Abstract
Macrophyte responses to anthropogenic pressures in two rivers of Central Spain were assessed to check if simple metrics can exhibit a greater discriminatory and explanatory power than complex indices at small spatial scales. Field surveys were undertaken during the summer of 2014 (Duraton River) and the spring of 2015 (Tajuña River). Aquatic macrophytes were sampled using a sampling square (45 × 45 cm). In the middle Duraton River, macrophytes responded positively to the presence of a hydropower dam and a small weir, with Myriophyllum spicatum and Potamogeton pectinatus being relatively favored. Index of Macrophytes (IM) was better than Macroscopic Aquatic Vegetation Index (MAVI) and Fluvial Macrophyte Index (FMI) in detecting these responses, showing positive and significant correlations with total coverage, species richness, and species diversity. In the upper Tajuña River, macrophytes responded both negatively and positively to the occurrence of a trout farm effluent and a small weir, with Leptodictyum riparium and Veronica anagallis-aquatica being relatively favored. Although IM, MAVI, and FMI detected both negative and positive responses, correlations of IM with total coverage, species richness, and species diversity were higher. Species evenness was not sensitive enough to detect either positive or negative responses of aquatic macrophytes along the study areas. Overall, traditional and simple metrics (species composition, total coverage, species richness, species diversity) exhibited a greater discriminatory and explanatory power than more recent and complex indices (IM, MAVI, FMI) when assessing responses of aquatic macrophytes to anthropogenic pressures at impacted specific sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic macrophytes are an important biotic component of rivers and streams (as well as of lakes, ponds, and wetlands). They diminish the erosion of river and stream beds, promote the sedimentation of suspended solids, take up nutrients from the water and sediments, contribute to the water oxygenation via photosynthesis, enhance the spatial heterogeneity and habitat complexity, and provide refuge, food resources, and breeding sites for freshwater fauna (Wetzel 2001; Kohler and Schneider 2003; Allan and Castillo 2007; Chambers et al. 2008; Dibble and Thomaz 2009; Ceschin et al. 2015). Major environmental factors influencing the distribution and abundance of aquatic macrophytes in rivers and streams are current velocity, flow regime, substrate characteristics, light availability (being affected by shading and water turbidity), and water quality (hardness, ionic content, pH, nutrients) (Riis et al. 2000; Bernez et al. 2004; Daniel et al. 2005, 2006; Franklin et al. 2008; Janauer et al. 2010; Steffen et al. 2014; Szoszkiewicz et al. 2017). Although these environmental factors greatly depend upon river order/size and catchment characteristics, they may also be affected by disturbance events (e.g., extreme weather conditions) and anthropogenic pressures (e.g., dams and weirs, channelization and urbanization, point and nonpoint pollution sources) that, accordingly, can affect aquatic macrophytes of lotic ecosystems.
Wiegleb et al. (2014), after analyzing patterns in dominance and growth forms of aquatic macrophytes over a period of 21 years in relation to changes in water chemistry, disturbance regime, and extreme weather conditions in two reference streams in the north-west German lowlands, concluded that the dynamics of riverine vascular plants at the observation site scale might be affected not only by physicochemical environmental factors, but also by stochastic interactions between macrophyte species and single disturbance events or weather extremes. And more recently, Bolpagni et al. (2016), after comparing riverine macrophyte diversity, cover data, spatial and temporal dynamics, and indicator and detector species with physical, chemical, and hydrological variables at 30 different river sites over a 3-year period (2009–2011), concluded that the presence of man-made barriers (e.g., dams, weirs) along river ecosystems can play an important role in structuring riverine macrophyte communities at small spatial scales because of their effects on fluvial hydro-geomorphology. Other studies have indeed showed that river damming can cause favorable effects on aquatic vegetation upstream and downstream from dams (Benitez-Mora and Camargo 2014; Tombolini et al. 2014; Ceschin et al. 2015; Abati et al. 2016).
Responses of macrophyte communities to anthropogenic pressures are usually assessed through changes in species composition, species coverage, and species richness (Suárez et al. 2005; Szoszkiewicz et al. 2006; Baattrup-Pedersen et al. 2008; Ceschin et al. 2010; Dodkins et al. 2012; Benitez-Mora and Camargo 2014; Abati et al. 2016). In addition, several macrophyte indices have been developed to assess the ecological/trophic status of rivers and streams in European countries. For example, the Mean Trophic Rank (Holmes et al. 1999) in the UK, the Trophic Index of Macrophytes (Schneider and Melzer 2003) in Germany, the Macrophyte Biological Index for Rivers (Haury et al. 2006) in France, the Macrophyte Index for Rivers (Szoszkiewicz et al. 2010) in Poland, the River Macrophyte Index (Kuhar et al. 2011) in Slovenia, and the Fluvial Macrophyte Index (Flor-Arnau et al. 2015) in Spain. On the whole, since the European Water Framework Directive was adopted in 2000 (Council of the European Communities 2000), the use of biotic indices based on aquatic macrophytes has expanded through time (Thiebaut et al. 2002; Schaumburg et al. 2004; Meilinger et al. 2005; Willby et al. 2009; Baattrup-Pedersen et al. 2013; Wiegleb et al. 2016).
