Abstract
In this paper, the algal cell density of cyanobacteria, green algae, and diatoms and their responses to the hydrochemical factors were analyzed to reveal the structural characteristics of water quality in an urban river. A total of nine sampling sites from upstream to downstream was explored in our study. At each site, the density of algae was identified every week during the wet season (June–October) from 2012 to 2017, and in situ detection was used for the relative 11 hydrochemical variables. The temporal and spatial characteristics of 14 variables were analyzed using a heatmap coupled with the cluster analysis method. The trend of each parameter was analyzed using the smoothing method with locally weighted regression. The nonmetric multidimensional scaling method was employed to detect the temporal and spatial similarities among algae along hydrochemical gradients. The responses of algal density to hydrochemical variables were analyzed using a redundancy analysis. The results showed that the water temperature (Wtemp), pH, dissolved oxygen (DO), cyanobacteria, and diatoms exhibited significant declining trends, and significant increasing trends were shown in the permanganate index, chemical oxygen demand, total nitrogen, ammonia nitrogen, and total phosphorus; the cyanobacteria exhibited certain differences with green algae and diatoms in summer and the downstream areas of the river. The temporal-spatial homogeneity of algal to hydrochemical variables showed the key influencing factors of Wtemp for cyanobacteria density, chlorophyll for green algae density, DO, and pH for diatoms. The results presented here are valuable for deepening our understanding of river ecosystem evaluations and effective environmental management, as well as an important reference for the sustainable development of aquatic biological resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater eutrophication is a global environmental challenge for the management of rivers, lakes, and reservoirs, as well as urban and agricultural areas, especially in China (Xia et al. 2010) and Europe (Anderson et al. 2002). Furthermore, eutrophication has also become a great international and domestic concern because of its economic and ecological consequences, such as frequent outbreaks of algal blooms that threaten the reliable supply of drinking water (Le et al. 2010; Pan et al. 2015) and the increase in nutrient concentrations that result in high productivity, which can further modify the structure of all aquatic communities (Mehner and Benndorf 1995). In particular, the structure and function of the phytoplankton community play important roles in maintaining the purification of urban landscape water, as well as the important link to the control and governance of water eutrophication. In particular, harmful cyanobacterial blooms have increased globally in frequency and intensity in recent decades. Eutrophication and warmer temperatures are often cited as key factors that promote these events (Davis et al. 2009; Hudnell and Dortch 2008; Paerl 2008), and cyanobacteria blooms have caused many problems, such as foul odors, decreased esthetic value, deterioration of water quality, and deoxygenation of water (Sigee et al. 1999). The green algae themselves are not harmful to marine environments, but their excessive growth can harm tourism, culture, transportation, and ecosystem health (Son et al. 2015a, b), and almost invariably they are found in all freshwater, even if in only small numbers and are greatest under sufficient nutrients (Sandgren 1988). Diatoms are important primary producers in freshwater ecosystems, and accumulating data indicate diatoms are sensitive to many environmental variables, including light, moisture conditions, temperature, current velocity, salinity, pH, dissolved oxygen, inorganic nutrients (carbon, phosphorus, nitrogen, silica), organic carbon, and organic nitrogen (Chen et al. 2016); therefore, they are considered powerful indicators of environmental changes, including acidification, eutrophication, and climatic changes (Dam et al. 1994). However, these situations are determined by several major factors, such as temperature, nutrients, and organic matter. Nitrogen and phosphorus are the most critical factors for maintaining the growth of algae. In urban rivers, the rapid urbanization of the population affects hydrosystems dramatically by altering hydrochemical conditions, hydrology, water quality, and aquatic biota (Newall and Walsh 2005). In particular, the changes in hydrochemical factors in urban rivers have great impacts on aquatic organisms, especially algae, and the cumulative changes to urbanization that impact rivers can be best reflected by anthropogenic activities (e.g., urban, industrial, and agricultural activities, and increasing exploitation of water resources) (Walker and Pan 2006).
In an urban river, the rapid urbanization of the population has caused a series of complex environmental problems, for example, the alteration of hydrosystems (Hopkins et al. 2015), the loosening of biogeochemical cycles (Pickett et al. 2011), the warming of urban meso-climate (Zhou et al. 2004, 2014), and the impairment of biodiversity (Grimm et al. 2008). Urbanization affects hydrosystems dramatically by altering hydrochemical conditions, hydrology, channel morphology, water quality, and aquatic biota (Newall and Walsh 2005). In addition, in urban areas, water quality degradation also may be associated with the increased area of impervious surfaces (Ren et al. 2003), with piped stormwater drainage systems (Walsh et al. 2016), and with effluent from wastewater systems (Gray and Becker 2002).
The changes in hydrochemical water quality in urban rivers have great impacts on aquatic organisms, and the impacts of the cumulative changes of urbanization on rivers can be best reflected by anthropogenic activities (e.g., urban, industrial, and agricultural activities and increasing exploitation of water resources) (Walker and Pan 2006). In a previous study, Singh et al. (2004) analyzed only data on water quality variables using different multivariate statistical techniques, Chang (2008) studied various water quality factors and focused on the relationship between water quality and changes in landscape factors in urban areas, and Jafari and Gunale (2006) mainly studied the hydrobiology of algae in an urban freshwater river. However, there is a lack of understanding of the response of algae density to the hydrochemical variations caused by both nature and anthropogenic activities. Therefore, we explored the variations in algae species and their responses to hydrochemical factors with an emphasis on an urban river ecosystem.
