Abstract
Indoor air quality is an increasing concern; it causes significant damage to health because it is recycled in confined environments for extended periods of time. Among the pollutants found in these environments, benzene, toluene, ethylbenzene, and xylenes (BTEX) are known for their potential toxic, mutagenic, and carcinogenic effects. This study monitored the BTEX concentrations in paint, carpentry, and varnish workplaces and evaluated the potential to cause adverse health effects on workers in these environments. Twenty samples were collected in workplaces, 20 samples were collected outside the area, and eight samples were taken of the products used. Samples were collected using coconut shell cartridges, and chemical analyses were performed by gas chromatography with mass spectrometry. Toluene presented higher indoor concentrations and indoor and outdoor ratios, indicating that the paint and varnish workplaces had significant BTEX sources. The highest benzene and toluene concentrations were obtained from the paint workshop, and higher concentrations of ethylbenzene and xylenes were obtained in the varnish workshop. The highest non-carcinogenic risks were obtained for m + p-xylenes in the varnish work place, and the second highest non-carcinogenic risk was also determined for the same workshop.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indoor air quality is an increasing concern; indoor concentrations of many pollutants are often higher than outdoor environments, and they can cause significant damage to health due to the long period of time, which people spend inside their homes, jobs, and recreation (Guo et al. 2000; Klinmalee et al. 2009).
The most important chemical compounds in indoor environments are volatile organic compounds (VOCs). These have a vapor pressure greater than 0.14 mmHg at 25 °C ranging from 2 to 12 carbon atoms (Maroni et al. 1995; Sarigiannis et al. 2011). VOCs are well known by the odor and toxic properties (Durmusoglu et al. 2010) and play a significant role in ozone formation in the troposphere and secondary aerosols. In indoor environments, VOCs have several emission sources such as paints, solvents, adhesives, furnishings, clothing, building materials, and cleaning supplies (Guo et al. 2000; Jones 1999; Guo et al. 2004; Lim et al. 2014).
Among the different VOCs commonly found in indoors environments can be highlight benzene, ethylbenzene, toluene, and xylenes (BTEX), which have high potential damage to human health (Ilgen et al. 2001). BTEX are well known to be toxic, mutagenic, and/or carcinogenic (Possanzini et al. 2002; Wilbur et al. 2008). Benzene was associated with higher rates of leukemia and tumors in the occupational workers. Studies suggest a link between benzene emitted by vehicles with cancer incidence; chronic exposure to toluene and xylenes have been associated with adverse effects on the nervous system, liver, and kidney; chronic exposure to ethylbenzene has been related to adverse effects on the kidneys and respiratory system (Hinwood et al. 2007).
Air quality standards are used to evaluate the pollutant concentrations inside workplaces, and these standards are essential to maintaining occupational health. In these environments, indoor air pollution is one of the leading causes of occupational diseases. In addition to work-related accidents in industrial environments, prolonged exposure to chemical pollutants from different sources is a major factor in the deterioration of workers’ health (Gioda and Aquino Neto 2003; Farshad et al. 2013).
The assessment of occupational exposure to chemicals, mainly BTEX, is performed based on the tolerance limits (TL), which are regulated in Brazil by the Ministry of Work and Employment through the Regulatory Standard (NR-15)—Activities and Unhealthy Operations (Ministry of Labor and Employment (MTE) 1995a, b). This standard defines TL as the concentration or maximum or minimum intensity that is related to the nature and duration of exposure to the agent at a level that will not cause harm to a worker’s health during his or her working life. Values are defined based on a working period of 48 h per week.
NR-15 lists the tolerance limits, which were based on the publication of the American Conference of Governmental Industrial Hygienists—ACGIH (2008). Note that this scientific association annually promotes a review; however, the correspondent Brazilian legislation remains unchanged for many years. When an evaluated substance is not listed in NR-15, the NR-9-Program for Environmental Risk Prevention (MTE 1994) allows the adoption of occupational exposure limit values established by ACGIH.
A threshold limit value (TLV) refers to the concentrations of a substance in the air and represents conditions under which it is believed that most workers can be exposed repeatedly, for a lifetime of work without adverse health effects (ACGIH 2008).
Table 1 shows a comparison between the parameters of BTEX exposure limits in Brazil by the ACGIH (2008) and other international organizations, such as the National Institute for Occupational Safety and Health (NIOSH 2003) and the Occupational Safety and Health Administration (OSHA 2016).
