Abstract
The objective of this research was to evaluate the interaction of landfill leachate of urban solid waste in clayey (CL) and sandy soils (SL) in order to determine physical and chemical parameters that can be used as indicators of soil contamination when there are faults in the landfill waterproofing. In the diffusion tests, compacted soil samples were placed in contact with leachate (methanogenic phase). The temporal analysis (200 days) considered the parameters pH, electrical conductivity (EC), alkalinity, nitrogen series, chemical oxygen demand (COD), solids and color for the leachate and pH, ΔpH, EC, total nitrogen (TN), chemical elements, and cation exchange capacity (CEC) for the soils. Correlation analysis and principal component analysis (PCA) were performed to results. It was observed that the studied soils have potential to attenuate chemicals present in the leachate; this indicates the possibility of using them as base in landfills. Correlation analysis and PCA carried out to CL showed that in a process of CL monitoring the pH would be the key parameter to indicate contamination of this soil, due to the high correlation of this parameter with the others analyzed. For the SL, the parameters pH, alkalinity, apparent color, and COD (total and filtered) could be used as indicators of contamination. In both soils, monitoring of concentrations of Ca, Mg, K, SB, V, and CTC can be used to indicate possible faults in the waterproofing system of the landfill.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disposing of urban solid waste (USW) in landfills is considered an efficient and economical waste disposal technique (Khattabi et al. 2002); however, it can exert impacts. Leachate, effluent originating from the infiltration of rainwater that percolates through solid waste carrying biological decomposition products and dissolved minerals, can contaminate the environment (soil, surface, and groundwater) and cause harm to human health if landfill management systems are not used (Longe and Enekwechi 2007; Parameswari and Mudgal 2014; Aboyeji and Eigbokhan 2016; Han et al. 2016; Ling and Zhang 2017; Kapelewska et al. 2017).
Soil under the landfill can be used as a secondary protective barrier to prevent migration of contaminants because of their low permeability and sorption properties (Nguyen et al. 2011), and also has low-cost installation. The recognized natural attenuation capacity of soil against chemical species in solution is mainly dependent on its texture, and those with a higher fine content are more reactive, as the particles found in the clay fraction attract circulating ions and promote their adsorption, while those with a higher particle size are more likely to leach (Rizzo and Lollo 2006; Cronan 2018).
Different technologies have been developed and applied in landfills with the objective of evaluating possible soil contamination due to faults in the waterproofing system of its base (Moreira et al. 2018; Shu et al. 2018) or to evaluate the levels of contamination of the soil and groundwater, due to percolation of the leachate in landfills without waterproofing (Xie et al. 2016; Akpan et al. 2018; Przydatek and Kanownik 2019), but up to now no studies are found that evaluate the interaction of the leachate with different soils, aiming to point out simplified parameters that could indicate the contamination of these, in case there is a problem of waterproofing at the base of the landfill.
Considering the above, the objective of this research was to evaluate the interaction by diffusion of USW leachate in soils of different textural classes by analyzing its physical and chemical characteristics and to indicate which of the analyzed parameters could be used as soil contamination indicators when there are failures in the landfill waterproofing system.
Materials and methods
Leachate and soils
The leachate used in this research was collected at the sanitary landfill in the city of Rolândia, Paraná, Brazil (23° 18′ 35″ S, 51° 22′ 09″ W, 730 m altitude; Fig. 1). The landfill has been in operation for 12 years and receives about 40 tonnes of household waste daily. According to the Köppen classification, the climate in the region is Cfa, humid subtropical, with hot and humid summers and cold and dry winters. The mean annual temperature is 22.5 °C and the mean annual rainfall is 1500 mm (Lopes et al. 2012). There was no rainfall in the 14 days prior to collection.
The soils used in the research were identified according to their predominant textural class: clayey and sand. Their physicochemical characterization was performed by Gonçalves et al. (2018).
Clay soil (CS) is composed of 56% clay, 24% silt, and 20% fine sand. Other physical characteristics of this soil are as follows: density of solids of 3.03 g cm−3 and plasticity index of 14. Its classification by the Unified Soil Classification System (USCS) is given as MH (silt of high compressibility), which is justified by its flocculated characteristic, mainly by acid pH and presence of low sodium content in its composition (De Melo et al. 2019). This soil was collected manually at a depth of 2 m at the Geotechnical Engineering Experimental Field, State University of Londrina (UEL), in the city of Londrina, Paraná, Brazil (23° 18′ 37″ S, 51° 09′ 46″ W, 585 m altitude; Fig 1).
Sandy soil (SS), whose granulometry presents 77% sand, with the rest of the composition being fine (10% silt and 13% clay), is classified as SC (sand clay) by the USCS. Among the other physical characteristics of this soil, it has a density of solids equal to 2.69 g cm−3 and a plasticity index of 16. This material was collected by digging a slope along the PR 376 - Km 37 highway, in the municipality of Mandaguaçu, Paraná, Brazil (23° 20′ 50″ S, 52° 05′ 3″ W, 580 m altitude; Fig 1).
