Abstract
Due to the widespread use of pesticides, human exposure to pesticides is possible and can potentially cause adverse impacts on public health. We measured 137 pesticides including organophosphorus, organochlorine, pyrethroid and carbamate pesticides together with various herbicides in 100 human blood samples collected from the general population in Beijing. The samples were analysed by triple quadrupole tandem gas chromatography-mass spectrometry. In total, 24 organochlorine pesticides, 5 pyrethroid pesticides and 6 organophosphorus pesticides were detected. The detection rates of HCB, α-HCH, β-HCH, γ-HCH, p,p′-DDE and quintozene were 99, 96, 74, 72, 96 and 95%, respectively. No statistically significant gender difference in the blood concentrations of the pesticides was found. Consistent with the trend of the increasing β-HCH, p,p′-DDE and quintozene concentrations with age, a strong positive correlation between the age and concentrations of β-BHC, p,p′-DDE and quintozene was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic pesticides have been widely used in agricultural production. Many public benefits have been gained from the use of synthetic pesticides, such as eradication of various diseases and pests in the fields, increase of agriculture yields and prevention of the propagation of carriers transmitting deadly diseases to humans. But despite the obvious advantages, the potential adverse effects on public health and the environment could be significant. Human beings are exposed to artificial chemicals from various environmental sources. The general human population is predominantly exposed to a range of pesticides through dietary intake, inhalation and drinking water (Margni et al. 2002). Because some pesticides are lipophilic and metabolically resistant, concerns have increased in the last few years regarding the toxicological and human health implications of these chemicals. Low-dose, long-term exposure and uptake of pesticides accumulatively among the general population can lead to chronic health issues, such as cancer, birth defects, reproductive disorders and Parkinson’s disease (Alavanja et al., 2013; Mostafalou and Abdollahi, 2013). A useful and common means of detecting human pesticide exposure is biological monitoring. Over the past few decades, methods have been established for the detection of various pesticides in human biological samples, such as blood, breast milk and urine, to assess exposure. Numerous analytical techniques, like chromatography and mass spectrometry, have been used as a part of these detection methods to precisely measure pesticides (Yusa et al., 2015). The applications of biological monitoring data are broad. These data record the universality of exposure to pesticides and can help us better understand the possible risks to humans. Meanwhile, the data can help us understand the main indicators of pesticide exposure in a particular population and identify potential pesticide exposure pathways. Furthermore, toxicological, epidemiological and molecular biological evidence can be obtained to confirm the relationship between specific pesticides and several diseases (Koureas et al., 2012). The very first step is to investigate the background biomonitoring data reflecting the internal exposure and body burden of the general population. Recently, several publications have appeared in which specific pesticide congeners have been identified and quantified in human milk in a Beijing population (Yu et al., 2006; Fujii et al., 2011; Song et al., 2013a; Song et al., 2013b). However, reports specific to the blood concentrations of pesticides, which can reflect exposure from all pathways, are few.
In this study, we developed a rapid screening GC-MS/MS method for a pesticide analysis of human blood plasma. We evaluated various pesticides including forbidden and restricted organochlorine pesticides which were proposed by the ministry of agriculture of the People’s Republic of China, early and modern organophosphorus pesticides, commonly used pyrethroid and carbamate pesticides, as well as multifarious herbicides. The aims of this study were to measure the background level of pesticides in the general population living in Beijing and to examine possible associations between pesticide levels and age and gender.
Materials and methods
Sample collection
The Ethics Committee of the Peking Union Medical College (PUMC) approved the study of contaminants in human blood. Briefly, blood from 52 female donors and 48 male donors was collected by the Peking Union Medical College Hospital (PUMCH). All the participants were from the Han nationality. The eligibility of the blood donors was based on the screening performed by nurses during the recruitment of the donors. And all the 100 donors were from ordinary physical examination population in hospital. The age of the blood donors ranged from 17 to 79. Children were excluded from participating. The donors were divided into six age groups: 17–29 (n = 17), 30–39 (n = 21), 40–49 (n = 26), 50–59 (n = 24), 60–69 (n = 8) and ≥ 70 (n = 4). Samples of human blood were gathered in heparinized tubes, stored at 4 °C, and then centrifuged for 10 min at 4000 rpm. The plasma supernatant was aspirated out and placed in a 2-mL glass vial kept at − 20 °C until extraction.
