Abstract
Monitoring of air quality using lichens as bioindicators on the basis of lichen diversity and frequency is limited along rural–urban ecosystems in tropics. This study attempted to assess and correlate the use of corticolous lichens with atmospheric SO2 and NO2 in such an ecosystem in Sabaragamuwa Province in Sri Lanka. Nine sampling locations, each having three subsampling sites with 162 Mangifera indica and Cocos nucifera trees, were selected for the study. The coverage and frequency of lichens found on selected trees were recorded by 400-cm2 grids and identified using taxonomic keys. SO2 and NO2 levels at each site were determined by “Ogawa” passive air samplers. Data of lichen diversity were used to formulate the index of atmospheric purity (IAP). The environmental parameters related to lichen colonization were measured using standard methods. Data were analyzed using MINITAB 17. The mapping of spatial distribution of lichens and air pollutants were done using inverse distance weighting surface interpolation of geographical information system based on IAP values. A negative correlation was observed between IAP and SO2 and NO2 levels. The presence of the genus Pyxine in almost all urban sites indicated that it could be used as a reliable pollutant tolerant indicator in urban ecosystems. In addition, the index-based mapping techniques could be used successfully to see the effect of atmospheric pollution in urban ecosystems. These results conclude that corticolous lichens have the potential to be used as bioindicators of air quality monitoring along rural–urban ecosystems of tropics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air quality monitoring is a systematic approach for studying the condition of the atmosphere, and it is the key component of air quality management. The concentration of pollutants and other parameters related to pollution describe the quality of the atmosphere. Monitoring and quantification of diverse atmospheric pollutants are usually done by means of physicochemical methods (Matusmoto and Mizoguchi 1995). Although these methods provide accurate and reliable data, the instruments required for such assays are expensive and do not provide monitoring at high intensity levels across large areas at different locations (Attanayaka and Wijeyaratne 2013). Nevertheless, biomonitoring is an appropriate tool for assessing the levels of atmospheric pollution (Smodis and Parr 1999). For this purpose, bioindicators are commonly used for the identification and qualitative determination of the levels of atmospheric pollution (Tonneijck and Posthumus 1987).
Among various bioindicators, lichens could be considered as a better group of organisms for air pollution monitoring (Ferry et al. 1973). Lichens are found from the tropics to polar regions, in built-up areas and even in extreme environments where a separate mycobiont and photobiont would be rare or non-existent (Weerakoon 2015). Monitoring the pollution status or health of the ecosystems using lichens has been carried out extensively for several decades, and a large body of literature has been published especially in temperate regions. Nevertheless, in tropical climates, limited information is available with regard to lichen diversity and air quality. Most of these limited researches have also been confined to forest ecosystems. Sri Lanka is one of the smallest, but biologically diverse countries in South Asian region. Consequently, it is recognized as a biodiversity hotspot of global and national importance. The availability of remarkable number of species of lichens in rural ecosystems is very much interesting. Nevertheless, urbanization followed by traffic congestion have contributed to the degradation of ambient air quality in many urban ecosystems. Thus, the present study was planned to assess and correlate the use of corticolous lichens as a potential biomonitoring tool in the estimation of atmospheric SO2 and NO2 along a rural–urban ecosystem in the Sabaragamuwa Province in Sri Lanka.
Methodology
Study area and subsampling sites
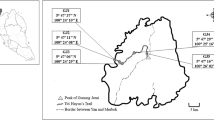
The study area was Kegalle urban council area (between N 070 42.622/ E 0790 49.072/ and N 070 15.335/ E 0800 20.410/) which is located in the Kegalle District in Sabaragamuwa Province, Sri Lanka. Nine study sites were selected randomly considering vegetation cover, land use patterns, and vehicular traffics. Selected sites covered urban, semi-urban, and rural ecosystems (Fig. 1). The sampling area of each site was about 0.25 km2, and all the sites were located within the same climatic zone. Each site consisted of three subsampling sites.
