Abstract
This study was aimed to determine the uptake and accumulation potential of a weed (Abutilon indicum L.) for phytoremediation of soil contaminated with cadmium. Plants were grown in soil spiked with 0, 2.5, 5, 10, 15, 20, 25 mg/kg Cd, individually. Plants sample (root and shoot) were analyzed for Cd content at 30, 60, and 90 days and accumulation trends were characterized. A steady increase in Cd accumulation with increasing metal concentration and exposure period was observed for all treatments. Accumulation of Cd in roots was found to be 4.3–7.7 times higher than that of shoots. Statistically significant difference (P ≤ 0.001) in mean metal content in root and shoot at successive days of study was recorded. Effect of Cd on growth and physiology was also evaluated. At higher Cd levels, root and shoot length and biomass of test plant were reduced significantly. Although, growth was delayed initially, it was comparable to control at the end of the study. Chlorophyll and proline content declined with the increase in Cd concentration at 30 and 60 days after treatment. However, at 90 days, values were more or less comparable to the control values showing the adaptability of test plant in Cd contamination. Considering the accumulation ability, BCF >1 (bioconcentration factor) and TF <1 (translocation factor) established A. indicum as a potential candidate plant for phytoremediation. Hence, phytoremediation employing indigenous weed species like A. indicum can be an ecologically viable option for sustainable and cost-effective management of heavy metal-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intense and inadequate use of fertilizers and pesticides in the soil, coupled with the increase in industrial and mining activity are the main reasons for the contamination of soil, waterways, and the water table by heavy metals (Malavolta 1994). Among the pollutants, Cadmium (Cd) is the major contaminant of soil poising significant environmental problems, including the risk of poisoning for humans. The background Cd level in agricultural soils is less than 1 mg/kg (Adriano 2001). High levels of metals in the soil can be phytotoxic. Poor plant growth and soil cover caused by metal toxicity can lead to metal mobilization in runoff water and subsequent deposition into nearby bodies of water. Thus, soil remediation is needed to eliminate risk to humans or the environment from toxic metals.
Technologies used for removal of heavy metals include reverse osmosis, ion exchange, chemical precipitation, solvent extraction, electro-dialysis, adsorption, etc. Most of these technologies are quite costly, energy intensive, and metal specific. It has been estimated that the cost of conventional remediating heavy metal-contaminated sites in the USA alone would exceed $7 billion (Salt et al. 1995). Contrary to this, phytoremediation offers a promising technology using plants to remediate or contain contaminants in soil, groundwater, surface water, or sediments (Miretzky et al. 2004). Phytoremediation involves phytoextraction, rhizofiltration, phytostabilization, and phytotransformation/phytodegradation. Phytostabilization is one of the strategies of phytoremediation that aims to reduce the mobility and bioavailability of pollutants in environment and not removing them. A significant fraction of metals can be stored within or adsorbed on the root surface contributing to long-term stabilization of pollutants.
Phytoremediation has recently become a subject of intense public and scientific interest and a topic of many recent researches (Igwe and Abia 2006). Ability to select plant species either resistant to or can accumulate great amounts of heavy metals would certainly facilitate reclamation of contaminated area (Lasat 2002). It has been demonstrated that wild native plants may be better phytoremediators for waste lands than the known metal hyperaccumulators like Thlaspi caerulescens and Alyssum bertolonii because these are slow growing with shallow root systems and low biomass. Even if the soil is naturally high in a particular metal, native plants often become adapted over time to the locally elevated levels so in the case of native flora and soils, metal toxicity issues, mostly, do not arise. Avena, Crotalaria, Crinum asiaticum and Calotropis procera, lemongrass, and other wild grasses have been reported for heavy metal bioindicatoring and phytoremedial purposes (Uraguchi et al. 2006; D’Souza et al. 2010; Varun et al. 2011). Allowing native species to remediate soils is an attractive proposition since a plant community comparable to that existing in the vicinity can be established. The outcome is, thus, both site remediation and ecological restoration.
Various criteria must be considered before selecting a plant species for phytoremedial purpose to achieve maximal phytoextraction. Species which can grow well with poor nutrient availability at contaminated sites, requiring less input in terms of cost and labor are preferable. Ideally, a plant that is suitable for phytoremediation should have a high above-soil biomass that acts as a sink for the contaminant. At present, there are nearly 450 known hyperaccumulators (Salt and Kramer 2000), but most are not appropriate for phytoextraction because of their slow growth and small size. Although weeds are considered undesirable or troublesome plants, many weeds and their relatives, occurring on mountains and other disturbed habitats, may be useful for phytoremediation (Lum et al. 2014). Many studies have shown that weed species had high accumulating abilities of heavy metals and so were used to remove heavy metals from polluted environment (Lum et al. 2014; Girdhar et al. 2014). Weed species such as black nightshade (Solanum nigrum), hemp (Cannabis sativa), and yellowcress (Rorippa globosa) have high endurance and accumulating ability to Cd (Ji et al. 2011; Shi et al. 2012; Wei and Twardowska 2013). Another species, giant ragweed (Ambrosia trifida) accumulates Cd and Zn in its tissue at levels that are two to three times greater than other plant species, suggesting the use of this plant for remediation of heavy metal-polluted soils (Peles et al. 1998).
This experiment was set up to identify a hardy weed plant that could tolerate concentrations of Cd in soil. Abutilon indium L. is a medium-sized weedy shrub with rapid growth and good biomass production. It was hypothesized that plants with high tolerance could then be tested for their phytoremediation potential. Thus, the aims of the present investigation are (i) to evaluate the phytoremedial potential of a weed, Abutilon indium, with respect to Cd and, (ii) to study the effect of Cd on its growth and physiology.
Material and Methods
Plant selection—Abutilon indicum L.
