Abstract
The purpose of this comparative analysis is the determination of the total quantity of metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb) in soil samples, above ground plant parts and tea made of plants Teucrium montanum and T. chamaedrys from different serpentine and calcareous habitats as well as of the total quantity of phenolic compounds and antioxidant activity. The obtained results showed that the quantities of certain metals (Mg, Fe, Ni and Mn) in the soil from the serpentine habitats were greater in comparison with other metals (Ca, Zn and Pb) which were more frequently found in the soil from the calcareous habitats. The results demonstrated that the analysed plant samples from the serpentine habitats contained higher quantity of Fe, Ni and Cr as opposed to the plant samples from the calcareous habitats which contained greater quantity of Ca and Zn. Although the studied species accumulate analysed metals in different quantities, depending on the substrate type, they are not hyperaccumulators of these metals. The use of these species from serpentine habitats for tea preparation is safe to a great extent, because in spite of the determined metal absorption by plant organs, the tea does not contain dangerous quantity of heavy metals. The results showed greater total quantity of phenolic compounds and the higher level of antioxidant activity in the plant samples from serpentine habitats in comparison with the samples from calcareous habitats, which is an indicator of one of the mechanisms of adaptation to the serpentine habitat conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Serpentine (ultramafic) soil represents a specific substrate as it has extreme chemical and physical characteristics. Disturbed mineral regime, the lack of essential nutrients (Ca, P, N, K), disturbed Ca to Mg ratio, greater quantities of heavy metals (Mn, Ni, Cr) and variation of pH values in the range from 5.5 to 9.58, i.e. from acidic to very strongly alkaline, are among the most important chemical characteristics. The significant physical characteristics of this type of soil are shallowness, skeletalness and specific water and temperature regime (Kruckeberg 1984; Brooks 1987; Brady et al. 2005; Alexander et al. 2007).
Calcareous soil is a soil that contains free calcium carbonate. This type of soil is characteristic of arid and semiarid areas as well as of humid and semihumid zones, particularly where their parent material is rich in CaCO3. On a chemical level, the presence of CaCO3 both induces alkaline reactions in calcareous soils and affects availability of N, P, K, Mg, Zn, Cu and Fe to plants. Carbonates in soil contribute to the pH buffering within the range of 7.5 to 8.5. Apart from chemical influence, CaCO3 in calcareous soil influences its physical properties as well (Lambers et al. 2008).
In order to maintain continued existence on the serpentine types of substrates, plants have to develop a series of anatomical, morphological and physiological adaptations, primarily due to high concentrations of heavy metals (Brooks 1998; Brady et al. 2005; Gall et al. 2015). The toxic effects of heavy metals on plants are predominantly increased production of reactive oxygen species and inhibition of proper functioning of essential groups in biomolecules due to dislocation of essential metal ions (Schützendübel and Polle 2002). Secondary metabolites take part in the process of adaptation of plant organisms to the ecological conditions of the environment, and their quantity in plant organs varies depending on both abiotic and biotic factors to which the plant is exposed. Biosynthetic pathways of secondary metabolites are to a certain extent under the influence of heavy metals in the soil, altitude, water regime in the habitat and radiation (Endt et al. 2002; Politycka and Adamska 2003), and it is confirmed that due to the presence of heavy metals, certain plants increase the synthesis of phenolics (Michalak 2006).
The genus Teucrium belongs to the family Lamiaceae. The species of the genus Teucrium are predominantly perennial, bushy or semi-bushy, rarely annual herbaceous plants. Some of the Teucrium species are medicinal and widely used in traditional medicine and pharmacology (Stanković 2012). Teucrium montanum L. is a perennial, shrub-like plant with half-ligneous branches, up to 25 cm high and inhabits thermophilic calcareous rocks, dry mountain meadows and edges of forests in Europe and Anatoly. T. montanum is used as a diuretic and in the treatment of digestive and respiratory diseases and possesses anti-inflammatory, antioxidative and antimicrobial effect (Stanković et al. 2011). Teucrium chamaedrys L. is a perennial herbaceous plant with half-ligneous and shrub-like low stem up to 30 cm high. It has little-branched stem with oval serrated leaves and tiny blooms on branch-tops. The plant inhabits several types of habitats such as rocky limestone areas, dry mountain meadows and pastures, and the edge of the sparse oak and pine forest is up to 1000 m above sea level in Central Europe, Mediterranean region and Western Asia. The species T. chamaedrys is applicable in the treatment of digestive disorders, abscesses, gout and conjunctivitis and in stimulation of fat and cellulite decomposition (Stanković et al. 2010).
The research encompasses the comparative analysis of the content of metals (Mn, Ni, Fe, Cu, Zn, Cr, Ca, Mg, K and Pb) in the soil, above-ground plant part samples and the tea prepared from the species T. montanum and T. chamaedrys from different habitats with serpentine and calcareous substrate. This implies that this research is theoretically and practically well-grounded, particularly in relation to the use of tea made of plants sampled from the serpentine substrate which naturally contains a significant amount of heavy metals. In addition, the goal of the presented comparative research was determined bearing in mind the fact that the intensity of the plant secondary metabolism and quantitative-qualitative content of secondary metabolites represent some of the mechanisms of adaptation to the stressful environmental conditions. Therefore, the principal aim of this study is the determination of the differences in quantity of the phenolic content and antioxidant activity of extracts of the selected plant species from habitats with serpentine soil in comparison to habitats with calcareous soil. The total quantity of phenols and flavonoids as well as antioxidant activity were measured in order to determine both the variability of the quantity of certain metals and secondary metabolites and the possible relation between the type of substrate and the quantity of metals in the soil, plants and tea by means of comparative analysis of the samples from the habitats with serpentine and calcareous substrate.
