Abstract
Increasing concerns have been raised about the toxicity of mercury (Hg) to humans, especially for those that consume a great amount of fish. High Hg concentrations have previously been measured in Amazonian waterbodies, both resulting from natural and anthropogenic sources. However, few studies have been conducted so far in Amazonian lakes that are fished by local populations. In addition, few of those studies included methylmercury (MeHg), the most toxic and bioaccumulative Hg form, and evaluated the influence of physico-chemical conditions and season on Hg dynamics. In the present study, total Hg (THg) and MeHg concentrations were measured in bottom sediment as well as in two fish and two crocodile species of the Amazonian Cuniã Lake. Bottom sediment MeHg concentrations were higher in the dry season than in the wet season, which is related to differences in physico-chemical (pH and electrical conductivity) conditions. Diet appeared to be related with animal tissue MeHg concentrations, with the herbivorous fish having lower MeHg levels than the predatory fish and crocodiles. Based on the measured tissue concentrations and published data on local person weight and fish consumption, MeHg risk to Cuniã Lake populations was estimated. Although the MeHg fish tissue concentrations did not exceed national and international standards, a significant risk to the local population is anticipated due to their high fish consumption rates.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns on mercury (Hg) toxicity initiated in the 1950s when severe health effects were noted in humans through consumption of fish contaminated with chemical waste released by the Chisso Corporation in Minamata (Japan) (Harada 1995; Kudo and Miyahara 1991; Kudo et al. 1998). In this millennium, these growing concerns were reflected in the initiation of several research projects, policy reports including the consideration of mercury as a priority substance in the Minamata Convention (EFSA 2015; EU 2008; UNEP 2013). Methylmercury (MeHg) is the most toxic Hg form and is produced through anaerobic microbial Hg methylation in sediments (Ullrich et al. 2001) after which it is quickly absorbed and accumulated by aquatic organisms like fish and aquatic macrophytes (Pestana et al. 2016; Vieira et al. 2018). The Amazon presents favorable characteristics for this process which concerns researchers around the world (Ha et al. 2017; Pestana et al. 2016; Vieira et al. 2018). Consumption of MeHg-contaminated fish is therefore the greatest entry route for humans, and people that rely on fish as their main protein source are especially expected to be at risk (FAO/WHO 2011).
The Madeira River in Brazil is one of the main Amazonian tributaries, and its riverside populations heavily depend on fish as their main income and food source (Hacon et al. 2014; Oliveira et al. 2010). This region is characterized by high environmental Hg levels, mostly occurring from natural sources such as geochemical characteristics of soils and biological decay (Roulet et al. 1998; Vieira et al. 2018). In addition, Artisanal Small-Scale Gold Mining (ASGM) in the Amazon region relied on Hg amalgamation to separate fine gold particles from riverbank and sediment components (Bastos et al. 2006; Pfeiffer and Lacerda 1988). During these mining operations, about 45% of Hg used was discharged directly in the Madeira River and the remaining 55% into the atmosphere (Pfeiffer and Lacerda 1988). Since most of the Hg emitted to the atmosphere is deposited within about 40 km from its source, most of the atmospheric Hg emissions were likely deposited (Bastos et al. 2006). Although mining activities have ceased, high Hg levels are still detected due to land-use changes like deforestation and the construction of hydroelectric dams, which lead to Hg remobilization (Pestana et al. 2019; Vieira et al. 2018). ASGM has indeed been considered to be the most significant source of Hg emissions worldwide (Donkor et al. 2006; Gammons et al. 2006; Gerson et al. 2018; Marrugo-Negrete et al. 2019; UNEP 2013).
Despite the great extent and importance of Amazonian water sources as fishing sites, the dynamics of Hg in these environments, and especially lakes, are still poorly understood (Brito et al. 2017). In addition, most existing studies focused on total Hg (THg) levels, whereas MeHg, the main Hg form both in terms of toxicity and bioaccumulation potential, has received much less attention (Brito et al. 2017; Roach et al. 2013). Since physico-chemical variables, seasonality, and stratification of lakes play an important role in the distribution and dynamics of MeHg, it thus becomes imperative to study MeHg distribution and bioaccumulation in Amazonian lakes (Brito et al. 2017; Pestana et al. 2016; Vieira et al. 2018).