The main aim of the present investigation was to assess responses of aquatic macrophytes to anthropogenic pressures in two rivers of Central Spain, using different metrics and indices and then comparing the obtained results. The initial hypothesis to be checked is that traditional and simple metrics (species composition, species coverage, species richness, species evenness, species diversity) can exhibit a greater discriminatory and explanatory power than more recent and complex indices (Index of Macrophytes, Macroscopic Aquatic Vegetation Index, Fluvial Macrophyte Index) when assessing anthropogenic pressures at small spatial scales.
Materials and methods
Study areas and sampling sites
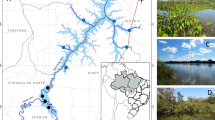
The first study area was located in the middle Duraton River (Segovia Province, Duero River Basin), in the vicinity of Burgomillodo Reservoir (Fig. 1), a deep-release hydropower impoundment with a total capacity of 15 hm3 and a maximum water depth of 40 m. To reinforce the re-oxygenation of hypolimnial waters released by the dam, a concrete weir (about 1 m high) was built about 150 m downstream from the dam. On the other hand, an industrial effluent entered Duraton River about 300 m downstream from Burgomillodo Dam (Fig. 1), discharging relatively high amounts of fluoride (F−) and suspended inorganic matter into the river.
Four sampling sites (D-1, D-2, D-3, and D-4) were selected along the first study area (Fig. 1). D-1 was placed upstream from Burgomillodo Dam, within the limits of Natural Park “Hoces Río Duratón.” This sampling site was used as a reference station. D-2 was placed about 0.2 km downstream from Burgomillodo Dam, subsequent to the small weir. D-3 and D-4 were placed about 0.1 and 2.2 km downstream from the industrial effluent, respectively. The river bottom was mainly composed of calcareous rocks (cobbles and pebbles) at all sampling sites, but with an apparent deposition of fine inorganic matter downstream from the industrial effluent.
The second study area was located in the upper Tajuña River (Guadalajara Province), a limestone river within the Tajo River Basin (Fig. 1). A flow-through trout (Oncorhynchus mykiss) farm was situated about 13 km downstream from the river source. Wastewater treatment involved simple sedimentation in a small settling pond without the adding of chemicals. In addition, a man-made weir (about 1 m high) was situated about 80 m downstream from the trout farm effluent to reinforce the sedimentation of suspended solids on the river bottom.
Four sampling sites (T-1, T-2, T-3, and T-4) were selected along the second study area (Fig. 1). T-1 was placed upstream from the trout farm, being used as a reference station. T-2, T-3, and T-4 were placed about 10, 100, and 1000 m downstream from the trout farm effluent, respectively. The river bottom was basically composed of calcareous rocks (cobbles and pebbles) at T-1, T-3, and T-4, but it was covered by a relatively thick layer of fine organic sediment at T-2.
These two study areas were selected because they comprise different anthropogenic pressures (particularly river damming and nutrient enrichment) that can be found in many rivers around the world.
Water sampling and analyses
Field surveys were undertaken in the summer of 2014 (middle Duraton River) and the spring of 2015 (upper Tajuña River). Current velocity, temperature, pH, conductivity, and dissolved oxygen were measured in situ at all sampling sites, using specific meters in accordance with standard methods (American Public Health Association 1998; Wetzel and Likens 2000). Water samples were also collected with clean polyethylene containers to analyze in the laboratory turbidity and fluoride (water samples from the middle Duraton River owing to the water pollution caused by the industrial effluent) and turbidity and inorganic nutrients (water samples from the upper Tajuña River owing to the water pollution caused by the trout farm effluent). Turbidity was directly analyzed with a standard turbidimeter (American Public Health Association 1998). To analyze fluoride, water samples from D-1, D-2, D-3, and D-4 sampling sites were first filtered through pre-rinsed 0.45 μm cellulose acetate filters, and then water filtrates were used to determine concentrations of fluoride ions (F−) by the standard SPADNS colorimetric method (American Public Health Association 1998). To analyze inorganic nutrients, water samples from T-1, T-2, T-3, and T-4 sampling sites were first filtered through pre-rinsed 0.45 μm cellulose acetate filters, and then water filtrates were used to determine concentrations of NH4-N, NO3-N, and PO4-P by spectrophotometry (American Public Health Association 1998).
Macrophyte sampling and analyses
Field surveys of aquatic macrophytes (excluding riparian helophytes) were also carried out in 2014 and 2015, always after finishing the respective water sampling and analyses. Species composition for the macrophyte community, along with the coverage (%) of each macrophyte species, was assessed at each sampling site by performing five river transects and examining either three (upper Tajuña River) or six (middle Duraton River) sampling squares (45 × 45 cm) per transect (Hauer and Lamberti 1996; Wetzel and Likens 2000). The number of sampling squares per transect was arbitrarily chosen, representing randomly chosen subsamples of each river transect. The taxonomic identification of aquatic macrophytes was conducted following Cirujano and Medina (2002) and Casas et al. (2006).