Several studies evaluated BTEX human exposure in occupational environments by combining the cancer risk calculations and adverse health effects (Farshad et al. 2013; Tunsaringkarn et al. 2012; Kumar et al. 2014). The characterization of human exposure is a critical component of environmental and occupational epidemiological studies (Weisel 2010), and it is an essential step in regulatory processes (Guo et al. 2004).
The potential risks to public health that are related to hazardous chemical exposure are severe, especially in developing countries, due to the shortage of resources for the chemical industry, risk management, and the growth in the production and use of chemicals (Farshad et al. 2013). Some professions are more at risk in terms of occupational health, such as those dealing directly and daily with chemicals, as in the case of artists and professionals who work with paints, glues, and solvents, among other BTEX sources (Sarigiannis et al. 2011). It is fundamental to carry out studies on air quality in these environments.
This study monitored BTEX concentrations in a very common work environment in paint, carpentry, and varnish workshops and evaluated the adverse health effects on workers from these environments due to exposure to these compounds. BTEX were determined in indoor air of these environments and also in the raw materials used.
Materials and methods
Study area
The carpentry, varnishing, and painting workplaces are used as study environments to collect indoor air belong to the preventive maintenance, corrective, and operational area from the Rio de Janeiro State University.
The workshops have natural ventilation through small windows to external area and artificial ventilation by fans. The rooms are equipped with wall hoods that promote a mechanical exhaust. The lighting is made artificially by fluorescent lamps.
Among the products used in the three workshops are alcohol, solvent, wax, water, and oil-based paints, contact glue, and turpentine. The exhaust equipment frequently were not working, and the procedures adopted are not correct in a occupational point of view, for example, many used products were found uncapped after use. As a reference, samples were collected from the outside of the open environment workshops from a distance of approximately 260 m and without the influence of the activities carried out in the workshops. Table 2 summarizes the characteristics and activities performed in the workshops.
Ten samples were collected from the main volatile products used in the workplaces to determine the BTEX concentrations by gas chromatography coupled to mass spectrometry (GC-MS). The solvents were conditioned in capped amber vials and stored under −4 °C prior to the analysis.
Twenty samples were collected inside each of the three workshops, and 20 samples were collected from the outdoor area. A total of 80 samples were collected between the months of December 2014 and February 2015 between the hours of 8 AM and 2 PM, which is a period of intensive work in these workshops.
To evaluate the atmospheric BTEX concentration in each of the areas, four air pumps (KNF UNMP 850KNDC) operated at 700 mL min−1 for a period of 3 h using double bed (100/50 mg) coconut shell cartridges (SKC 226-01) according to the methodologies described in several works (Klinmalee et al. 2009; Rodrigues et al. 2007; Ras et al. 2010; Ward et al. 2009; Dewangan et al. 2013; Martins et al. 2014; de Castro et al. 2015; Corrêa et al. 2012a).
Sample extraction was performed with 1000 μL of HPLC grade dichloromethane at −20 °C for 10 min in an ultrasonic bath (Martins et al. 2014; Corrêa et al. 2012a). Chemical analyses were performed using a Varian 450GC coupled to a Varian MS220 mass spectrometer with an HP 5 MS capillary column (30 m, 0.32 mm, 0.25 μm) using Heliumat 1.2 mL min−1. Samples were analyzed in the liquid phase with a splitless injection of 1.0 μL at 200 °C. The column temperature began at 40 °C for 4 min, and it was heated at 10 °C min−1until 220 °C (Corrêa et al. 2012a). The transfer line, manifold, and ion trap were operated at 280, 50, and 250°C, respectively, using 50 μA. Selective ion monitoring operated using 78, 91, and 109 m/z as principal ions.
The analysis of solvents was performed by headspace technique placing 100 mg of each sample into a 10-mL vial, which was sealed and stabilized at 70 °C for 15 min at 500 rpm. Then, 1000 μL of the headspace was sampled with a syringe at 70 °C, and analyses were performed under the same conditions as for the liquid extracts.
The analytical curves were performed using a standard mixture of 2000 mg L−1 of each BTEX sample in methanol (Supelco 47993) in triplicate injections at the following concentrations: 100, 200, 500, 1000, 2000, 4000, and 20,000 μg L−1, as well as a solvent blank. The detection and quantification limit for all BTEX samples were 12 and 35 μg L−1, respectively. The determination coefficients for all BTEX samples were greater than 0.99.