Material and methods
Plant material
T. montanum and T. chamaedrys were sampled from the natural habitats with serpentine (the mountains Goč and Stolovi) and calcareous soil (the mountains Kopaonik and Durmitor), during the period of flowering in 2013 (Table 1). The samples were identified at the Department of Biology and Ecology, Faculty of Science, University of Kragujevac. Aboveground plant parts were dried in the dark room at room temperature after which they were ground in a blender and stored in dark sealed containers.
Sampling of soil for analysis
Apart from the plant species, the soil was sampled from the same localities for the purpose of the comparative analysis of the quantity of metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb). The sampling and laboratory preparation of the soil samples were carried out in accordance with the described procedure for soil sampling (Kastori et al. 2006).
Chemicals
Methanol, perchloric acid (HClO4), nitric acid (НNО3) and natrium hydrogen carbonate (NaHCO3) were purchased from “Zorka pharma” Šabac, Serbia. Hydrogen peroxide (H2O2) was obtained from “Meilab” Belgrade, Serbia. Gallic acid, rutin hydrate and 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent were bought from Sigma Chemicals Co., St Louis, MO, USA. Folin-Ciocalteu phenol reagent and aluminium chloride hexahydrate (AlCl3 × 6H2O) were obtained from Fluka Chemie AG, Buchs, Switzerland.
Preparation of plant extracts and plant material
Dried plant samples were ground to obtain plant powder. For the plant extract preparation, the prepared plant powder (10 g) was mixed with 250 mL of methanol and kept at room temperature. After 24 h, the extract was refined through Whatman no. 1 filter paper. The plant extracts were evaporated under vacuum at 40 °C using a rotary evaporator. The extracts were preserved in sterile sample tubes at 4 °C. In order to determine the phenolic content and antioxidant activity in plant material, for start solution preparation, each powdered sample was dissolved in methanol at a concentration of 1 mg mL−1 and filtered after 24 h.
Determination of total phenolics in the plant extracts and plant material
The total quantity of phenolic compounds in the plant extracts and plant material was determined using spectrophotometric method (Singleton et al. 1999). The samples were prepared by compounding 0.5 mL of methanolic solution (1 mg mL−1) of extract or start solution obtained from plant material, 2.5 mL of 10% Folin-Ciocalteu reagent dissolved in water and 2.5 mL of 7.5% NaHCO3. Prepared samples were incubated at 45 °C for 15 min. After the incubation, the absorbance was measured at 765 nm of wavelength. Three test samples were prepared for the calculation of mean value of absorbance. The similar procedure was repeated for the dissolution of gallic acid on which basis the calibration line was constructed. The obtained values of the quantity of phenolics in the plant extracts and plant material were expressed in terms of gallic acid equivalent (mg of GA g−1 of extract and mg of GA g−1 of plant material).
Determination of total flavonoids in the plant extracts and plant material
The total quantity of flavonoids in the plant extracts and plant material was determined using spectrophotometric method (Quettier et al. 2000). The sample consisted of 1 mL of methanolic solution of the extract or start solution obtained from plant material and 1 mL of 2% AlCl3 solution dissolved in methanol. The samples were incubated for 1 h at room temperature. After the incubation, the absorbance was measured at 415 nm of wavelength. Three test samples were prepared in order to obtain the mean value of absorbance. The similar procedure was repeated for the solution of rutin on the basis of which the calibration line was constructed. The total content of flavonoids in the plant extract was read from the calibration line, and the values were expressed in terms of rutin equivalent (mg of Ru g−1 of extract and mg of Ru g−1 of plant material).
Determination of antioxidant activity of the plant extracts and plant material
The capability of plant extracts and plant material to neutralize DPPH radicals was determined by spectrophotometric method (Stanković et al. 2015). The solution of the plant extract and of the start solution obtained from the plant material was prepared in methanol. Diluted solutions (1 mL of each) were mixed with 1 mL of DPPH methanolic solution. After the addition of reagent and the sample incubation, the absorbance was measured at wavelength of 517 nm. Inhibition was calculated in percents using the equation (Eq. (1)). IC50 values (μg mL−1) were estimated from the percentage inhibition versus concentration plot, using a non-linear regression algorithm. In presented results, antioxidant efficiency of the extract increased with the decreasing of IC50 values. The results were represented as mean value ± standard deviation (n = 3).
Determination of the quantity of metals by atomic absorption spectrometry
The quantification of metals (Mg, Ca, K, Ni, Fe, Cr, Mn, Zn, Cu and Pb) in plants and soil samples was performed using atomic absorption spectrometric method. For the determination of the quantity of metals in the soil, 1 g of soil sample previously dried in a microwave was dissolved by HNO3 and HClO4 and evaporated to certain volume. After cooling, 12 mL of distilled water was added. The mixture was filtrated and placed in sample vials. The quantity of metals in the plants was determined using 1 g of the prepared plant material which was afterwards dissolved by HNO3 and H2O2. The mixture was evaporated to certain volume. After cooling, 12 mL of distilled water was added. Thus, the prepared mixture was filtrated and placed in sample vials (Shah et al. 2013). For the determination of the quantity of metals in the tea, 100 mL of boiling distilled water was mixed with 1 g of the prepared plant material. The mixture was left to cool at room temperature for 10 min and then filtered to obtain a clear solution for further processing. Consequently, the quantity of metals in all three groups of samples was measured in the same conditions. In order to determine the exact quantity of metals, an atomic absorption spectrophotometre Perkin Elmer Company Model 3300/96 with MHS-10 hydride system was used. Each measurement was performed in triplicate after which the mean values were calculated. The quantity of metals in the analysed samples was expressed in terms of mg kg−1.
Bioaccumulation factor
Bioaccumulation factor is used for the determination of the quantity of metals accumulated by plants from the soil, and it represents the ratio between the content of a metal in a plant and its content in the soil (Pandy and Tripathi 2010). Bioaccumulation factor was calculated using the appropriate equation (Eq. (2)):
Statistical analysis
Statistical analysis of the results of the measurements was performed using a SPSS (Chicago, Illinois) statistical software package (SPSS for Windows, version XIV, 2010).