The Fenhe River is the second largest tributary of the Yellow River and is located in the most prosperous position of an urban area. In recent years, eutrophication has been of great concern because the sharp decline in water quality and massive cyanobacterial blooms has caused serious problems to the water ecosystem and human health. Changes in the algae of large temperate freshwater rivers have long been recognized to provide a good indicator of the trophic status and environmental quality of the system. The control of the principal environmental factors on algal cell density is essential in such systems. Therefore, the algae density has a rapid response to hydrochemical factors, and studies are necessary to identify the bioindicators and to clarify the abiotic variables. However, previous studies have been restricted to a limited period of time or a one-time algae bloom event (Ayana et al. 2015; Lu et al. 2017; Son et al. 2015a, b). In this study, we explored the variations in three kinds of algae and their responses to hydrochemical factors with an emphasis on an urban river ecosystem. The objectives of our study are as follows: (1) to elaborate the temporal and spatial characteristics of hydrochemical variables and algal cell density; (2) to describe the differences of algal cell density at monthly and spatial scales; and (3) to identify main hydrochemical factors that influence the algal cell density at month and spatial scale. The analysis of the temporal and spatial distribution of algal species and the complex relationship of algal cell density with hydrochemical factors can provide scientific evidence relevant to water quality assessment and pollution control. These results can aid in identifying improved methods for controlling eutrophication in the river.
Material and methods
Study area
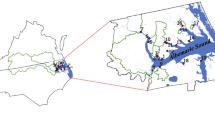
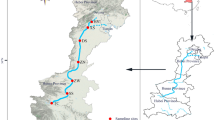
Fenhe River is the first tributary in the middle reach of the Yellow River with 39,000 km2 of area and a total length of approximately 710 km. The selected study area is located in Taiyuan, Shanxi, northern China (37° 80′ N, 112° 55′ E) (Fig. 1). The area has a semi-humid continental monsoon climate and an average annual precipitation of 450~510 mm. The nature of the river is a semi-enclosed water body. The surface width is 150 m, the buffer zone on both sides wetland is 50 m, and the total length is 20.5 km, including the construction of a 13.5 km water storage project from Xiangyun Bridge (S1) to Shengli Bridge (S7) and a 7 km artificial wetland from Shengli Bridge (S7) to Chaicun Bridge (S9).
In our study area, nine sampling sites were selected. Sites S7, S8, and S9 are in the old industrialized region. Sites S2 and S5 are in the transition area. Sites S1, S3, S4, and S6 belong to the new economic development zone, located in downstream areas of the river and have just been exploited in recent years; the surrounding areas are mostly schools, parks, residents, etc. The population flows more because of its landscape, and it is facing a serious environmental protection to counteract the worst water pollution.
Sampling and analysis
To acquire the best information that can express the responses of freshwater algae to hydrochemical factors in an appropriate way with the practicalities of sampling and analysis, nine sampling locations scattered in the main stations from upstream to downstream were selected in the study (Fig. 1). The samples were investigated every week during the wet season from June 2012 to October 2017. Weekly sampling strategy was determined taking into account the uncertainty of algal blooms in Fenhe river. The water temperature (Wtemp), air temperature (Airtemp), pH, dissolved oxygen (DO), permanganate index (CODMn), chemical oxygen demand (CODCr), total nitrogen (TN), ammonia nitrogen (NH4+-N), total phosphorus (TP), chlorophyll (Chl), and transparency (SD) were determined according to the corresponding standard methods, as shown in Table 1 (Wang et al. 2017).
Quantitative samples of the algae were collected using 10 L or 20 L of raw water sampling concentrated to approximately 50 mL through a 25# plankton net (mesh diameter of 64 μm) in 100 mL vials and fixed immediately with a 15% Lugol’s solution (Haande et al. 2011). Then, the algal cell density was counted in a cell counting chamber (0.1 mL) using a microscope (OlympusBX51) at an eyepiece magnification of × 10 and an objective magnification of × 40. The cell numbers of different algae species in 100 random fields were determined (Zhao et al. 2015). Under the microscope, cyanobacteria that can be identified based on their morphology are mostly unicellular and filamentous, and a few have false branches or true branches without chromatophores; green algae range in size from microscopic unicellular organisms to large globular colonies with extensive filamentous growths and have a flagellum; the identification of diatoms is based on the morphology of diatom shells and the pattern on the shell surface, they have a high siliceous cell wall, and contain yellow-green or yellow-brown chromatophores in the freshwater environment (Bellinger and Sigee 2010).
Statistical analyses
After all variables were determined, the weekly water quality data and the algal cell density data were aggregated, categorized, and transformed into an Excel-format database in time. These data were subsequently used for statistical analyses. The data structure was analyzed using boxplots, which were performed with the “ggpubr” library. The heatmap method coupled with a cluster analysis was employed to illustrate the temporal and spatial characteristic of each parameter. The heatmap plot was implemented with the “Pheatmap” library. The cluster method was a Pearson correlation based on the Euclidean distance of Ward’s method. The trend analysis of each parameter was conducted using the smooth method with locally weighted regression (LOESS), which was implemented with the “ggpubr” library. The relevant R2/p values of the fitted curve are shown in the trend figure.
The nonmetric multidimensional scaling (NMDS) method was completed using the “vegan” library, which indicated the similarity of the algae species along hydrochemical gradients in temporal and spatial patterns in the urban river system. The fit of the NMDS analysis results was measured by the stress coefficient, with a perfect fit degree (stress < 0.05), better fit (stress < 0.1), general fit (stress < 0.2), and poor fit (stress > 0. 3). A permutational multivariate analysis of variance (PermANOVA) was used to identify the differences of the three kinds of algae temporally and spatially (95% confidence level), which adopted the “vegan” library.