Macrophyte metrics and indices
Following macrophyte sampling and analyses, abundance and richness metrics were estimated for the whole macrophyte community at each sampling site: abundance is expressed as the cover percentage of all aquatic macrophytes per river transect (i.e., total macrophyte coverage); richness is expressed as the total number of macrophyte species per river transect (i.e., species richness). Species evenness and species diversity were also calculated using Camargo’s (1992, 1993, 1995) indices of community structure, in which species evenness is the complement of species dominance (species evenness = 1 – species dominance), and species diversity is the resulting product of multiplying species richness by species evenness (species diversity = species richness × species evenness). Lastly, the Index of Macrophytes (Suárez et al. 2005), the Macroscopic Aquatic Vegetation Index (Moreno et al. 2006), and the more recent Fluvial Macrophyte Index (Flor-Arnau et al. 2015) were calculated. These three biotic indices based on aquatic macrophytes have been developed to assess the effects of organic pollution and nutrient enrichment on the ecological/trophic status of rivers and streams in the Iberian Peninsula.
The Index of Macrophytes (IM; Suárez et al. 2005) is based on the differential sensitivity of a relatively small number of key indicator genera of algae and angiosperms, plus two general taxonomic groups of bryophytes (mosses and liverworts): IM can take values from less than 3 (very bad ecological/trophic status) to more than 30 (excellent ecological/trophic status). The Macroscopic Aquatic Vegetation Index (MAVI; Moreno et al. 2006) is based on the differential sensitivity of 50 taxa (36 genera of algae, 13 genera of angiosperms, and 1 genus of pteridophytes), plus two general taxonomic groups of bryophytes (mosses and liverworts): MAVI can exhibit values from less than 2.5 (very poor ecological/trophic status) to more than 6 (excellent ecological/trophic status). The Fluvial Macrophyte Index (FMI; Flor-Arnau et al. 2015) is based on the differential sensitivity of 124 taxa (50 species of angiosperms, 31 species/genera of algae, 30 species of mosses, 8 species of liverworts, and 5 species of pteridophytes): FMI can take values from less than 4.5 (very poor ecological/trophic status) to more than 14 (excellent ecological/trophic status).
Statistical analyses
Since parametric methods usually have more statistical power than nonparametric methods to detect that the null hypothesis is false (i.e., to avoid a type II error) (Sokal and Rohlf 1995), t tests were performed to check significant (P < 0.05) differences between mean values of physicochemical and biological parameters (including metrics and indices) at reference stations and mean values of physicochemical and biological parameters at impacted/polluted sampling sites. Besides, relationships between macrophyte metrics and indices along the study areas were examined by Pearson’s correlation analyses. Normality and homoscedasticity were assumed for physicochemical and biological data (Sokal and Rohlf 1995).
Results
No significant (P > 0.05) differences between the reference station (D-1) and impacted/polluted sampling sites (D-2, D-3, and D-4) were found for current velocity, temperature, pH, conductivity, and dissolved oxygen in the first study area (Table 1). However, fluoride concentrations and turbidity levels significantly (P < 0.05) increased downstream from the industrial effluent (Table 1), with D-3 exhibiting the highest mean values (0.6 mg F− L−1 and 55.2 NTU). In the second study area, no significant (P > 0.05) differences between the reference station (T-1) and impacted/polluted sampling sites (T-2, T-3, and T-4) were found for temperature, pH, and conductivity (Table 1). However, concentrations of inorganic nutrients (ammonium, nitrate, and phosphate) significantly (P < 0.05) increased downstream from the trout farm effluent, whereas concentrations of dissolved oxygen significantly (P < 0.05) decreased (Table 1). In addition, turbidity was significantly (P < 0.05) higher at T-2 than at T-1, whereas current velocity was significantly (P < 0.05) lower (Table 1), this fact reinforcing the sedimentation of suspended solids at T-2.
The aquatic moss Fontinalis antipyretica and the angiosperm Potamogeton pectinatus were found at all sampling sites along the first study area (Table 2), with F. antipyretica being the most abundant macrophyte species at D-1 (12.4%) and P. pectinatus being the most abundant macrophyte species at D-2 (20.5%), D-3 (25.7%), and D-4 (22.4%). The aquatic moss Platyhypnidium riparioides was found at D-1 (6.7%), only, and the angiosperm Apium nodiflorum was found at D-1 (4.9%) and D-2 (7.3%) (Table 2). The angiosperm Myriophyllum spicatum and the filamentous green algae Cladophora sp. were found at D-2, D-3, and D-4 (Table 2). However, while the mean cover percentage of Cladophora sp. increased (from 3.4 to 12.0%) with increasing downstream distance from Burgomillodo Dam, the mean cover percentage of M. spicatum decreased (from 15.2 to 7.8%) (Table 2).
The angiosperm Lemna minor was found at all sampling sites along the second study area (Table 2), exhibiting its highest coverage at T-1 (10.6%). However, the most abundant macrophyte species at the reference station (T-1) was A. nodiflorum (38.5%), with its coverage significantly (P < 0.05) decreasing downstream from the trout farm effluent, particularly at T-2 and T-3 where it was absent (Table 2). Although the aquatic moss Leptodictyum riparium also was absent at T-2, its coverage was higher at T-3 (8.2%) and T-4 (16.8%) than at T-1 (3.9%) (Table 2). Cladophora sp. was found at T-2 (6.7%) and T-3 (7.2%), being the predominant macrophyte species at T-2 (Table 2). The angiosperm Veronica anagallis-aquatica was found at T-3 (16.5%) and T-4 (35.7%), being the most abundant macrophyte species at these two sampling sites (Table 2).