The aim of the present study was to study THg and MeHg concentrations in bottom sediment and aquatic organisms of the Amazonian Lake Cuniã. Physico-chemical water variables were also measured to evaluate their possible influence of the measured sediment and organism metal concentrations. Sampling was conducted both in the dry and the wet season to evaluate a possible influence of season on THg and MeHg dynamics. Organism tissue samples consisted of two fish (Mylossoma aureum and Cichla monoculus) and two crocodile (Melanosuchus niger and Caiman crocodilus) species. Since these organisms are known to be among the organisms that are consumed daily, a human risk assessment was conducted based on their tissue concentrations.
Materials and methods
Study area
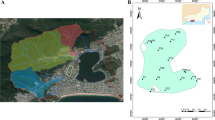
The study area is located in the Federal Conservation Unit Extractive Reserve of Cuniã Lake (RESEX; State of Rondônia, Brazil). The RESEX is formed by more than 60 lakes and by a floodplain (“igarapé”) of the main lake (Lake Cuniã) that connects it to the Madeira River. Lake Cuniã has a surface area of 18,000 ha and is connected to the Madeira River by a 42-km long channel, which contributes to the seasonal oscillation of the lake water level (Goulding 1999). The local communities of Cuniã are riverside communities with fishing and nut extractivism as their main subsistence activities (Carvalho et al. 2019). Seven sediment sampling sites were selected across Lake Cuniã (08° 19′ 10.9″ S, 63° 30′ 01.9″ W), which is located 130 km downstream the city of Porto Velho (Gomes et al. 2017) (Fig. 1).
Total mercury and methylmercury extraction and analyses in sediment and animals
Sediment samples were collected in the marginal zone of the lake in the first 5 to 10 cm of depth with a plastic spoon and stored in plastic pots. After collection, the sediment samples were transported on ice in cool boxes to the laboratory. For THg determination, sediment subsamples were first subjected to a gravimetric process to select the fraction smaller than 0.075 mm. This fraction was dried at 40 °C, macerated, and stored in polyethylene bottles until further analysis. Aliquots of 0.5 g (dry weight) were weighed in duplicate and transferred to a hot plate at 80 °C with addition of 5 mL aqua regia (HCl:HNO3 = 3:1, Merck®). After about 30 min, 6 mL of KMnO4 (5%, Merck®) was added to maintain Hg in the Hg2+ (oxidized) form. After extraction and cooling of the samples, excess of oxidant was withdrawn with drops of hydroxylamine hydrochloride (12%, Merck®); then, they were gravity filtered through a cellulose filter (Whatman 44, NJ, USA) and filled to a volume of 15 mL with ultra-pure water. To extract MeHg, sediment subsamples were lyophilized after which about 0.5 g (dry weight) was transferred to the teflon tubes. MeHg extraction was performed by the addition of 5 mL of a KOH:methanol mixture (25:75% w/v, Merck®) and keeping them at 70 °C for 6 h in which they were shaken every hour for about 10 s. Subsequently, samples were stabilized in the dark for 48 h and ethylated by adding 30 μL of the sample and 50 μL of 1% NaBEt4 to 200 μL acetic acid and sodium acetate buffer solution (pH 4.5).