Risks to human health
The workers’ health risks from exposure to BTEX in the areas studied was assessed (Vilavert et al. 2012). Equation 1 was used to calculate the risk, as suggested by the Risk Assessment Guidance for Superfund developed by the U.S.EPA (1989).
where CE is the exposure concentration (μg m−3), CA is the contaminant concentration in the air (μg m−3), ET is exposure time (hours day−1), FE is the frequency of exposure (day year−1), ED is the exposure duration (years), and AT is the average time (lifetime in years × 365 days year−1 × 24 h day−1).
Using Eq. 2 and the EC value calculated for each pollutant, the danger coefficient (HQ) was estimated and was compared to the inhalation reference concentration (RFC) with exposure concentration (EC) (U.S.EPA 1989):
where HQ is the danger coefficient, EC the exposure concentration (μg m−3), and toxicity value (mg m−3) is the inhalation reference concentration (RFC). By calculation, it was possible to estimate the probability of not contracting cancer, using Eq. 3.
where CR is the carcinogenic risk and IRU inhalation risk unit.
Results and discussion
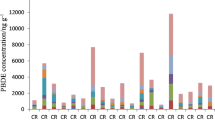
Figure 1 shows the results for the BTEX in the four workplaces evaluated. The highest concentrations for benzene and toluene which were observed in paint workshop were 3.6 and 788.3 μg m3, respectively. For ethylbenzene and m + p-xylenes, the highest concentrations found in the varnish workshop were 284.0, 484.6, and 265.9 μg m−3. The high standard deviations are explained by the different activities undertaken, the several emission sources, and the manner that each worker used the products. The high number of samples is considered to be representative for evaluating the workers’ exposure. The lower standard deviation was found for benzene in the three other workshops, indicating that the solvents used are not significant sources of benzene. For outdoor samples, the standard deviation obtained for each of BTEX samples indicates that there is a single source, which is likely vehicular emission from tailpipe and evaporative emissions because the testing site is a large parking lot.
In the three workshops evaluated, toluene was the most abundant compound, representing 43, 49, and 69 % for varnish, paint, and carpentry workshops, respectively. In the external environment, ethylbenzene and toluene were the most representative BTEX values, with 32 % of each.
Benzene concentrations were close to those found in different studies (Corrêa et al. 2012a; Corrêa and Arbilla 2007), except for samples collected in landfill areas (Durmusoglu et al. 2010; Corrêa et al. 2012b). The highest BTEX concentrations at the landfill area exceeded the maximum value obtained in the three workshops.
The correlation between BTEX values showed characteristic profiles. Benzene only had high correlation (>0.70) in the paint workshop, with correlations of 0.86 and 0.87 with m + p-xylenes and o-xylene, respectively. The benzene-toluene correlation was low in all sites. Compounds that showed correlations greater than 0.85 were ethylbenzene and xylenes in all three workshops, indicating that each sample came from the same source. Toluene only showed a high relationship with ethylbenzene and xylenes in the carpentry workshop; this relationship was possibly due to the use of contact adhesive, which is the only product that is exclusively used in carpentry.
Table 3 shows the ratio of BTEX concentrations obtained in the indoor and outdoor environments. For benzene, the higher ratio was obtained in the paint workshop. The highest TEX ratios were found at varnish and paint workshops, where all BTEX concentrations were also higher. The high values for the indoor/outdoor ratio indicate the existence of significant internal sources of these compounds.
According to Guo et al. (2004), the ratio value between the indoor (I) and outdoor sources (O) is a determining factor for the identification of BTEX sources, and it is a better parameter than absolute concentrations. If I/O > 1, there must be indoor sources of pollution (Massolo et al. 2010; Yoon et al. 2011), and a detailed investigation must be done to prevent workers’ exposure.
In comparison to the study by Guo et al. (2004), which was performed in different environments such as offices, homes, schools, shopping malls, and restaurants in Hong Kong, i.e., places where the vehicular air pollution was a significant source of VOCs, the values found in this study were higher, probably due to the use of organic solvents in the workshops emitting BTEX.
The values of I/O obtained by Vilavert et al. (2012) were higher than 400, establishing a positive correlation for indoor-outdoor BTEX concentrations, making the existence of significant occupational exposure levels within the installation evident.