The two soils present quartz, iron oxide, and kaolinite (mineral of type 1:1) in their composition, differing only in their proportions of occurrence.
Soil contamination
Contamination of the bottom liner of a sanitary landfill was simulated using diffusion cells (adapted from Barone 1989). The test consists of leaving the contaminating solution in direct contact with a compacted and saturated soil specimen, configuring no hydraulic flow. These conditions favor the diffusion process to the detriment of the other physical processes, culminating in the best appreciation of the phenomenon (Johnson et al., 1989; Leite et al. 2003).
Soil cylinders were compacted with normal Proctor energy. The optimum compaction moisture for soils had already been determined by Gonçalves et al. (2018). In order to ensure statistical similarity between the compacted soil cylinders, the parameters for the deviation of the moisture content were controlled (± 1.5 % in relation to the optimum compaction moisture) and degree of compaction (± 5.0 %).
The specimens for the diffusion test were obtained by cutting the compacted cylinders at a height of approximately 4.5 cm, totaling 20 specimens for each soil. Each specimen was placed in a diffusion cell (ϕinternal = 10 cm, mean height = 12 cm) and saturated by capillarity using water.
After saturating the specimens, the diffusion cells were sealed in the lower part and 500 cm3 of leachate was transferred to each cell, making a column of leachate on the soil (6.4-cm blade).
Monitoring leached and contaminated soil characteristics
On previously determined days, a diffusion cell of each type of soil was randomly chosen to analyze the time variation of leachate and soil characteristics throughout the diffusion process.
Leachate samples in contact with clay soil (CL) and leachate in contact with sandy soil (SL) were analyzed using pH, alkalinity, electrical conductivity (EC), biochemical oxygen demand (BOD), chemical oxygen demand (COD—total and filtered), solids series, nitrogen series, and color (true and apparent) parameters (Apha 2005).
After removal, soil specimens were left outdoors for moisture reduction. The pH analysis (CaCl2, H2O, KCl), EC, organic carbon, total nitrogen (TN), and elements (Ca, Mg, K, Al, H + Al) were performed, whose methodologies are described by Pavan et al. (1991) and Tedesco et al. (1995). Based on these analyses, the base saturation (V%), cation exchange capacity (CEC pH 7.0), and ΔpH (the difference between the pH in KCl 1 mol L−1 and the pH in water) were calculated.
The experiment took 200 days, with increasing time intervals between sample withdrawals. The leachate and soil parameters were analyzed by temporal diffusion interaction curves.
Statistical analysis
As the objective of evaluating the associations among the analyzed variables, as well as their correlation and identifying which main parameters could be used as indicators to evaluate soil contamination, the multivariate statistical analysis—principal component analysis (PCA) and correlation analysis (correlation matrix)—was used to evaluate 14 out of the 18 parameters monitored in the leachate (pH, EC, total alkalinity, apparent and true color, nitrogen series, COD, and solids series) and 14 out of the 16 parameters monitored for soil (pH(H2O), pH(KCl), ΔpH, EC, C, N, P, Ca, Mg, K, SB, H + Al, V, and CEC). These analyses were performed using the Statistica 10 statistical software.
Results and discussion
The physical-chemical characterization of the leachate and soils used in this research is presented in Table 1.
The physical-chemical characteristics of the leachate are highly variable throughout the life of the landfills (Naveen et al. 2017). The characteristics of the deposited residues, the age of the landfill, and the climatic conditions of the place have a significant effect on its composition (Kawai et al. 2012; Tałałaj et al. 2016; Mishra and Tiwary 2018; VAHABIAN et al. 2019). The leachate used in this research presented high pH values, alkalinity, total nitrogen, NH4+-N, color, and total fixed solids (TFS)/total solids (TS) ratio, as well as the low values for BOD/COD ratios, which is expected for a leachate from a landfill which has been in operation for more than 10 years (methanogenic phase), as discussed by Spagni and Marsili-Libelli (2009) and Oliveira et al. (2017), who studied leachates that had the same characteristics.
For both soils, the ΔpH is negative which indicates the predominance of surface negative charges. Sposito (1989) in his book, where provides insight into the chemical behavior of pollutants in soils, indicates that soils that have low base saturation, such as those analyzed in this research (Table 1), have a lower adsorption of basic cations, Ca2+, Mg2+, and K+, in the soil colloids when compared with the exchange points occupied by the acid cations, H+ and Al3+, which indicates the retention potential of these compounds initially in the analyzed soils.
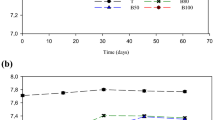
Figure 2 shows variations in EC and pH of the leachate (CL and SL) and the soils (CS and SS) throughout the diffusion test.
The EC of both CL and SL decreased (up to the 66th day of the study) (Fig. 2A, B). Its decrease was more significant for the clay soil, indicating that the cations of the solution (Ca+2, Mg2+, K+, Na+ among others) were adsorbed to the soil more significantly. After that, the trend reversed, culminating in the increase in EC. The retention takes place over a period that corresponds to the saturation potential of soil salts. From this point on, the complexity and variety of elements present in the medium can influence desorption of soil surface elements (Sposito 1989; Cronan 2018).