A total of 30 muscle tissue of animals were sampled and analyzed for THg and MeHg. Regarding fish, five M. aureum individuals were sampled in both the dry and the rainy season, whereas five and three C. monoculus individuals were considered in the dry and rainy season, respectively. The crocodiles were captured only in the dry season, as proposed in the management plan of the unit and also due to difficulties in catching these animals during the rainy season: eight M. niger and four C. crocodilus. All animals were sacrificed to control the crocodile population sizes by RESEX with authorization of the Brazilian governmental organization ICMBio (Chico Mendes Institute for Biodiversity Conservation) n° 48977-1. Animal samples were stored in a falcon tube and stored in cool boxes on ice until arrival to the laboratory. For THg determination, muscle tissue samples of 0.5 g (wet weight) were used for M. aureum, whereas 0.2-g (wet weight) samples were used for the other three species. The chemical digestion was performed by the addition of 1 mL H2O2 (Merck®) and 4 mL of HNO3:H2SO4 mixture (1:1, Merck®). Samples were manually homogenized and transferred to a block digester for 30 min at a temperature of 70 °C. After cooling, 5 mL of KMnO4 solution (5% Merck®) was added and returned to the digester block for another 20 min at 70 °C. After cooling, the samples were covered with plastic film to prevent contamination and remained at room temperature for 12 h to stabilize, after which drops of hydroxylamine hydrochloride solution was added (12%, Merck®). For the determination of MeHg, 0.1 and 0.05-g (wet weight) muscle tissue samples of M. aureum and the other three organisms, respectively, were transferred to a 14-mL falcon tubes. Extraction was performed by adding 3 mL of a 1:4 KOH:methanol (w/v) mixture to the tubes and keeping them in a drying oven at 70 °C for 6 h with stirring every hour. Thereafter, samples were stabilized for 48 h in the dark and ethylated as described above.
The extracted samples were analyzed for THg and MeHg through cold vapor–coupled atomic absorption spectrophotometry (CV-AAS, FIMS-400, PerkinElmer, Germany) as described in Bastos et al. (1998). Under these analytical conditions, the detection limits for THg and MeHg were 0.0029 and 0.0003 mg/kg sediment and < 0.008 and < 0.001 mg/kg for the test organisms, respectively. For quality control, internal reference sediment samples with known THg (ref. SS2; 0.28 mg/kg dry weight) and MeHg (ref. IAEA356; 0.0055 mg/kg dry weight) were also analyzed and indicated a recovery of 104% and 96%, respectively. Similarly, recovery for a fish tissue reference sample (ref. DORM-2; 4.64 mg THg/kg dry weight and 4.47 mg MeHg/kg dry weight) also indicated adequate values for THg (97%) and MeHg (93%). Throughout the rest of this manuscript, sediment and animal THg and MeHg concentrations are expressed as mg/kg dry weight and mg/kg wet weight, respectively.
Abiotic water variables and sediment organic matter content
To improve relating the sediment metal concentrations to the abiotic conditions, water quality variables and sediment organic matter content were determined on the same sampling days as those for sediment metal quantification. The physico-chemical variables’ temperature (T), electrical conductivity (EC), dissolved oxygen (DO), and pH were measured in triplicate using a calibrated multiparameter sensor (YSI 556 MPS). For sediment organic matter determination, the samples were stored in plastic pots and kept in a thermal bag containing ice during transport. In the laboratory, the sediment samples were oven-dried at 60 °C for 12 h. Subsequently, 5-g dry sediment was combusted at 550 °C for 5 h (Quimis® model Q318M25T) to determine organic matter content by gravimetric process (Maitland 1979).
Statistical analysis
The statistical analysis of potential differences in the values of THg and MeHg between the tissues of the four test species was conducted through one-way ANOVA using SigmaPlot v11.0 software (Systat 2008). Firstly, normality of the data was tested using the Shapiro-Wilk test. A Tukey’s test was carried out when differences were obtained in data that followed a normal distribution. If data were not normally distributed and data transformation did not correct for normality, a Kruskal-Wallis test was applied to the data followed by the multiple comparisons Dunn’s method. In all statistical tests, a significance level of 95% (p ≤ 0.05) was adopted.
Results and discussion
Mercury and methylmercury concentrations in sediment
THg sediment concentrations were comparable between the dry and rainy season and averaged 0.097 ± 0.034 mg/kg and 0.11 ± 0.024 mg/kg, respectively. A similar sediment THg concentration (0.098 mg/kg) was previously recorded by Bastos et al. (2006) in the Cuniã Lake. Only 0.29 to 0.96% of the THg was in the MeHg form (Table 1). In natural environments, anaerobic bacteria in sediments are known to methylate Hg to MeHg, which is subsequently quickly distributed and accumulated throughout the aquatic food web (Pestana et al. 2016; Vieira et al. 2018).