Results
The quantity of metals in the soil, plants and tea
The results of the comparative analysis of the quantity of metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb) in the soil samples as well as in the samples of the aboveground plant parts and the tea from the plant material of the species T. montanum from the locality with serpentine (the mountains Goč and Stolovi) and calcareous (the mountains Kopaonik and Durmitor) soil are presented in Table 2. Table 2 indicates the quantity range of the analysed metals in the soil, plant samples and tea. The quantity of Fe, Ni, Mn, Cu, Cr and Pb in the tea is below the limit of detection.
The mean value of the content of the analysed elements for the species T. montanum in the serpentine soil was ordered in the following way: Mg > Fe > K > Ca > Ni > Mn > Cr > Pb > Zn > Cu; in the plants, К > Mg > Ca > Fe > Ni > Mn > Cr > Zn > Cu > Pb and in the tea, K > Mg > Ca > Zn. The mean value of the content of the analysed elements in the calcareous soil was ordered as follows: Fe > Ca > Mg > K > Mn > Cr > Zn > Ni > Pb > Cu; in the plants, K > Ca > Mg > Fe > Zn > Mn > Ni > Cr > Cu > Pb and in the tea, K > Ca > Mg > Zn.
The obtained results of the research showed that the quantities of certain metals (Mg, Fe, Ni and Mn) in the soil samples from the serpentine habitats (the mountains Goč and Stolovi) were greater in comparison with other metals (Ca, Zn and Pb) which were more frequently found in calcareous soil (the mountains Durmitor and Kopaonik). The results demonstrated that the plant samples from the substrate contained higher quantity of Fe, Ni, and Cr in the aboveground organs as opposed to the same organs of the plants on the calcareous soil which contained greater quantity of Ca and Zn. The results for the tea suggested that the species T. montanum on calcareous soils contained greater quantities of Ca, K and Zn in comparison with the plant samples from the serpentine soils which had greater quantities of Mg. When it comes to the rest of the metals, the values were below the limit of detection in both cases.
The results of the comparative analysis of the quantitity of metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb) in the soil samples as well as in the samples of both the aboveground plant parts and the tea of the plant material of the species T. chamaedrys from the serpentine locality (Goč mountain) and calcareous locality (Kopaonik mountain) are presented in Table 3.
The mean value of the content of the analysed elements for the species T. chamaedrys in the serpentine soil was ordered as follows: Fe > Mg > Ca > K > Mn > Ni > Cr > Zn > Pb > Cu; in the plants, К > Ca > Mg > Fe > Mn > Ni > Zn > Cr > Cu > Pb and in the tea, K > Ca > Mg > Zn. The mean value of the content of the analysed elements in the calcareous soil was ordered in the following manner: Fe > Ca > Mg > K > Mn > Cr > Zn > Ni > Pb > Cu; in the plants, K > Ca > Mg > Fe >Mn > Zn > Ni > Cr > Cu > Pb and in the tea, K > Ca > Mg > Zn.
The research results for the species T. chamaedrys indicated that the quantities of certain metals (Mg, Fe, Ni, Cr and Mn) in the serpentine soil samples (Goč mountain) were greater in comparison with other metals (Ca, К, Zn and Pb) more frequently found in calcareous soil (Kopaonik mountain). The results showed that the plant species on the serpentine soil contained higher quantity of Fe, Ni and Cr in the aboveground plant organs as opposed to the same organs of the plants on the calcareous soil which had greater quantity of Mg, Ca, Mn and Zn. The results for the tea revealed that the species T. chamaedrys from calcareous soils contained greater quantities of Mg and K when compared with the species sampled from serpentine soils which were more abundant in Ca and Zn. When it comes to the other metals, the values were below the limit of detection in both cases.
Correlation between the quantity of metals in the soil, plant and tea samples
The results obtained using the Pearson correlation test for the species T. montanum showed that in terms of the content of metals, there was the correlation between the quantity of metals in the soil, plant material and tea. Pearson correlation was not applicable for the obtained results for species T. chamaedrys due to the number of localities in which the sampling was performed. The results for the correlation are presented in Table 4.
Analysis of metal bioaccumulation factor
Bioaccumulation factor (BAF) defined as the ratio between contents of a certain element in a plant and soil was calculated for each of the analysed metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb). Bioaccumulation factors for the species T. montanum from both serpentine and calcareous localities are presented in Table 5. Bioaccumulation factors for the species T. chamaedrys from both serpentine and calcareous localities are shown in Table 6.
The quantity of total phenolic compounds and flavonoids: the level of antioxidant activity
The total quantity of phenolic compounds and flavonoids and the level of antioxidant activity in the extracts and plant material of the species T. montanum from serpentine (the mountains Goč and Stolovi) and calcareous habitats (the mountains Kopaonik and Durmitor) are presented in Table 7.
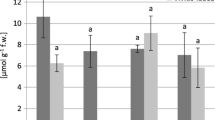
The total content of phenolic compounds in the plant extracts of the species T. montanum from serpentine localities ranged from 160.21 to 190.20 mg GA g−1 of the extract, whereas the quantity of phenolics from calcareous localities was 143.42 and 148.21 mg GA g−1 of the extract. The values of the quantity of flavonoids in the plant extracts of the species T. montanum from serpentine localities were from 53.82 to 54.19 mg Ru g−1 of the extract, while the flavonoid content obtained from the extracts made of the samples from calcareous localities reached the values 46.50 and 49.53 mg Ru g−1 of the extract. Antioxidant activity of the extracts of the species T. montanum from serpentine localities ranged from 47.99 to 53.91 μg mL−1, while the antioxidant values for the extracts from calcareous locality were 60.30 and 61.69 μg mL−1.