Lichen sampling
A total of 162 individual trees (81 Mangifera indica and 81 Cocos nucifera) was mapped covering all three ecosystems using GARMIN (etrex 10) GPS. A 400-cm2 (20 cm × 20 cm) transparent quadrate was placed randomly at north and south directions at 1.5 m height of the bole of each tree which had a diameter at breast height (DBH) greater than 10 cm to quantitatively determine the coverage and the frequency of lichens on vertical trees. The number of lichen species observed within the grid and the number of grid units in which a particular species was observed were counted. Lichen species with a diameter less than 3 mm were not recorded. The lichen collection was stored in 20 °C in a laboratory of the Department of Zoology and Environmental Management, University of Kelaniya, for identification.
Identification of lichens
The specimens were identified up to generic level and where possible to species level using several field keys (Weerakoon 2015; Wolseley and Chimonides 2007; Bungartz et al. 2010; Awasthi 1988, 1991; Barkman 1958). Microscopic observations of freehand sections of thallus and fruiting bodies were also made in order to follow the keys. Some lichen species were identified by extensive matching with correctly identified specimens deposited at the National Herbarium, National Botanic Gardens, Peradeniya-Sri Lanka.
Species richness and total abundance
Species richness is the total number of species in an assemblage or a sample while total abundance is the total number of individuals of all species recorded within the study area (Colwel 2009). Thus, species richness was determined using the number of lichen species observed within the grid and the number of grid units in which a particular species was observed. The total abundance was estimated for each site separately by adding all the individuals of each species present in the particular site.
Species diversity and evenness
Lichen diversity of each site was determined using Shannon’s diversity index, and the distribution of individuals of a species was determined using Pielou’s evenness index.
Shannon–Wiener diversity index (H′)
where
- Pi :
-
The proportion of individuals in the “ith” taxon of the community
- s :
-
Total number of taxa in the community
Pielou’s evenness index (J)
where
- H1:
-
Shannon–Wiener diversity index
- S:
-
The number of species in the community or species richness
Index of atmospheric purity
The index of atmospheric purity (IAP) gives an evaluation of the level of atmospheric pollution, which is based on the number (n), frequency (f), and tolerance of the lichens present in the area under study. The IAP values were calculated as described by Conti (2008):
where
- n :
-
Number of species recorded
- Q :
-
Ecological index (the average number of species, which coexisted with each species)
- f :
-
Cover or frequency of each species
The Q (for any particular species) was calculated by adding together the number of species present (growing) at various sites and then dividing this sum by the total number of sites where that species occurred (LeBlanc et al. 1974). The cover or frequency of each species (f) was calculated using a transparent quadrat that was used for the sampling of lichens. The frequency–coverage (f) and the ecological index (Q) data of lichens were compiled and tabulated separately for each of the nine sites to calculate the IAP.
Percentage canopy cover
Over story canopy cover was determined in each lichen sampling event. For this purpose, each selected tree was measured using canopy cover grid which consists of 100 blank squares. The canopy cover grid sheet was placed on the ground directly below the canopy of the sampling tree. The number of squares that were shaded were counted. Percentage Canopy cover was calculated as follows:
Bark pH
The bark pieces (about 0.5 g) were soaked in 5.0 mL of 0.025 M KCl (potassium chloride) solution. After soaking, they were kept for approximately 8 h at 20 °C. The pH values were determined using a calibrated standard pH electrode (model: 6011A).
Light intensity
Lux meter (digital model—DLM 530) was used to measure the light intensity at each selected tree at the time of sampling.
Geographical data treatment
ArcGIS 10.2.2 software package was used to perform surface interpolation for all sampling sites using estimated IAP values to measure the impact of atmospheric pollution in the study area.
Atmospheric pollutants—determination of SO2 concentrations
Coating, assembling, and installing of “Ogawa” passive air sampler
The “Ogawa” passive air samplers (Ogawa and Co., USA) were installed at selected all subsampling sites. The samplers were loaded with coated filter pads by modifying the methodology described by Hirano and Maeda (1996). For this purpose, triethanolamine (TEA) (22.30 mL), ethylene glycol (3.60 mL), and acetone (50%, 50 mL) were added to a 100-mL volumetric flask and the mixture was topped up with the distilled water for the preparation of 100 mL of coating reagent. Whatman GF/B (47 mm) filter papers were mechanically cut according to the appropriate size, washed with deionized water using suction filtration technique, and kept in a desiccator to dry. The dried filter papers were coated with coating reagent prepared (100 μL) using a micropipette. After the coating process, they were further dried in a desiccator. The coated filter paper was placed in the relevant places of each chamber of the “Ogawa” passive air sampler. The passive air samplers were fixed to the windshields and exposed to air for a period of 1 month. Windshields were fixed to suitable places that were located away from the roadsides and other emission sources as described by Hirano and Maeda (1996) in order to prevent the direct contact of filter pads with emissions. All the samplers were installed at a height of about 3 m from the ground level (Fig. 2).