Sediment MeHg concentrations were approximately two times higher in the dry season (1.1 ± 0.58 μg/kg) than in the rainy season (0.56 ± 0.28 μg/kg) (one-way ANOVA; Tukey test; p < 0.05). This is likely to be correlated with the physico-chemical water characteristics, which are known to influence the methylation of Hg to MeHg in sediments (Vieira et al. 2018). From Table 2, it may be deducted that the EC and pH values were lower (one-way ANOVA; Tukey test; p < 0.05) in the dry season when compared with the rainy season. These variables have indeed been reported to influence MeHg production in sediments (Brito et al. 2017; Pestana et al. 2016; Vieira et al. 2018), and a significant correlation of EC (r = 0.47; p < 0.05) and pH (r = 0.62; p < 0.05) with MeHg concentrations was found (Fig. 2). The pH values measured in Cuniã Lake presented acidic characteristics, with mean pH values of 4.6 ± 0.25 and 5.1 ± 0.22 in the dry and rainy season, respectively. A previous study in two other Amazonian floodplains (“igarapé”—Saracá and Caranã) found values very close to those denoted in the present study for Lake Cuniã, with a minimum pH of 4.1 and a maximum pH of 4.8 (Callisto and Esteves 1998). Bozelli (2000) found pH values between 5.5 and 6.2 in Lake Batata, in the western region of the State of Pará, on the Trombetas River. According to Esteves and Marinho (2011), Amazonian lakes have pH values around 5 since the soils possess acidic characteristics, and due to dark waters that are rich in humic substances. Since acidic pH may increase the bioavailability of Hg for bacterial absorption (Kelly et al. 2003) and stimulate methylmercury production (Gilmour and Henry 1991), this may at least partly explain the higher MeHg sediment concentrations measured in the dry season.
Metal concentrations in fish and crocodiles
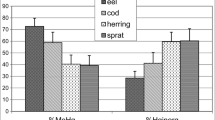
The THg and MeHg concentrations measured in the two fish and two crocodile species are presented in Fig. 3. Contrarily to the sediment, MeHg accounted for the bulk (84% to 94%) of THg in all organisms. The high %MeHg in the biota is because MeHg biomagnifies through food chains, while inorganic mercury does not (Pestana et al. 2019). This is related with the fact that MeHg is lipophilic, facilitating its penetration through biological membranes and the intestine barrier and transportation into viscera (Golovanova et al. 2008). In line with this, other studies have also demonstrated that the greatest part (typically > 80%) of THg in fish is in the MeHg form (Bastos et al. 2016; Kehrig et al. 2009; Mourão 2016).
Both THg and MeHg concentrations were substantially lower in the fish M. aureum when compared with the other three species. Feeding habits are known to greatly influence Hg levels, with herbivorous fish like M. aureum having much lower Hg concentrations than predatory species like the other fish (C. monoculus) and the crocodile species (M. niger and C. crocodilus) evaluated in the present study (Bastos et al. 2016; Oliveira et al. 2010).
As discussed in the previous section, differences in physico-chemical conditions may have favored MeHg production during the dry season over the rainy season. For M. aureum, however, no differences in MeHg tissue concentrations were encountered between the seasons (dry season 36 ± 7.4 mg/kg; rainy season 33 ± 14 mg/kg). Interestingly, average MeHg tissue concentrations for C. monoculus were twice as high in the dry season (0.96 ± 0.37 mg/kg) than in the rainy season (0.50 ± 0.017 mg/kg), although this difference was not statistically significant due to high variation in the dry season values (one-way ANOVA; Dunn’s method; p > 0.05). Greater Hg bioaccumulation in predatory fish during the dry season has previously been reported and was hypothesized to result from a greater fish prey density (and hence greater predation rates) in the lower dry season water volume (Dorea et al. 2006; Hylander et al. 2000).
Human risk assessment and concluding remarks
Based on the Hg fish concentrations determined in the present study and some literature data, the risk of Hg contamination via fish consumption to the local population was assessed. Fish consumption in the study area has previously been determined to average 388 g/person/day (Cerdeira et al. 1997, 369 g/person/day; Oliveira et al. 2010, 406 g/person/day). Since no detailed information is currently available on fish diet or MeHg concentrations in all fish consumed, the average concentrations measured in M. aureum (0.099 μg/g fish) and C. monoculus (0.74 μg/g fish) were taken as best- and worst-case scenarios, respectively. With an average local person weight of 68 kg (Mourão 2016; Pedrosa 2018), an average weekly MeHg intake of respectively 4.0 and 30 μg/kg bw was calculated. These values are 3 to 23 times higher than the tolerable weekly intake (TWI) of 1.3 μg/kg bw/week established by the CONTAM panel (EFSA 2015).