Mean values of total coverage, species richness, species diversity, and IM significantly (P < 0.05) increased downstream from Burgomillodo Dam, especially at D-2 (Table 3). Conversely, mean values of MAVI and FMI significantly (P < 0.05) decreased downstream from Burgomillodo Dam, particularly at D-4 (Table 3). No significant (P > 0.05) differences between the reference station (D-1) and impacted/polluted sampling sites (D-2, D-3, and D-4) were found for mean values of species evenness (Table 3). In the second study area, mean values of total coverage, species richness, species diversity, IM, and MAVI were significantly (P < 0.05) lower at T-2 than at T-1, but with mean values of total coverage, species richness, species diversity, and FMI being significantly (P < 0.05) higher at T-4 than at T-1, and with total coverage being significantly (P < 0.05) lower at T-3 than at T-1 (Table 3). No significant (P > 0.05) differences between the reference station (T-1) and impacted/polluted sampling sites (T-2, T-3, and T-4) were found for mean values of species evenness (Table 3).
In the first study area, total coverage, species richness, species diversity, and IM exhibited significant (P < 0.05) and positive correlations between them (Table 4), but nonsignificant (P > 0.05) and negative correlations with species evenness, MAVI, and FMI (Table 4). The correlation coefficient between MAVI and FMI was significant (P < 0.05) and positive (Table 4). In the second study area, total coverage, species richness, species diversity, IM, MAVI, and FMI exhibited significant (P < 0.05) and positive correlations between them (Table 4). However, correlation coefficients of IM with total coverage, species richness, and species diversity were higher than those of MAVI and FMI (Table 4). Correlations of species evenness with the other macrophyte metrics and indices were negative and significant (P < 0.05), excepting with MAVI (P > 0.05) (Table 4).
Discussion
Responses of the riverine macrophyte community to anthropogenic pressures in the first study area (middle Duraton River) should be considered as positive owing to significant increases in total coverage, species richness, and species diversity at D-2, D-3, and D-4 with regard to the reference station D-1 (Table 3). Recent studies focusing on riverine macrophyte communities in other dammed rivers and streams (Benitez-Mora and Camargo 2014; Abati et al. 2016) have reported that species coverage and species richness increased downstream from dams as a likely consequence of higher river-bed width (increasing light availability), reduced river-flow force (increasing substrate stability), and/or increased nutrient concentrations. Because previous studies (Gonzalo and Camargo 2013) found no significant difference between sampling sites, upstream and downstream from Burgomillodo Reservoir, regarding inorganic nutrients, the most probable cause of the observed changes in the riverine macrophyte community downstream from Burgomillodo Dam would be higher river-bed width, positively affecting light availability and also substrate stability. In addition, the impounded area between Burgomillodo Dam and the concrete weir (built to reinforce the re-oxygenation of hypolimnial waters released by the dam) would be a source of aquatic macrophytes for downstream sampling sites since it was inhabited by a great amount of hydrophytes (P. pectinatus and M. spicatum, primarily).
Significant increases in total coverage, species richness, and species diversity however were lower at D-3 and D-4 than at D-2 because of the water pollution caused by the industrial effluent at downstream sampling sites (Table 1), although Cladophora sp., F. antipyretica, and P. pectinatus apparently tolerated relatively well the increased fluoride concentrations and turbidity levels at D-3 and D-4 (Table 2). Previous studies have already showed that Cladophora sp. and P. pectinatus may be relatively tolerant to anthropogenic pressures in lotic ecosystems, exhibiting low scores (i.e., low sensitivity) within macrophyte indices (Suárez et al. 2005; Haury et al. 2006; Moreno et al. 2006; Willby et al. 2009; Kuhar et al. 2011; Baattrup-Pedersen et al. 2013; Flor-Arnau et al. 2015). F. antipyretica might also be considered as a tolerant macrophyte species to anthropogenic pressures since numerous studies have showed that this aquatic moss has wide ecological ranges, showing great ability to accumulate high amounts of trace elements and, accordingly, being often used as an indicator species for the biomonitoring of river pollution (Tipping et al. 2008; Ceschin et al. 2012; Gonzalo and Camargo 2013; Gecheva and Yurukova 2014; Debén et al. 2015). IM, MAVI, and FMI however give relatively high scores (i.e., high sensitivity) to this aquatic moss (Suárez et al. 2005; Moreno et al. 2006; Flor-Arnau et al. 2015).
In spite of the detected positive responses of aquatic macrophytes to anthropogenic pressures in the middle Duraton River, MAVI and FMI significantly decreased their values downstream from Burgomillodo Dam, particularly at D-4 (Table 3), exhibiting negative (but nonsignificant) correlations with total coverage, species richness, and species diversity along the first study area (Table 4). This finding is a little surprising since these two macrophyte indices have been basically developed to assess the effects of organic pollution and nutrient enrichment on the ecological/trophic status of rivers and streams (Moreno et al. 2006; Flor-Arnau et al. 2015), being (in theory) much less affected by other anthropogenic pressures. In contrast, IM significantly increased its values downstream from Burgomillodo Dam, particularly at D-2 (Table 3), exhibiting positive and significant correlations with total coverage, species richness, and species diversity along the first study area (Table 4).