For the soils, the EC increased throughout the experiment (Fig. 2A, B) and reached values 10 to 15 times higher than the values obtained in the characterization. This occurrence can be correlated with the contribution of ions by the soil-leachate contact. Leite et al. (2003), when evaluating the diffusion of specific substances in soil, also observed an increase in EC over time, indicating that ions were being released either by precipitation processes or by the ion addition of the soil contamination.
Koda et al. (2017) used the electrical resistivity method to investigate the possible migration pathways of pollutants in landfills. The objective of this study was to analyze the spatial migration of pollution for further design of the reclamation and restoration plans. These authors point out in their methodology that there is a positive correlation between soil EC and the amount of dissolved elements in their solution, i.e., the higher the contribution of polluting solution ions, the higher the soil EC. This characteristic, when properly evaluated and based on analytical determinations, can be used as important information for the evaluation of soil contamination.
Considering the pH, it can be seen from Fig. 2C and D that there was a variation in the values of this parameter for both the leachate and the soils analyzed. These variations can be explained by observing other parameters, such as the alkalinity and the nitrogen series of the leachate during the experiment (Fig. 3) and the variation of the evaluated elements in the soil (Fig. 7).
The conditions of the diffusion test (presence of traces of oxygen in the medium, average temperature of 21 ± 30 °C) and the high concentration of nitrogen present in the leachate could have interfered in the variation of the pH values, initially at 7.7 and at the end of the 200 days at 4.6 for CL and 6.1 for SL.
The primary nitrogen transformations in the soil, such as nitrogen immobilization, ammonification, nitrification, denitrification, and biological fixation, are important processes for maintaining nitrogen supplementation in the medium (Levy-Booth et al. 2014; Wan et al. 2015) and it is difficult to find a soil that does not contain nitrifying microorganisms. As there was a decrease in the concentration of NH4+-N and the presence of NO2−-N and NO3−-N (Fig. 3C–F) in the leachate in contact with both soils, the nitrification process was observed in both.
In the conventional process of nitrification in the soil, the NH4+-N is oxidized to NO2−-N by the action of ammonia-oxidizing bacteria (AOB; Nitrosomonas sp.) or archaea (ammonia-oxidizing archaea (AOA)), and NO2−-N can be oxidized to NO3−-N by the action of nitrite-oxidizing bacteria (NOB; Nitrobacter sp.) (Norton et al. 2002; Prosser and Nicol 2008; Yin et al. 2017). In the NH4+-N oxidation, about 7.1 mg CaCO3 is consumed every 1 mg ammonia nitrogen oxidized, due to the release process of H+ ions in the medium (EPA 1993). Considering the above, the decrease in pH for both soils may be partially explained by the alkalinity consumption.
Almost all of the alkalinity present in the leachate was due to bicarbonates and carbonates (Fig. 3A). Its decrease was quite significant in the first 30 days and less significant after this period, regardless of the type of soil analyzed. At the end of the experiment, more than 99 and 95% of the total alkalinity of the CL and SL assays were removed, respectively.
Thirty days after the experiment, it can be observed in Fig. 3C and D that 89 and 67% of NH4+-N were consumed from CL and SL, respectively. Considering the amount of alkalinity consumed in the leachate during this period, it would be possible to nitrify about 555 mg NH4+-N/L of the CL and about 406 mg NH4+-N/L of the SL, which would correspond to a removal percentage of NH4+-N/L of 76% in the CL and 56% in the SL. From the balance between the oxidized NH4+-N concentration and the NO2−-N and NO3−-N values, there is a deficit in the concentrations of the components of the nitrogen series, which indicates the occurrence of other processes, such as volatilization of ammonia, soil adsorption, or even denitrification (reduction to N2).
In the soil, the NH4+-N adsorption process is usually faster than the adsorption of the oxidized nitrogen compounds and the adsorption rates of these compounds are affected by the pH of the medium. Lijklema (1972) sought to develop a simulation model that allowed the calculation of the pH in an activated sludge reactor operating under dynamic conditions. In his research, the author explains that in acidic and neutral environmental conditions, ammonia is found in the form of ammonium ion NH4+-N, while in alkaline conditions, with pH above 8.5, some of the ammonia can be released into the atmosphere. Thus, CS, which maintained the pH of the CL between the neutral and acidic range for a longer time due to its buffering power and greater number of active adsorption sites, was able to retain more ammonium ions, whereas SL the volatilization loss was probably higher.
Figure 4 shows the variation of the total nitrogen concentration removed (TNremoved) from the leachate and the nitrogen adsorbed (TNadsorbed) in the soil during the diffusion test. The TNremoved from the leachate were obtained based on the difference between the initial total nitrogen (TN) (total Kjeldahl nitrogen (TKN) and oxidized nitrogen) in the leachate and its variation over the days. The TNadsorbed in the soil were obtained based on the difference between the initial concentration of TN present in the soil and its concentrations obtained over time.