Interestingly, the fish THg concentrations for M. aureum were generally below the maximum levels set nationally (Brazil: ANVISA 2013) and internationally (EC 2006) for fish in general (0.5 mg/kg) and predatory fish (1.0 mg/kg), respectively. Several other studies in the Amazonian region also demonstrated that local fish THg concentrations adhered to these standards. In the European scientific opinion underlying these standards, however, mean daily consumption of fish and seafood products in the EU ranging 10 g (the Netherlands) and 80 g (Norway) per person (70 to 560 g/week) were considered (EFSA 2004).
The comparisons of THg concentrations for predatory and non-predatory Amazonian fish are recorded in literature with difference in the concentrations found depending on the species; however, predators generally have higher concentrations of HgT (Pimentel et al. 1995; Bastos et al. 2008). In previous studies conducted in the region, the values found are very similar to those of the present study for carnivorous and omnivorous organisms. For example, Bastos et al. (2015) found average concentrations of THg 51–1242 μg/kg for a wide variety of carnivorous fish, whereas Bastos et al. (2016) presented values of 0.5 mg/kg THg in predatory males and females in Calophysus macropterus. Hacon et al. (2014) in turn reported 1.64 μg/g THg values for carnivorous fish Pinirampus sp. Values of 1.53 μg/kg THg for the predatory species Cichla monoculus were recorded by Rabito et al. (2011).
This study demonstrates that a sole verification of fish MeHg concentrations in Amazonian villages with their standards is not sufficient. Through their high fish consumption rates, these people are likely to be at risk. In line with this, several studies have reported that hair of local people contained Hg concentrations and almost all contained concentrations well above the safe concentration established by WHO (< 6.0 μg/g; e.g., Bastos et al. 2006; Hacon et al. 2014; Lima 2018; Mourão 2016; Carvalho et al. 2019; Azevedo et al. 2019). Such studies also associated losses in the neuropsychological performance of locals in the Madeira River region to mercury exposure through the consumption of fish (Lima 2018 and references therein).
Several other studies in other countries have demonstrated a correlation between gold mining activities, environmental contamination of Hg, and the human exposure through consumption of local fish, e.g., Ghana (Donkor et al. 2006), Peru (Gammons et al. 2006), Senegal (Gerson et al. 2018), and Colombia (Marrugo-Negrete et al. 2019). Subsequently, this does not merely deserve attention in the Amazon as evaluated in the present study, but also in other areas with (historical) gold mining activities.
Conclusion
This study provides new insights into the influence of physico-chemical conditions and season on Hg dynamics in Amazonian lakes that are fished by local population. In addition, unlike previous studies, we also evaluated MeHg, the most toxic and bioaccumulative Hg form. Bottom sediment MeHg concentrations were higher in the dry season than in the wet season, which is related to differences in physico-chemical (pH and electrical conductivity) conditions. In addition, feeding habit appeared to be related with animal tissue MeHg concentrations, with the herbivorous fish having lower MeHg levels than the predatory fish and crocodiles. Lastly, although the MeHg fish tissue concentrations did not exceed national and international standards, a significant risk to the local population is anticipated due to their high fish consumption rates.
Thus, future studies are needed to better estimate the human MeHg exposure in this and other (historical) gold mining areas including (i) fish (species) consumption pattern determination, (ii) human body sample analyses, and (iii) assessment of the frequency of known MeHg-related toxicological symptoms. In addition, monitoring a greater number of locally consumed fish may provide insights into species that should be avoided. Informing and training local fishermen on such results and establishing local environmental and health science–based guidelines may aid in reducing the exposure of Amazonian people to unacceptable MeHg exposure (UNEP 2013).
References
ANVISA, 2013. Resolução RDC N° 42, de 29 de agosto de 2013 - Dispõe sobre o Regulamento Técnico MERCOSUL sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos. Agência Nacional de Vigilância Sanitária, Ministério da Saúde. p. 17.