The total content of phenolic compounds in the plant material of the species T. montanum from the serpentine localities was from 44.07 to 66.52 mg GA g−1 of the plant material, whereas the quantity of phenolics obtained in the samples from calcareous habitats was 37.11 and 39.63 mg GA g−1of the plant material. The values of the quantity of flavonoids in the plant material of the species T. montanum from serpentine localities ranged from 19.07 to 20.90 mg Ru g−1 of the plant material, while the values for the plant material from calcareous localities were 15.38 and 17.57 mg Ru g−1 of the plant material. Antioxidant activity of the plant material of the species T. montanum from serpentine localities ranged from 177.99 to 263.07 μg mL−1, while the antioxidant values of the plant material from calcareous localities were 302.56 and 315.98 μg mL−1 .
The results indicated greater total quantity of both total phenolic compounds and flavonoids as well as the higher level of antioxidant activity in the extracts and plant material from the species T. montanum sampled on serpentine habitats in comparison with the samples from calcareous habitats. The total quantity of phenolics and flavonoids as well as the level of antioxidant activity in the extracts and plant material of the species T. chamaedrys sampled on serpentine (Goč mountain) and calcareous habitats (Kopaonik mountain) are shown in Table 8.
The total content of phenolic compounds in the plant extracts of the species T. chamaedrys from serpentine locality was 255.10 mg GA g−1 of the extract, whereas the quantity of phenolics from calcareous locality was of 225.97 mg GA g−1 of the extract. The value of the quantity of flavonoids in the plant extracts of the species T. chamaedrys from serpentine locality was 94.93 mg Ru g−1 of the extract, while the value for flavonoids of the extract from the calcareous locality was 66.38 mg Ru g−1 of the extract. The value of antioxidant activity of the extract of the species T. chamaedrys from serpentine locality was 38.76 μg mL−1, while the value for antioxidant activity of the extract from the sample from the calcareous locality was 44.99 μg mL−1.
The total content of phenolic compounds in the plant material of the species T. chamaedrys from serpentine locality was 92.84 mg GA g−1 of the plant material, whereas the quantity of phenolics in the plant material from calcareous locality reached the value of 84.18 mg GA g−1 of the plant material. The value for flavonoid content of the plant material of the species T. chamaedrys from serpentine locality was 34.40 mg Ru g−1 of the plant material, while the plant material from calcareous soil showed the value of 29.81 mg Ru g−1 of the plant material. The value of antioxidant activity of the aboveground plant material of the species T. chamaedrys from serpentine locality was 92.49 μg mL−1, while the plant material from calcareous locality showed the value for antioxidant activity of 102.21 μg mL−1.
Correlation between the quantity of total phenolic compounds, flavonoids and antioxidant activity
The results concerned with the total quantity of phenolic compounds and flavonoids as well as with the values of antioxidant activity of the extracts and plant material of the species T. montanum and T. chamaedrys were statistically analysed in order to determine the degree of correlation. The values of the correlation indicated the multilevel relation between the parameters subjected to analysis. The results of the analysis are presented in Table 9.
Discussion
The examination of the content of metals (Mg, Ca, K, Ni, Fe, Mn, Zn, Cu, Cr and Pb) in the species T. montanum and T. chamaedrys sampled on serpentine and calcareous habitats showed the difference in the quantity of metals in the soil, plant material and tea (Tables 2 and 3). The samples of serpentine soil contained a higher content of Mg in comparison with the samples of calcareous soil. The obtained results for the quantities of Mg in the soil are in accordance with the values other authors provided. Those ranged from 10,060 to 118,400 mg Mg kg−1 in serpentine soils (Ghaderian et al. 2007). The content of Mg in plants sampled on serpentine habitats may reach well over 7000 mg Mg kg−1 (Lombini et al. 1998). The higher content of Mg in plants from serpentine habitats is due to both the greater quantities of this metal in the soil and the substantial quantity of Mg in its minerals (Brooks 1987).
The content of Ca matches the range of values given by other authors. The range covered values from 1895 to 5750 mg kg−1 (Ghaderian et al. 2007). In the serpentine soil samples, the ratio between the content of Ca and Mg was smaller than one. The data approximately matched the data provided in other studies (Brady et al. 2005). Serpentine plants adapted to the low content of Ca in this particular type of soil (Brooks 1987; Brady et al. 2005). The favourable level of Ca in plant tissues is 5 g kg−1 (Shallari et al. 1998). It is a well-known fact that if there is a sufficent content of Ca in the soil, its content in the plant stem of the plant growing on that soil ranges from 0.1 to 5% in dry plant material (Marschner 1995), whereas the content of Ca in the plants growing on ultramafic soil reaches the percentage of up to 0.8% (Kataeva et al. 2004).
The total content of K in the soil varies from 0.2 to 3%. K is a macroelement vital for plant physiological processes. The plants store large quantities of K in their organs. The content of this metal in the aboveground plant parts may reach from 4 to 5% (Kastori et al. 2013).
The content of Ni in the soil depends upon both its content in the parent rock and pedogenetic processes. The previous studies revealed that, on average, the values of Ni content in the soils all over the word vary from 13 to 37 mg kg−1 (Kabata-Pendias 2011). Other authors claim that the total content of Ni in serpentine soils ranges from 500 to 8000 mg kg−1 (Ghaderian et al. 2007). The values obtained in this research confirm the same content of Ni. The values of quantity of Ni in the species T. montanum (Table 2) from the locality Žička reka cross the limit of toxicity (100 mg Ni kg−1), while the values for the other samples are in accordance with the results by other authors (Brooks 1987; Chaney et al. 2008).
According to the general soil analyses, the content of Fe in the soil reaches up to 3.5% (Kabata-Pendias 2011). The obtained results confirmed the data which demonstrate that the serpentine soils contain greater content of Fe (Ghaderian et al. 2007; Reeves et al. 2007; Bech et al. 2008). It was demonstrated that the plants growing on serpentine soils contain the Fe in content ranging from 2127 to 3580 mg kg−1 (Johnston and Proctor 1977). The results of this research showed that the plants contained smaller quantity of Fe in comparison with the expected values, the only exception being the plant samples from the locality Žička reka with the content of Fe within the already established range of values.