Reagents and apparatus
The individual standard stock solutions of 137 pesticides (concentration 100 μg/mL, > 98.8% purity) were obtained from the Agriculture Environmental Protection of Institute (China). N-hexane (HPLC grade) was purchased from Sigma (USA). Acetonitrile (AR), cyclohexane (AR), ethyl acetate (AR) and anhydrous sodium sulfate (AR) were all purchased from Beijing Chemical Works (China).
The gas chromatography-mass spectrometer system consisted of a Varian 450 GC and a 300 triple quadrupole MS (Bruker Daltonics Inc., USA). The chromatographic separation was accomplished using a Varian capillary column VF-5 MS (60 m × 0.25 mm × 0.25 μm) with 5% phenyl polysiloxane as the non-polar stationary phase (Bruker Daltonics Inc., USA). Meanwhile, the electronic balance (PL203/01, Mettler Toledo Inc., Switzerland), the vortex mixer (WH-1, Shanghai huxi analytical instruments, China), the vacuum pump (SHB-B, Zhengzhou greatwall scientific company, China), the rotary evaporator (Laborota 4000/4, Heidolph instruments, Germany) and the centrifuge (80–2, Jintan medical-equipment company, China) were used.
Sample preparation technique
The procedures for the sample preparation, including the extraction and clean-up, were based on a liquid solvent extraction method. A mixture of ethyl acetate and cyclohexane was a common solvent applied in a multi-residue pesticide analysis using QuEChERS methodology (Steiniger et al., 2010; Wilkowska and Biziuk, 2011). Briefly, the plasma was thawed at 4 °C, and then, a precise 0.5 mL plasma sample was mixed with 1 mL acetonitrile in a 5 mL stoppered test tube, vibrated and centrifuged. The supernatant was transferred to another test tube and extracted with 3 × 1 mL ethyl acetate/cyclohexane (v/v, 3:1). The organic phase was desiccated using an appropriate amount of anhydrous sodium sulfate and concentrated to 0.5 mL with mild nitrogen blowing.
Instrumental analysis
The conditions for the GC analysis were as follows. The injector temperature was maintained at 250 °C in split-less injection mode, and the volume of the injection was 1 μL. Pure helium (99.999%) was used as the carrier gas with a constant flow of 1 mL/min. The column temperature was 120 °C at the start, held for 1 min, increased to 150 °C at a rate of 8 °C/min, held for 2 min, then increased to 205 °C at 2 °C/min, held for 6 min, increased to 270 °C at 5 °C/min and finally increased to 280 °C at 1 °C/min and maintained for 30 min.
The triple quadrupole tandem MS system was manipulated using the ionisation mode of electron impact (EI), and the electron energy was 70 eV. The SIM width was 0.7 amu. The temperature of the transfer line was set at 250 °C, while the ion source and manifold were 220 and 40 °C, respectively. The collision gas was high-purity argon (99.999%), and the pressure was kept at 2.0 mTorr.
Table 1 lists the determination parameters used in this paper, including the time segments, retention times, qualification and quantification ions and the collision energies for each pesticide. The determination of the concentrations of the pesticide residues was performed using an external standard method. The concentration of the standard solution of the matrix-matched pesticide was 10 ng/mL. The peak area of the quantitative ion for each pesticide was determined using the standards and then applied to measure the pesticides in the unknown samples.
Quality assurance/quality control
A solvent blank (ethyl acetate: cyclohexane v/v, 3:1) was periodically processed through the full analytic procedure to detect any background interference. Meanwhile, the 12 samples of each set contained a method blank sample to monitor the background pollution. To assure the proper recognition and quantification of the object compounds, some quality control specifications were employed. First, the retention times for the determination of the reference compounds should match. Second, the proportions of two typical ions should be no more than 15% of the theoretical values. Finally, the signal to noise ratio of the selected ions should be greater than 3. The congener was excluded if all of these conditions were not met.
Statistical analysis
The statistical software package SPSS 17.0 was used to analyse the data. If the detected concentrations of the compounds were lower than the LODs, the values applied to the statistical analysis were equal to one-half of the LODs of the analytical method, and the computations of the median, mean and sum were set at zero. P-P plots were used to test the normality of the variables, and the Kolmogorov-Smirnov test was used to validate the normality. The result of a chi-squared test was used to determine the relationship among categorical variables. A non-parametric u-test was used to compare the continuous variables with non-normal distributions, while the Spearmen-rho coefficient was used to measure the correlation of two continuous variables.