In the study by Jung et al. (Jung et al. 2010) that was conducted in the neighborhood of an airport, BTEX I/O ratios above 1 were found, which were mainly for toluene due to chemical products used for cleaning. According to Jung et al. (2010), the ratio of benzene and toluene has been widely used as an emission source indicator. The presence of benzene is assigned to vehicular emissions, while the presence of toluene is also allocated to vehicular emissions from tailpipe and evaporative emissions. Generally, several authors that highlight the origin of these compounds in the atmosphere use the toluene and benzene (T/B) ratios. Values of T/B above 4 indicate predominant sources of evaporative emissions and similar solvents (Bravo et al. 2002; Chan et al. 2002). The T/B values were 81.73, 41.42, and 190.49 for paint, carpentry, and varnish workshops, respectively, indicating that there was a significant contribution by the products used.
Chemical analysis of the products used in the workplaces
Table 4 presents the concentrations found for the raw materials used in each workshop, and Table 5 shows the chemical composition of the raw material used. Samples 1 to 4 presented higher TEX concentrations, and these raw materials are the most commonly used in the paint and varnishing workshops. This finding is indicative of a positive correlation with the results found previously. Benzene was not found in the raw material, indicating that the I/O ratios are correct.
Human health risks
The carcinogenic and non-cancer risks due to continuous BTEX exposure were assessed from the benzene results for each of workplace. Table 6 presents the average, maximum, and minimum risks for the three studied workshop types. The carcinogenic risk for the varnish workshop was 2.58 × 10−7. This value was lower than that found by Vilavert et al. (2012), who studied locations near an organic waste treatment plant, as well as the value found by Dutta et al. (2009) in a bus station near roads and houses; however, the value was higher than the carcinogenic risk calculated in a landfill by Palmiotto et al. (2014).
For the varnish workshops, the higher non-carcinogenic risks for the m + p-xylenes and o-xylene were 0.088 and 0.048, respectively. Toluene is the most abundant BTEX in the three evaluated workshops, and it had the highest non-carcinogenic risk in the paint workshop.
According to LaGrega et al. (1994), the carcinogenic risk can be considered indifferent when it is less than 1.00 × 10−6, but it is considered significant when it is greater than 1.00 × 10−3 (Rodrics et al. 1987); the non-carcinogenic risk can be considered indifferent when it is equal to 1.0 (LaGrega et al. 1994).
When comparing non-carcinogenic risk values, it can be observed that values were below 1.0 in all workshops, indicating that there was no non-carcinogenic risk. In the case of carcinogenic risk, the mean values ranged from 9.19 × 10−7 and 5.7 × 10−7, and the carcinogenic potential also was considered indifferent. Maximum values were just above 1.00 × 10−6 and may be considered more expressive. Table 6 shows the carcinogenic and non-carcinogenic risks of BTEX exposure in the workshops.
The approach used here was the only possible due to the data set obtained in these workplaces. A more robust methodology is detailed in the works of Sarigiannis and Gotti (2008), Sarigiannis et al. (2009), and Karakitsios et al. (2013). They developed a methodology for assessment the determinants that comprise the overall leukemia risk due to benzene exposure and how these are affected by the other pollutants. An integrated modeling environment was constructed with several variables, such as traffic emissions, dispersion models, human exposure models, and a coupled internal dose/biology-based dose-response risk assessment model, in order to assess the benzene-imposed leukemia risk, as much as the impact of traffic fleet renewal and smoking banning to these levels. This may result in lower or greater toxicity of mixtures than what would be expected by summing the toxicity of individual chemicals.
Conclusions
In all of the workshops evaluated, toluene concentrations were higher. The samples showed a high standard deviation, indicating that different work routines are critical to the evaluation; thus, a high sample number was necessary to obtain a confidence value. I/O ratios indicated that paint and varnish workshops have higher BTEX concentrations.
The highest concentrations of benzene and toluene were obtained in the paint workshop, and higher concentrations of ethylbenzene, m + p-xylenes, and o-xylene were obtained in the varnish workshop. The raw materials with the highest TEX concentrations were those used in the painting and varnish workshops.
The first and second non-carcinogenic risks were obtained for the varnish workshop for m + p-xylenes. The carcinogenic risk average value and all non-carcinogenic values can be considered indifferent for all workplaces. The maximum carcinogenic risk values can be considered expressive.
