Abstract
Until recently, exposure pathways of concern for cyanotoxins have focused on recreational exposure, drinking water, and dermal contact. Exposure to cyanotoxins through fish consumption is a relatively new area of investigation. To address this concern, microcystins and other cyanotoxins were analyzed in fish collected from nine Washington lakes with recurrent toxic blooms using two types of enzyme-linked immunosorbent assays (ELISAs) and liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS). Microcystins or microcystin-like compounds were elevated in fish liver relative to muscle and other tissues (liver > gut > muscle). Microcystin concentrations in fish fillet samples using anti-Adda ELISA (range 6.3–11 μg/kg wet weight) were consistently higher in all fish species than concentrations using anti-microcystin (MC)-leucine-arginine (LR) ELISA (range 0.25–2.4 μg/kg wet weight). MC-leucine-alanine (LA) was the only variant detected in fish (2.5–12 μg/kg in four livers) among the nine variants analyzed by LC-MS/MS. Fish fillets showed no accumulation of the MCs targeted by LC-MS/MS. Other cyanotoxins analyzed (anatoxin-a, saxitoxin, domoic acid, and okadaic acid) were not detected in fish. Based on this and evidence from other studies, we believe that people can safely consume two 8-oz fish fillet meals per week from lakes with blooms producing MCs (clean the fish and discard viscera).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria (blue-green algae) are known to produce toxins that can cause gastrointestinal, neurological, dermal, and respiratory health effects (NOAA 2014). The cyanobacteria genera Microcystis is the most common nuisance cyanobacteria in freshwaters and has a history of causing water quality problems by producing microcystins that can remain in the water for days or weeks after a bloom has disappeared (Codd and Bell 1996, Cousins et al. 1996, Lahti et al. 1997). Microcystins (MCs) primarily affect the liver, and if they are liver carcinogens as researchers suspect, continuous, low-level exposure through any route could prove significant (WHO 2003, Fitzgeorge et al. 1994, Botana 2007). Another potent cyanotoxin, anatoxin-a, is produced by several genera and affects organisms through neurological impacts (Botana 2007, Huisman et al. 2005, Falconer 2005). A third cyanotoxin, saxitoxin, is a neurotoxin not only primarily associated with paralytic shellfish poisoning (PSP or “red tide”) in marine waters but also produced by cyanobacteria in freshwater (Aràoz et al. 2010, Lagos et al. 1999, Pomati et al. 2000, Castro et al. 2004, van Apeldoorn et al. 2007). These three cyanotoxins have been detected in Washington lakes.
While the primary human health exposure pathways for toxic cyanobacteria are drinking water and recreational exposures (Stone and Bress 2007), a poorly studied but potentially important exposure route for humans is consumption of fish containing cyanotoxins (Stone and Bress 2007, Kann 2008, Wilson et al. 2008). Freshwater and brackish water fish are known to accumulate MCs in their tissues, including muscle, liver, and other organs (Kotak et al. 1996, Magalhães et al. 2001, Sipiä et al. 2001, Xie et al. 2005, Ibelings et al. 2005, Gkelis et al. 2006, Wood et al. 2006, Wilson et al. 2008, Kann 2008). Of further concern regarding cyanotoxin exposure to humans through fish consumption is evidence that MCs are heat stable and do not break down during cooking (Harada et al. 1996). Fish appear to take up MC quickly (Tencala and Dietrich 1997, Magalhães et al. 2001, Dyble et al. 2011), and although rapid loss of MC in muscle and liver tissue occurs once fish are removed from exposure (Adamovsky et al. 2007, Dyble et al. 2011), consuming contaminated fish has been identified as a potential human health concern. Minimal research has been conducted on MC transfer between seafood and humans, but evidence shows that transfer occurs with MC detected in the blood serum of fishermen who consumed contaminated fish (Chen et al. 2009).
In the past several years, toxicity events in Washington, USA, lakes have increased (Hamel 2009, Jacoby et al. 2015). To address environmental and public health concerns related to increased blooms, Washington developed a freshwater algae control program funded by the state legislature that provides for toxicity tests of water samples from lakes with blooms (Hamel 2009, DOH 2008 and 2011). Data from 5 years of regular sampling demonstrate that MCs are the most abundant toxins in regional lakes, followed by anatoxin-a, saxitoxins, and cylindrospermopsins (Hardy 2013; Trainer and Hardy 2015). While toxic blooms have been observed during all months of the year, higher MC concentrations generally occur from September through November. One unaddressed concern is the potential to impact human health by consuming fish from regional lakes experiencing toxic blooms. All but one county in the state have lowland lakes stocked with fish species that are caught for human consumption.
In 2008, Washington State Department of Ecology (Ecology) conducted a pilot project to test for presence of MCs, anatoxin-a, and saxitoxins using enzyme-linked immunosorbent assays (ELISA) in fish from six Washington lakes experiencing cyanobacterial blooms (Johnson 2010a). Significant MC concentrations appeared to be present in fish from most lakes surveyed. Yet there were concerns that anti-MC-leucine-arginine (LR) ELISAs (Abraxis) were underestimating MC concentrations indicated in part because of low recovery of spiked control samples (Johnson 2010a). Anatoxin-a, analyzed by high-pressure liquid chromatography (HPLC), was not detected. This neurotoxin may be too unstable to accumulate or is simply not taken up by fish. A third cyanotoxin, saxitoxin, was tentatively identified by ELISA in fish from one lake. The 2008 report recommended further sampling and analysis to obtain accurate and verifiable data on MCs and saxitoxins in fish from state lakes.