Analysis of SO2
Analysis of SO2 was done as described by Hirano and Maeda (1996). The trapped SO2 in the exposed filter papers was extracted into 10 mL of color-developing reagent (sulfamic acid 1 mL, formaldehyde 2 mL, pararosaniline 2 mL, and water 5 mL) to develop the color, and the absorbance was measured at 500 nm using UV spectrophotometer (model: UV-1650PC). The concentration of SO2 was then calculated from a calibration plot with known concentrations of TEA solution.
Determination of NO2 concentrations
Coating, assembling, and installing of the “Ogawa” passive sampler
The same type of the “Ogawa” passive air samplers and windshields were used to trap NO2. Sampling procedure of NO2 was similar to that of SO2. The samplers were loaded with coated filter pads by modifying the methodology described by Hirano and Maeda (1996). For this purpose, 6.1 g of sodium iodide, 0.880 g of sodium hydroxide, and 3.6 g of ethylene glycol were added to a 100-mL volumetric flask and was topped up with methanol solution (1:3 ratio) for the preparation of 100 mL of coating reagent. Whatman 150-mm filter papers were then mechanically cut according to the appropriate size, were washed using de-ionized water using suction filtration technique, and were kept in a desiccator to dry. The dried filter papers were coated with 50 μL coating reagent using a micropipette. After the coating, they were further dried in a desiccator. The dried coated filter paper was placed in the passive air sampler and installed at sampling site.
Analysis of NO2
Analysis of SO2 was done as described by Hirano and Maeda (1996). The trapped NO2 was extracted into 10 mL of Saltzman reagent. The absorbance was measured at 545-nm wave length using UV spectrophotometer (model: UV-1650PC). The concentration of NO2 was calculated against a calibration plot with known concentrations of sodium nitrite.
Results
Species richness and total abundance
A total of 89 lichen species representing 25 genera and 15 families from nine sampling locations were found (Fig. 3). The genus Graphis of the family Graphidaceae was the most dominant lichen genera in the study area followed by Cryptothecia of the family Arthoniaceae, Arthonia of the family Arthoniaceae, Pyrenula of the family Physciaceae, respectively.
The highest lichen richness was observed from rural ecosystem (77 species), while 36 species were observed from semi-urban ecosystem and 35 species were recorded from urban ecosystem, respectively. In addition, number of genera and number of families were gradually decreased from rural to urban (Fig. 4). The selected rural ecosystem (RU1, RU2, and RU3) represented 80% of the total genera recorded while urban ecosystem (U1, U2, and U3) represented 52% of the total genera.
Of the recorded lichen genera, ten (10) genera including Arthonia, Chrysothrix, Cryptothecia, Dirinaria, Myriotrema, Pertusaria, Pyrenula, Pyxine, Sarcographa, and Graphis were recorded from all three ecosystems, while Megalospora, Parmotrama, and Porina were found from both rural and the semi-urban ecosystems. Leptogium, Physcia, and Platythecium genera were recorded from both semi-urban and the urban ecosystems (Fig. 5). In addition, seven (07) lichen genera including Cresponia, Heterodermia, Pyrenocarp, Chapsa, Dictyonema, Lecanora, and Ocellularia were only found from selected rural ecosystems and Collema and Leptotrema genera were only found in semi-urban ecosystems.
In rural ecosystem, the most distributed lichen genera were Cryptothecia, Graphis, and Pertusatia. In semi-urban ecosystem, the most distributed lichen genera was Cryptothecia. Nevertheless, the abundance of Graphis was comparatively low. Pyxine was the second highest abundant in semi-urban ecosystem, and it was the dominant lichen genus found in urban ecosystem. The abundance of Cryptothecia and Graphis species in urban ecosystem were remarkably reduced when compared with rural and urban ecosystems.