As can be seen in Fig. 4, throughout the assay, there were variations in the TN concentration in both soils. In the CS, there was an increase in the TNadsorbed with subsequent decrease in the adsorption potential of this compound, leaving a final NT concentration in the soil practically equal to initial. For the SS, TN concentration increased less significantly with subsequent stabilization. Considering Figs. 3 and 4 simultaneously, it can be confirmed that the process of TN removal of leachate by routes such as nitrification and denitrification was more intense in CL than in SL (TNconverted).
Figure 5 shows the variation of COD, color, and solids series of the leachate throughout the diffusion test. From this figure (Fig. 5A), it is observed that there was practically no difference between the concentrations of CODT and CODF in each of the days of analysis, which is explained by the low concentration of particulate organic matter present in the leachate, as discussed by Oliveira et al. (2017) who used leachate collected in the same site as the one used in this study. The CODT decreased, reaching approximately 200 and 900 mg L−1 in the CL and SL, respectively, at the end of 200 days, respectively, with 90% and 50% reductions.
The apparent (and true) color removal tendency of the leachate (Fig. 5B) can also be seen in Fig. 6. On the first day, the apparent color reduction for both soils was close to 20%. The true color reduction was more significant for the CL, approximately 35%, in detriment of a reduction of just over 25% for SL. Reductions of apparent color of 95 and 82% and true color of 97 and 83% were achieved for clay and sandy soils, respectively, at the end of the 200 days of study.
The analysis of the solids series for CL and SL showed a tendency to reduce solid concentration, which is more expressive for CL (Fig. 5C). Cronan (2018) reviewing the physical and chemical characteristics of the soils, together with the main biogeochemical processes that influence the underground cycle of the elements in the ecosystems of the hydrographic basins, points out that cations and anions are chemical substances that can be retained and conserved in the soil under different conditions. The retention mechanism usually involves a dynamic electromagnetic attraction between a charged ion and a colloidal surface with an opposite electrical charge. The soils used in this experiment had a predominance of negative charges (shown by the initial ΔpH), which favors their affinity with positive charge chemical elements, such as NH4+-N, Ca2+, Mg2+, K+, H+, and Al3+. The selectivity for adsorption of these exchangeable cations to clay minerals is described by the Lyotropic series and ranges from the weak binding of some monovalent cations to the strongest binding of cations, such as H+ and Al3+.
According to the Lyotropic series, NH4+-N is a cation that has high affinity with the clay soil. This feature may explain why TN concentration increased more rapidly in CS than in SS (Fig. 4). Krčmar et al. (2018) performed assessment of the municipal landfill pollution impact on soil and shallow groundwater in Serbia during the period from 2014 to 2016. These authors indicate that some substances migrate more easily from the leachate to the soil, such as chlorides, TN, and COD. This fact is mainly related to the chemical characteristics of these compounds and also to their high concentration in the leachate, which makes them possible indicators of soil contamination in landfills.
Considering the temporal analysis of the other elements (Fig. 7), a gradual increase of K+ was observed. This was due to the fact that this cation has the same adsorption potential as the ammonium ion, causing competition for active adsorption sites between them. This relationship is also clear when it is observed that only with the occurrence of other processes such as nitrification, K+ can be assimilated. Concerning the other elements, a significant increase in Ca2+ and Mg2+ cations was observed already on the first day of analysis. It should be noted that the highest apparent assimilation of Ca2+ over Mg2+ is due to the higher initial concentration of this element in the leachate at the moment of contamination (Table 1).
The hydrogen-aluminum sum followed a tendency contrary to the other elements analyzed, for both types of soils, with a significant reduction in the concentration over time, especially in the first days. This occurrence is probably due to the increase of pH in the soil. The availability of aluminum decreases when the pH of the medium increases. Tedesco et al. (1995) explains that when the soil starts to have a neutral to alkaline reaction, the greater fraction of the pH-dependent loads become available for cation exchange, starting to be occupied by bases, which, in this research, led to the observation of a significant contribution of soil solution bases to the soil in the early days.
Acid soils with base saturation (V) below 50%, as they were initially CS and SS, have their surface charges dominated by hydrogen or aluminum ions. On the other hand, they demonstrate a deficiency of adsorbed metallic cations. Corroborating with the observation in the decrease of the H + Al sum, an increase in elements from the leachate and an increase in the pH of the soil, the V of the two soils, reached approximately 70% at the end of the 200 days of study.
Regarding monitoring the soil CEC, shown in Fig. 8, this parameter increased for both soils over time. Sposito (1989) explains that the increase in the pH of the soils directly implies the increase in their CEC due to their dependent character loads. It is known that the pH of the SS increased significantly more than that of CS (Fig. 2B). However, it should be emphasized that the more granula nature, together with the lower retention of cations, makes SS more susceptible to nutrient losses due to leaching. Thus, even with the fine fraction of the two soils being similar, its smaller proportional amount of fine grains allowed for a less significant variation of its CEC.