Based on results from the 2008 pilot study showing uptake of cyanotoxins by fish and the potential for adverse human health effects though this exposure route, we hypothesized that MC and saxitoxin accumulations in freshwater fish are a human health concern. We developed a comprehensive study in 2010 and 2011 to screen fish muscle and liver tissue from Washington lakes to detect presence or absence of MC and saxitoxin collected during or immediately following cyanobacteria blooms. If MC presence was found in fish tissues, a human health assessment based on exposure through fish consumption would be performed. Objectives of this investigation were to
-
1.
Determine if MC accumulates in freshwater fish exposed to cyanotoxins in temperate lowland lakes using ELISA and liquid chromatography coupled with mass spectrometry/mass spectrometry (LC-MS/MS).
-
2.
Evaluate two ELISA methods for MCs in fish tissue to determine if these methods accurately detect MC in fish fillets and/or liver.
-
3.
Test for the presence of saxitoxin in fish from a known saxitoxin-producing lake.
-
4.
Determine if the presence of cyanotoxins in fish tissue represents a public health concern.
Methods
Project description
During summer and fall (May, July, August, September, and October) of 2010, we collected fish fillet samples from eight Washington lakes experiencing significant cyanobacteria blooms. Saxitoxin was analyzed in fish from two lakes using ELISA. We analyzed samples for MC by two ELISA methods and LC-MS/MS. The results in 2010 showed that the ELISA and LC-MS/MS analytical methods produced different results when analyzing the same tissue sample. In 2011, a second set of tissue and liver samples was collected to determine if ELISA and LC-MS/MS could correctly assess the presence or absence of MCs and to determine if the two methods were comparable (Johnson et al. 2013). Samples from 2011 included four archived fish muscle homogenates provided by California Department of Fish and Game (CDFG) to compare results between laboratories; these samples had previously tested positive for MC by LC-MS/MS (2007).
Samples were analyzed by the CDFG Water Pollution Control Laboratory (LC-MS/MS) and the King County Environmental Laboratory (KCEL) (ELISA). This study followed a Quality Assurance Project Plan (Johnson 2010b) developed in accordance with Ecology’s guidance (Lombard and Kirchmer 2004).
Sampling methods
Lakes sampled
Fish were collected from lakes listed (Table 1). For logistical reasons, field work focused mainly on western Washington lakes. One reservoir in eastern Washington, Lake Spokane (Spokane River), was also sampled (Figs. 1 and 2).
Washington fish collection
It is not possible to predict when or where cyanobacteria blooms will occur. The trigger for fish sampling in our study was detection of high levels of MCs (>6 ppb) in algae samples collected through Ecology’s Freshwater Algae Control Program.
We attempted to collect fish soon after high toxin levels (>6 ppb) were reported. Popular sport fish such as rainbow trout, bass, and perch as well as bottom-feeding species such as suckers and bullheads were targeted with one to three fish species collected from each lake. Composite samples were analyzed to provide a cost-effective estimate of toxin concentrations. Where possible, each sample consisted of pooled tissues from five individual fish. In some instances, only a few fish were encountered during collection efforts, and sample size was lower. Fish were collected by electroshocking, gill net, or hook and line, following Environmental Assessment Program (EAP) standard operating procedures (SOP) (Sandvik 2006a). For fish without size limits, fish large enough to be retained for consumption were collected. Fish selected for analysis were killed by a blow to the head. Fish were put in new plastic bags and placed on ice soon after collection then transported to Ecology
All fish samples analyzed in 2010 samples were muscle fillets because human health concern was the impetus for this study (Table 2). In 2011, fish fillets and liver were analyzed in an effort to provide laboratories with material that had a range of MC concentrations (Table 3).
Control fish
Three types of fish samples were analyzed by anti-Adda ELISA and LC-MS/MS in 2011:
-
1.
Fillet and
-
2.
Liver samples from fish collected in western Washington lakes with cyanobacteria blooms. Washington Department of Fish and Wildlife (WDFW) Arlington Fish Hatchery was the source of the rainbow trout sampled in Ketchum Lake; samples were collected at the hatchery approximately 1 week before Ketchum Lake was stocked and are considered a control.
-
3.
Archived yellow perch fillet samples from Klamath River (CA) reservoirs obtained through CDFG and were known to test positive for specific MCs (Table 3) (Kann 2008). CDFG provided these known positive MC samples to Ecology where they were re-homogenized, split into separate aliquots, and sent to KCEL and CDFG.
Fish preparation
At Ecology headquarters, each fish was given a unique identifying number and its length and weight were recorded. Fish were individually wrapped in aluminum foil, put in plastic bags, and kept frozen pending preparation of tissue samples.
Tissue samples were prepared following the EAP SOP (Sandvik, 2006b). Techniques to minimize contamination were used. Staff wore non-talc nitrile gloves and worked on heavy-duty aluminum foil or a polyethylene cutting board. Gloves and foil were changed between samples; the cutting board was cleaned between samples.
In 2010, the fish were scaled, except for bullheads where the skin was discarded because it is not eaten. In 2011, all fish fillet samples were skinless in an effort to improve sample homogeneity. For both years, the entire fillet from one or both sides was removed with stainless steel knives and homogenized in a KitchenAid blender. Fish liver samples were homogenized with an IKA-Ultra-Turrax dispersion tool.
All tissues were homogenized to uniform color and consistency. Fillet homogenates were placed in 2 or 4 oz. glass jars cleaned to EPA (1990) QA/QC specifications. The smaller liver and CDFG homogenates were put in small plastic vials. Each homogenate was split into two aliquots, one for CDFG and one for KCEL. Some liver samples were too small to split and were analyzed by CDFG only.