As confirmed in numerous studies, the content of Mn in serpentine soils is higher than in other types of soil (Ghaderian et al. 2007). The expected range of values of Mn content is from 411 to 550 mg kg−1 (Kabata-Pendias 2011). Some authors claim that the majority of plants contain from 20 up to 300 mg kg−1 of Mn (Pais and Jones 2000).
The content of Zn in the soil varies from 10 to 300 mg kg−1 with an average of 50 mg kg−1 (Montilla et al. 2003; Escarré et al. 2011). In the majority of cases, the plant contains from 15 to 150 mg kg−1, whereas the value of 300 mg Zn kg−1 stands out as the maximum possible content according to the hitherto established results (Brunetti et al. 2009).
The range of content of Cu covers the values from 14 to 109 mg kg−1 (on average 38.9 mg kg−1). Our results are in accordance with the data the previous analyses confirmed—the content of Cu in the plant stems reaches the content of up to 20 mg kg−1 (Kabata-Pendias 2011).
According to Ghaderian et al. (2007), serpentine soils contain form 60 to 265 mg Cr kg−1. The content of Cr in plants varies from 0.2 to 4 mg kg−1. However, certain studies conducted up to now proved that certain plants growing on serpentine soils may contain even up to 100 mg Cr kg−1 (Kabata-Pendias 2011). This research provides ample evidence in favour of so high content of Cr in plants.
The mean value of Pb in soil varies from 18 to 32 mg kg−1. However, it has been recorded that the content of Pb in unpolluted habitats reached even 70 mg kg−1. The content of Pb in the plants growing on unpolluted soil ranges from 0.05 to 3.0 mg kg−1 (Kabata-Pendias 2011). Other established values of Pb in plants varied from 10 to 25 mg kg−1 (Carranza-Ălvarez et al. 2008). The values of quantity of Pb in the samples taken from the abovementioned localities included in this research practically did not depart from the values obtained so far (Reeves et al. 2007; Bech et al. 2008; Kabata-Pendias 2011).
On the basis of the results of the analysis, the comparisons between content of metals in serpentine and calcareous soils have been performed. By means of this comparison, it has been established that there are significantly greater quantities of some metals (Mg, Fe, Ni and Mn) in serpentine soil whereas the other metals observed (Ca, Zn and Pb) were found in greater quantities in calcareous soil. The outcome of the comparison is in accordance with general claim that certain metals are more frequent in a certain type of soil.
The results demonstrated that the analysed plant samples from the serpentine habitats contained higher quantity of Fe, Ni and Cr as opposed to the plant samples from the calcareous habitats which contained greater quantity of Ca and Zn. This implies that the quantity of metals in plants depends on the quantity of metals in soil.
However, when it comes to the species T. montanum and T. chamaedrys from serpentine habitats, the quantity of Mg exceeded the quantity of Ca, thus confirming one more time that the ratio of the quantities of Ca/Mg in serpentine soils is smaller than one (Brady et al. 2005). The absorption and accumulation of Mg, Fe, Ni and Mn in serpentine soil and Ca, Zn, Cu and Pb in calcareous soil is predominantly influenced by the characteristics of soil (Kabata-Pendias 2011).
The in-depth analysis of the tea of medicinal species showed that the content of certain metals (Mg, Ca, K and Zn) were in accordance with the results of other studies of medicinal plants (Li et al. 2015; Seenivasan et al. 2016). The values of other metals (Ni, Fe, Mn, Cu, Cr and Pb) were below the detection limit. On the basis of the obtained results, it is deducible that geological substrate and the soil formed on it influence the quantity of metals in tea. The tea of the species T. montanum and T. chamaedrys, obtained by means of standard procedure, did not contain heavy metals in spite of the serpentine or calcareous habitats from which the samples were collected. As plant organs absorb metals, the tea does not contain heavy metals and is therefore safe to use. This conclusion is supported by the result that the tea obtained from the aboveground plant parts, regardless of the quantity of metals in the soil and plant material, contains only minerals necessarily to be taken in on a daily basis in human nutrition.
There was a significant correlation between the quantity of metals in the soil, plants and tea. Table 4 shows only values greater than R = ±0.850. The observations established the link between the quantities of Mg, Ni, Zn and Ca in the soil. Lyon et al. (1968) proved the correlation between the content of Mg and that of heavy metals in serpentine plants. The quantity of Ni, Fe, Mn, Cr and Co in woody serpentine plants correlate as well (Lazarus et al. 2011). Also, significant correlations between the content of some trace elements in soil and their accumulation in plant parts were established (Kosiorek et al. 2016). The results of this research do not depart from the generally acknowledged findings.
It has been established that both the contents of Mn and Cu in the soil and those of Mn and Fe in the plants correlate. The studies confirmed the interractions between Mn and other elements (Kabata-Pendias 2011), such as the following: antagonistic and synergistic effects of Mn in the process of absorption of Cd and Pb; antagonistic effects of Mn in the process of K, Na and N absorption and the substantial impact Zn makes on the weaker absorption of Mn (Kabata-Pendias 2011). One of the crucial points in general research in this field was the discovery of both the synergetic interraction between Cu and K and the antagonistic relation between Cu and Mn. The antagonistic interaction between Cu and Zn implies that the greater absorption of one element disturbs the freer absorption of the other element (Arias et al. 2006), which is a consequence of the same carrier involved in the absorption mechanisms of these elements. A number of studies ascertained the synergistic interaction between Cr and other elements (Ca, Mg, Fe, Mn, Cu) (Dong et al. 2007). The research further supports the previous findings. Other scientists have confirmed the existence of correlations between certain metals (Yan et al. 2012; Gonneau et al. 2014).