A redundancy analysis (RDA) is a direct gradient sorting method in ecology and is used to imply the relationship between the variables and multiple responses. This study used an RDA to analyze the responses of different algal cell densities to environment variables at a monthly scale and at a spatial scale. The analysis was performed by the “vegan” library. The relative permutation test was used to verify the significance of variables temporally and spatially (with a significance level of p < 0.05).
All statistics are based on R languages (version 3.4.3; R Project Statistical Computing, Vienna, Austria).
Results and discussion
Descriptive statistics for hydrochemical variables and algal cell density
Descriptive statistics for temporal and spatial changes are shown in boxplots (Fig. 2) and in Table S1. The results conveyed that the pH values varied between 7.30 and 9.66, with an average value of 8.31, which manifested a range of alkaline to basic characteristics. The above values usually indicate the presence of carbonates of calcium and magnesium in alkaline water (Begum and Ramaiah 2009). The Wtemp ranged from 11.9 to 29.1 °C throughout the monitoring period. The changes in Wtemp could originate from anthropogenic impacts, particularly from reservoir operations and industry (Hrdinka et al. 2012). DO and CODMn varied from 1.54 to 12.80 mg/L and from 0.92 to 16.40 mg/L, respectively. The TN, NH4+-N, and TP ranged from 0.46 to 12.2 with an average of 2.01 mg/L, from 0.1 to 8.05 with an average of 0.63 mg/L, and from 0.01 to 3.25 with an average of 0.1 mg/L, respectively. Eutrophication guidelines for freshwater give TP concentration ranges of < 0.01 and > 0.02 mg/L, and TN < 0.2 and > 0.5 mg/L for oligotrophic and eutrophic status according to the USEPA standards, respectively (Eisner et al. 2016). However, the average values of TN and TP of the river had already exceeded the threshold value of a eutrophic state and resulted in water quality in a medium eutrophication level. In addition, from the data structure, we concluded that NH4+-N and TP were highest at site S1, which was located in the downstream area of the river near the urban center. The increases in pollution generation due to human population, domestic activities, agriculture, industrial activities, and hospitals (Carpenter et al. 1998; Jayaswal et al. 2018), which the municipalities have no options to treat on-site due to the lack of a sewage and municipal wastewater treatment facility (Kannel et al. 2007; Mahmood et al. 2018), loading rates could reach the downstream areas of freshwater ecosystems and resulted in high nutrient and organic matter concentrations. These results are consistent with previous studies that revealed that the chemical and physical parameters of water indicated that the nutrient concentrations of the urban downstream sites were the highest and were significantly different from urban upstream and reference stream sites (Barrenha et al. 2018; Chen et al. 2016). Values of TN and NH4+-N were highest in June 2017, and the TP appeared to peak at site S1 in July 2012. CODCr and Chl showed relatively high fluctuations in the range of 5.98 to 70.00 mg/L and 0.55 to 209.00 mg/m3, respectively.
In the Fenhe River ecosystem, the main algal species were identified, including cyanobacteria, green algae, and diatoms. Their cell density was generally in the following order in this area: cyanobacteria > green algae > diatoms. They had wide ranges from 0.2 to 478.5 × 106, 0.56 to 82.8 × 106, and 0.02 to 36.6 × 106 cells/L, respectively. The highest cyanobacteria and diatom cell densities occurred at site S5 in July 2012, and the minimum values appeared at site S4 in August. For green algae, the peak value occurred at site S6 in July 2017, and the minimum value appeared at site S3. In terms of the annual community composition, the primary dominant species were Oscillatoria tenuis, Merismopedia tenuissima of Cyanophyta; Scenedesmus quadricauda of Chlorophyta; and Synedra acusvar, Cyclotella meneghiniana of Bacillariophyta.
Algal blooms could pose a serious risk to water quality and the structure and function of aquatic ecosystems. Developing a greater understanding of the hydrochemical variables control on the timing, magnitude and duration of blooms is essential for the effective management of aquatic ecosystems and phytoplankton development. This study has highlighted that to gain understanding of the factors controlling algal bloom dynamics, hydrochemical variables and biological monitoring data need to be acquired at high-frequency timescales that are commensurate with the rapid phytoplankton dynamics that typically occur in rivers. Therefore, based on the data in our results, the strategy of sampling duration (weekly) was used because of its effectiveness for analyzing hydrologic characteristic and this strategy was deemed appropriate to capture algal blooms.
The temporal and spatial characteristics of parameters
Three clusters were arbitrarily divided in both temporal and spatial scales by a cluster analysis in the heatmap (Fig. 3). Each cluster was combined based on the correlation of variables. Temporally, cluster-1 included TN and NH4+-N, and their concentrations were higher in 2017. Cluster-2 included pH, TP, CODMn, CODCr, and Chl. It was difficult to identify the individual groups that came from a single source, as they reflected a complex assimilation of certain industrial sewage (CODMn, CODCr, and TP) and the wastewater treatment plant (TN and NH4+-N), whereas cluster-3 included SD, DO, three kinds of algae, Wtemp, and Airtemp, which demonstrated a strong correlation between temperature and algal cell density. Spatially, cluster-1 was at site S9, where the concentrations of TN and DO were highest with little organic matter, the cyanobacteria cell density was lowest, and the water quality was good. The reason is that DO is the main factor that affects the growth of algae and is an important index of water self-purification ability. Cluster-2 consisted of sites S1 and S4, and they were located near the factory and enterprise of the bustling area, where the concentrations of TN, TP, CODCr, and CODMn were highest. Cluster-3 included sites S2, S3, S5, S6, S7, and S8. There were many tourists and residents living around these stations, and the discharge of domestic waste water could result in the high cyanobacteria cell density.