Contradictory behaviors of macrophyte indices confronting the same anthropogenic pressures are not unusual owing to differences in macrophyte scores, sophistication degree, and calculation methodology (Suárez et al. 2005; Moreno et al. 2006; Fabris et al. 2009; Birk and Willby 2010; Kuhar et al. 2011; Demars et al. 2012; Flor-Arnau et al. 2015; Wiegleb et al. 2016). Concerning species evenness, the absence of significant differences between impacted/polluted sampling sites (D-2, D-3, and D-4) and the reference station (D-1) (Table 3) clearly indicates a lower discriminatory power of species evenness to assess anthropogenic pressures at small spatial scales. Abati et al. (2016) however found that the species evenness of macrophyte assemblages in mountain streams tended to decrease downstream from dams because of the differential effect of stream regulation on the relative abundances of macrophyte species.
Responses of the riverine macrophyte community to anthropogenic pressures in the second study area (upper Tajuña River) should be considered as negative owing to significant decreases in total coverage, species richness, and species diversity at T-2 with regard to the reference station T-1 (Table 3). These decreases mainly were a consequence of the absence of A. nodiflorum at T-2 (Table 2). Besides, responses of the riverine macrophyte community to anthropogenic pressures in the second study area can also be considered as positive because of significant increases in total coverage, species richness, and species diversity at T-4 with reference to T-1 (Table 3). These increases were a clear consequence of the increased abundances of V. anagallis-aquatica and L. riparium at T-4 (Table 2).
The main causes of the observed changes in the riverine macrophyte community downstream from the trout farm effluent would be the deposition of suspended solids on the river bottom, particularly at T-2 where it was covered by a relatively thick layer of organic sediment, and the increased concentrations of nitrate, ammonium, and phosphate (Table 1). Physicochemical alterations downstream from trout farm effluents often involve increases in nutrient and turbidity levels and deposition of suspended solids (Boaventura et al. 1997; Pulatsu et al. 2004; Bartoli et al. 2007; Ruiz-Zarzuela et al. 2009; Guilpart et al. 2012; Lalonde et al. 2015). In addition, other studies have showed that substrate characteristics and concentrations of inorganic nutrients are major environmental factors affecting riverine macrophyte communities (Barendregt and Bio 2003; Daniel et al. 2005, 2006; Baattrup-Pedersen et al. 2008; Steffen et al. 2014; Szoszkiewicz et al. 2017): nutrient enrichment usually enhances macrophyte coverage, whereas fine organic sediments tend to decrease the abundance and diversity of aquatic macrophytes.
Both negative and positive responses of the riverine macrophyte community to the trout farm effluent in the upper Tajuña River were relatively well detected by IM, MAVI, and FMI, exhibiting lower values at T-2 than at T-1 and higher values at T-4 than at T-1 (Table 3). Furthermore, these three macrophyte indices exhibited positive and significant correlations with total coverage, species richness, and species diversity along the second study area (Table 4), although correlation coefficients of IM were higher than those of MAVI and FMI. Nevertheless, the behavior of IM, MAVI, and FMI appeared to be unreasonable regarding the main reason for their development: to assess the effects of organic pollution and nutrient enrichment on the ecological/trophic status of rivers and streams (Suárez et al. 2005; Moreno et al. 2006; Flor-Arnau et al. 2015). Because significant increases in the concentration of inorganic nutrients (nitrate, ammonium, and phosphate) downstream from the trout farm effluent occurred not only at T-2, but also at T-3 and T-4 (Table 1), values of IM, MAVI, and FMI at the reference station (T-1) should be higher not only than their respective values at T-2 (Table 3), but also than their respective values at T-3 and T-4. In consequence, lower values of IM, MAVI, and FMI at T-2 than at T-1 were surely due to the great alteration of substrate characteristics at T-2 by the deposition of suspended solids, whereas higher values of IM, MAVI, and FMI at T-4 than at T-1 were surely due to the particular score systems of these macrophyte indices. Actually, while A. nodiflorum and V. anagallis-aquatica exhibit the same middle score within IM (Suárez et al. 2005) and MAVI (Moreno et al. 2006), FMI gives A. nodiflorum the half of the score for V. anagallis-aquatica (Flor-Arnau et al. 2015). Furthermore, L. riparium exhibits higher scores than A. nodiflorum and V. anagallis-aquatica within IM, MAVI, and FMI, but certain studies (Ceschin et al. 2012; Gecheva et al. 2017) have reported that freshwater eutrophication can favor this aquatic moss.
Conclusions
Taking into account the obtained results and their subsequent discussion, the following conclusions may be supported: (1) downstream responses of the riverine macrophyte community to anthropogenic pressures in the middle Duraton River may be regarded as positive owing to significant increases in total coverage, species richness, and species diversity, although these positive responses declined to some extent downstream from the industrial effluent; (2) IM was the best macrophyte index in detecting positive responses of aquatic macrophytes along the first study area, with MAVI and FMI being less suitable; (3) downstream responses of the riverine macrophyte community to anthropogenic pressures in the upper Tajuña River may be regarded as negative owing to significant decreases in total coverage, species richness, and species diversity at the nearest downstream sampling site, but also as positive because of significant increases in total coverage, species richness, and species diversity at the farthest downstream sampling site; (4) IM was the best macrophyte index in detecting positive and negative responses of aquatic macrophytes along the second study area, with MAVI and FMI being less suitable; (5) species evenness was not sensitive enough to detect either positive or negative responses of aquatic macrophytes along the study areas.