Results and discussion
Method validation
The results suggest that the separation of all 137 pesticides was successful because of the high selectivity and sensitivity of the detection. The calibration curve of each compound was calculated using the concentration peak area obtained through the injection of matrix-matched pesticide standard solutions in GC-MS/MS. The results from a method validation study are shown in Table 1. The linear relationships of the calibration curves were well within the correlation coefficients (r2), ranging from 0.9002 to 0.9998 in the study of serial concentrations. The limits of detection (LODs) were calculated using an S/N of 3, and the results were between 0.4 and 21.6 ng/mL. The limits of quantification (LOQs) were calculated using an S/N of 10, and the results were between 1.4 and 71.9 ng/mL. The accuracy of the full methodology was evaluated by recovery experiments, which were implemented using additive concentrations at three levels: 10, 50 and 200 ng/mL. Meanwhile, the precision of the method was determined using six replicates at each concentration and expressed using the relative standard deviations (RSDs). As shown in Table 1, the recovery results of most pesticides ranged from 70 to 120%, and the RSD results ranged from 0.6 to 20.0%. Because of the acceptable method validation results, it is clear that the method established in this paper is exact and reliable based on the European Council, Document No.SANCO 825/00 (European Commission, 2010).
Concentrations of pesticides in blood plasma
The 100 plasma samples were analysed with GC-MS/MS for the residues of organochlorine pesticides and organophosphorus, carbamate, and pyrethroid insecticides. The results are shown in Table 2. In total, 24 organochlorine pesticides, 5 pyrethroid pesticides and 6 organophosphorus pesticides were detected with detection rates between 1.0 and 99.0%. The detection frequency of 35 positive pesticides is shown in Fig. 1. Most of the positive OCPs were persistent organic pollutants. HCB, α-HCH, β-HCH, γ-HCH, p,p′-DDE and quintozene were measured at ratios of 99, 96, 74, 72, 96 and 95%, respectively. In the present study, the concentrations of HCB ranged from 1.74 to 6.01 ng/mL. The plasma levels of α-HCH, β-HCH, γ-HCH and δ-HCH ranged from 3.35 to 5.36, from 2.45 to 14.19, from 3.25 to 6.59 and from 5.09 to 7.65 with a mean of 3.65, 2.83, 2.58 and 0.84 ng/mL, respectively. Among the four analysed HCH isomers (α-, β-, γ- and δ-HCH), it was obvious that α-HCH was the most common isomer, and this finding was inconsistent with a former study (Zamir et al., 2009; Freire et al., 2014; Caba et al., 2015). For the DDT isomers (p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE and p,p′-DDD), p,p′-DDE was the most abundant, which was consistent with previous results (Bedi et al., 2015; Koureas et al., 2016). The concentrations of p,p′-DDE ranged from 2.85 to 13.36 ng/mL with a mean of 4.89 ng/mL. The concentrations of quintozene and aldrin were in the range of 3.35 to 5.36 and 2.66 to 56.48 ng/mL, respectively. Compared with organochlorine pesticides, the detection rates of organophosphorus and pyrethroid pesticides were much lower. Bifenthrin, tetramethrin, fenpropathrin, cyfluthrin and deltamethrin were detected at rates of 11, 4, 2, 4 and 2%, respectively. The concentrations of bifenthrin ranged from 0.93 to 3.39 ng/mL. Positive OPPs included terbufos, methyl-parathion, parathion-ethyl, methidathion, phosalone and coumaphos with detection rates lower than 5%. The concentrations of parathion-ethyl (1.61–2.28 ng/mL) were much lower than those reported in previous studies with a mean of 2900 ng/mL (Park et al., 2009), which represented acute fatality cases. The observed trends for the total DDT, HCH and HCB were comparatively lower than those from earlier reports; see Table 3.
– no detail information mentioned
Pesticide distribution by gender and age
On the basis of the gender and age groups, the results of 100 plasma samples were categorised and interpreted. According to the determined frequency and concentration, a comparison of the results for a total of 35 pesticides was performed. The gender comparisons of the detection frequency of 35 pesticides are illustrated in Fig. 1 and Table 2. Previous research indicated that the concentrations of several OCPs, such as α-HCH, β-HCH, γ-HCH, aldrin, heptachlor, o,p′-DDE and p,p′-DDE, were observably higher in males than those in females (Wang et al., 2013). However, the present study found no statistically significant difference between men and women with regard to the pesticide concentration (p > 0.05) based on the Mann-Whitney tests.