Trend variations
To quantify the trends of hydrochemical factors and dynamics of algal cell density, we performed a trend analysis (Fig. 4). The pH and DO showed a significantly descending trend (p < 0.01). CODMn, CODCr, TN, NH4+-N, and TP indicated significantly upward tendencies (p < 0.01). Cyanobacteria and diatom cell densities also revealed significantly decreasing trends (p < 0.01), while the green algae cell density exhibited an increasing trend. The increasing trend of organic matter and nutrient contents aroused our attention. The decreases in cyanobacteria and diatom cell density, as well as the increase in green algae cell density, deserve further research and discussion.
Temporal-spatial similarity of algal cell density to the hydrochemical gradient
The NMDS method was used to detect the similarity among algae along hydrochemical gradients in temporal and spatial patterns in an urban river system. As shown in Fig. S1, the nonparametric fitted curve degree was higher (R2 = 0. 966) than the linear fit (R2 = 0. 866), which indicated that with the survey data of algal cell density with a nonlinear structure, NMDS could better extract the information of the algal nonlinear agglomeration distribution from the data.
Temporally, the PermANOVA significance (p = 0.001, R2 = 0.131) and stress value (stress = 0.185) manifested a superior fitting effect of the NMDS analysis (Fig. 5a). The NMDS ordination plot showed a clear separation of cyanobacteria cell density with other algae in July and August. Although the green algae and diatoms were assembled close together and largely overlapped, a certain difference was observed between green algae and diatoms in October. Bracher et al. (2008) also concluded that in summer, diatoms were subjected to high losses, whereas they were favored in October, and green algae cell density was counted in very small numbers in October, which demonstrated the difference between diatoms and green algae.
Spatially, the PermANOVA revealed the significance (p = 0.001, R2 = 0.09) and the stress value (stress = 0.185) indicated a better fitting effect of the NMDS analysis (Fig. 5b). The NMDS plot showed that the cyanobacteria cell density had a distinct separation with green algae and diatoms based on the downstream sites (S1, S3, and S6). These sites, located in downstream areas of the river, were exploited in recent years, and the surrounding areas were mostly schools, parks, and residential areas, which caused water eutrophication and generated the massive growth of algae, and thus, the water body was seriously polluted. A small discrepancy between green algae and diatom density was found in the upstream areas of the river (S5, S7, and S8).
The responses of algal cell density to hydrochemical variables
An RDA was performed to analyze the responses of algal cell density to hydrochemical variables temporally and spatially, as well as to further understand the driving factors that controlled the growth of algae. Before the analysis, a detrended correspondence analysis (DCA) was used to test the suitability of the RDA. The results of the DCA demonstrated that the gradient length of first axis was less than 2 units, which confirmed that the RDA was suitable for analyzing our data. The variance in response variables explained by the hydrochemical factors was fairly high, as manifested by the eigenvalues obtained for the first two axes in the RDA ordination. In a distinct ordination with the RDA, the hydrochemical variables explained 92.68% and 90.48% of the total variance in the data temporally and spatially, respectively. The application of forward selection using the permutation test for all constrained eigenvalues from the RDA ordinations showed significance at the p < 0.001 level (Table 1).
Temporally, the RDA plot indicated Wtemp, Airtemp, pH, CODCr, Chl, and DO were closely related to the algal cell density with the first component axis 1 (77.64% of variance) (Fig. 6a). The Monte Carlo permutation test showed Wtemp, Airtemp, CODMn, CODCr, Chl, DO, pH, TN, and NH4+-N were also all significant above the 99.9% confidence level (Table 1). In particular, Wtemp, Airtemp, CODMn, and Chl were the primary hydrochemical factors to change the cyanobacteria and green algae cell densities. The cyanobacteria cell density was closer to the Wtemp and Airtemp, which indicated the driving factor of cyanobacteria was temperature. Furthermore, the temporal cluster showed that algal cell density, Wtemp, and Airtemp were gathered into a group (Fig. 3a), and the RDA plot indicated July was highly correlated with cyanobacteria because of the influence of temperature. Therefore, the growth of this group could be attributed to the high temperature freshwater discharge (Harsha and Malammanavar 2004), and as the seasonal temperatures increase from 10 to 30 °C in freshwater ecosystems, the phytoplankton group with the highest growth rates generally shifts from diatoms to green algae to cyanobacteria (Butterwick et al. 2005; Canale and Vogel 1974; Reynolds 1997). The latest experiment also demonstrated cyanobacteria have the highest thermal optimum, followed by green algae and diatoms (Nalley et al. 2018). And in our study area, cyanobacteria could be in a dormant period in winter and massively gathered when the temperature rises in June, whereas the growth and reproduction of diatoms increased in autumn. We can come to a consistent conclusion by combining the data structure with previous studies, and the highest cyanobacteria cell density was observed during summer periods, particularly in July, while green algae cell density decreased to some extent in August in an urban river (De et al. 2005). However, certain evidence also showed that in a nonurban area, relative proportions of cyanobacteria declined in summer, whereas diatoms were comparatively enhanced (Bahnwart et al. 1998). From the RDA plot, we could conclude the response of green algae cell density to Chl, and the response of diatom cell density to DO and pH. Diatom cell density also had a negative correlation with TN and NH4+-N. George et al. (2012) also revealed the strong negative correlation of diatoms with salinity and strong positive correlation with DO in freshwater. The combination of the temporal cluster with the RDA obtained strong correlations between Wtemp and Airtemp, TN and NH4+-N, and CODCr and Chl.