Tissue samples were refrozen for overnight shipment with chain-of-custody record to the analyzing laboratories. Samples were maintained at or near freezing during shipment. All samples were submitted blind to the laboratories.
Analysis methods
Microcystins
LC-MS/MS and ELISA (Envirologix, Abraxis, and Bio Scientific) were used to detect MC in fish samples (Table 4). ELISAs allow for congener-independent detection of MCs without cross-reacting to non-algal compounds. Two types of ELISA kits were initially used for the 2010 samples: Envirologix™ anti MC-LR (Portland, ME, USA) and Abraxis LLC anti-Adda (Warminster, PA, USA). The relative responses (cross-reactivities) of the two ELISA tests with the different MC variants are listed (Table 5). The anti MC-LR ELISA has good cross-reactivity with MC variants MC-LR, MC-arginine-arginine (RR), and tyrosine-arginine (YR) but has poorer reactivity with MC-leucine-tyrosine (LY) and MC-leucine-alanine (LA) (Ueno et al. 1996, Carmichael and An 1999). Since the anti-MC-LR ELISA responds primarily to the MC-LR variant, it may underestimate MC content in fish tissues.
The indirect competitive anti-Adda ELISA was developed to quantify all MC variants (over 90 MC variants have been reported in environmental samples, Welker and von Döhren 2006) by analyzing the constituent amino acid Adda (using the β-amino acid 6E-Adda for antibody recognition). Anti-Adda ELISAs have good cross-reactivity with at least 80 % of the known MCs, due to the presence of 6E-Adda (Fischer et al. 2001). Because a wider range of MCs is targeted, anti-Adda ELISA is the more frequent choice for fish tissue work. However, anti-Adda ELISA is more susceptible to background/matrix effects and false positives (Meriluoto and Spoof 2007) which may lead to overestimation of actual toxicity (McEliney and Lawton 2004, Preece et al. 2015).
LC-MS/MS is the preferred method for cyanotoxin detection since it can quantify specific MC variants whereas ELISA can only determine MC presence and cannot differentiate between specific variants. However, within current capabilities, ELISA offers the best option for public health agencies because the method is relatively fast and inexpensive, requires minimum sample processing, and is commercially available.
CDFG recently validated its LC-MS/MS method for analyzing MCs in water and tissue (Mekebri et al. 2009). Nine MC variants (MC-LR, MC-desmethyl-LR, MC-RR, MC-desmethyl-RR, MC-YR, MC-LA, MC-LW, MC-LF, MC-LY) and several other algae toxins (anatoxin-a, domoic acid, okadaic acid) are quantified. The L, R, Y, A, F, and W designations stand for the MC variable amino acids leucine, arginine, tyrosine, alanine, phenylalanine, and tryptophan, respectively. MC-LR, MC-RR, MC-YR, and MC-LA are the most important toxins of human health concern associated with blue-green blooms (EPA 2006).
Quality control (QC) samples included method blanks, spiked blanks, matrix spikes, matrix spike duplicate, surrogate spikes, and laboratory duplicates, as appropriate. Results were within the QC limits established by the analyzing laboratories.
Statistics
Data normality was tested using the Shapiro-Wilkes test prior to additional statistical analyses. ELISA data showed a gamma distribution, so associations between the dependent variable MC (μg/kg) and the predictor ELISA type were tested with a generalized linear model (GLM) (Kutner et al. 2004, McCullagh and Nelder 1989). Tissue type was tested with ANOVA to determine if the various fish tissues accumulated MCs differentially. Significance was assessed α = 0.05.
Results
Microcystins and saxitoxin in fish tissues—2010
ELISA—microcystins
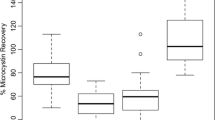
KCEL initially analyzed fish fillet samples by anti-MC-LR ELISA. However, MC-LR matrix spike recoveries were low and inconsistent (21–34 %) (Table 6). A subset of seven samples was then analyzed by anti-Adda ELISA where more acceptable recoveries were achieved (65 %) (Table 6).
MCs were present in all samples measured with anti-Adda ELISA—6.3 to 11.0 μg/kg—and were significantly higher than anti-MC-LR ELISA results (p = 0.001). Due to different cross-reactivities (see Table 6), close agreement between anti-MC-LR and anti-Adda ELISA would only be expected when analyzing purified MC-LR, yet even MC-LR spiked tissue samples yielded poor results. Although acceptable MC recoveries were found in QC samples, we were concerned that the anti-Adda ELISA was not accurately measuring MCs in fish samples. For example, one Anderson Lake sample analyzed in duplicate differed by almost a factor of two, indicating that precision of the anti-Adda ELISA may be poor (Table 6).
Ketchum Lake yellow perch gut, liver, and muscle tissues collected from waters experiencing blue-green blooms were found to contain MCs (Table 7). MC levels in liver and gut were significantly (p = 0.002) higher than either muscle or skin. The pattern liver > gut > muscle/skin generally fits expectations of how bioaccumulative chemicals would be taken up by fish following ingestion.
Enzyme-linked immunosorbent assays—saxitoxins
Saxitoxin was analyzed in selected fish fillet samples collected from Waughop Lake, a lake with blooms known to produce saxitoxin, and Ketchum Lake in 2010. An ELISA kit that specifically recognizes saxitoxin as well as other PSP toxins to a lesser degree was used (detection limit for saxitoxin is 0.015 ng/mL). No saxitoxin was detected at or above approximately 0.5 to 0.8 μg/kg (Table 8).