Lichen diversity and evenness
According to the Shannon–Wiener diversity index (H′), selected rural ecosystem showed the highest lichen diversities (> 2.0) than semi-urban or urban ecosystems (Table 1). The Pielou’s evenness index which describes the evenness of distribution of lichens within the study area showed the highest values from rural ecosystem (> 0.65) and when moving into the urban ecosystem, the evenness showed a decreasing trend.
Index of atmospheric purity and atmospheric pollutants
Selected urban ecosystem showed high level of atmospheric pollution. The moderate level of pollution was recorded from SU1, SU2, and SU3, respectively. The highest IAP was recorded from RU3 (90.26) which indicated the lowest level of atmospheric pollution (Fig. 6). Both NO2 and SO2 levels in the ambient air were decreased when moving from urban to rural ecosystems (Fig. 7).
Environmental parameters related to lichen diversity
The mean bark pH values of C. nucifera and M. indica sampled in the study area were acidic and varied from 4.26 to 5.58. Nevertheless, a remarkable pH variation was not observed from different ecosystems. On the other hand, the canopy cover of the selected two tree types showed a significant increase (p ≤ 0.05; one-way ANOVA) along urban–rural ecosystems (Table 2).
In general, when moving away from urban to rural ecosystems, the mean lichen diversity showed an increasing trend. The highest diversity index of 2.9324 was recorded from RU3 on C. nucifera trees, and the lowest lichen diversity index of 1.3836 was recorded from U1 on M. indica trees (Table 2).
Considering air pollutants, NO2 levels in urban ecosystem were significantly higher than that of the semi-urban or rural ecosystems (p ≤ 0.05). The lowest ambient air pollutant concentrations (in terms of NO2 and SO2) were found from rural ecosystem. The lowest NO2 concentration was recorded from RU1 (9.32 ± 2.81 μg/m3) while the lowest SO2 level (0.062 ± 0.06 μg/m3) was recorded from RU3.
The relationship between the lichen diversity indices, atmospheric pollutants, and environmental parameters
SO2 concentrations of the study area has a positive relationship with NO2 concentrations, and the correlation coefficient showed very high significant difference at p ≤ 0.05 level (Table 3). Correlation coefficients between lichen diversity and bark pH of the three two plant species were not significantly different. Percentage canopy cover of C. nucifera has a negative correlation with both NO2 and SO2 concentrations. A positive correlation between the Shannon–Wiener diversity index of M. indica and the percentage canopy cover of M. indica was observed.
Index of atmospheric purity (IAP) showed a negative correlation with Shannon–Wiener diversity index of C. nucifera and M. indica at p ≤ 0.01 significant level, and the IAP values increased when decreasing the NO2 and SO2 concentration from rural to urban ecosystems. A very high positive correlation between the Shannon–Wiener diversity index of C. nucifera and M. indica and IAP values of the study area at p ≤ 0.05 significant level and the positive correlation between IAP values and the Pielou’s evenness index (J) of Cocos nucifera and M. indica were observed (Table 3). The Shannon–Wiener diversity index of C. nucifera and M. indica has a negative correlation with SO2 and NO2 concentrations. The Pielou’s evenness index (J) of C. nucifera has a negative relationship with NO2 concentrations. Both NO2 and SO2 concentrations also showed a very high negative relationship at p ≤ 0.05 level with the Pielou’s evenness index (J) of M. indica (Table 3).
Geographical data treatment
The surface interpolation of IAP values using inverse distance weighting (IDW) method of geographical information system was able to locate the most disturbed zones and the most undisturbed zones in the study area. Surface interpolation is the estimation of surface values at unsampled points based on known surface values of surrounding points. One of the most common approaches in biomonitoring with lichens is by means of index of atmospheric purity (IAP). Thus, the areas with greatest disturbance to the lichen diversity and the highest atmospheric pollution levels were indicated in red while the green color indicated the lowest disturbance to the lichen communities and the lowest levels of atmospheric pollution. Moderate levels of atmospheric pollution levels indicated in yellow and pale orange colors in the map (Fig. 8).