Spatially, the RDA-manifested Wtemp, Airtemp, pH, CODCr, Chl, and DO were highly correlated with the first component axis 1 (73.83% of variance) (Fig. 6b). The Monte Carlo permutation test demonstrated that Wtemp, Airtemp, CODMn, CODCr, Chl, DO, pH, TN, and NH4+-N were all extremely significant (Table 1). In particular, Wtemp and Airtemp were highly correlated with cyanobacteria, Chl was significantly correlated with green algae, while DO and pH were important in determining fluctuations in diatoms. The spatial cluster also indicated the close connection between pH and diatoms (Fig. 3b). The RDA showed site S6 located in the downstream area had a strong correlation with cyanobacteria and green algae cell densities, while the diatoms were distributed in the upstream areas of the river (S5 and S7), and the NMDS indicated the differences among algae in the upstream and downstream areas of the river. These results could be well verified in the classification of cluster analysis that cyanobacteria and green algal cell densities had the higher value at site S6 and diatom cell density was highest at sites S5 and S7. The increase in algal cell density in the downstream site could be explained by the extra time for phytoplankton to develop as well as greater inputs of high organic pollution from cities and agricultural lands (Varol and Şen 2018). The upstream areas can introduce new water resources from the Fenhe reservoir, with better water quality and fast flowing. Laboratory experiments revealed that the fast flowing could be the driving factor accounting for the decrease of cyanobacteria and the increase of diatoms (Yu et al. 2015). These conclusions are different from those in other nonurban areas; for example, Bahnwart et al. (1998) concluded cyanobacteria and diatom species were subjected to large density losses along fast flowing, shallow upstream river sections, whereas green algae density was favored, and diatom cell density benefited from low flow velocity and increased water depth in the downstream areas of rivers. The RDA plot (Fig. 6b) revealed that site S1 was mainly affected by TN and NH4+-N, especially S1 located at the downstream sewage treatment plant. Therefore, the biological oxidation of urban effluents and industrial waste from the wastewater treatment plant may be the most important source of nutrients (Pesce et al. 2008; Sharma et al. 2008) (Table 2).
The responses of algal cell density to hydrochemical variables among years
The responses of algal cell density to hydrochemical variables among years are shown in Fig. 7. The Wtemp was the primary contribution variables for the total cell density and the cell density of the different algal groups in 2012 and 2013. Interestingly, we found that there was a negative relationship between nutrients and cyanobacteria cell density but not-significant effect on green algae and diatoms in 2014. The negative relationship was also found in nutrient-rich lakes at TP levels in excess of 0.1 mg/L and TN levels in excess of 3 mg/L (Filstrup and Downing 2017; Filstrup et al. 2018). The high nutrient levels significantly negative affect total cell density in this study might be attributable to the suppression of photosynthesis and growth due to high ammonium levels (Glibert et al. 2016). And the NH4+-N also had a positively correlated to TN and TP. The concentration of organic matter was considered beneficial to the growth and reproduction of cyanobacteria and green algae, whereas negative effect on diatoms in 2015, 2016, and 2017.
The water sampling surveys were carried out during summer and fall (from July to October), whereas the absence of data (such as the construction of sampling site) between upstream and downstream sampling sites could lead to the lack of obvious differences in algal cell density. In our study, there were some shortcomings, for instance, the cause of such change may be locally or regionally driven. In addition, the discussion above has been mainly based on our present knowledge and understanding, although imperfect, and we know that the urban environment is a dynamic one and will be subject to noteworthy changes in the decades to come due to the impact of local, regional, and global changes. The most critical of these changes is a trend for an increase in urban population and industry, and such growth is likely to generate an increase in contaminants in urban rivers and increased pressure on water resources.
Conclusions
We analyzed the changes of 11 hydrochemical parameters and algal cell density using multivariate statistical methods over 6 years, and the results are summarized as follows:
-
(1)
The algal species of the urban river are mainly cyanobacteria, green algae, and diatoms. The cyanobacteria are the main group, followed by green algae and diatoms. During the period of study, the TN and TP exceeded the standard values as a whole, especially at sites S8 and S1, which had the highest values of TN and TP, respectively, eventually leading the water body to a mild eutrophic state or moderate eutrophication status.
-
(2)
The concentrations of TN, NH4+-N, CODMn, and CODCr were high at sites S1 and S4, which were located in the most prosperous position of the urban area, and the two areas had relatively higher pollution because of anthropogenic activity and industrial waste. The trend analysis showed that TN, NH4+-N, TP, CODMn, and CODCr had significantly upward tendencies, and cyanobacteria and diatoms showed decreasing tendencies.
-
(3)
As seen from the NMDS analysis, the cyanobacteria exhibited differences with green algae and diatoms in certain months (July and August) because of the increasing temperature, and at the downstream sites, (S1, S3, and S6) under the influences of human activities and wastewater treatment plant. A small discrepancy existed between green algae and diatom cell density in October and at the upstream sites (S5, S7, and S8).
-
(4)
The RDA indicated that temperature, Chl, DO, and pH were the key driving factors for the change in algal cell density, especially the response of cyanobacteria cell density to temperature, the response of green algae cell density to Chl, and the response of diatom cell density to DO and pH temporally and spatially.