Overall, traditional and simple metrics (species composition, species coverage, species richness, species diversity) exhibited greater discriminatory and explanatory power than more recent and complex indices (IM, MAVI, FMI) when assessing macrophyte responses to anthropogenic pressures at impacted specific sites (i.e., at small spatial scales). Moreover, because the present study has showed that IM, MAVI, and FMI may be significantly affected not only by organic pollution and nutrient enrichment, but also by other anthropogenic pressures (dam, small weirs, industrial effluent), thereby increasing the difficulty and uncertainties for the proper interpretation of their behavior, the ecological/trophic status of rivers and streams should not be directly determined according to the numerical value of these macrophyte indices, at least at impacted specific sites. These conclusions and recommendations agree in general with those of other related studies (Demars et al. 2012; Demars 2013; Bolpagni et al. 2016; Wiegleb et al. 2016).
References
Abati, S., Minciardi, M. R., Ciadamidaro, S., Fattorini, S., & Ceschin, S. (2016). Response of macrophyte communities to flow regulation in mountain streams. Environmental Monitoring and Assessment, 188, 414–426.
Allan, J. D., & Castillo, M. M. (2007). Stream ecology: structure and function of running waters. Dordrecht: Springer.
American Public Health Association (1998). Standard methods for the examination of water and wastewater (the twentieth edition). Washington, DC: APHA-AWWA-WPCF.
Baattrup-Pedersen, A., Springe, G., Riis, T., Larsen, S. E., SandJensen, K., & Larsen, L. M. K. (2008). The search for reference conditions for stream vegetation in northern Europe. Freshwater Biology, 53, 1890–1901.
Baattrup-Pedersen, A., Larsen, S. E., & Riis, T. (2013). From expert judgment to supervised classification: a new approach to assess ecological status in lowland streams. Science of the Total Environment, 447, 116–122.
Barendregt, A., & Bio, A. M. F. (2003). Relevant variables to predict macrophyte communities in running waters. Ecological Modelling, 160, 205–217.
Bartoli, M., Nizzoli, D., Longhi, D., Laini, A., & Viaroli, P. (2007). Impact of a trout farm on the water quality of an Apennine creek from daily budgets of nutrients. Chemistry and Ecology, 23, 1–11.
Benitez-Mora, A., & Camargo, J. A. (2014). Ecological responses of aquatic macrophytes and benthic macroinvertebrates to dams in the Henares River Basin (Central Spain). Hydrobiologia, 728, 167–178.
Bernez, I., Daniel, H., Haury, J., & Ferreira, M. T. (2004). Combined effects of environmental factors and regulation on macrophyte vegetation along three rivers in western France. River Research and Applications, 20, 43–59.
Birk, S., & Willby, N. (2010). Towards harmonization of ecological quality classification: establishing common grounds in European macrophyte assessment for rivers. Hydrobiologia, 652, 149–163.
Boaventura, R., Pedro, A. M., Coimbra, J., & Lencastre, E. (1997). Trout farm effluents: characterization and impact on the receiving streams. Environmental Pollution, 95, 379–387.
Bolpagni, R., Racchetti, E., & Laini, A. (2016). Fragmentation and groundwater supply as major drivers of algal and plant diversity and relative cover dynamics along a highly modified lowland river. Science of the Total Environment, 568, 875–884.
Camargo, J. A. (1992). Temporal and spatial variations in dominance, diversity and biotic índices along a limestone stream receiving a trout farm effluent. Water, Air, and Soil Pollution, 63, 343–359.
Camargo, J. A. (1993). Macrobenthic surveys as a valuable tool for assessing freshwater quality in the Iberian Peninsula. Environmental Monitoring and Assessment, 24, 71–90.
Camargo, J. A. (1995). On measuring species evenness and other associated parameters of community structure. Oikos, 74, 538–542.
Casas, C., Brugués, M., Cros, R. M., & Sérgio, C. (2006). Handbook of mosses of the Iberian Peninsula and the Balearic Islands. Barcelona: Institut d’Estudis Catalans.
Ceschin, S., Salerno, G., Bisceglie, S., & Kumbaric, A. (2010). Temporal floristic variations as indicator of environmental changes in the Tiber River in Rome. Aquatic Ecology, 44, 93–100.
Ceschin, S., Aleffi, M., Bisceglie, S., Savo, V., & Zuccarello, V. (2012). Aquatic bryophytes as ecological indicators of the water quality status in the Tiber river basin (Italy). Ecological Indicators, 14, 74–81.
Ceschin, S., Tombolini, I., Abati, S., & Zuccarello, V. (2015). The effect of river damming on vegetation: it is always unfavourable? A case study from the River Tiber (Italy). Environmental Monitoring and Assessment, 187, 301. https://doi.org/10.1007/s10661-015-4521-7.