Liquid chromatography coupled with mass spectrometry/mass spectrometry
Twenty fish fillet samples were analyzed by LC-MS/MS for nine MC variants (limit of detection 1.0 μg/kg). No variants were detected at or above the method detection limit of 0.5 μg/kg. Anatoxin-a, domoic acid, and okadaic acid were also not detected (Table 9).
Microcystins in fish tissues—2011
Enzyme-linked immunosorbent assays—microcystins
Due to inconsistent recoveries by the anti-MC-LR ELISA in 2010, only the anti-Adda ELISA was used for 2011 analyses. Based on ELISA, MC levels were distinctly different between Washington and California samples (Fig. 3). Concentrations ranged from 2.0 to 5.9 μg/kg in Washington fillets, 5.5 to 35 μg/kg in California fillets, and 35 to 132 μg/kg in Washington livers.
Liquid chromatography coupled with mass spectrometry/mass spectrometry
LC-MS/MS detected MC-LA in Washington fish livers (Table 10). Concentrations ranged from 2.5 to 14.0 μg/kg. Other MC variants, anatoxin-a, domoic acid, and okadaic acid were below method detection limit of 0.5 μg/kg (Table 9).
There was evidence of a correlation between the ELISA response and MC-LA concentrations in largemouth bass liver (Fig. 4). Again, the much higher levels determined by ELISA suggest the presence of other MCs or MC-like compounds not analyzed by LC-MS/MS, or they were possibly false positives. For unknown reasons, the only other fish liver sample analyzed by both methods (rainbow trout) had widely disparate results (Fig. 4). The large difference in results could be due to matrix effects with the ELISA or the low RBT sample size (n = 1). A larger sample size may provide better information on why the RBT liver was such an outlier.
CDFG identified significant concentrations of desmethyl-LR (80.0–422.0 μg/kg) in the California perch fillets when the samples were originally analyzed in 2007. When re-analyzed in 2011, no MC was detected, so CDFG reviewed their 2007 LC-MS/MS output. A desmethyl-LR standard had not been available at that time (2007), and it had been quantified based on MC-LR, the parent compound. A standard has since been obtained, allowing CDFG to determine that desmethyl-LR had been misidentified and the peak was due to an unknown MC for which we do not have a standard.
Anatoxin-a, domoic acid, and okadaic acid were not detected. LC-MS/MS detection limits for fish tissue were 5.0, 2.0, and 1.0 μg/kg, respectively.
Health assessment
We used the non-cancer endpoint to calculate the MC concentration in fish that would allow for eight meals per month (two meals/week) that coincides with the American Heart Association recommendation of two fish meals a week (Kris-Etherton et al. 2002):
where
-
TDI = tolerable daily intake (TDI) for MC—0.04 μg/kg day
-
BW = body weight (15 kg or 33 lbs: child) (60 kg or 132 lbs: adult)
-
Consumption rate = g/day
-
Conc.fish = mean concentration of MC in fish (μg/kg)
-
Meal size = 4 oz or 0.1135 kg; 8 oz or 0.227 kg
-
Month = 30.44 days
-
mg/kg = parts per million (ppm)
-
μg/kg = parts per billion (ppb)
The number of 8-oz meals per month converts to grams per day:
Thus, the MC concentration in fish that would allow a 15-kg person (child) to safely consume eight meals per month (meal size of 4 oz for a child) is 20 μg/kg. Using the same assumptions except for a larger meal size (8-oz meal size for an adult) and a larger BW (60 kg, adult female) results in a concentration of 40 μg/kg MC concentration in fish tissue.
Discussion
A number of studies have now evaluated MC accumulation by fish (Gkelis et al. 2006, Wood et al. 2006, Poste et al. 2011, Papadimitriou et al. 2012, Romo et al. 2012). Most of these efforts have employed HPLC, anti-Adda-ELISA, LC-MS, or, more recently, LC-MS/MS. HPLC is subject to false positives (Ohio EPA 2012), and LC-MS has been shown to overestimate MC concentrations compared to LC-MS/MS (Kohoutek et al. 2010; Berry et al. 2011). We compared findings from Ecology’s 2008 and 2010–2011 samples with other recent investigations where anti-Adda ELISA has been used to analyze freshwater fish potentially affected by cyanobacteria blooms (Table 11). All of these studies from other areas of the world using anti-Adda ELISA have found similar levels in fish muscle, with average concentrations confined to the relatively narrow range of 5.0 to 14.0 μg/kg MC. Higher levels are consistently measured in liver, averaging between 50 and 200 μg/kg MC.
We found that anti-Adda ELISA was able to differentiate between low, moderate, and high levels (muscle/skin < gut < liver) of MCs. These results suggest that the anti-Adda ELISA can determine MC presence when higher levels of the toxin are present. Therefore, anti-Adda ELISA appears to be a useful tool for screening fish tissue samples but only when concentrations are elevated (>10 μg/kg, Gies-Asteggiante et al. 2011). A recent study comparing anti-Adda ELISA with LC-MS/MS for detecting MC in fish tissue samples concluded that anti-Adda ELISA was able to correctly assess the presence or absence of MC-LR, MC-LA, MC-YR, and MC-RR when concentrations were greater than 10 μg/kg but that it had shortcomings for quantitative purposes (Gies-Asteggiante et al. 2011). The study suggests that anti-Adda ELISA could be used as an initial screening tool for fish followed by confirmation of presumed positive samples by LC-MS/MS or, conversely, as confirmation for LC-MS/MS results.