This study can be extended to other aquatic areas in the world that currently suffer from or have suffered from water pollution crises with regard to urban sustainability. The data analysis in this study could be easily replicated for convenience and availability. The algal density response to hydrochemical variables could also be conducted in other similar regions. Therefore, the implications of this work could be more global. Moreover, the results presented here are valuable for deepening our understanding of algae variations in changing environments and can be used to develop a practical strategy for effective implementation of river management.
References
Anderson, D. M., Glibert, P. M., Burkholder, J. M., Rabalais, N. N., & Nixon, S. W. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4), 704–726. https://doi.org/10.1007/BF02804901.
Ayana, E. K., Worqlul, A. W., & Steenhuis, T. S. (2015). Evaluation of stream water quality data generated from MODIS images in modeling total suspended solid emission to a freshwater lake. Science of the Total Environment, 523, 170–177. https://doi.org/10.1016/j.scitotenv.2015.03.132.
Bahnwart, M., Hübener, T., & Schubert, H. (1998). Downstream changes in phytoplankton composition and biomass in a lowland river–lake system (Warnow River, Germany). Hydrobiologia, 391(1–3), 99–111. https://doi.org/10.1016/j.scitotenv.2005.09.078.
Barrenha, P. I. I., Tanaka, M. O., Hanai, F. Y., Pantano, G., Moraes, G. H., Xavier, C., Awan, A. T., Grosseli, G. M., Fadini, P. S., & Mozeto, A. A. (2018). Multivariate analyses of the effect of an urban wastewater treatment plant on spatial and temporal variation of water quality and nutrient distribution of a tropical mid-order river. Environmental Monitoring and Assessment, 190(1), 43–58. https://doi.org/10.1007/s10661-017-6386-4.
Begum, A., & Ramaiah, M. (2009). Heavy metal pollution and chemical profile of cauvery river water. Journal of Chemistry, 6(1), 47–52. https://doi.org/10.1155/2009/154610.
Bellinger, E.G., & Sigee, D.C. (2010). Freshwater Algae: Identification and Use as Bioindicators: Wiley illustration.
Bracher, A., Vountas, M., Dinter, T., Burrows, J. P., Ttgers, R. R., & Peeken, I. (2008). Quantitative observation of cyanobacteria and diatoms from space using PhytoDOAS on SCIAMACHY data. Biogeosciences Discussions, 5(6), 751–764. https://doi.org/10.5194/bg-6-751-2009.
Butterwick, C., Heaney, S. I., & Talling, J. F. (2005). Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Freshwater Biology, 50(2), 291–300. https://doi.org/10.1111/j.1365-2427.2004.01317.x.
Canale, R. P., & Vogel, A. H. (1974). Effects of temperature on phytoplankton growth. Journal of the Environmental Engineering Division, 100, 231–241.
Carpenter, S. R., Caraco, N. F., Correll, D. L., Howarth, R. W., Sharpley, A. N., & Smith, V. H. (1998). Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications, 8(3), 559–568. https://doi.org/10.1890/1051-0761(1998)008.
Chang, H. (2008). Spatial analysis of water quality trends in the Han River basin, South Korea. Water Research, 42(13), 3285–3304. https://doi.org/10.1016/j.watres.2008.04.006.
Chen, X., Zhou, W., Pickett, S. T. A., Li, W., Han, L., & Ren, Y. (2016). Diatoms are better indicators of urban stream conditions: a case study in Beijing, China. Ecological Indicators, 60, 265–274. https://doi.org/10.1016/j.ecolind.2015.06.039.
Dam, H. V., Mertens, A., & Sinkeldam, J. (1994). A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherland Journal of Aquatic Ecology, 28(1), 117–133. https://doi.org/10.1007/BF02334251.
Davis, T. W., Berry, D. L., Boyer, G. L., & Gobler, C. J. (2009). The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae, 8(5), 715–725. https://doi.org/10.1016/j.hal.2009.02.004.
De, P. R., Faraloni, C., Sili, C., & Vincenzini, M. (2005). Populations of exopolysaccharide-producing cyanobacteria and diatoms in the mucilaginous benthic aggregates of the Tyrrhenian Sea (Tuscan archipelago). Science of the Total Environment, 353(1–3), 360–368. https://doi.org/10.1016/j.scitotenv.2005.09.078.
Eisner, L. B., Gann, J. C., Ladd, C., Cieciel, K. D., & Mordy, C. W. (2016). Late summer/early fall phytoplankton biomass (chlorophyll a) in the eastern Bering Sea: Spatial and temporal variations and factors affecting chlorophyll a concentrations. Deep Sea Research Part II Topical Studies in Oceanography, 134(1), 100–114. https://doi.org/10.1016/j.dsr2.2015.07.012.
Filstrup, C. T., & Downing, J. A. (2017). Relationship of chlorophyll to phosphorus and nitrogen in nutrient-rich lakes. Inland Waters, 7(4), 385–400. https://doi.org/10.1080/20442041.2017.1375176.
Filstrup, C. T., Wagner, T., Oliver, S. K., Stow, C. A., Webster, K. E., Stanley, E. H., & Downing, J. A. (2018). Evidence for regional nitrogen stress on chlorophyll a in lakes across large landscape and climate gradients. Limnology & Oceanography, 63(S1), 324–339. https://doi.org/10.1002/lno.10742.
George, B., Kumar, J. I. N., & Kumar, R. N. (2012). Study on the influence of hydro-chemical parameters on phytoplankton distribution along Tapi estuarine area of Gulf of Khambhat, India. Egyptian Journal of Aquatic Research, 38(3), 157–170. https://doi.org/10.1016/j.ejar.2012.12.010.