Some suggestions to enhance air quality and minimize health effects in the workplaces evaluated are as follows: increase the ambient and local ventilation, substitute solvent-based products with water-based products, and establish routines for maintaining the closure of the containers after use.
References
ACGIH. (2008). Threshold limit value and biological exposure. American Conference of Governmental Industrial Hygienists: USA.
Bravo, H., Sosa, R., Sánchez, P., Bueno, E., & González, R. (2002). Concentrations of benzene and toluene in the atmosphere of the southwestern area at the Mexico City Metropolitan Zone. Atmospheric Environment, 36, 3843–3849. doi:10.1016/S1352-2310(02)00292-3.
Chan, C. Y., Chan, L. Y., Wang, X. M., Liu, Y. M., Lee, S. C., Zou, S. C., Sheng, G. Y., & Fu, J. M. (2002). Volatile organic compounds in roadside microenvironments of metropolitan Hong Kong. Atmospheric Environment, 36, 2039–2047. doi:10.1016/S1352-2310(02)00097-3.
Corrêa, S. M., & Arbilla, G. (2007). A two-year of aromatic hydrocarbons monitoring at the downtown area of the city of Rio de Janeiro. Journal of the Brazilian Chemical Society, 18, 539–543. doi:10.1590/S0103-50532007000300007.
Corrêa, S. M., Arbilla, G., Marques, M. R. C., & Oliveira, K. M. P. G. (2012a). The impact of BTEX emissions from gas stations into the atmosphere. Atmospheric Pollution Research, 3, 163–169. doi:10.5094/APR.2012.016.
Corrêa, S. M., Souza, C. V., Sodré, E. D., & Teixeira, J. R. (2012b). Volatile organic compound emissions from a landfill, plume dispersion and the tropospheric ozone modeling. Journal of the Brazilian Chemical Society, 23, 496–504. doi:10.1590/S0103-50532012000300017.
de Castro, B. P., Machado, G. S., Bauerfeldt, G. F., Fortes, J. D. N., & Martins, E. M. (2015). Assessment of the BTEX concentrations and reactivity in a confined parking area in Rio de Janeiro, Brazil. Atmospheric Environment, 104, 22–26. doi:10.1016/j.atmosenv.2015.01.013.
Dewangan, S., Chakrabarty, R., Zielinska, B., & Pervez, S. (2013). Emission of volatile organic compounds from religious and ritual activities in India. Environmental Monitoring and Assessment, 185, 9279–9286. doi:10.1007/s10661-013-3250-z.
Durmusoglu, E., Taspinar, F., & Karademir, A. (2010). Health risk assessment of BTEX emissions in the landfill environment. Journal of Hazardous Materials, 176, 870–877. doi:10.1016/j.jhazmat.2009.11.117.
Dutta, C., Som, D., Chatterjee, A., Mukherjee, A. K., Jana, T. K., & Sen, S. (2009). Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environmental Monitoring and Assessment, 148, 97–107. doi:10.1007/s10661-007-0142-0.
Farshad, A., Oliaei, H. K., Mirkazemi, R., & Bakand, S. (2013). Risk assessment of benzene, toluene, ethylbenzene and xylenes (BTEX) in paint plants of two automotive industries in Iran by using the COSHH guideline. European Science Journal, 3, 270–276.
Gioda, A., & Aquino Neto, F. R. (2003). Comments on studies of industrial and non-industrial environments in Brazil: a comparative approach. Cadernos de Saúde Pública, 19, 1389–1397.
Guo, H., Murray, F., & Wilkinson, S. (2000). Evaluation of total volatile organic compound emissions from adhesives based on chamber tests. Journal of the Air & Waste Management Association, 50, 199–206. doi:10.1080/10473289.2000.10464006.
Guo, H., Lee, S. C., Chan, L. Y., & Li, W. M. (2004). Risk assessment of exposure to volatile organic compounds in different indoor environments. Environmental Research, 94, 57–66. doi:10.1016/S0013-9351(03)00035-5.
Hinwood, A. L., Rodriguez, C., Runnion, T., Farrar, D., Murray, F., Horton, A., & Galbally, I. (2007). Risk factors for increased BTEX exposure in four Australian cities. Chemosphere, 66, 533–541. doi:10.1016/j.chemosphere.2006.05.040.