Perch fillets analyzed by CDFG using LC-MS/MS originally demonstrating significant concentrations of desmethyl-LR were re-analyzed using a standard to determine that the peak was due to an unknown MC. Inconsistent anti-Adda ELISA results in fish tissues may also be related to unknown MCs.
In our study, MC-LA was the only variant detected in fish among the nine MCs analyzed by LC-MS/MS. The detections—2.5 to 12 μg/kg—were in four fish liver samples. Fish fillets showed no accumulation of the MCs targeted in the analysis. This finding is consistent with recent studies done elsewhere (Berry et al. 2011, Romo et al. 2012, Papadimitriou et al. 2010, Poste et al. 2011). There was some limited evidence of a correspondence between anti-Adda ELISA and MC-LA concentrations in fish liver (Fig. 4).
Kohoutek et al. (2010) used LC-MS/MS to conduct an extensive study of MCs in 148 edible fish tissue samples from five reservoirs with dense cyanobacterial blooms in Europe. All samples were below the limits of detection (1.2 μg/kg for RR and 5.4 μg/kg for YR and LR). Other recent LC-MS/MS studies that have also found little or no accumulation of important MC variants in fish muscle include CH2M Hill (2009), DeBlois et al. (2009), Prendergast and Foster (2010), Bruno et al. (2006), and (Ohio EPA 2012). These finding are consistent with LC-MS/MS results from this study where target MCs were not detected (<1.0 μg/kg) in 30 fish fillets.
Both ELISA and LC-MS/MS underestimate total MC content in fish tissues due to their inability to extract enzyme-bound toxin (Berry et al. 2011). Studies cited in Berry et al. (2011) suggest that the total concentration could be an order of magnitude higher. While it has been assumed that bound MCs are relatively benign, there is recent evidence that they may become bioavailable in the digestive system of fish consumers (Smith et al. 2010). The extent of underestimation and its relevance to human health remain to be determined in future studies.
Waughop Lake is one of two lakes in Washington producing saxitoxin at levels of potential human health concern for recreational users of the lake. Saxitoxin was first reported in state algae samples in 2009 (Hamel 2009). Seven other Washington lakes have produced levels of saxitoxin just above the MDL during the past 5 years (Hardy 2013). Saxitoxin was tentatively identified at low levels (<1 μg/kg) in fish samples collected from Waughop in 2008 and analyzed by ELISA (Johnson, 2010a) but was not detected in any 2011 fish samples although saxitoxin was observed slightly above MDL in the lake from June to September that year.
Anatoxin-a, domoic acid, and okadaic acid were not detected in fish tissues in this study. LC-MS/MS detection limits for fish tissue were 5.0, 2.0, and 1.0 μg/kg, respectively.
Our health risk assessment for MCs shows that all concentrations in fish muscle tissue as determined by methods used in this study (anti-MC-LR ELISA, anti-Adda ELISA, and LC-MS/MS) fall below critical human health levels based on consuming eight meals per month for a child (20 μg/kg, assuming 4-oz meals) and for an adult (40 μg/kg, assuming 8-oz meals). The highest estimated concentration of MCs in fish from Washington lakes was 11 ppb in a rainbow trout muscle sample from Anderson Lake. Four of six yellow perch muscle samples from California analyzed using anti-Adda ELISA were higher than 20 μg/kg (Table 10). Interestingly, several studies from North America and Europe found muscle tissue concentrations of MCs as estimated by anti-Adda ELISA to be below our estimated critical human health levels for a child (Table 11). Based on our study and others, we believe that humans can safely consume two 8-oz fish fillet meals per week of fish from lakes with blooms producing MCs in regional lakes. The exception appears to be yellow perch in California waterbodies with higher levels of MCs that may be of concern to children consuming fish fillets.
Concentrations of MCs from the gut (plus contents) and liver were consistently higher than critical values in our study (Tables 7 and 10). Higher concentrations of MCs in liver were also found in studies from Mexico, Spain, Greece, and Canada (Table 11) as well as Lake Erie (Wilson et al. 2008). Further, higher liver concentrations were found across all species tested (rainbow trout, largemouth bass, yellow perch, carp, mullet, and additional species tested in Canada). Thus, our consumption advice for fish from lakes with cyanobacterial blooms also recommends that consumers clean the fish and discard viscera.
In summary, we confirmed MC in several freshwater fish species from temperate lakes using two types of ELISA and LC-MS/MS, with higher concentrations in liver than in the gut or muscle tissue. We recommend that ELISA should be used only to screen fish tissue since we found the assays to produce poor recoveries, false positives, and inconsistent results when analyzing the same sample. Future studies could address different extraction methods to investigate if results are improved when analyzing MC in fish tissues. For example, Preece et al. (2015) demonstrated direct monoclonal ELISAs combined with a short solvent extraction result in fewer false positives than the polyclonal ELISAs that we used in our study. We also recommend that LC-MS/MS be used in conjunction with ELISA to confirm results of initial screening. Finally, our results indicate that MC levels in fish muscle tissue collected from lowland lakes in Washington State are not of human health concern at this time.
References
Adamovsky, O., Kipp, R., Hilscherova, K., Babica, P., Palikova, M., Paskova, V., Navratil, S., Marsalek, B., & Blaha, L. (2007). Microcystin kinetics (bioaccumulation and elimination) and biochemical responses in common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix) exposed to toxic cyanobacterial blooms. Environmental Toxicology and Chemistry, 26(12), 2687–2693.
Aràoz, R., Molgo, J., & de Marsac, N.T. (2010). Neurotoxic cyanobacterial toxins. Toxicon, 56(5), 813–828.