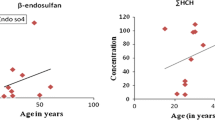
The correlations between age and pesticide concentration were evaluated using the Spearman chi-squared test. Spearman rho coefficients and p values are shown in Table 2. Consistent with the trend of increasing β-HCH, p,p′-DDE and quintozene concentrations with age (Fig. 2), strong correlations between age and the concentrations of β-BHC, p,p′-DDE and quintozene were observed. Plasma quintozene concentration and age were positively correlated (Spearman’ rho = 0.200, p < 0.05). Plasma β-HCH concentration and age were positively correlated (Spearman’ rho = 0.277, p < 0.05). Plasma p,p′-DDE concentration and age were positively correlated (Spearman’ rho = 0.211, p < 0.05). These results indicated that plasma β-HCH, p,p′-DDE and quintozene concentrations increased with age, which was consistent with previous results (Jakszyn et al. 2009). The reason of this phenomenon might be that the older had a greater chance for high levels of exposure to these persistent chemicals, whereas they also had a longer time to accumulate these chemicals in their body.
Conclusion
The data presented in this study indicated low exposure of the general population in Beijing to pesticides in comparison to human populations in other countries. No statistically significant difference in the gender-related concentrations was found. Consistent with the trend of the increasing β-HCH, p,p′-DDE and quintozene concentrations with age, strong correlations between age and concentrations of β-BHC, p,p′-DDE and quintozene were observed. A limitation associated with this study was the small sample size. Further research into the specific sources and routes of exposure is warranted.
Abbreviations
- OCP:
-
Organochlorine pesticide
- OPP:
-
Organophosphorus pesticide
- PP:
-
Pyrethroid pesticide
- GC:
-
Gas chromatography
- MS:
-
Mass spectroscopy
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
References
Alavanja, M. C. R., Ross, M. K., & Bonner, M. R. (2013). Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA: cancer. Jornal dos Clinicos, 63, 120–142. https://doi.org/10.3322/caac.21170/full.
Bedi, J. S., Gill, J. P., Kaur, P., Sharma, A., & Aulakh, R. S. (2015). Evaluation of pesticide residues in human blood samples from Punjab (India). Veterinary World., 8(1), 66–71. https://doi.org/10.14202/vetworld.2015.66-71.
Bjermo, H., Darnerud, P. O., Lignell, S., Pearson, M., Rantakokko, P., Nälsén, C., Enghardt Barbieri, H., Kiviranta, H., Lindroos, A. K., & Glynn, A. (2013). Fish intake and breastfeeding time are associated with serum concentrations of organochlorines in a Swedish population. Environment International, 51, 88–96. https://doi.org/10.1016/j.envint.2012.10.010.
Bhatnagar, V. K., Kashyap, R., Zaidi, S. S. A., Kulkarni, P. K., & Saiyed, H. N. (2004). Levels of DDT, HCH, and HCB residues in human blood in Ahmedabad, India. Bulletin of Environmental Contamination and Toxicology, 72, 261–265. https://doi.org/10.1007/s00128-003-9049-9.
Caba, M., Meza, E., Waliszewski, S. M., & Martínez-Valenzuela, C. (2015). Inverse correlation among organochlorine pesticide levels to total lipid serum contents: a preliminary study in Veracruz, Mexico. Environmental Monitoring and Assessment, 187(7), 467. https://doi.org/10.1007/s10661-015-4694-0.
Dirtu, A. C., Cernat, R., Dragan, D., Mocanu, R., Van Grieken, R., Neels, H., & Covaci, A. (2006). Organohalogenated pollutants in human serum from Iassy, Romania and their relation with age and gender. Environment International, 32, 797–803. https://doi.org/10.1016/j.envint.2006.04.002.
European Commission, 2010. Document No.SANCO 825/00: Guidance document on pesticide residue: analytical methods. http://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_825-00_v8.pdf
Freire, C., Koifman, R. J., Sarcinelli, P. N., Rosa, A. C., Clapauch, R., & Koifman, S. (2014). Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. International Journal of Hygiene and Environmental Health, 217(2–3), 370–378. https://doi.org/10.1016/j.ijheh.2013.07.012.
Fujii, Y., Haraguchi, K., Harada, K. H., Hitomi, T., Inoue, K., Itoh, Y., Watanabe, T., Takenaka, K., Uehara, S., Yang, H. R., Kim, M. Y., Moon, C. S., Kim, H. S., Wang, P., Liu, A., Hung, N. N., Koizumi, A., et al. (2011). Detection of dicofol and related pesticides in human breast milk from China, Korea and Japan. Chemosphere, 82, 25–31. https://doi.org/10.1016/j.chemosphere.2010.10.036.