The species T. montanum and T. chamaedrys on serpentine and calcareous soil show BAF values higher than 1 for K, whereas in the case of serpentine soil, BAF values exceed 1 for both species for Ca (Tables 5 and 6). When it comes to other metals, the values of BAF were below 1. The value of BAF in plants strongly implicates the possibility of their application in phytoextraction. BAF values above 2 are regarded as extremely high (Pandy and Tripathi 2010).
The presence of heavy metals in the particular type of soil affects the quantity of metals in plants. This fact explicates the difference in the quantity of secondary metabolites in plants sampled on calcareous soil (localities Kopaonik and Durmitor) and serpentine soil (localities Goč, Žička reka and Kamenica) with significantly higher values of secondary metabolites in serpentine plants (Tables 7 and 8). Numerous studies confirmed that the existence of heavy metals in soil causes increased synthesis of secondary metabolites (Pavlova 2009). This research further corroborates this claim. The content of phenolics both in the extracts and in the plant material of the species T. montanum and T. chamaedrys from serpentine soil is significantly higher than in those samples from calcareous localities (Tables 7 and 8).
The experiments showed that certain plant species increase the synthesis of phenolic compounds in the presence of heavy metals (Michalak 2006; Stanković 2011; Veličković et al. 2014). The higher content of phenolics is the part of plant response to the effects of heavy metals in the soil and consequently to the unfavourable conditions resulting from these effects (Lavid et al. 2001). The increase in the content of heavy metals, in some cases, causes higher content of phenolic compounds (Hamid et al. 2010). The content of phenolic compounds in the samples of wheat increases due to the very exposure of the samples to the higher content of Cu in the soil medium (Ganeva and Zozikova 2007). The antioxidant properties of phenolic compounds are directly attributable to their ability to both chelate ions of transition metals and inhibit the reactions caused by superoxides (Rice-Evans et al. 1997).
The results showed the greater quantity of flavonoids in both the extracts and plant material of the species T. montanum and T. chamaedrys sampled on serpentine localities in comparison to the calcareous samples (Tables 7 and 8). Flavonoids build complexes with heavy metals which is why they have a crucial role in the adaptation of plants to the adverse effects of heavy metals and such like environmental conditions stemming from these effects (Michalak 2006; Korkina 2007). It is considered that antioxidant activity is among multiple protective functions flavonoids have (Rusak et al. 2005). Among others, the previously mentioned function of protecting plants from the stress caused by heavy metals in soil and plant organs proved to be very important (Kim et al. 1999; Michalak 2006).
The research showed the higher values of the antioxidant activity both in the extracts and plant material of the species T. montanum and T. chamaedrys sampled on serpentine localities in comparison with the calcareous samples (Tables 7 and 8). Among other mechanisms, antioxidant capacity of secondary metabolites is of great importance when it comes to the adaptation of plants on serpentine substrate to heavy metals in soil. Therefore, the higher level of the antioxidant activity represents the principal response of the plant species from the genus Atriplex to the increased content of heavy metals in plant organs (Kachout et al. 2009).
In addition to other ways, plants adjust their secondary metabolism as a response to the environmental conditions. The shallowness of serpentine soils implies that such soils are characterized with low capacity to retain water. Therefore, the water regime in such habitats is highly unbalanced (Brady et al. 2005). The unbalanced water regime further leads to the increased synthesis of secondary metabolites in plants (Khan et al. 2010). Due to the soil structure and disturbed water regime serpentine, soils are infallibly arid (Kruckeberg 1984; Brooks 1987). Temperature stress in the plant species growing on arid, serpentine soils considerably influences the greater production of flavonoids (Rivero et al. 2001). The obtained values for the correlation between the quantity of phenolic compounds and antioxidant activity imply that the secondary metabolites from the group of phenolic compounds represent the principal active substances which serve as carriers of antioxidant activity (Table 9). Other numerous studies have established and confirmed the correlation between phenolic compounds and antioxidant activity (Piluzza and Bullitta 2011; Stanković 2011).
Conclusions
Conducted comparative analysis of the content of metals in the soil samples, aboveground plant parts and tea prepared from the species T. montanum and T. chamaedrys sampled from serpentine and calcareous habitats indicate that variability of their quantity depends on the soil type. Results showed that the contents of Mg, Fe, Ni and Mn in the soil samples from the serpentine habitats were greater in comparison with Ca, Zn and Pb which were more frequently found in the soil samples from the habitats with calcareous soil. Analysed plants from serpentine habitats contained higher quantity of Fe, Ni and Cr as opposed to the plant samples from the calcareous habitats which contained greater quantity of Ca and Zn. Regardless of the observed presence of metals in the aboveground plants parts, analysed plant species cannot be declared as metal-hyperaccumulating plants. Results for the metal content in the tea samples from T. montanum and T. chamaedrys showed that, in spite of the detected metal absorption by plant organs, the tea does not contain dangerous quantity of analysed metals. The analysis indicate the greater content of phenolic compounds and antioxidant activity level in the plant samples from the serpentine habitats in comparison to the samples from analysed calcareous habitats.
References
Alexander, E. B., Coleman, R. G., Keeler-Wolf, T., & Harrison, S. P. (2007). Serpentine geoecology of western North America. New York: Oxford University Press Inc..
Arias, M., Perez-Novo, C., Lopez, E., & Soto, B. (2006). Competitive adsorption and desorption of copper and zinc in acid soils. Geoderma Regional, 133(3–4), 151–159.
Bech, J., Tume, P., Longan, L., Reverter, F., & Tempio, M. (2008). Concentration of Cd, Cu, Pb, Zn, Al, and Fe in soils of Manresa, NE Spain. Environmental Monitoring and Assessment, 145(1–3), 257–266.
Brady, U. K., Kruckeberg, R. A., & Bradshaw, J. H. D. (2005). Evolutionary ecology of plant adaptation to serpentine soils. Annual Review of Ecology, Evolution and Systematics, 36, 243–266.