Discussion
The present study attempted to assess and correlate the use of corticolous lichens with atmospheric SO2 and NO2 along rural–urban ecosystems in Kegalle in Sabaragamuwa Province in Sri Lanka. In the ecosystems studied, air pollution with respect to SO2 and NO2 could be highly attributed to traffic congestion, which is again related to land use pattern to some extent. Increase of clearance together with vehicular emissions to the atmosphere mainly in urban ecosystem and also some semi-urban areas has exerted an unavoidable pressure on the ambient air quality and the health of habitats and organisms including lichens. Belnap et al. (2006) reported that lichens can respond rapidly to environmental changes through both reductions and increments in cover. Nevertheless, Hawksworth and Rose (1970) observed an increase in abundance due to the induced resistance to any particular type of pollutant in Toronto, Canada. The present results revealed that urban ecosystem contained the lowest lichen species richness. Occurrence of many lichen species were higher in rural ecosystem than semi-urban or urban ecosystems. Similar results were also observed from a study carried out by Das (2008) in India.
When considering the semi-urban ecosystem studied, the genus Chryptothecia was the dominant lichen species followed by the genus Pyxine. According to the lichen distribution of three selected urban sites, the genus Pyxine was the pre-dominant lichen species. A trend of decline of the genus Chyptothecia and the genus Graphis was observed in urban ecosystem. Hence, it could clearly be attributed that most lichen species were replaced by weedy lichens including Pyxine spp. along with higher environmental disturbances. Genus Pyxine was frequently found on the bark of trees especially along heavily polluted roadsides in urban ecosystems and also some semi-urban sites. Wolseley and Aguirre-Hudson (2007) stated that these species are often found to be tolerant of atmospheric pollutants such as oxides of sulfur and used as environmental bioindicators to assess air quality. Many studies that had been carried out in different countries described Pyxine spp. along with Dirinaria spp. is tolerant of air pollution in urban sites (Saipunkaew et al. 2005). A global increase in members of the Physciaceae (including the genus Pyxine) had also been linked to climate change. Some Pyxine spp. growing inside the industrial area is the most tolerant and has accumulated higher levels of all the heavy metals analyzed (Asta et al. 2002).
Lichen diversity indices can be taken as estimates of environmental quality and stress that are prevailing in the area, where higher values correspond to good quality with low stress and low values indicate poor quality and high stress (Asta et al. 2002). The Shannon–Wiener index and Pielou’s evenness index values expressed a decreasing pattern from rural to urban ecosystems. The highest lichen diversity which was recorded from rural ecosystem (RU1, RU2, and RU3) indicated that lichens may rapidly colonize on available substrata, where fragments of undisturbed areas remain. The low level of disturbance and comparatively high moisture caused low level of environmental stress for epiphytic lichen development and increase in lichen diversity in rural ecosystems. Low diversity recorded from the selected urban sites indicated that the natural vegetation has been removed and replaced by urban infrastructure and those urban sites and some semi-urban sites located in close proximity to a main road and indicated the possible air pollution due to vehicular emissions, which could have influenced on the reduction of lichens diversity resulting no source of lichen propagules or suitable substrata for colonization. Low diversity in those areas may have resulted since the recovery of specialist lichen species is slow due to poor dispersal with the unsuitable environmental conditions and absence of specialized habitats for development of their thalli. Accordingly, the Pielou’s evenness index values showed higher values in rural ecosystem when compared with urban ecosystem which indicated that the existing lichen species were evenly distributed throughout the areas unless the urban ecosystem that were predominated by small number of specific lichen species (e.g., Pyxine) which can tolerate the harsh environmental conditions in disturbed selected urban sampling sites.