Guo, H., Jin, Y. L., Cheng, Y. B., Leaderer, B., Lin, S. B., Holford, T. R., Qiu, J., Zhang, Y. W., Shi, K. C. H., Zhu, Y., Niu, J. J., Bassig, B. A., Xu, S. H. Q. Z. B., Li, Y. H., Hu, X. B., Chen, Q., & Zheng, T. Z. H. (2014). Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere, 110, 1–7. https://doi.org/10.1016/j.chemosphere.2014.02.017.
Hassine, S. B., Hammami, B., Ameur, W. B., Megdiche, E. Y., Barhoumi, B., Abidi, R. E., & Driss, M. R. (2014). Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and their relation with age, gender, and BMI for the general population of Bizerte, Tunisia. Environmental Science and Pollution Research International, 21(10), 6303–6313. https://doi.org/10.1007/s11356-013-1480-9.
Jaraczewska, K., Lulek, J., Covaci, A., Voorspoels, S., Kaluba-Skotarczak, A., Drews, K., & Schepens, P. (2006). Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Science of the Total Environment, 372, 20–31. https://doi.org/10.1016/j.scitotenv.2006.03.030.
Jakszyn, P., Goñi, F., Etxeandia, A., Vives, A., Millán, E., López, R., Amiano, P., Ardanaz, E., Barricarte, A., Chirlaque, M. D., Dorronsoro, M., Larrañaga, N., Martínez, C., Navarro, C., Rodríguez, L., Sánchez, M. J., Tormo, M. J., González, C. A., & Agudo, A. (2009). Serum levels of organochlorine pesticides in healthy adults from five regions of Spain. Chemosphere, 76, 1518–1524. https://doi.org/10.1016/j.chemosphere.2009.05.048.
Kim, M., Song, N. R., Hong, J., Lee, J., & Pyo, H. (2013). Quantitative analysis of organochlorine pesticides in human serum using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Chemosphere, 92, 279–285. https://doi.org/10.1016/j.chemosphere.2013.02.052.
Koureas, M., Karagkouni, F., Rakitskii, V., Hadjichristodoulou, C., Tsatsakis, A., & Tsakalof, A. (2016). Serum levels of organochlorine pesticides in the general population of Thessaly, Greece, determined by HS-SPME GC–MS method. Environmental Research, 148, 318–321. https://doi.org/10.1016/j.envres.2016.04.015.
Koureas, M., Tsakalof, A., Tsatsakis, A., & Hadjichristodoulou, C. (2012). Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicology Letters, 210, 155–168. https://doi.org/10.1016/j.toxlet.2011.10.007.
Luzardo, O. P., Boada, L. D., Carranza, C., Ruiz-Suárez, N., Henríquez-Hernández, L. A., Valerón, P. F., Zumbado, M., Camacho, M., & Arellano, J. L. (2014). Socioeconomic development as a determinant of the levels of organochlorine pesticides and PCBs in the inhabitants of western and central African countries. Science of the Total Environment, 497-498, 97–105. https://doi.org/10.1016/j.scitotenv.2014.07.124.
Margni, M., Rossier, D., Crettaz, P., & Jolliet, O. (2002). Life cycle impact assessment of pesticides on human health and ecosystems. Agriculture, Ecosystems and Environment, 93, 379–392. https://doi.org/10.1016/S0167-8809(01)00336-X.
Mostafalou, S., & Abdollahi, M. (2013). Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicology and Applied Pharmacology, 268, 157–177. https://doi.org/10.1016/j.taap.2013.01.025.
Mariscal-Arcas, M., Lopez-Martinez, C., Granada, A., Olea, N., Lorenzo-Tovar, M. L., & Olea-Serrano, F. (2010). Organochlorine pesticides in umbilical cord blood serum of women from southern Spain and adherence to the Mediterranean diet. Food and Chemical Toxicology, 48(5), 1311–1315. https://doi.org/10.1016/j.fct.2010.02.029.
Park, M. J., In, S. W., Lee, S. K., Choi, W. K., Park, Y. S., & Chung, H. S. (2009). Postmortem blood concentrations of organophosphorus pesticides. Forensic Science International, 184(1–3), 28–31. https://doi.org/10.1016/j.forsciint.2008.11.008.