Brooks, R. R. (1987). Serpentine and its vegetation: a multidisciplinary approach. In T. R. Dudley (Ed.), Ecology, phytogeography, and physiology series. Oregon, Portland: Dioscorides Press.
Brooks, R. R. (1998). Geobotany and hyperaccumulators. In R. R. Brooks (Ed.), Plants that hyperaccumulate heavy metal (pp. 55–94). Wallingford: CAB International.
Brunetti, G., Soler-Rovira, P., Farrag, K., & Senesi, N. (2009). Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region—southern Italy. Plant and Soil, 318, 285–298.
Carranza-Ălvarez, C., Alonso-Castro, A. J., Alfaro-De La Torre, M. C., & Garciá De La Cruz, R. F. (2008). Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosĭ, Mexico. Water, Air & Soil Pollution, 188(1), 297–309.
Chaney, R. L., Chen, K. Y., Li, Y. M., Angle, J. S., & Baker, A. J. M. (2008). Effects of calcium on nickel tolerance and accumulation in Alyssum species and cabbage grown in nutrient solution. Plant and Soil, 311, 131–140.
Dong, J., Wu, F. B., Huang, R. G., & Zhang, G. P. (2007). A chromium-tolerant plant growing in Cr contaminated land. International Journal of Phytoremediation, 9(3), 167–179.
Endt, D. V., Kijne, J. W., & Memelink, J. (2002). Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry, 61(2), 107–114.
Escarré, J. C., Lefébvre, C., Raboteau, S., Dos Santos, A., Gruber, W., Marel, J. C. C., et al. (2011). Heavy metal concentration survey in soils and plants of the Les Malines mining district (southern France): implications for soil restoration. Water, Air and Soil Pollution, 216, 485–504.
Gall, E. J., Boyd, S. R., & Rajakaruna, N. (2015). Transfer of heavy metals through terrestrial food webs: a review. Environmental Monitoring and Assessment, 187, 201–222.
Ganeva, G., & Zozikova, E. (2007). Effect of increasing Cu2+ concentrations on growth and content of free phenols in two lines of wheat (Triticum aestivum) with different tolerance. General and Applied Plant Physiology, 33(1–2), 75–82.
Ghaderian, A. M., Mohtadi, A., Rahiminejad, R., Reeves, R. D., & Baker, A. J. M. (2007). Hyperaccumulation of nickel by two Alyssum species from the serpentine soils of Iran. Plant and Soil, 293, 91–97.
Gonneau, C., Genevois, N., Frérot, H., Sirguey, C., & Sterckeman, T. (2014). Variation of trace metal accumulation, major nutrient uptake and growth parameters and their correlations in 22 populations of Noccaea caerulescens. Plant and Soil, 384, 271–287.
Hamid, N., Bukhari, N., & Jawaid, F. (2010). Physiological responses of Phaseolus vulgaris to different lead concentrations. Pakistan Journal of Botany, 42(1), 239–246.
Johnston, W. R., & Proctor, J. (1977). Metal concentrations in plants and soils from two British serpentine sites. Plant and Soil, 46(1), 275–278.
Kabata-Pendias, A. (2011). Trace elements in soils and plants (4th ed.). New York, London, Taylor and Francis Group Boca Ration: CRC Press.
Kachout, S. S., Mansoura, B. A., Leclerc, C. J., Mechergui, R., Rejeb, N. M., & Ouerghi, Z. (2009). Effects of heavy metals on antioxidant activities of Atriplex hortensis and A. rosea. Journal of Food, Agriculture and Environment, 7(3–4), 938–945.
Kastori, R., Kádár, I., & Sekulić, P. (2006). Sampling soil and plants in noncontaminated and contaminated sites. Novi Sad: Institute of Field and Vegetable Crops.
Kastori, R., Ilin, Ž., Maksimović, I., & Putnik-Delić, M. (2013). Potassium in plant nutrition potassium and vegetables. Serbia, Novi Sad: Faculty of Agriculture of Novi Sad.
Kataeva, M. N., Alexeeva-Popova, N. V., Drozdova, I. V., & Beljaeva, A. I. (2004). Chemical composition of soils and plant species in the polar Urals as influence by rock type. Geoderma Regional, 122(2–4), 257–268.
Khan, M. A. M., Ulrichs, C., & Mewis, I. (2010). Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomologia Experimentalis et Applicata, 137(3), 229–236.
Kim, M. S., Kim, C., Jo, H. D., & Ryu, W. Y. (1999). Effect of fungal elicitor and heavy metals on the production of flavonol glycosides in cell cultures of Ginkgo biloba. Journal of Microbiology and Biotechnology, 9, 661–667.
Korkina, L. G. (2007). Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cellular and Molecular Biology, 53(1), 15–25.
Kosiorek, M., Modrzewska, B., & Wyszkowski, M. (2016). Levels of selected trace elements in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula L.), and Norway maple (Acer platanoides L.) in an urbanized environment. Environmental Monitoring and Assessment, 188(10), 598.
Kruckeberg, A. R. (1984). California serpentines: Flora, vegetation, geology, soils and management problems. Berkeley: University of California Press.
Lambers, H., Stuart Chapin III, F., & Pons, L. T. (2008). Plant physiological ecology. New York: Springer.
Lavid, N., Schwartz, A., Yarden, O., & Tel-Or, E. (2001). The involvement of polyphenols and peroxidase activities in heavy metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta Medica, 212(3), 323–331.
Lazarus, B. E., Richards, J. H., Claassen, V. P., O'Dell, R. E., & Ferrell, M. A. (2011). Species specific plant-soil interaction influence plant distribution on serpentine soils. Plant and Soil, 342, 327–344.
Li, L., Fu, L. Q., Achal, V., & Liu, Y. (2015). A comparison of the pontential health risk of aluminium and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environmental Monitoring and Assessment, 187, 228–240.