Glibert, P. M., Wilkerson, F. P., Dugdale, R. C., Raven, J. A., Dupont, C. L., Leavitt, P. R., Parker, A. E., Burkholder, J. A. M., & Kana, T. M. (2016). Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnology & Oceanography, 61(1), 284–300. https://doi.org/10.1002/lno.10203.
Gray, S. R., & Becker, N. S. C. (2002). Contaminant flows in urban residential water systems. Urban Water, 4(4), 331–346. https://doi.org/10.1016/S1462-0758(02)00033-X.
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J., Bai, X., & Briggs, J. M. (2008). Global change and the ecology of cities. Science, 319(5864), 756–760. https://doi.org/10.1126/science.1150195.
Haande, S., Rohrlack, T., Semyalo, R. P., Brettum, P., Edvardsen, B., Lyche-Solheim, A., Sørensen, K., & Larsson, P. (2011). Phytoplankton dynamics and cyanobacterial dominance in Murchison Bay of Lake Victoria (Uganda) in relation to environmental conditions. Limnologica - Ecology and Management of Inland Waters, 41(1), 20–29. https://doi.org/10.1016/j.limno.2010.04.001.
Harsha, T. S., & Malammanavar, S. G. (2004). Assessment of phytoplankton density in relation to environmental variables in Gopalaswamy pond at Chitradurga, Karnataka. Journal of Environmental Biology, 25(1), 113–116.
Hopkins, K. G., Morse, N. B., Bain, D. J., Bettez, N. D., Grimm, N. B., Morse, J. L., Palta, M. M., Shuster, W. D., Bratt, A. R., & Suchy, A. K. (2015). Assessment of regional variation in streamflow responses to urbanization and the persistence of physiography. Environmental Science & Technology, 49(5), 2724–2732. https://doi.org/10.1021/es505389y.
Hrdinka, T., Novický, O., Hanslík, E., & Rieder, M. (2012). Possible impacts of floods and droughts on water quality. Journal of Hydro-Environment Research, 6(2), 145–150. https://doi.org/10.1016/j.jher.2012.01.008.
Hudnell, H. K., & Dortch, Q. (2008). Chapter 2: a synopsis of research needs identified at the interagency, international symposium on cyanobacterial harmful algal blooms (ISOC-HAB). Advances in Experimental Medicine & Biology, 619(6), 17–44. https://doi.org/10.1007/978-0-387-75865-7_2.
Jafari, N. G., & Gunale, V. R. (2006). Hydrobiological study of algae of an urban freshwater river. Journal of Applied Sciences & Environmental Management, 10(2), 153–158.
Jayaswal, K., Sahu, V., & Gurjar, B. (2018). Water pollution, human health and remediation. Water Remediation (pp. 11–27). Singapore: Springer. https://doi.org/10.1007/978-981-10-7551-3_2.
Kannel, P. R., Lee, S., Kanel, S. R., & Khan, S. P. (2007). Chemometric application in classification and assessment of monitoring locations of an urban river system. Analytica Chimica Acta, 582(2), 390–399. https://doi.org/10.1016/j.aca.2006.09.006.
Le, C., Zha, Y., Li, Y., Sun, D., Lu, H., & Yin, B. (2010). Eutrophication of lake waters in China: cost, causes, and control. Environmental Management, 45(4), 662–668. https://doi.org/10.1007/s00267-010-9440-3.
Lu, X., Song, S., Lu, Y., Wang, T., Liu, Z., Li, Q., Zhang, M., Suriyanarayanan, S., & Jenkins, A. (2017). Response of phytoplankton commun-ity to water quality in local alpine glacial lake of Xinjiang Tianchi, China: potential drivers a-nd management implications. Environmental Science Processes & Impacts, 19(10), 1300–1311. https://doi.org/10.1039/C7EM00180K.
Mahmood, S., Sharif, F., Rahman, A., & Khan, A. U. (2018). Analysis and forecasting of municipal solid waste in Nankana City using geo-spatial techniques. Environmental Monitoring and Assessment, 190(5), 275–288. https://doi.org/10.1007/s10661-018-6631-5.
Mehner, T., & Benndorf, J. (1995). Eutrophication; a summary of observed effects and possible solutions. Aqua, 44, 35–44.
Nalley, J. O., O'Donnell, D. R., & Litchman, E. (2018). Temperature effects on growth rates and fatty acid content in freshwater algae and cyanobacteria. Algal Research, 35, 500–507. https://doi.org/10.1016/j.algal.2018.09.018.
Newall, P., & Walsh, C. J. (2005). Response of epilithic diatom assemblages to urbanization influences. Hydrobiologia, 532(1–3), 53–67. https://doi.org/10.1007/s10750-004-9014-6.
Paerl, H. (2008). Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. Oxygen Transport to Tissue XXXIII, 619, 217–237. https://doi.org/10.1007/978-0-387-75865-7_10.
Pan, B. Z., Wang, H. Z., Pusch, M. T., & Wang, H. J. (2015). Macroinvertebrate responses to regime shifts caused by eutrophication in subtropical shallow lakes. Freshwater Science, 34(3), 942–952. https://doi.org/10.1086/682077.
Pesce, S., Fajon, C., Bardot, C., Bonnemoy, F., Portelli, C., & Bohatier, J. (2008). Longitudinal changes in microbial planktonic communities of a French river in relation to pesticide and nutrient inputs. Aquatic Toxicology, 86(3), 352–360. https://doi.org/10.1016/j.aquatox.2007.11.016.