Berry, J. P., Lee, E., Walton, K., Wilson, A., & Bernal-Brooks, F. (2011). Bioaccumulation of microcystins by fish associated with a persistent cyanobacterial bloom in Lago de Patzcuaro (Michoacan, Mexico). Environmental Toxicology and Chemistry, 30(7), 621–1628.
Botana, L. (2007). Phycotoxins. Chemistry and biochemistry. Blackwell Publishing. Ames, Iowa 50014, USA. ISBN-13: 978-0-8138-2700-1. 345 pp.
Bruno, M., Fiori, M., Mattei, D., Melchiorre, S., Messineo, V., Volpi, F., Bogialli, S., & Nazzari, M. (2006). ELISA and LC-MS/MS methods for determining cyanobacterial toxins in blue-green algae food supplements. Natural Product Research, 20(9), 827–835.
Carmichael, W., & An, J. (1999). Using an enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of microcystins and nodularins. Natural Toxins, 7(6), 377–385.
Castro, D., Vera, D., Lagos, N., Garcia, C., & Vasquez, M. (2004). The effect of temperature on growth and production of paralytic shellfish poisoning toxins by the cyanobacterium Cylindrospermopsis raciborskii C10. Toxicon, 44(5), 483–489.
CH2M Hill. (2009). Analysis of microcystins in resident fish and mussel tissues in the vicinity of the Klamath hydroelectric Project in 2008. Prepared by CH2M Hill Inc. for PacifiCorp Energy. Portland, Oregon, USA. December 2009.
Chen, J., Xie, P., Li, L., & Xu, J. (2009). First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicological Sciences, 108(1), 81–89.
Codd, G. A., & Bell, S. G. (1996). The occurrence and fate of blue-green algal toxins in freshwaters. R and D Report 29. National Rivers Authority (p. 30). London: Her Majesty’s Stationery Office.
Cousins, I.T., Bealing, D.J., James, H.A., & Sutton, A. (1996). Biodegradation of microcystin-LR by indigenous mixed bacterial populations. Water Research, 30(2), 481–485.
DeBlois, C., Cote, G., Grenon, M-C., Sarrasin, B., & Berryman, D. (2009). Analytical method for monitoring of total cyanotoxins in surface water and fish tissues by LC-MS/MS. www.ceaeq.gouv.qc.ca/documents/publications/affiches/pittcon09_cyano.pdf.
DOH. (2008). Washington State recreational guidance for microcystins (provisional) and anatoxin-a (interim/provisional). Washington State Department of Health. Olympia, WA. http://www.doh.wa.gov/Portals/1/Documents/4400/334-177-recguide.pdf. Accessed 20 Jan 2015.
DOH. (2011). Washington State provisional recreational guidance for cylindrospermopsin and saxitoxin. DOH 332–118, October 2011. Washington State Department of Health. Olympia, WA. http://www.doh.wa.gov/Portals/1/Documents/4400/332-118-CylindroSax%20Report.pdf. Accessed 20 Jan 2015.
Dyble, J., Gossiaux, D., Landrum, P., Kashian, D., & Pothoven, S. (2011). A kinetic study of accumulation and elimination of microcystin-LR in yellow perch (Perca flavescens) tissue and implications for human fish consumption. Marine Drugs, 9(12), 2553–2571.
EPA. (1990). Specifications and guidance for obtaining contaminant-free sample containers. U.S. Environmental Protection Agency. OSWER Directive #93240.0-05. Washington DC 20460.
EPA. (2006). Toxicological reviews of cyanobacterial toxins: microcystins LR, RR, YR and LA (external review draft). Washington, DC: U.S. Environmental Protection Agency. EPA/600/R-06/139.
Falconer, I. (2005). Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. CRC Press. Boca Raton, Florida, USA. ISBN 0-415-31879-3. 279 pp.
Fischer, W., Garthwaite, I., Miles, C., Ross, K., Aggen, J., Chamberlin, A., Towers, N., & Dietrich, D. (2001). Congener-independent immunoassay for microcystins and nodularins. Environmental Science and Technology, 35(24), 4849–4856. doi:10.1021/es0111821.
Fitzgeorge, R., Clark, S., & Keevil, C. (1994). Routes of intoxication. In G. A. Codd, T. M. Jefferies, C. W. Keevil, & P. Potter (Eds.), Detection methods for cyanobacterial toxins (pp. 69–74). Cambridge: Royal Society of Chemistry.
Gies-Asteggiante, L., Lehotay, S. J., Fortis, L. L., Paoli, G., Wijey, C., & Heinzen, H. (2011). Development and validation of a rapid method for microcystins in fish and comparing LC-MS/MS results with ELISA. Analytical and Bioanalytical Chemistry, 401, 2617–2630.
Gkelis, S., Lanaras, T., & Sivonen, K. (2006). The presence of MCs and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters. Aquatic Toxicology, 78, 32–41.
Hamel, K. 2009. Freshwater algae control program: report to the Washington State Legislature (2008–2009). Washington State Department of Ecology, Olympia, WA. Publication No. 09-10-082. www.ecy.wa.gov/biblio/0910082.html.
Harada, K.I., Tsuji, K., & Watanabe, M.F. (1996). Stability of microcystins from cyanobacteria III. Effect of pH and temperature. Phycologia, 35(6 Supplement), 83–88.
Hardy, F. J. (2013). Final progress report: harmful algae bloom-related illness surveillance system (HABISS). Olympia (WA): Washington Department of Health.