Azevedo, L. S., Pestana, I. A., da Costa Nery, A. F., Bastos, W. R., & Souza, C. M. M. (2019). Variation in Hg accumulation between demersal and pelagic fish from Puruzinho Lake, Brazilian Amazon. Ecotoxicology, 28(10), 1143–1149.
Bastos, W. R., Malm, O., Pfeiffer, W. C., & Cleary, D. (1998). Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon. Brazil Ciência Cultura, 50, 255–260.
Bastos, W. R., Gomes, J. P. O., Oliveira, R. C., Almeida, R., Nascimento, E. L., Bernardi, J. V. E., & Pfeiffer, W. C. (2006). Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Science of the Total Environment, 368, 344–351.
Bastos, W. R., Rebelo, M. D. F., Fonseca, M. D. F., Almeida, R. D., & Malm, O. (2008). A description of mercury in fishes from the Madeira River Basin, Amazon, Brazil. Acta Amazonica, 38(3), 431–438.
Bastos, W. R., Dórea, J. G., Bernardi, J. V. E., Lauthartte, L. C., Mussy, M. H., Lacerda, L. D., & Malm, O. (2015). Mercury in fish of the Madeira river (temporal and spatial assessment), Brazilian Amazon. Environmental Research, 140, 191–197.
Bastos, W. R., Dórea, J. G., Bernardi, J. V. E., Manzatto, A. G., Mussy, M. H., Lauthartte, L. C., & Malm, O. (2016). Sex-related mercury bioaccumulation in fish from the Madeira River, Amazon. Environmental Research, 144, 73–80.
Bozelli, R.L., (2000). Lago Batata: impacto e recuperação de um ecossistema amazônico. Doctoral thesis Universidade Federal.
Brito, B. C., Forsberg, B. R., Kasper, D., Amaral, J. H., de Vasconcelos, M. R., de Sousa, O. P., & Bastos, W. R. (2017). The influence of inundation and lake morphometry on the dynamics of mercury in the water and plankton in an Amazon floodplain lake. Hydrobiologia, 790, 35–48.
Callisto, M., & Esteves, F. A. (1998). Biomonitoramento da macrofauna bentônica de Chironomidae (Diptera) em dois igarapés amazônicos sob influência das atividades de uma mineração de bauxita. Oecologia Brasiliensis, 5(1), 20.
Carvalho, L. V. B., Hacon, S. S., Vega, C. M., Vieira, J. A., Larentis, A. L., Mattos, R. C. O. C., Valente, D., Costa-Amaral, I. C., Mourão, D. S., Silva, G. P., Beatriz, F. A., & Oliveira, B. F. A. (2019). Oxidative stress levels induced by mercury exposure in Amazon juvenile populations in Brazil. International Journal of Environmental Research and Public Health, 16, 2682.
Cerdeira, R. G. P., Ruffino, M. L., & Isaac, V. J. (1997). Consumo de pescado e outros alimentos pela população ribeirinha do Lago Grande de Monte Alegre, PA-Brasil. Acta Amazônica, 27, 213–228.
Donkor, A. K., Bonzongo, J. C., Nartey, V. K., & Adotey, D. K. (2006). Mercury in different environmental compartments of the Pra River Basin, Ghana. Science of the Total Environment, 368, 164–176.
Dorea, J. G., Barbosa, A. C., & Silva, G. S. (2006). Fish mercury bioaccumulation as a function of feeding behavior and hydrological cycles of the Rio Negro, Amazon. Comparative Biochemistry and Physiology, Part C, 142, 275–283.
EC. (2006). Commission Regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L, 364, 5–24.
EFSA. (2004). Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to mercury and methylmercury in food. EFSA Journal, 34, 1–14.
EFSA. (2015). Scientific Opinion. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA Journal, 13, 3982.
Esteves, F. A., & Marinho, C. C. (2011). Carbono inorgânico. In Fundamentos de limnologia (3rd ed., p. 790). Rio de Janeiro: Interciência.
EU. (2008). Directive 2008/105/EC of the European Parliament and the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Official Journal of the European Union L, 348, 84–97.
