. C., Lomash, V., & Srivastava, N. (2010). Monocrotophos induced lipid peroxidation and oxidative DNA damage in rat tissues. Pesticide Biochemistry and Physiology, 97(3), 214–222. https://doi.org/10.1016/j.pestbp.2010.02.004
Yu, Z., Jiang, A. L. (2011). Evaluation of oxidative stress responses and neurotoxicity potential of methamidophos in Mytilus edulis Advanced Materials Research 343–344, 795 801. https://doi.org/10.4028/www.scientific.net/AMR.343-344.795
Acknowledgements
The authors wish to express their gratitude to Ms. Moreska Costa for editing the manuscript.
Author information
Authors and Affiliations
Contributions
All the authors have contributed to the scientific study, analysis and preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dias, R., D’Costa, A., Praveen Kumar, M. et al. DNA damage and biochemical responses in estuarine bivalve Donax incarnatus (Gmelin, 1791) exposed to sub-lethal concentrations of an organophosphate pesticide monocrotophos. Environ Monit Assess 193, 317 (2021). https://doi.org/10.1007/s10661-021-09103-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09103-0