Ilgen, E., Karfich, N., Levsen, K., Angerer, J., Schneider, P., Heinrich, J., Wichmann, H., Dunemann, L., & Begerow, J. (2001). Aromatic hydrocarbons in the atmospheric environment: part I. Indoor versus outdoor sources, the influence of traffic. Atmospheric Environment, 35, 1235–1252. doi:10.1016/S1352-2310(00)00388-5.
Jones, A. P. (1999). Indoor air quality and health. Atmospheric Environment, 33, 4535–4564. doi:10.1016/S1352-2310(99)00272-1.
Jung, K. H., Artigas, F., & Shin, J. Y. (2010). Personal, indoor, and outdoor exposure to VOCs in the immediate vicinity of a local airport. Environ Monit Assess, 173, 555–567. doi:10.1007/s10661-010-1404-9.
Karakitsios, S. P., Sarigiannis, D. A., Gotti, A., Kassomenos, P. A., & Pilidis, G. A. (2013). A methodological frame for assessing benzene induced leukemia risk mitigation due to policy measures. Science of the Total Environment, 443, 549–558.
Klinmalee, A., Srimongkol, K., & Kim Oanh, N. T. (2009). Indoor air pollution levels in public buildings in Thailand and exposure assessment. Environmental Monitoring and Assessment, 156, 581–594. doi:10.1007/s10661-008-0507-z.
Kumar, A., Singh, B. P., Punia, M., Singh, D., Kumar, K., & Jain, V. K. (2014). Assessment of indoor air concentrations of VOCs and their associated health risks in the library of Jawaharlal Nehru University, New Delhi. Environmental Science and Pollution Research International, 21, 2240–2248. doi:10.1007/s11356-013-2150-7.
LaGrega, M. D., Buckingham, P. L., & Evans, J. C. (1994). Hazardous waste management. New York: McGraw Hill. ISBN 0-07-019552-8.
Lim, S. K., Shin, H. S., Yoon, K. S., Kwack, S. J., Um, Y. M., Hyeon, J. H., Lee, B. M., et al. (2014). Risk assessment of volatile organic compounds benzene, toluene, ethylbenzene, and xylene (BTEX) in consumer products. Journal of Toxicology and Environmental Health A, 77, 1502–1521. doi:10.1080/15287394.2014.955905.
Maroni, M., Seifert, B., & Lindvall, T. (1995). Indoor air quality—a comprehensive reference book. Amsterdam: Elsevier. ISBN 0444816429.
Martins, E. M., Quiterio, S. L., Corrêa, S. M., Fortes, J. D. N., Monteiro, M., & Prestes, B. (2014). BTEX inside a spinning classroom. Cadernos de Saúde Coletiva, 22, 218–220. doi:10.1590/1414-462X201400020017.
Massolo, L., Rehwagen, M., Porta, A., Ronco, A., Herbarth, O., & Mueller, A. (2010). Indoor-outdoor distribution and risk assessment of volatile organic compounds in the atmosphere of industrial and urban areas. Environmental Toxicology, 25, 339–349. doi:10.1002/tox.20504.
Ministry of Labor and Employment (MTE) (1994) Regulatory Standard (NR 9). Prevention program of environmental risks. http://sislex.previdencia.gov.br/paginas/05/mtb/9.htm. Accessed 28 May 2015.
Ministry of Labor and Employment (MTE) (1995a) Normative Instruction N. 1. Assessment of benzene concentrations in workplaces relating to Annex 13-A Benzene, of Regulatory Standard n. 15—unhealthy activities and operations. http://www.lex.com.br/doc_10174_INSTRUCAO_NORMATIVA_N_1_DE_20_DE_DEZEMBRO_DE_1995.aspx. Accessed 08 Jul 2015.
Ministry of Labor and Employment (MTE) (1995b) Ordinance no. 14. Change the item of carcinogenic substances of Annex 13, of Regulatory Standard no. 15—unhealthy activities and operations. http://sislex.previdencia.gov.br/paginas/05/mtb/15.htm. Accessed Jul 2015.
NIOSH. (2003). Manual of analytical methods (NMAM) (4th ed.). National Institute for Occupational Safety and Health, Accessed 28 May 2015.
OSHA. (2016). Occupational Safety and Health Administration. https://www.osha.gov/dsg/annotatedpels/tablez-1.html. Accessed Oct 2016.