FAO/WHO. (2011). Report of the joint FAO/WHO expert consultation on the risks and benefits of fish consumption. In Rome, food and agriculture organization of the united nations (p. 50). Geneva: World Health Organization.
Gammons, C. H., Slotton, D. G., Gerbrandt, B., Weight, W., Young, C. A., McNearny, R. L., Cámac, E., Calderón, R., & Tapia, H. (2006). Mercury concentrations of fish, river water, and sediment in the Rıo Ramis-Lake Titicaca watershed, Peru. Science of the Total Environment, 368, 637–648.
Gerson, J. R., Driscoll, C. T., Hsu-Kim, H., & Bernhardt, E. S. (2018). Senegalese artisanal gold mining leads to elevated total mercury and methylmercury concentrations in soils, sediments, and rivers. Science of the Anthropocene, 6, 11.
Gilmour, C. C., & Henry, E. A. (1991). Mercury methylation in aquatic systems affected by acid deposition. Environmental Pollution, 71(2–4), 131–169.
Golovanova, I. L., Komov, V. T., & Gremyatchikh, V. A. (2008). Hydrolysis of carbohydrates in roach (Rutilus rutilus (L.)) at different levels of mercury accumulation. Inland Water Biology, 1(3), 296.
Gomes, D. F., Sanches, N. A. O., Sahm, L. H., & Gorni, G. R. (2017). Aquatic oligochaeta (Annelida: Clitellata) in extractive reserve Lake Cuniã, Western Brazilian Amazon. Biota Neotropica, 17, 1.
Goulding, M. (1999). Introduction. In C. Padoch, J. M. Ayres, M. Pinedo-Vazquez, & A. Henderson (Eds.), Várzea: Diversity, development, and the conservation of Amazonian’s white waters floodplain (pp. 3–6). Nova York: New York botanical garden press.
Ha, E., Basu, N., Bose-O’Reilly, S., Dórea, J. G., McSorley, E., Sakamoto, M., & Chan, H. M. (2017). Current progress on understanding the impact of mercury on human health. Environmental Research, 152, 419–433.
Hacon, S., Dórea, J., Fonseca, M., Oliveira, B., Mourão, D., Ruiz, C., & Bastos, W. (2014). The influence of changes in lifestyle and mercury exposure in riverine populations of the Madeira river (Amazon basin) near a hydroelectric project. Int. J. Environmental Research and Public Health, 11, 2437–2455.
Harada, M. (1995). Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology, 25, 1–24.
Hylander, L. D., Pinto, F. N., Guimaraes, J. R., Meili, M., Oliveira, L. J., & Silva, E. D. C. (2000). Fish mercury concentration in the Alto Pantanal, Brazil: Influence of season and water parameters. Science of the Total Environment, 261, 9–20.
Kehrig, H. A., Palermo, E. F., Seixas, T. G., Santos, H. S., Malm, O., & Akagi, H. (2009). Methyl and total mercury found in two man-made Amazonian reservoirs. Journal of the Brazilian Chemical Society, 20, 1142–1152.
Kelly, C. A., Rudd, J. W., & Holoka, M. H. (2003). Effect of pH on mercury uptake by an aquatic bacterium: Implications for Hg cycling. Environmental Science & Technology, 37(13), 2941–2946.
Kudo, A., & Miyahara, S. (1991). A case history; Minamata mercury pollution in Japan-from loss of human lives to decontamination. Water Science & Technology, 23, 283–290.
Kudo, A., Fujikawa, Y., Miyahara, S., Zheng, J., Takigami, H., Sugahara, M., & Muramatsu, T. (1998). Lessons from Minamata mercury pollution, Japan after a continuous 22 years of observation. Water Science & Technology, 38, 187–193.
Lima, C.S., (2018). Efeitos neuropsicológicos da exposição ao mercúrio em crianças e adolescentes da região do rio Madeira. Doctoral thesis Federal University of Bahia, Salvador, Brazil. p. 105.
Maitland, P. S. (1979). The distribution of zoobenthos and sediments in Loch Leven, Kinross, Scotland. Archiv für Hydrobiologie, 85, 98–125.
Marrugo-Negrete, J., Durango-Hernández, J., Calao-Ramos, C., Urango-Cárdenas, I., & Díez, S. (2019). Mercury levels and genotoxic effect in caimans from tropical ecosystems impacted by gold mining. Science of the Total Environment, 664, 899–907.