Porta, M., de Basea, M. B., Benavides, F. G., López, T., Fernandez, E., Marco, E., Alguacil, J., Grimalt, J. O., & Puigdomènech, E. (2008). Differences in serum concentrations of organochlorine compounds by occupational social class in pancreatic cancer. Environmental Research, 108, 370–379. https://doi.org/10.1016/j.envres.2008.06.010.
Song, S., Ma, J., Tian, Q., Tong, L., & Guo, X. (2013a). Hexachlorobenzene in human milk collected from Beijing, China. Chemosphere, 91, 145–149. https://doi.org/10.1016/j.chemosphere.2012.12.019.
Song, S., Ma, X., Tong, L., Tian, Q., Huang, Y., Yin, S., & Sun, H. (2013b). Residue levels of hexachlorocyclohexane and dichlorodiphenyltrichloroethane in human milk collected from Beijing. Environmental Monitoring and Assessment, 185, 7225–7229. https://doi.org/10.1007/s10661-013-3096-4.
Steiniger, D., Lu, G., Butler, J., Phillips, E., Fintschenko, Y., et al. (2010). Determination of multiresidue pesticides in green tea by using a modified QuEChERS extraction and ion-trap gas chromatography/mass spectrometry. Journal of AOAC International, 93, 1169–1179.
Saoudi, A., Fréry, N., Zeghnoun, A., Bidondo, M. L., Deschamps, V., Göen, T., Garnier, R., & Guldner, L. (2014). Serum levels of organochlorine pesticides in the French adult population: the French national nutrition and health study (ENNS), 2006–2007. Science of the Total Environment, 472, 1089–1099. https://doi.org/10.1016/j.scitotenv.2013.11.044.
Thomas, G. O., Wilkinson, M., Hodson, S., & Jones, K. C. (2006). Organohalogen chemicals in human blood from the United Kingdom. Environmental Pollution, 141, 30–41. https://doi.org/10.1016/j.envpol.2005.08.027.
Wang, H. S., Chen, Z. J., Wei, W., Man, Y. B., Giesy, J. P., Du, J., Zhang, G., Wong, C. K., & Wong, M. H. (2013). Concentrations of organochlorine pesticides (OCPs) in human blood plasma from Hong Kong: markers of exposure and sources from fish. Environment International, 54, 18–25. https://doi.org/10.1016/j.envint.2013.01.003.
Wilkowska, A., & Biziuk, M. (2011). Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chemistry, 125, 803–812. https://doi.org/10.1016/j.foodchem.2010.09.094.
Waliszewski, S. M., Caba, M., Herrero-Mercado, M., Saldariaga-Norena, H., Meza, E., Zepeda, R., Martinez-Valenzuela, C., Gomez, A. S., & Villalobos, P. R. (2012). Organochlorine pesticide residue levels in blood serum of inhabitants from Veracruz, Mexico. Environmental Monitoring and Assessment, 184, 5613–5621. https://doi.org/10.1007/s10661-011-2366-2.
Yusa, V., Millet, M., Coscolla, C., & Roca, M. (2015). Analytical methods for human biomonitoring of pesticides. A review. Analytica Chimica Acta, 891, 15–31. https://doi.org/10.1016/j.aca.2015.05.032.
Yu, H. F., Zhao, X. D., Zhao, J. H., Zhu, Z. Q., & Zhao, Z. (2006). Continuous surveillance of organochlorine pesticides in human milk from 1983 to 1998 in Beijing, China. International Journal of Environmental Health Research, 16, 21–26. https://doi.org/10.1080/09603120500397615.
Zamir, R., Athanasiadou, M., Nahar, N., Mamun, M. I. R., Mosihuzzaman, M., & Bergman, Å. (2009). Persistent organohalogen contaminants in plasma from groups of humans with different occupations in Bangladesh. Chemosphere, 74, 453–459. https://doi.org/10.1016/j.chemosphere.2008.09.043.
Acknowledgements
We thank Dr. Wu Wei who works at the Peking Union Medical College Hospital for providing us with the blood samples. Gratitude is also expressed to all the donors who collaborated with the study and provided blood samples.
Funding
This study was funded by the China Food and Drug Administration; the Item Number was ZG2016–2.
Author information
Authors and Affiliations
Ethics declarations
Ethics approval for this study was granted by The Ethics Committee of the Peking Union Medical College (PUMC).
Rights and permissions
About this article
Cite this article
Li, J., Wang, P., Shi, S. et al. Background biomonitoring of residue levels of 137 pesticides in the blood plasma of the general population in Beijing. Environ Monit Assess 190, 315 (2018). https://doi.org/10.1007/s10661-018-6694-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6694-3