Several researchers have identified land transport as the main cause of pollution in urban areas of Sri Lanka (Yalegama 2004). This could be further confirmed by the observed negative significant NO2 and SO2 levels over lichen diversity studied. Hawksworth and Rose (1970) and Richardson (1988) reported that epiphytic lichens are sensitive to phytotoxic gases mainly NOx and SOx. According to Skye (1958), a correlation of air pollution data with the distribution of epiphytic lichens in highly disturbed areas showed a weaker growth than in areas which were pristine; the pattern of atmospheric pollution confirmed closely to the distribution of lichens. Nevertheless, concentrations of air pollutants can be affected adversely or beneficially on the growth of the lichen thallus. Conti and Cecchetti (2001) stated that lichen membrane proteins may be damaged by the presence of SO2 and the lichen enzymes can have considerable damage in the presence of high levels of SO2, which may cause a reduction in protein biosynthesis in some lichens; or there may be negative effect on the nutritional interchange between symbionts with, as a consequence, an alteration of their delicately balance. In addition, Gonzalez-Tezero et al. (1995) stated that SO2 is a powerful catalysts of lipid membrane peroxidation such as O3 and NO2. As such, both SO2 and NO2 may cause a reduction of pH of lichen thalli.
In the present study, the highest monthly concentration of NO2 were recorded from U2 location and SO2 were recorded from U1 sampling location (29.15 μg/m3 NO2 and 1.024 μg/m3 SO2, respectively) which were located in highly disturbed urban area. Nevertheless, SO2 and NO2 levels were very much lower in the sparsely disturbed rural ecosystem. The lowest monthly NO2 were recorded from RU1 site and SO2 concentrations were recorded from RU3 site (9.32 μg/m3 NO2 and 0.062 μg/m3 SO2, respectively). When the effects of SO2 and NO2 are taken together, SO2 has a significant positive correlation with NO2.
The sensitivity of the photobiont of lichens to the conditions of the environment, especially temperature and moisture, is a critical factor in the survival of the lichen–algal symbiosis (Wolseley and Aguirre-Hudson 2007). Nevertheless, the study carried out by Attanayaka and Wijeyaratne (2013) reported that exposure of corticolous lichens to light seems to have no significant effect on the lichen diversity examined in their study. But, studies have shown that different lichen families have preferences either for light or shaded conditions (Attanayaka and Wijeyaratne 2013). In the present study, over story canopy cover was measured to see the correlation with lichen diversity. The Shannon–Wiener diversity index values for lichens of M. indica showed a significant positive correlation with the percentage canopy cover of M. indica trees, and there was a significant negative correlation between ambient NO2 and SO2 levels and percentage canopy cover of C. nucifera trees. Basically, it indicates that the reduction of lichen diversity could be resulted with the decline of canopy cover. Evidently, the percentage canopy cover was significantly reduced (Table 3) when moving from rural to urban ecosystems within the study area. Hence, the decline of canopy cover can contribute to direct contact of vehicular emissions with lichens that are available on vegetation, and in turn, it may dramatically reduce the species diversity of lichens.
Many researchers have used IAP to monitor the effects of atmospheric pollutants (especially SO2 and NO2) on living organisms as a quantitative method (Krick and Loppi 2002). IAP showed a significant negative correlation with both ambient NO2 and SO2 levels. Therefore, IAP in the present study gave lower IAP values for highly disturbed urban ecosystem which were having higher air pollutant concentrations, while the rural ecosystem showed higher IAP values indicating the less air pollutant concentrations. There was a significant positive correlation between IAP values and Shannon–Wiener diversity indices and Pielou’s evenness indices of the sampling locations within the study area which indicate that increase in lichen diversity in a particular area has higher IAP values and has better ambient air quality.
The compositional changes in lichen communities were correlated with the changes in levels of atmospheric pollution. IAP gives an evaluation of the level of atmospheric pollution, which is based on the number, frequency, and tolerance of the lichens present in the study area. Conti and Cecchetti (2001) categorized pollution level of atmosphere considering IAP values in tropics (Table 4).
Accordingly, all the selected urban sites fall into level B category, which means a high level of pollution. Selected three semi-urban areas have moderate level of pollution and can be categorized under level C based on the above classification. RU1 rural site falls into the level D which indicates low level of pollution having 41.60 of IAP while other two rural areas; RU2 and RU3 expressed the very low levels of pollution (60.63, 90.26 of IAP, respectively) that can be categorized under level E. In the present study, there was no any “lichen desert” recorded (area with no lichens/IAP = 0).