Palmiotto, M., Fattore, E., Paiano, V., Celeste, G., Colombo, A., & Davoli, E. (2014). Influence of a municipal solid waste landfill in the surrounding environment: toxicological risk and odor nuisance effects. Environmental International, 68, 16–24. doi:10.1016/j.envint.2014.03.004.
Possanzini, M., Di Palo, V., & Cecinato, A. (2002). Sources and photodecomposition of formaldehyde and acetaldehyde in Rome ambient air. Atmospheric Environment, 36, 3195–3201. doi:10.1016/S1352-2310(02)00192-9.
Ras, M. R., Marcé, R. M., & Borrull, F. (2010). Volatile organic compounds in air at urban and industrial areas in the Tarragona region by thermal desorption and gas chromatography-mass spectrometry. Environmental Monitoring and Assessment, 161, 389–402. doi:10.1007/s10661-009-0755-6.
Rodrics, J. V., Brett, S. M., & Wrenn, G. C. (1987). Significant risk decisions in federal regulatory agencies. Regulatory Toxicology and Pharmacology, 7, 307–320. doi:10.1016/0273-2300(87)90038-9.
Rodrigues, F., Milas, I., Martins, E. M., Arbilla, G., Bauerfeldt, G. F., & Paula, M. D. (2007). Experimental and theorical study of the air quality in a suburban industrial-residential area in Rio de Janeiro, Brazil. Journal of the Brazilian Chemical Society, 18, 342–351. doi:10.1590/S0103-50532007000200015.
Sarigiannis, D. A., & Gotti, A. (2008). Biology-based dose-response models for health risk assessment of chemical mixtures. Fresenius Environmental Bulletin, 17, 1439–1451.
Sarigiannis, D., Gotti, A., Cimino Reale, G., & Marafante, E. (2009). Reflections on new directions for risk assessment of environmental chemical mixtures. International Journal of Risk Assessment and Management, 13, 216–241.
Sarigiannis, D. A., Karakitsios, S. P., Gotti, A., Liakos, I. L., & Katsoyiannis, A. (2011). Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environmental International, 37, 743–765. doi:10.1016/j.envint.2011.01.005.
Tunsaringkarn, T., Siriwong, W., Rungsiyothin, A., & Nopparatbundit, S. (2012). Occupational exposure of gasoline station workers to BTEX compounds in Bangkok, Thailand. International Journal of Occupational Environmental Medicine, 3, 117–125.
U.S.EPA (1989) Risk assessment guidance for superfund. Volume I—human health evaluation manual (part A) EPA/540/1-89/002. Washington D.C. 20450.
Vilavert, L., Nadal, M., Figueras, M. J., & Domingo, J. L. (2012). Volatile organic compounds and bioaerosols in the vicinity of a municipal waste organic fraction treatment plant. Human health risks. Environmental Science Pollution Research, 19, 96–104. doi:10.1007/s11356-011-0547-8.
Ward, T. J., Underberg, H., Jones, D., Hamilton, R. F., & Adams, E. (2009). Indoor/ambient residential air toxics results in rural western Montana. Environmental Monitoring and Assessment, 153, 119–126. doi:10.1007/s10661-008-0342-2.
Weisel, C. P. (2010). Benzene exposure: an overview of monitoring methods and their findings. Chemico-Biological Interactions, 184, 58–66. doi:10.1016/j.cbi.2009.12.030.
Wilbur, S., Wohlers, D., Paikoff, S., Keith, L. S., & Faroon, O. (2008). ATSDR evaluation of health effects of benzene and relevance to public health. Toxicology Industrial Health, 24, 263–398. doi:10.1177/0748233708090910.
Yoon, C., Lee, K., & Park, D. (2011). Indoor air quality differences between urban and rural preschools in Korea. Environmental Science and Pollution Research, 18, 333–345. doi:10.1007/s11356-010-0377-0.
Acknowledgments
The authors wish to thank the Research Support Foundation of the State of Rio de Janeiro (FAPERJ) and National Council for Scientific and Technological Development (CNPq) for the financial support and research grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, E.M., Borba, P.F.d.S., dos Santos, N.E. et al. The relationship between solvent use and BTEX concentrations in occupational environments. Environ Monit Assess 188, 608 (2016). https://doi.org/10.1007/s10661-016-5621-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5621-8