Moreover, a correlation can be made between the CEC of the soils, leachate color removals, and the presence of humic substances (mainly humic and fulvic acids) and the pH of both of them. Humic substances can be found in soils, wastewater, and sediments with stable organic matter (Mendonça and Rowell 1996; Canellas et al. 2005). The humic colloids present in the soil have high CEC and have pH-dependent solubility. For the leachate, it is known that in the methanogenic phase there is an accumulation of humic and fulvic acids of difficult biological degradation, which contributes to its dark coloration (Fig. 6(a)) (Yong et al. 1992). Although the fractions were not differentiated in this study, maintaining pH above 4 for both soils, the CEC of its humic fraction was maintained. The tendency of this parameter to increase throughout the experiment allowed the soil surface exchange sites to progressively ionize, favoring the continuous interaction between soil and leachate (Cronan 2018). Corroborating with this tendency for the CEC of the two soils, at the end of the 200 days of study, the CS showed (Fig. 6(f)) a greater removal of color than SS. This fact, in addition to the other parameters analyzed, shows that in the clay soil there was a more significant interaction of the leachate with the soil, differently to the sandy soil.
In general, it can be pointed out that there was interaction between the both soils and the leachate over time, however in different intensities. Even though the CS has a higher potential for interaction, the SS used in this research also presented attenuation potential. Possibly this result is related to mineralogy of the fine fraction of both soils, which is similar, differing only in the proportion of occurrence. It should also be noted that, in addition to the presumed differences in the interaction of leachate with different textured soils, the physicochemical complexity of the leachate generated in landfills (Christensen et al. 2001; Kjeldsen et al. 2003; Arunbabu et al. 2017) shows the difficulty of working with such a heterogeneous environment.
Based on the analysis of the interaction by diffusion of the leachate into the soils, some parameters could be indicated to evaluate the quality of the medium in the occurrence of some failures in the landfill waterproofing system. Figure 9 shows the correlation circles of the analyzed parameters in the leachate in contact with the soils: CL (Fig. 9A) and SL (Fig. 9B).
The PCA performed on the parameters for CL showed that the first two principal components (PC) explain about 79.00% of the data and 60.60% of the total variability explained by PC1 and 18.41% by PC2 (Fig. 9A). All the analyzed parameters, except for EC, TS, TFS, and TVS, exerted a strong influence on the constitution of PC1. The total alkalinity, color (apparent and true), TKN, NH4+-N, NO2−-N, and COD (total and filtered) marked the negative quadrant of this PC, and they were positively correlated and anticorrelated to pH and NO3−-N (Table 2). In relation to PC2, the parameters that most influenced its constitution were EC, TS, and TVS, both of which were present in the negative quadrant.
For SL (Fig. 9B), the first two PCs were able to describe 79.00% of the data and 49.89% of the total variability explained by PC1 and 17.53% by PC2. The parameters that most influenced the construction of PC1 were as follows: pH, total alkalinity, true color, TKN, NH4+-N, NO2−-N, TS, TVS, and TFS. Regarding PC2, the parameters that exerted most influence in its construction were EC, NO2---N, and COD (total and filtered). In general, the parameters that most influenced the constitution of PC1 were as follows: pH, total alkalinity, true color, TKN, NH4+-N, and NO2−-N. In the case of PC2, EC it was the main parameter for both media analyzed.
From PCA and correlation analysis (Tables 2 and 3) applied to the evaluated parameters, it is possible to indicate that for the CL the most important parameter in the monitoring of a possible contamination of the soil would be the pH, due to its high correlation with the evaluated parameters, which indicates that its alteration could signal some problem in the system of waterproofing of the landfill and possible contamination of the soil with the leachate. For SL, pH, total alkalinity, apparent color, and COD (total and filtered) could be used as the key parameters to indicate the contamination of this type of soil with the studied effluent.
Shu et al. (2018) sought to investigate the transport behavior of pollutants commonly found in leachate generated in landfills (Cd (II), DQO, and dichlorodiphenyltrichloroethane (DDT)) through typical landfill barrier systems, using a geotechnical centrifuge and numerical modeling. The authors indicated that, independently of the studied condition, COD can be used as a key parameter to indicate that the containment barrier of the landfill has been violated, being this result attributed mainly to the displacement velocity of the same in relation to the evaluated compounds.
Using the PCA and the correlation analysis applied to the analyzed parameters in the soils, it could be observed that all the parameters exerted a strong influence on the constitution of the PC1, and was mostly located in the negative quadrant of this PC obtained for both soils. The Ca, Mg, K, SB, V, and CEC parameters were the ones that most influenced the construction of PC1 and presented the highest values of correlation, which is due to the dependence among them. If it was possible to made a prospection process to monitor the soils, for the studied condition, the analysis of the mentioned parameters could be used to evaluate the contamination. It is also worth mentioning that in the case of CS, 4 other parameters could also be used in isolation: pHwater, pHKCl, ΔpH, and H + Al.