Mapping of lichen diversity using values of IAP is an attractive approach. Showing spatial distribution patterns of the studied descriptors, maps of lichen biodiversity, or abundance enable a quick and clear identification of areas with different levels of disturbance (Pinho et al. 2004; Asta et al. 2002). Spatial mapping of lichen diversity or associated measurements had been extensively used both in research and applicative lichen biomonitoring works (Pinho et al. 2004). GIS software is an ideal tool for interpolating the estimated values of the response variable such as IAP values related to the lichen diversity in non-measured parts of the study area. Unlike IAP zones demarcated according to the quality level categorization of IAP (Conti and Cecchetti 2001), the zone map in the present study showed a better visual presentation due to use of interpolation with the help of GIS software (ArcMap 10.2.2.). According to the resultant map, large amount of air pollutants (NO2 and SO2) were concentrated around urban ecosystem which indicate in U1, U2, and U3 and it resulted to the declining of lichen diversity. Semi-urban areas showed moderate pollution levels which indicate in SU1, SU2, and SU3 expressing the intermediate lichen diversities. Rural ecosystem showed lower level of accumulation of air pollutants with low level of traffic conditions or transportation and less urbanization. RU3 sampling site and adjacent areas showed the lowest air pollution and the conditions in RU2 site was more or less similar to RU3 site. Nevertheless, RU1 site showed a higher pollution level than other rural sites and it could be attributed to the accumulation of some amount of vehicular emissions coming from a bypass road.
Conclusion
The present study clearly revealed that vehicular emissions have an influence on the diversity and distribution of lichens in the study area. The presence of the genus Pyxine in almost all urban sites indicated that it could be used as a reliable pollutant tolerant indicator in urban ecosystems, while the genus Graphis was very sensitive for ambient air pollutants. Results further revealed that the index-based mapping techniques could be used successfully to see the effect of atmospheric pollution along rural–urban ecosystems. These results conclude that corticolous lichens have the potential to be used as bioindicators of ambient air quality monitoring along rural–urban ecosystems of tropics.
References
Asta, J., Erhardt, W., Ferretti, M., Fornasier, F., Kirschbaum, U., Nimis, P.L., Purvis, O.W., Pirintsos, S., Scheidegger, C., Van Haluwyn, C., Wirth, V., et al. (2002). European guideline for mapping lichen diversity as an indicator of environmental stress. London. The British Lichen Society. pp. 273–279. In: Nimis, P.L., Scheidegger, C. & Wolseley, P.A. (eds), Monitoring with Lichens - Monitoring Lichens, Kluwer Academic Publishers, Dordrecht, Boston, London.
Attanayaka, A. N. P. M., & Wijeyaratne, S. C. (2013). Corticolous lichen diversity, a potential indicator for monitoring air pollution in tropics. Journal of the National Science Foundation of Sri Lanka, 41, 131–140.
Awasthi, D.D. (1988). A key to the macro lichens of India, Nepal and Sri Lanka, pp. 120. Cramer, India.
Awasthi, D.D. (1991). A key to the micro lichens of India, Nepal and Sri Lanka, pp. 80. Cramer, India.
Barkman J.J. (1958). Phytosociology and ecology of cryptogamic epiphytes. Van Gorcum & Company, Assen, The Netherlands.
Belnap, J., Phillips, S. L., & Troxler, T. (2006). Soil lichen and moss cover and species richness can be highly dynamic: The effects of invasion by the annual exotic grass Bromus fectorum, precipitation, and temperature on biological soil crusts in SE Utah. Applied Soil Ecology, 32, 63–76.
Bungartz, F., Lücking, R., & Aptroot, A. (2010). The family Graphidaceae (Ostropales, Lecanoromycetes) in the Galapagos Islands. Nova Hedwigia, 90, 1–44.
Colwel, R.K. (2009). Biodiversity: Concepts, patterns, and measurements. The Princeton guide to eclogy, pp.257–263.
Conti M.E. (2008). Biological monitoring: Theory & applications. Wit Press, Sothhampton, Boston.
Conti, M. E., & Cecchetti, G. (2001). Biological monitoring: Lichens bioindicators of air pollution assessment: A review. Environmental Pollution, 114, 471–492.
Das, P. (2008). Lichen flora of Cachar District (southern Assam) with reference to occurrence, distribution and its role as environmental bioindicators. Ph.D. thesis. In Assam University. India: Silchar.