Mourão, D.S., (2016). Avaliação da exposição ao mercúrio em comunidades ribeirinhas de Porto Velho, Rondônia. Doctoral thesis Escola Nacional de Saúde Pública Sergio Arouca, Rio de Janeiro. p. 94.
Oliveira, R. C., Dórea, J. G., Bernardi, J. V., Bastos, W. R., Almeida, R., & Manzatto, Â. G. (2010). Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): Impact on hair mercury. Annals of Human. Biology, 37, 629–642.
Pedrosa, O.P., (2018). Estudo prospectivo do estado de saúde de uma população ribeirinha da Amazônia brasileira. Doctoral thesis Federal University of Rondônia, Porto Velho. pp. 137.
Pestana, I. A., Bastos, W. R., Almeida, M. G., de Carvalho, D. P., Rezende, C. E., & Souza, C. M. M. (2016). Spatial-temporal dynamics and sources of total Hg in a hydroelectric reservoir in the Western Amazon, Brazil. Environmental Science and Pollution Research, 23, 9640–9648.
Pestana, I. A., Azevedo, L. S., Bastos, W. R., & de Souza, C. M. M. (2019). The impact of hydroelectric dams on mercury dynamics in South America: A review. Chemosphere, 219, 546–556.
Pfeiffer, W. C., & Lacerda, L. D. (1988). Mercury inputs into the Amazon region, Brazil. Environmental Technology, 4, 325–330.
Pimentel, T., Forsberg, B. R., Padovani, C. (1995). Contaminação Mercurial Em Peixes do Rio Madeira: Resultados e Recomendações.
Rabito, I., Bastos, W. R., Almeida, R., Anjos, A., de Holanda, Í. B. B., Galvão, R. C. F., & de Oliveira Ribeiro, C. A. (2011). Mercury and DDT exposure risk to fish-eating human populations in Amazon. Environment International, 37(1), 56–65.
Roach, K. A., Jacobsen, N. F., Fiorello, C. V., Stronza, A., & Winemiller, K. O. (2013). Gold mining and mercury bioaccumulation in a floodplain lake and main channel of the Tambopata river, Perú. J. Journal of Environmental Protection, 4, 51–60.
Roulet, M., Lucotte, M., Saint-Aubin, A., Tran, S., Rheault, I., Farella, N., & Mergler, D. (1998). The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos River Valley, Pará state, Brazil. Science of the Total Environment, 223, 1–24.
Systat. (2008). Systat software, incorporation sigma plot for Windows version 11.0. Statistics for user’s guide (p. 578). Chicago: Systat Software Inc.
Ullrich, S. M., Tanton, T. W., & Abdrashitova, S. A. (2001). Mercury in the aquatic environment: A review of factors affecting methylation. Critical Reviews in Environmental Science and Technology, 31, 241–293.
UNEP. (2013). Minamata Convention on mercury-text and annexes. United Nations Environment Programme. Available via: http://wedocs.unep.org/handle/20.500.11822/8541. Accessed 2 Aug 2019.
Vieira, M., Bernardi, J. V., Dórea, J. G., Rocha, B. C., Ribeiro, R., & Zara, L. F. (2018). Distribution and availability of mercury and methylmercury in different waters from the Rio Madeira Basin, Amazon. Environmental Polllution, 235, 771–779.
Funding
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for collection assistance, especially to the coordinator of the Reserve, Lucas Henrique Sahm, for assistance in the collection of data. The authors also acknowledge the support from the São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP) for their financial support (process number 2017/24126-4) through a postdoc grant for the R. Moreira and the Portuguese Government (Foundation for Science and Technology - FCT) through a postdoc grant for the M. Daam (SFRH/BPD/109199/2015) and the research unit CENSE (UID/AMB/04085/2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, D.F., Moreira, R.A., Sanches, N.A.O. et al. Dynamics of (total and methyl) mercury in sediment, fish, and crocodiles in an Amazonian Lake and risk assessment of fish consumption to the local population. Environ Monit Assess 192, 101 (2020). https://doi.org/10.1007/s10661-020-8066-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8066-z