Huisman, J., Matthijs, H., & Visser, P. (2005). Harmful cyanobacteria. Netherlands: AQEC 3. Springer. 241 pp. ISBN 1-4020-3009-6.
Ibelings, B. W., Bruning, K., de Jonge, J., Wolfstein, K., Pires, L. M. D., Postma, J., & Burger, T. (2005). Distribution of microcystins in a lake foodweb: no evidence for biomagnification. Microbial Ecology, 49(4), 487–500.
Jacoby, J, Burghdoff, M., Williams, G., Read, L., & Hardy, F.J. (2015). An evaluation of factors associated with microcystins in nine lakes in the Puget Sound Region (Washington, USA). Inland Waters. In Press.
Johnson, A. (2010a). Blue-green algae toxins in Washington lakes: screening fish tissues for microcystins and anatoxin-a. Washington State Department of Ecology, Olympia, WA. Publication No. 10-03-011. https://fortress.wa.gov/ecy/publications/summarypages/1003011.html.
Johnson, A. (2010b). Quality assurance project plan: blue-green algae toxins in fish and sediment from Washington Lakes: microcystins and Saxitoxin. Washington State Department of Ecology, Olympia, WA. Publication No. 10-03-112. https://fortress.wa.gov/ecy/publications/SummaryPages/1003112.html.
Johnson, A., Friese, M., & Coots, R. (2013). Microcystins and other blue-green algae toxins analyzed in fish and sediment from Washington lakes. Environmental Assessment Program. Ecology Publication No. 13-03-001.
Kann, J. (2008). Microcystin bioaccumulation in Klamath River fish and freshwater mussel tissue: preliminary 2007 results. Technical memorandum prepared for Karuk Tribe of California. Ashland OR: Aquatic Ecosystem Sciences LLC.
Kohoutek, J., Adamovský, O., Oravec, M., Šimek, Z., Palíková, M., Kopp, R., & Bláha, L. (2010). LC-MS analyses of microcystins in fish tissues overestimate toxin levels—critical comparison with LC-MS/MS. Analytical and Bioanalytical Chemistry, 398, 1231–1237.
Kotak, B. G., Zurawell, R. W., Prepas, E. E., & Holmes, C. F. (1996). Microcystin-LR concentration in aquatic food web compartments from lakes of varying trophic status. Canadian Journal of Fisheries and Aquatic Sciences, 53, 1974–1985.
Kris-Etherton, P. M., Harris, W. S., & Appel, L. J. (2002). AHA scientific statement: fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation, 106, 2747–2757.
Kutner, M. H., Nachtsheim, C. J., & Neter, J. (2004). Applied linear regression models. New York: McGraw-Hill/Irwin.
Lagos, N., Onodera, H., Zagatto, P. A., Andrinolo, D., Azevedo, S. M. F. Q., & Oshima, Y. (1999). The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon, 37(10), 1359–1373.
Lahti, K., Rapala, J., Fardig, M., Niemela, M., & Sivonen, K. (1997). Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Research Volume, 31(5), 1005–1012.
Loftin, K., Graham, J., Rosen, B., & St. Amand, A. (2010). Analytical methods for cyanotoxin detection and impacts on data interpretation. Workshop: guidelines for design, sampling, analysis, and interpretation for cyanobacterial studies, 2010 National Water Quality Monitoring Conference, Denver, CO. April 26, 2010. U.S. Geological Survey and Phycotech. http://ks.water.usgs.gov/static_pages/studies/water_quality/cyanobacteria/loftin-analytical-Methods.pdf. Accessed 3 March 2015.
Lombard, S., & Kirchmer, C. (2004). Guidelines for preparing quality assurance project plans for environmental studies. Washington State Department of Ecology, Olympia, WA. Publication No. 04-03-030. https://fortress.wa.gov/ecy/publications/summarypages/0403030.html.
Magalhães, V. F., Soares, R. M., & Azevedo, S. (2001). Microcystin contamination in fish from the Jacarepagua Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon, 39(7), 1077–1085.
McCullagh, P., & Nelder, J. A. (1989). Generalized linear models. London: Chapman and Hall.
McEliney, J., & Lawton, L. (2004). Detection of the cyanobacterial hepatotoxins microcystins. Toxicology and Applied Pharmacology, 203, 219–230.
Mekebri, A., Blondina, G. J., & Crane, D. B. (2009). Method validation of microcystins in water and tissue by enhanced liquid chromatography tandem mass spectrometry. Journal of Chromatography, 1216, 3147–3155.
Meriluoto, J. A. O., & Spoof, L. E. M. (2007). Cyanotoxins: sampling, sample processing and toxin uptake. In H. K. Hudnel (Ed.), Cyanobacterial harmful algal blooms state of the science and research needs (pp. 483–499). New York: Springer Press.
NOAA. (2014). Harmful algal blooms [website]. Washington, DC: National Oceanic and Atmospheric Administration. (updated 1 April 2014).http://oceanservice.noaa.gov/hazards/hab. Accessed 10 Feb 2015.
Ohio EPA. (2012). Ohio 2012 integrated report, section 1: considerations for future lists. www.epa.ohio.gov/portals/35/tmdl/2012IntReport/IR12SectionIfinal.pdf.
Papadimitriou, T., Kagalou, I., Bacopoulos, V., & Leonardos, I. (2010). Accumulation of microcystins in water and fish tissues: an estimation of risks associated with microcystins in most of the Greek Lakes. Environment Toxicology, (25), 418–427.
Papadimitriou, T., Kagalou, I., & Leonardos, I. D. (2012). Seasonally accumulation of microcystins in the various tissues of an endemic and protected fish species (Rutilus panosi) with different sizes. Clean-Soil Air Water, 40(4), 402–407.