Ferry, B. W., Backley, M. S., Hawkswarth, D. L., et al. (1973). Air pollution and lichens. London: The Athlone Press.
Gonzalez-Tezero, M. R., Martinez- Lirola, M. J., Casares- Porcel, M., Molero-Mesa, J., et al. (1995). Three lichens used in popular medicine in eastern Andalucia (Spain). Economic Botany, 49, 96–98.
Hawksworth, D. L., & Rose, F. (1970). Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature, 227, 145–148.
Hirano, K., & Maeda, H. (1996). Monitoring methods of NO2, and SO2 in ambient air using a diffusion sampler. Yokohama City Research Institute for Environmental Science, Yokohama, Japan.
Krick, R., & Loppi, S. (2002). The IAP approach. In P. L. Nimis, C. Scheidegger, & P. A. Wolseley (Eds.), Monitoring with lichens – monitoring lichens (pp. 21–37). The Netherlands: Kluwer Academic Publishers.
LeBlanc, F., Robitaille, G., & Rao, D. N. N. (1974). Biological response of lichens and bryophytes to environmental pollution in the Murdochville copper mine area, Quebec. Hattori Botanical Laboratory, 38, 405–433.
Matusmoto, M. & Mizoguchi, T. (1995). A simple and simultaneous measurement method of sulphur dioxide in atmosphere using molecular diffusion sampler, International Seminar on the Simple Measuring and Evaluation Method on Air Pollution, Japan Society of Air Pollution (JSAP) and Environmental Research and Training Center (ERTC), Pathumthani, Thailand.
Pinho, P., Augusto, S., Branquinho, C., Bio, A., Pereira, M. J., Soares, A., Catarino, F., et al. (2004). Mapping lichen diversity as a first step for air quality assessment. Journal of Atmospheric Chemistry, 49, 377–389.
Richardson, D. H. S. (1988). Understanding the pollution sensitivity of lichens. Botanical Journal of the Linnean Society, 96, 31–43.
Saipunkaew, W., Wolseley, P., & Chimonides, P. J. (2005). Epiphytic lichens as indicator of environment health in the vicinity of Chiang Mai city, Thailand. The Lichenologist, 37(4), 345–356. https://doi.org/10.1017/S0024282905014994.
Skye, E. (1958). The influence of air pollution on the fruiticolous and foliaceous lichen flora around the shale-oil works at Krarntop in the province of Narke. Svensk Bot. Tidskr. 52: 133–190. 345–356.
Smodis, B., & Parr, R. M. (1999). Biomonitoring of air pollution as exemplified by recent IAEA programs. Biological Trace Element Research, 71(1), 257–266.
Tonneijk, A. E. G., & Pasthumus, A. C. (1987). Use of indicator plants for biological monitoring of effects of air pollution: The Dutch approach. VDI Berichte, 609, 205–216.
Weerakoon, G. (2015) Fascinating lichens of Sri Lanka. Dilmah Conservation, Sri Lanka.
Wolseley, P.A. & Aguirre-Hudson, B. (2007). Lichens as indicators of environmental changes in the tropical forests of Thailand. http://www.jstor .org/locate/envpol. Accesed 23 March 2017.
Wolseley, P., & Chimonides, J. (2007). Corticolous lichen and moss communities in lowland dipterocarp forests under differing management regimes. Bibliotheca Lichenologica., 95, 583–603.
Yalegama, S.S.B. (2004). Impact of emission standards on particulate pollution from diesel vehicles, Proceedings of the First National Symposium on Air Quality Management in Sri Lanka, 2–3 December, Air Resource Management Centre and Ministry of Environment and Natural Resources, Sri Lanka.
Acknowledgements
The authors acknowledge Dr. Udeni Jayalal of the Department of Natural Resources, Sabaragamuwa University of Sri Lanka, for sharing knowledge to identify lichens and the National Building Research Organization and the Department of Zoology and Environmental Management of the University of Kelaniya in Sri Lanka, for providing field and laboratory facilities, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yatawara, M., Dayananda, N. Use of corticolous lichens for the assessment of ambient air quality along rural–urban ecosystems of tropics: a study in Sri Lanka. Environ Monit Assess 191, 179 (2019). https://doi.org/10.1007/s10661-019-7334-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7334-2