Lombini, A., Dinelli, E., Ferrari, C., & Simoni, A. (1998). Plant-soil relationships in the serpentine screes of Mt. Prinzera (northern Apennines, Italy). Journal of Geochemical Exploration, 64(1), 19–33.
Lyon, G. L., Brooke, R. R., Peterson, P. J., & Butler, G. W. (1968). Trace elements in a New Zealand serpentine flora. Plant and Soil, 29, 225.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd ed.). London: Academic Press.
Michalak, A. (2006). Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15(4), 523–530.
Montilla, I., Parra, M. A., & Torrent, J. (2003). Zinc phytotoxicity to oilseed rape grown on zinc-loaded substrates consisting of Fe oxide-coated and calcite sand. Plant and Soil, 257(1), 227–236.
Pais, I., & Jones, J. B. (2000). The handbook of trace elements. Florida: St. Luice Press.
Pandy, P., & Tripathi, K. (2010). Bioaccumulation of heavy metal in soil and different plant parts of Albizia procera (Roxb.) seedling. The Bioscan, 5, 263–266.
Pavlova, D. (2009). Morphological variation in Teucrium chamaedrys in serpentine and non-serpentine populations. Soil and biota of serpentine: a world view. Northeast Naturalist, 16(5), 39–55.
Piluzza, G., & Bullitta, S. (2011). Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the mediteranean area. Pharmaceutical Biology, 49(3), 250–247.
Politycka, B., & Adamska, D. (2003). Release of phenolic compounds from apple residues decomposing in soil and the influence of temperature on their degradation. Polish Journal of Environmental Studies, 12(1), 95–98.
Quettier, D. C., Gressier, B., Vasseur, J., Dine, T., Brunet, C., Luyckx, M. C., et al. (2000). Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. Journal of Ethnopharmacology, 72(1–2), 35–42.
Reeves, R. D., Baker, A. J. M., Becquer, T., Echevarria, G., & Miranda, Z. J. G. (2007). The flora and biogeochemistry of the ultramafic soils of Goiás state Brazil. Plant and Soil, 293(1), 107–119.
Rice-Evans, C. A., Miller, N. J., & Paganga, G. (1997). Antioxidant properties of phenolic compounds. Trends in Plant Science, 2(4), 152–159.
Rivero, R. M., Ruiz, J. M., Garcia, P. C., Lopez-Lefebre, L. R., Sanchez, E., & Romero, L. (2001). Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Science, 160(2), 315–321.
Rusak, G., Gutzeit, H., & Ludwig-Müller, J. (2005). Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutrition Research, 25(2), 143–155.
Schützendübel, A., & Polle, A. (2002). Plant responses to abiotic stresses: heavy metal–induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany, 53(372), 1351–1365.
Seenivasan, S., Anderson, A. T., & Muraleedharan, N. (2016). Heavy metal content in tea soils and their distribution in different parts of tea plants, Camellia sinensis (L). O. Kuntze. Environmental Monitoring and Assessment, 188, 428–436.
Shah, A., Niaz, A., Ullah, N., Rehman, A., Akhlaq, M., Zakir, M., et al. (2013) Comparative study of heavy metals in soil and selected medicinal plants. Journal of Chemistry, 1 ̶ 5. doi:10.1155/2013/621265
Shallari, S., Schwartz, C., Hasko, A., & Morel, J. L. (1998). Heavy metals in soils and plants of serpentine and industrial sites of Albania. Science of the Total Environment, 209(2–3), 133–142.
Singleton, V. L., Orthofer, R., & Lamuela, R. R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Stanković, S. M. (2011). Ecological study of Teucrium montanum L.—population, phenological and plant part variability of secondary metabolites concentration. International botanical congress Botanikertagung 2011. Berlin. Germany. Conference book 255.
Stanković, S. M. (2012). Biological effects of secondary metabolites of species from the genus Teucrium L. of Serbian flora. Kragujevac, University of Kragujevac, Dissertation.
Stanković, M., Topuzović, M., Solujić, S., & Mihajlović, V. (2010). Antioxidant activity and concentration of phenols and flavonoids in the whole plant and plant parts of Teucrium chamaerdys L. var. glanduliferum Haussk. Journal of Medicinal Plants Research, 4(20), 2092–2098.
Stanković, M., Nicifirović, N., Topuzović, M., & Solujić, S. (2011). Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, f. supinum (L.) Reichenb. Biotechnology & Biotechnological Equipment, 25(1), 2222–2227.
Stanković, S. M., Petrović, M., Godjevac, D., & Dajić-Stevanović, Z. (2015). Screening inland halophytes from the Central Balkan for their antioxidant activity in relation to total phenolic compounds and flavonoids: are there any prospective medicinal plants? Journal of Arid Environments, 120, 26–32.
Veličković, M. J., Dimitrijević, S. D., Mitić, S. S., Mitić, N. M., & Kostić, A. D. (2014). The determination of the phenolic composition, antioxidative activity and heavy metals in the extracts of Calendula officinalis L. Advanced Technologies, 3(2), 46–51.
Yan, X., Zhang, F., Zeng, C., Zhang, M., Devcota, P. L., & Yao, T. (2012). Relationship between heavy metal concentrations in soils and grasses of roadside farmland in Nepal. International Journal of Environmental Research and Public Health, 9(9), 3209–3226.
Acknowledgements
This investigation was supported by Ministry of Science and Technological Development of the Republic of Serbia (III41010). The authors acknowledge Ana Vučićević for manuscript lecturing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zlatić, N.M., Stanković, M.S. & Simić, Z.S. Secondary metabolites and metal content dynamics in Teucrium montanum L. and Teucrium chamaedrys L. from habitats with serpentine and calcareous substrate. Environ Monit Assess 189, 110 (2017). https://doi.org/10.1007/s10661-017-5831-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5831-8