Pomati, F., Sacchi, S., Rossetti, C., Giovannardi, S., Onodera, H., Oshima, Y., & Neilan, B. A. (2000). The freshwater cyanobacterium Planktothrix sp. FP1: molecular identification and detection of paralytic shellfish poisoning toxins. Journal of Phycology, 36(3), 553–562.
Poste, A., Hecky, R., & Guildford, S. (2011). Evaluating microcystin exposure risk through fish consumption. Environmental Science and Technology, 45(13), 5806–5811.
Preece, E., Moore, B., Swanson, M, & Hardy, F.J. (2015). Identifying best methods for routine ELISA detection of microcystin in seafood. Environment Monitoring Assessment, (187:12). doi:10.107/s10661-014-4255-y.
Prendergast, L., & Foster, K. (2010). Technical memorandum: analysis of microcystin in fish in Copco and Iron Gate Reservoirs in 2009. Portland OR: Pacific Energy Corp.
Romo, S., Fernandez, F., Ouahid, Y., & Baron-Sola, A. (2012). Assessment of microcystins in lake water and fish (Mugilidae, Liza sp.) in the largest Spanish coastal lake. Environment Monitoring Assessment., (184), 939–949. doi:10.1007/s10661-011-2011-0
Sandvik, P. 2006a. Standard operating procedure for field collection, processing and preservation of finfish samples at the time of collection in the field version 1.0. Washington State Department of Ecology, Olympia, WA. SOP Number EAP009. www.ecy.wa.gov/programs/eap/quality.html.
Sandvik, P. (2006b). Standard operating procedures for resecting finfish whole body, body parts or tissue samples. Version 1.0. Washington State Department of Ecology, Olympia, WA. SOP Number EAP007. www.ecy.wa.gov/programs/eap/quality.html.
Sipiä, V. O., Kankaanpää, H. T., Flinkman, J., Lahti, K., & Meriluoto, J. A. O. (2001). Time dependent accumulation of cyanobacterial hepatotoxins in flounders (Platichthys flesus) and mussels (Mytilus edulis) from the northern Baltic Sea. Environmental Toxicology, 16(4), 330–336.
Smith, J. L., Schulz, K. L., Zimba, P. V., & Boyer, G. L. (2010). Possible mechanism for the foodweb transfer of covalently bound microcystins. Ecotoxicology and Environmental Safety, 73(5), 757–761.
Stone, D., & Bress, W. (2007). Addressing public health risks for cyanobacteria in recreational freshwaters: Oregon and Vermont Framework. Integrated Environmental Assessment and Management, 3(1), 137–143.
Tencala, F., & Dietrich, D. (1997). Biochemical characterization of microcystin toxicity in rainbow trout (Oncorhynchus mykiss). Toxicon, 35, 583–595.
Trainer, V. L. & Hardy, F. J. (2015). Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins, 7(4), 1206--1234.
Ueno, Y., Nagata, S., Tsutsumi, T., Hasegawa, A., Watanabe, M., Park, H., Chen, G., Chen, G., & Yu, S. (1996). Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis, 17(6), 1317–1321.
Van Apeldoorn, M.E., van Egmond, H.P., Speijers, G.J.A, & Bakker, G.J.A. (2007). Toxins of cyanobacteria. Molecular Nutrition Food Research, 51(1), 7–60.
Welker, M., & Von Döhren, H. (2006). Cyanobacterial peptides—nature’s own combinatorial biosynthesis. FEM Microbiol Review, 30(4), 530–563.
WHO. (2003). Guidelines for safe recreational water environments. Volume 1: coastal and fresh waters (p. 219). Geneva: World Health Organization.
Wilson, A., Gossiaux, D. C., Hook, T. O., Berry, J. P., Landrum, P. F., Dyble, J., & Guildford, S. J. (2008). Evaluation of the human health threat associated with the hepatotoxin, microcystin, in the muscle and liver tissues of yellow perch (Perca flavescens). Canadian Journal of Fisheries and Aquatic Sciences, 65, 1487–1497.
Wood, S. A., Briggs, L. R., Sprosen, J., Ruck, J. G., Wear, R. G., Holland, P. T., & Bloxham, M. (2006). Changes in concentrations of microcystins in rainbow trout, freshwater mussels, and cyanobacteria in Lakes Rotoiti and Rotoehu. Environmental Toxicology, 21(3), 205–222.
Xie, L. Q., Xie, P., Guo, L. G., Li, L., Miyabara, Y., & Park, H. D. (2005). Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu China. Environmental Toxicology, 20(3), 293–300.
Acknowledgments
Washington Department of Ecology (Ecology) funded this work under the Environmental Assessment Program and Water Quality Program (Lizbeth Seebacher). We would like to thank Randy Coots and Michael Friese, Ecology, John Berry, Florida International University, and Gail Cho and Patricia Bucknell, California Department of Fish and Game, for their work on the original report. Jim Buckley, Francis Sweeney, and Gabriela Hannach of King County Environmental Laboratory (KCEL) conducted ELISA analyses and provided advice. Robert Arnold, Adam Couto, Richard Eltrich, Thom Johnson, Larry Phillips, Scott Meechan, and Ecology staff provided fish samples for the project and we thank them.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views or policies of the Washington State Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardy, F.J., Johnson, A., Hamel, K. et al. Cyanotoxin bioaccumulation in freshwater fish, Washington State, USA. Environ Monit Assess 187, 667 (2015). https://doi.org/10.1007/s10661-015-4875-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4875-x