Chambers, P. A., Lacoul, P., Murphy, K. J., & Thomaz, S. M. (2008). Global diversity of aquatic macrophytes in freshwater. Hydrobiologia, 595, 9–26.
Cirujano, S., & Medina, L. (2002). Plantas acuáticas de las lagunas y humedales de Castilla-La Mancha. Madrid: Real Jardín Botánico (CSIC) y Junta de Comunidades (Castilla La Mancha).
Council of the European Communities. (2000). Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy. Official Journal of the European Communities, L327, 1–73.
Daniel, H., Bernez, I., Haury, J., & Le Coeur, D. (2005). The ability of aquatic macrophytes to assess fish farm pollution in two salmon rivers. Hydrobiologia, 551, 183–191.
Daniel, H., Bernez, I., & Haury, J. (2006). Relationships between macrophytic vegetation and physical features of river habitats: the need for a morphological approach. Hydrobiologia, 270, 11–17.
Debén, S., Aboal, J. R., Carballeira, A., Cesa, M., Real, C., & Fernández, J. A. (2015). Inland water quality monitoring with native bryophytes: a methodological review. Ecological Indicators, 53, 115–124.
Demars, B. O. L. (2013). Uncertainties in biotic indicators and a corrigendum to Ponader et al. (2007): implications for biomonitoring. Ecological Indicators, 27, 44–47.
Demars, B. O. L., Potts, J. M., Trémolières, M., Thiébaut, G., Gougelin, N., & Nordmann, V. (2012). River macrophyte indices: not the Holy Grail! Freshwater Biology, 57, 1745–1759.
Dibble, E. D., & Thomaz, S. M. (2009). Use of fractal dimension to assess habitat complexity and its influence on dominant invertebrates inhabiting tropical and temperate macrophytes. Journal of Freshwater Ecology, 24, 93–102.
Dodkins, I., Aguiar, F., Rivaes, R., Albuquerque, A., RodríguezGonzález, P., & Ferreira, M. T. (2012). Measuring ecological change of aquatic macrophytes in Mediterranean rivers. Limnologica, 42, 95–107.
Fabris, M., Schneider, S., & Melzer, A. (2009). Macrophyte-based bioindication in rivers: a comparative evaluation of the reference index (RI) and the trophic index of macrophytes (TIM). Limnologica, 39, 40–55.
Flor-Arnau, N., Real, M., González, G., Cambra Sánchez, J., Moreno, J. L., Solà, C., & Munné, A. (2015). Índice de Macrófitos Fluviales (IMF), una nueva herramienta para evaluar el estado ecológico de los ríos mediterráneos. Limnetica, 34, 95–114.
Franklin, P., Dunbar, M. J., & Whitehead, P. (2008). Flow controls on lowland river macrophytes: a review. Science of the Total Environment, 400, 369–378.
Gecheva, G., & Yurukova, L. (2014). Water pollutant monitoring with aquatic bryophytes: a review. Environmental Chemistry Letters, 12, 49–61.
Gecheva, G., Pall, K., & Hristeva, Y. (2017). Bryophyte communities’ responses to environmental factors in highly seasonal rivers. Botany Letters, 164, 79–91.
Gonzalo, C., & Camargo, J. A. (2013). The impact of an industrial effluent on the water quality, submersed macrophytes and benthic macroinvertebrates in a dammed river of Central Spain. Chemosphere, 93, 117–124.
Guilpart, A., Roussel, J. M., Aubin, J., Caquet, T., Marle, M., & LeBris, H. (2012). The use of benthic invertebrate community and water quality analyses to assess ecological consequences of fish farm effluents in rivers. Ecological Indicators, 23, 356–365.
Hauer, F. R., & Lamberti, G. A. (Eds.). (1996). Methods in stream ecology. San Diego (CA): Academic Press.
Haury, J., Peltre, M. C., Trémolières, M., Barbe, J., Thiébaut, G., & Bernez, I. (2006). A new method to assess water trophy and organic pollution—the Macrophyte Biological Index for Rivers (IBMR): its application to different types of river and pollution. Hydrobiologia, 570, 153–158.
Holmes, N.T.H., Newman, J.R., Chadd, S., Rouen, K.J., Saint, L., & Dawson, F.H. (1999). Mean trophic rank: a user’s manual. R&D technical report E38. Bristol (UK): Environment Agency.
Janauer, G. A., Schmidt-Mumm, U., & Schmidt, B. (2010). Aquatic macrophytes and water current velocity in the Danube River. Ecological Engineering, 36, 1138–1145.
Kohler, A., & Schneider, S. (2003). Macrophytes as bioindicators. Archiv für Hydrobiologie Supplement 147. Large Rivers, 14, 17–31.
Kuhar, U., Germ, M., Gaberscik, A., & Urbanic, G. (2011). Development of a River Macrophyte Index (RMI) for assessing river ecological status. Limnologica, 41, 235–243.
Lalonde, B. A., Ernst, W., & Garron, C. (2015). Chemical and physical characterisation of effluents from land-based fish farms in Atlantic Canada. Aquaculture International, 23, 535–546.
Meilinger, P., Schneider, S., & Melzer, A. (2005). The reference index method for the macrophyte-based assessment of rivers: a contribution to the implementation of the European water framework directive in Germany. International Review of Hydrobiology, 90, 322–342.