Samadder et al. (2017) evaluated soil samples contaminated with leachate, collected at different depths in the periphery of a landfill that does not have any coating of its base and leachate treatment system. From the correlation analysis applied to the parameters analyzed in the soil, these also indicated positive correlation values among the parameters Ca, Mg, and K, which confirms that the monitoring of any of these parameters in the soil can indicate the contamination of the same with leachate, and consequent failure of the waterproofing barrier. Mouhoun-Chouaki et al. (2019) evaluating the effects of solid waste discharge on soil quality in a landfill located in Algeria indicated an increase in organic matter and heavy metals (Cu, Zn, Cd, Pb, Ni, and Cr) in contaminated soil, which shows, as in the case of leachate, the possibility of using the organic matter concentration as a possible parameter to indicate soil contamination.
Conclusion
From the temporal analysis of the interaction by diffusion of the leachate in the soils of different textural classes and through the multivariate statistical analysis, PCA and correlation analysis, it was verified that there was interaction of the chemical compounds evaluated with both soils analyzed (sandy and clayey), being the intensity of this interaction dependent on the intrinsic characteristics of each one.
The clay soil (CS) was the one that showed the greatest potential of interaction, mainly due to its composition (soil with high fine content and greater Fe and Al oxides portions—more reactive with the environment), but the studied sandy soil (SS) also presented attenuation potential of contaminants, which indicates the possibility of using it in landfill bases.
It was also noted that some substances migrated more easily from the leachate to the soil, such as N-NH4+ and organic matter (COD). This fact is mainly related to the chemical characteristics of these compounds and also to their high concentration in the leachate.
From the correlation analysis and PCA of the 14 parameters evaluated in the leachate and soil, the following conclusions can be reached: for the monitoring of the leachate, when it is in contact with clay soils (CL), as the one used in this research, the most important parameter to be monitored would be pH, due to its high correlation with the evaluated parameters. For the leachate in contact with the sandy soil (SL), pH, alkalinity, apparent color, and COD (total and filtered) could be used as the key parameters to indicate the contamination of this type of soil with the studied effluent.
For soils, the statistical analysis indicates that the monitoring of the concentration of Ca, Mg, K, SB, base saturation (V), and CEC could be used to evaluate the contamination of these. It should also be noted that in the case of clay soil (CS), four other parameters could also be used in isolation to investigate the contamination of this soil: pHwater, pHKCl, ΔpH, and H + Al.
References
Aboyeji, O. S., & Eigbokhan, S. F. (2016). Evaluations of groundwater contamination by leachates around Olusosun open dumpsite in Lagos metropolis, southwest Nigeria. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2016.09.002.
Akpan, A. E., Ugbaja, A. N., Okoyeh, E. I., & George, N. J. (2018). Assessment of spatial distribution of contaminants and their levels in soil and water resources of Calabar, Nigeria using geophysical and geological data. Environment and Earth Science, 77, 13. https://doi.org/10.1007/s12665-017-7189-1.
APHA – American Public Health Association. (2005). Standard methods for the examination of water and wastewater (Vol. 21, p. 1082). Washington: APHA, AWWA, WPCF.
Arunbabu, K., Indu, S., & Ramasamy, E. V. (2017). Leachate pollution index as an effective tool in determining the phytotoxicity of municipal solid waste leachate. Waste Management. https://doi.org/10.1016/j.wasman.2017.07.012.
Barone, F. S. (1989) Determination of diffusion and adsorption coefficients for some contaminants in clayey soil and rock: laboratory determination and field evaluation. Doctoral thesis – University of Western Ontario.
Canellas, L. P., Zandonadi, D. B., Médici, L. O., Peres, L. E. P., Olivares, F. L., & Façanha, A. R. (2005). Bioatividade de substâncias húmicas: ação sobre desenvolvimento e metabolismo das plantas. In L. P. Canellas & G. A. Santos (Eds.), Humosfera: tratado preliminar sobre a química das substâncias húmicas (pp. 224–243). Campos dos Goytacazes: CCTA, UENF.
Christensen, T. H., Kjeldsen, P., Bjerg, P. L., Jensen, D. L., Christensen, J. B., Baun, A., Albrechtsen, H. J., & Heron, G. (2001). Review: biogeochemistry of landfill leachate plumes. Applied Geochemistry. https://doi.org/10.1016/S0883-2927(00)00082-2.
Cronan, C.S. (2018) Soil biogeochemistry. In: Ecosystem biogeochemistry. Springer Textbooks in Earth Sciences, Geography and Environment. Springer. https://doi.org/10.1007/978-3-319-66444-6_2
De Melo, T. R., Figueiredo, A., Machado, W., & Tavares Filho, J. (2019). Changes on soil structural stability after in natura and composted chicken manure application. International Journal of Recycling of Organic Waste in Agriculture, 8, 1–6. https://doi.org/10.1007/s40093-019-0250-1.
EPA – Environmental Protection Agency. (1993). Manual: Nitrogen. Cincinnati: Ohio.
Gonçalves, F., Souza, C. H. U., Tahira, F. S., Fernandes, F., & Teixeira, R. S. (2018). Incremento de lodo de ETA em barreiras impermeabilizantes de aterro sanitário. Revista DAE. https://doi.org/10.4322/dae.2016.018.