Pickett, S. T., Cadenasso, M. L., Grove, J. M., Boone, C. G., Groffman, P. M., Irwin, E., et al. (2011). Urban ecological systems: scientific foundations and a decade of progress. Journal of Environmental Management, 92(3), 331–362. https://doi.org/10.1016/j.jenvman.2010.08.022.
Ren, W., Zhong, Y., Meligrana, J., Anderson, B., Watt, W. E., Chen, J., & Leung, H. L. (2003). Urbanization, land use, and water quality in Shanghai: 1947–1996. Environment International, 29(5), 649–659. https://doi.org/10.1016/S0160-4120(03)00051-5.
Reynolds, C. S. (1997). Successional development, energetics and diversity in planktonic communities. New York: Springer.
Sandgren, C. D. (1988). Growth and reproductive strategies of freshwater phytoplankton. Cambridge: Cambridge University Press.
Sharma, S., Dixit, S., Jain, P., Shah, K. W., & Vishwakarma, R. (2008). Statistical evaluation of hydrobiological parameters of Narmada River water at Hoshangabad City, India. Environmental Monitoring and Assessment, 143(1–3), 195–202. https://doi.org/10.1007/s10661-007-9968-8.
Sigee, D. C., Glenn, R., Andrews, M. J., Bellinger, E. G., Butler, R. D., Epton, H. A. S., & Hendry, R. D. (1999). Biological control of cyanobacteria: principles and possibilities. Hydrobiologia, 395-396(1), 161–172. https://doi.org/10.1023/A:1017097502124.
Singh, K. P., Malik, A., Mohan, D., & Sinha, S. (2004). Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)— a case study. Water Research, 38(18), 3980–3992. https://doi.org/10.1023/A:1017097502124.
Son, Y. B., Choi, B. J., Yong, H. K., & Park, Y. G. (2015a). Tracing floating green algae blooms in the Yellow Sea and the East China Sea using GOCI satellite data and Lagrangian transport simulations. Remote Sensing of Environment, 156(156), 21–33. https://doi.org/10.1016/j.rse.2014.09.024.
Son, J. H., Kim, S., & Carlson, K. H. (2015b). Effects of wildfire on river water quality and riverbed sediment phosphorus. Water Air & Soil Pollution, 226(3), 26. https://doi.org/10.1007/s11270-014-2269-2.
Varol, M., & Şen, B. (2018). Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River, Turkey. River Research & Applications, 34(1), 13–23. https://doi.org/10.1002/rra.3223.
Walker, C. E., & Pan, Y. (2006). Using diatom assemblages to assess urban stream conditions. Hydrobiologia, 561(1), 179–189. https://doi.org/10.1007/1-4020-5070-4_13.
Walsh, C. J., Booth, D. B., Burns, M. J., Fletcher, T. D., Hale, R. L., Lan, N. H., et al. (2016). Principles for urban stormwater management to protect stream ecosystems. Freshwater Science, 35, 398–411. https://doi.org/10.1086/685284.
Wang, X., Zhang, F., Kung, H. T., Ghulam, A., Trumbo, A. L., Yang, J., Ren, Y., & Jing, Y. (2017). Evaluation and estimation of surface water quality in an arid region based on EEM-PARAFAC and 3D fluorescence spectral index: a case study of the Ebinur Lake watershed, China. Catena, 155, 62–74. https://doi.org/10.1016/j.catena.2017.03.006.
Xia, J., Cheng, S., Hao, X., Xia, R., & Liu, X. (2010). Potential impacts and challenges of climate change on water quality and ecosystem: case studies in representative rivers in China. Journal of Resources and Ecology, 1(1), 31–35. https://doi.org/10.3969/j.issn.1674-764x.2010.01.004.
Yu, Q., Chen, Y., Liu, Z., Giesen, N. V. D., & Zhu, D. (2015). The influence of a eutrophic lake to the river downstream: spatiotemporal algal composition changes and the driving factors. Water, 7(5), 2184–2201. https://doi.org/10.3390/w7052184.
Zhao, H. J., Wang, Y., Yang, L. L., Yuan, L. W., & Peng, D. C. (2015). Relationship between phytoplankton and environmental factors in landscape water supplemented with reclaimed water. Ecological Indicators, 58, 113–121. https://doi.org/10.1016/j.ecolind.2015.03.033.
Zhou, G., Minakawa, N., Githeko, A. K., & Yan, G. (2004). Association between climate variability and malaria epidemics in the east African highlands. Proceedings of the National Academy of Sciences of the United States of America, 101(8), 2375–2380. https://doi.org/10.1073/pnas.0308714100.
Zhou, W., Qian, Y., Li, X., Li, W., & Han, L. (2014). Relationships between land cover and the surfa-ce urban heat island: seasonal variability and effects of spatial and thematic resolution of land cover data on predicting land surface temperatures. Landscape Ecology, 29(1), 153–167. https://doi.org/10.1007/s10980-013-9950-5.
Funding
This work was supported by the Social Development Foundation of Shanxi (No. 201603D321001; No. 201603D321008), the National Natural Sciences Foundation of China (No.41401020; No. 41601202), and the Fund for Shanxi “1331 Project” Key Innovative Research Team.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 283 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Wang, F., Lv, J. et al. Responses of freshwater algal cell density to hydrochemical variables in an urban aquatic ecosystem, northern China. Environ Monit Assess 191, 29 (2019). https://doi.org/10.1007/s10661-018-7177-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7177-2