Moreno, J. L., Navarro, C., & De las Heras, J. (2006). Propuesta de un índice de vegetación acuática (IVAM) para la evaluacion del estado trófico de los ríos de Castilla-La Mancha: comparación con otros índices bióticos. Limnetica, 25, 821–838.
Pulatsu, S., Rad, F., Koksal, G., Aydm, F., Benli, A. C. K., & Topcu, A. (2004). The impact of rainbow trout effluents on water quality of Kaqrasu stream, Turkey. Turkish Journal of Fisheries and Aquatic Sciences, 4, 9–15.
Riis, T., Sand-Jensen, K., & Vestergaard, O. (2000). Plant communities in lowland Danish streams: species composition and environmental factors. Aquatic Botany, 66, 255–272.
Ruiz-Zarzuela, I., Halaihel, N., Balcázar, J. L., Ortega, C., Vendrell, D., Pérez, T., Alonso, J. L., & de Blas, I. (2009). Effect of fish farming on the water quality of rivers in northeast Spain. WST, 60, 663–671.
Schaumburg, J., Schranz, C., Foerster, J., Gutowski, A., Hofmann, G., Meilinger, P., Schneider, S., & Schmedtje, U. (2004). Ecological classification of macrophytes and phytobenthos for rivers in Germany according to the Water Framework Directive. Limnologica, 34, 283–301.
Schneider, S., & Melzer, A. (2003). The trophic index of macrophytes (TIM): a new tool for indicating the trophic state of running waters. International Review of Hydrobiology, 88, 49–67.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry: the principles and practice of statistics in biological research (the third edition). New York: Freeman.
Steffen, K., Leuschner, C., Müller, U., Wiegleb, G., & Becker, T. (2014). Relationship between macrophyte vegetation and physical and chemical conditions in northwest German running waters. Aquatic Botany, 113, 46–55.
Suárez, M. L., Mellado, A., Sánchez-Montoya, M. M., & Vidal-Abarca, M. R. (2005). Propuesta de un índice de macrófitos (IM) para evaluar la calidad ecológica de los ríos de la cuenca del Segura. Limnetica, 24, 305–318.
Szoszkiewicz, K., Ferreira, T., Korte, T., Baattrup-Pedersen, A., Davy-Bowker, J., & O’Hare, M. (2006). European river plant communities: the importance of organic pollution and the usefulness of existing macrophyte metrics. Hydrobiologia, 566, 211–234.
Szoszkiewicz, K., Jusik, S., Lawniczak, A. E., & Zgoła, T. (2010). Macrophyte development in unimpacted lowland rivers in Poland. Hydrobiologia, 656, 117–131.
Szoszkiewicz, K., Budka, A., Pietruczuk, K., Kayzer, D., & Gebler, D. (2017). Is the macrophyte diversification along the trophic gradient distinct enough for river monitoring? Environmental Monitoring and Assessment, 189, 4. https://doi.org/10.1007/s10661-016-5710-8.
Thiebaut, G., Guérold, F., & Muller, S. (2002). Are trophic and diversity indices based on macrophyte communities pertinent tools to monitor water quality. Water Research, 36, 3602–3610.
Tipping, E., Vincent, C. D., Lawlor, A. J., & Lofts, S. (2008). Metal accumulation by stream bryophytes, related to chemical speciation. Environmental Pollution, 156, 936–943.
Tombolini, I., Caneva, G., Cancellieri, L., Abati, S., & Ceschin, S. (2014). Damming effects on upstream riparian and aquatic vegetation: the case study of Nazzano (Tiber River, central Italy). Knowledge and Management of Aquatic Ecosystems, (412), 03. https://doi.org/10.1051/kmae/2013085.
Wetzel, R. G. (2001). Limnology: lake and river ecosystems (the third edition). San Diego (CA): Academic Press.
Wetzel, R. G., & Likens, G. E. (2000). Limnological analyses (the third edition). New York: Springer.
Wiegleb, G., Bröring, U., Filetti, M., Brux, H., & Herr, W. (2014). Long-term dynamics of macrophyte dominance and growth-form types in two north-west German lowland streams. Freshwater Biology, 59, 1012–1025.
Wiegleb, G., Gebler, D., van de Weyer, K., & Birk, S. (2016). Comparative test of ecological assessment methods of lowland streams based on long-term monitoring data of macrophytes. Science of the Total Environment, 541, 1269–1281.
Willby, N. J., Pitt, J. A., & Phillips, G. (2009). The ecological classification of UK rivers using aquatic macrophytes. UK environment agency science reports, project SC010080 /SR1. Bristol: Environment Agency.
Acknowledgements
The University of Alcala provided logistical support for carrying out field and laboratory studies. Valuable comments and suggestions by two anonymous reviewers are gratefully acknowledged.
Funding
Funds for this research came from the Spanish Ministry of Science and Innovation (Research project CGL2011-28585).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camargo, J.A. Responses of aquatic macrophytes to anthropogenic pressures: comparison between macrophyte metrics and indices. Environ Monit Assess 190, 173 (2018). https://doi.org/10.1007/s10661-018-6549-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6549-y