Han, Z., Ma, H., Shi, G., He, L., Wei, L., & Shi, Q. (2016). A review of groundwater contamination near municipal solid waste landfill sites in China. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2016.06.201.
Johnson, R. L., Cherry, J. A., Pankow, J. F. (1989). Diffusive contaminant transport in natural clay: a field example and implications for clay-lined waste disposal sites. Environmental Science & Technology, 23 (3), 340–349
Kapelewska, J., Kotowska, U., Karpińska, J., Kowalczuk, D., Arciszewska, A., & Świrydo, A. (2017). Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchemical Journal. https://doi.org/10.1016/j.microc.2017.11.008.
Kawai, M., Purwanti, I. F., Nagao, N., Slamet, A. H., & Toda, T. (2012). Seasonal variation in chemical properties and degradability by anaerobic digestion of landfill leachate at Benowo in Surabaya, Indonesia. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2012.06.022.
Khattabi, H., Aleya, L., & Mania, J. (2002). Changes in the quality of landfill leachates from recent and aged municipal solid waste. Waste Management & Research. https://doi.org/10.1177/0734247X0202000407.
Kjeldsen, P., Barlaz, M. A., Rooker, A. P., Baun, A., Ledin, A., & Christensen, T. H. (2003). Present and long-term composition of MSW landfill leachate: a review. Critical Reviews in Environmental Science and Technology, 32(4), 297–336.
Koda, E., Tkaczyk, A., Lech, M., & Osinski, P. (2017). Application of electrical resistivity data sets for the evaluation of the pollution concentration level within landfill subsoil. Applied Sciences, 7, 262. https://doi.org/10.3390/app7030262.
Krčmar, D., Tenodi, S., Grba, N., Kerkez, D., Watson, M., Rončević, S., & Dalmacija, B. (2018). Preremedial assessment of the municipal landfill pollution impact on soil and shallow groundwater in Subotica, Serbia. Science of the Total Environment, 615(2018), 1341–1354. https://doi.org/10.1016/j.scitotenv.2017.09.283.
Leite, A. L., Paraguassú, A. B., & Rowe, R. K. (2003). Sorption of Cd, K, F and Cl on some tropical soils. Canadian Geotechnical Journal, 40(3), 629-642.https://doi.org/10.1139/t03-011.
Levy-Booth, D. J., Pr Escott, C. E., & Grayston, S. J. (2014). Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biology and Biochemistry. https://doi.org/10.1016/j.soilbio.2014.03.021.
Lijklema, L. (1972). Factors affecting pH change in alkaline waste water treatment – III: a dynamic simulation. Water Research. https://doi.org/10.1016/0043-1354(72)90091-7.
Ling, C., & Zhang, Q. (2017). Evaluation of surface water and groundwater contamination in a MSW landfill area using hydrochemical analysis and electrical resistivity tomography: a case study in Sichuan province, Southwest China. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-017-5832-7.
Longe, E. O., & Enekwechi, L. O. (2007). Investigation on potential groundwater impacts and influence of local hydrogeology on natural attenuation of leachate at a municipal landfill. International journal of Environmental Science and Technology. https://doi.org/10.1007/BF03325971.
Lopes, D. D., Silva, S. M. C. P., Fernandes, F., Teixeira, R. S., Celligoi, A., & Dall’antônia, L. H. (2012). Geophysical technique and groundwater monitoring to detect leachate contamination in the surrounding area of a landfill e Londrina (PR - Brazil). Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2012.05.028.
Mendonça, E. S., & Rowell, P. L. (1996). Mineral and organic fractions of two oxisols and their influence on effective cation - exchange capacity. Soil Science Society of America Journal. https://doi.org/10.2136/sssaj1996.03615995006000060038x.
Mishra, S., & Tiwary, D. (2018). Leachate characterization and evaluation of leachate pollution potential of urban municipal landfill sites. International Journal of Environment and Waste Management. https://doi.org/10.1504/IJEWM.2018.093431.
Moreira, C. A., Helene, L. P. I., Nogara, P., & Ilha, L. M. (2018). Analysis of leaks from geomembrane in a sanitary landfill through models of electrical resistivity tomography in South Brazil. Environment and Earth Science, 77(7). https://doi.org/10.1007/s12665-017-7180-x.
Mouhoun-Chouaki, S., Derridj, A., Tazdaït, D., & Salah-Tazdaït, R. (2019). A study of the impact of municipal solid waste on some soil physicochemical properties: the case of the landfill of Ain-El-Hammam Municipality, Algeria. Applied and Environmental Soil Science. https://doi.org/10.1155/2019/3560456.
Naveen, B. P., Mahapatra, D. M., Sitharam, T. G., Sivapullaiah, P. V., & Ramachandra, T. V. (2017). Physico-chemical and biological characterization of urban municipal landfill leachate. Environmental Pollution. https://doi.org/10.1016/j.envpol.2016.09.002.
Nguyen, T.-B., Lim, J., & Choi, H. (2011). Numerical modeling of diffusion for volatile organic compounds through composite landfill liner systems. Journal of Civil Engineering. https://doi.org/10.1007/s12205-011-1293-7.
Norton, J., Alzerreca, J., Suwa, Y., & Klotz, M. (2002). Diversity of ammonia monooxygenase operon in autotrophic ammonia oxidizing bacteria. Archives of Microbiology. https://doi.org/10.1007/s00203-001-0369-z.
Oliveira, A. C. D. G., Correa, C. Z., Prates, K. V. M. C., & Lopes, D. D. (2017). Nitrifying, denitrifying and heterotrophic biomass present in moving bed-reactor. American Journal of Environmental Sciences. https://doi.org/10.3844/ajessp.2017.47.57.
Parameswari, K., & Mudgal, B. V. (2014). Geochemical investigation of groundwater contamination in Perungudi dumpsite, South India. Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-013-0832-6.
Pavan, M. A., Blocj, M. F., Zempulski, H. C., Miyazawa, M., & Zocoler, D. C. (1991). Manual de análise química do solo. Paraná: Instituto Agronômico do Paraná, Londrina.
Prosser, J. I., & Nicol, G. W. (2008). Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environmental Microbiology. https://doi.org/10.1111/j.1462-2920.2008.01775.x.
Przydatek, G., & Kanownik, W. (2019). Impact of small municipal solid waste landfill on groundwater. Quality. Environ Monit Assess, 191, 169. https://doi.org/10.1007/s10661-019-7279-5.
Rizzo, R. P., & Lollo, J. A. (2006). Capacidade de retenção de barreiras de proteção produzidas com solo arenoso estabilizado quimicamente. Engenharia Sanitaria e Ambiental. https://doi.org/10.1590/S1413-41522006000300008.
Samadder, S. R., Prabhakar, R., Khan, D., Kishan, D., & Chauhan, M. S. (2017). Analysis of the contaminants released from municipal solid waste landfill site: a case study. Science of the Total Environment, 580, 593–601. https://doi.org/10.1016/j.scitotenv.2016.12.003.
Shu, S., Zhu, W., Wang, S., Ng, C. W. W., Chen, Y. M., & Chiu, A. C. F. (2018). Leachate breakthrough mechanism and key pollutant indicator of municipal solid waste landfill barrier systems: centrifuge and numerical modeling approach. Science of the Total Environment, 612, 1123–1131. https://doi.org/10.1016/j.scitotenv.2017.08.185.
Spagni, A., & Marsili-Libelli, S. (2009). Nitrogen removal via nitrite in a sequencing batch reactor treating sanitary landfill leachate. Bioresource Technology. https://doi.org/10.1016/j.biortech.2008.06.064.
Sposito, G. (1989). The chemistry of soils. New York: Oxford University Press.
Tałałaj, I. A., Biedka, P., Walery, M. J., & Leszczyński, J. (2016). Monitoring Of leachate quality at a selected municipal landfill site in Podlasie, Poland. Journal of Ecological Engineering. https://doi.org/10.12911/22998993/63477.
Tedesco, M. J., et al. (1995). Análises de solo, plantas e outros materiais (2.Ed ed.). Porto Alegre: UFRGS 174 p.
Vahabian, M., Hassanzadeh, Y., & Marofi, S. (2019). Assessment of landfill leachate in semi-arid climate and its impact on the groundwater quality case study: Hamedan, Iran. Environmental Monitoring and Assessment, 191, 109. https://doi.org/10.1007/s10661-019-7215-8.
Wan, G. X., Han, C., Zh An, G. J., Hu An, G. Q., Den, G. H., Den, G. Y., & Zh Ong, W. (2015). Long term fertilization effects on active ammonia oxidizers in an acidic up land soil in China. Soil Biology and Biochemistry. https://doi.org/10.1016/j.soilbio.2015.02.013.
Xie, H., Chen, Y., Thomas, H. R., Sedighi, M., Masum, S. A., & Ran, Q. (2016). Contaminant transport in the sub-surface soil of an uncontrolled landfill site in China: site investigation and two-dimensional numerical analysis. Environmental Science and Pollution Research, 23, 2566–2575. https://doi.org/10.1007/s11356-015-5504-5.
Yin, C., Fan, F. L., Son, G. A. L., Fan, X. P., Din, G. H., Ran, W., Qiu, H. Z., & Lian, G. Y. C. (2017). The response patterns of community traits of N2O emission-related functional guilds to temperature across different arable soils under inorganic fertilization. Soil Biology and Biochemistry. https://doi.org/10.1016/j.soilbio.2017.01.022.
Yong, R. N., Mohamed, A. M. O., & Warkentin, B. P. (1992). Principles of contaminant tranport in soils (p. 327). Amsterdam: Elsevier Science Publishers B. V.
Acknowledgments
The authors would like to thank the Coordination of Improvement of Higher Level Personnel (CAPES) for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonçalves, F., Correa, C.Z., Lopes, D.D. et al. Monitoring of the process of waste landfill leachate diffusion in clay and sandy soil. Environ Monit Assess 191, 577 (2019). https://doi.org/10.1007/s10661-019-7720-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7720-9