Abutilon indicum, commonly known as “country mallow”, is a medium-sized perennial weed shrub belonging to family Malvaceae, about 2 m tall; stem green, terete, and pubescent. Leaves simple, ovate-orbicular, base cordate, margins crenate to dentate, apex acute, velvety, sparsely stellate-hairy above, glaucous beneath; petioles 2–6-cm long; stipules up to 4-mm long, linear. Flowers axillary, solitary; pedicel 4–5-cm long, jointed very near the top. Calyx 5, pubescent with minute stellate hairs. Corolla 5, 2–2.5 cm across, orange-yellowish. Staminal column 6 mm long. Ovary globose, densely pubescent with silvery white hairs; styles 15–20 of 1-cm long; stigma capitate. Fruits circular in shape, consisting of 11–20 radiating hairy carpels, brown when dry; each carpel is flattened, somewhat boat shaped. Seeds ovate or sub-orbicular, 3-mm long, dull black.
Experimental design
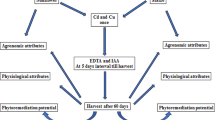
Seeds of Abutilon indicum were collected from uncontaminated soil. Seeds were first sterilized in 0.1 % HgCl2 and then allowed to germinate in a germination tray. After germination, seedlings were transplanted to pots having 5 kg garden soil (loam) with a layer of crocks and gravel of about 1.5 in. depth at the bottom. Soil pH was 6.3, electrical conductivity 0.46 dS/m, and organic matter content 1.84 %. Single plant per pot was maintained. The seedlings were first allowed to grow for 4 weeks after which Cd was added to pots at varying concentrations (0, 2.5, 5, 10, 15, 20, 25 mg/kg soil) as aqueous solution using CdCl2·5H2O salt. Pots were kept in a random block design and watered as and when required in such a way as to prevent loss of contaminants by leaching. A plastic tray was kept below the treatment pot to collect any leachate, which was returned to the pots at next watering. The whole experiment was conducted in green house for 3 months. Any symptoms of metal toxicity exhibited by plants were visually noted during the whole experimental period. At each sampling date, i.e., 30, 60, and 90 days after treatment (DAT) plants were harvested and taken for metal uptake analysis. Plant growth parameters like root length, shoot length, and biomass, and physiological parameters like total chlorophyll and proline content were also determined.
Collection of samples
At each sampling, plants were harvested, washed with tap water to remove adhering soil particles. Samples were further washed thoroughly three to four times with distilled water and finally with de-ionized water and allowed to drip dry completely in a dust-free chamber at room temperature and further used for analysis.
Cadmium analysis
For Cd content in soil, 0.5 g soil sample was digested using a wet digestion method with HNO3 and HClO4 (3:1 ratio) and boiled on a hot plate for 20 min. Plant samples were analyzed by dry ash method where the samples were ashed in a muffle furnace and 0.5 g cooled ash was dissolved in HNO3 and boiled for 20 min on a hot plate. The filtrate in each case was analyzed for Cd content by atomic absorption spectrophotometer (AAnalyst100, Perkin Elmer, USA) using an air-acetylene flame.
Biochemical analysis
Chlorophyll content
Chlorophyll content in A. indicum leaf samples was determined on fresh weight basis. 40 mg fresh leaves were placed in 10 ml 80 % acetone in a sealed dark bottle in a refrigerator. After 5 days, absorbance of the solution was measured by a spectrophotometer at different wavelengths, i.e., 480, 510, 630, 645, 652, and 665 nm, and chlorophyll content was calculated using relevant formula (Arnon 1949).
Determination of proline content
Amount of proline in plants was determined according to method given by Bates et al. (1973). 500 mg of fresh leaves were taken and crushed with 10 ml of 3 % sulphosalicylic acid. This was then centrifuged at 3000 rpm for 15 min. 2 ml of supernatant was taken in a test tube and 2 ml each of ninhydrin and glacial acetic acid were added. The solution was boiled in water bath for 30 min and then transferred into an ice bath. After 30 min, 4 ml toluene was added and the test tube was shaken vigorously. The upper red chromophore (toluene layer) was taken and absorbance was calculated at 520 nm. Toluene was used as blank reference.
Assessment of phytoremedial potential of plant
Phytoremedial potential of plants is also influenced by the mobility and availability of contaminants in soil and plants. To assess the phytoaccumulation potential of plants some factors have been employed by workers based on simple ratios of contaminant concentration in plant parts and growth matrix. These were calculated as follows:
Statistical analysis
Pearson’s coefficient for correlation was statistically analyzed at a significance level of P < 0.05 and P < 0.01. The statistical significance of differences among means was determined by one-way analysis of variance (ANOVA).
Results and discussion
Uptake in A. indicum
Cd uptake in roots and shoots of A. indicum is shown in Table 1. Cd content significantly increased with increasing metal concentration and the exposure period at all the testing days. A steady increase in Cd uptake and accumulation was observed for all treatments. Roots showed a progressive accumulation of Cd as a function of the external medium. Cd was preferentially accumulated in roots. Significantly, a maximum Cd uptake of 43.7 mg/kg (in root) and 10.05 mg/kg (in shoot) was observed at highest Cd treatment (25 mg/kg) at 90 DAT. At all the sampling, maximum uptake of Cd was observed in highest dose. Accumulation of Cd in roots was found to be 4.3–7.7 times higher than that of shoots. Metal accumulation in root tissue can be accomplished either through deposition of the metal ions along the cell wall and/or inside the cell in the vacuoles. Cd was not observed in the control plants at any sampling stage. One-way analysis of variance (ANOVA) indicates a statistical significant difference (P ≤ 0.001) in the mean metal content in shoots and roots of A. indicum in response to Cd content. A. indicum was found to tolerate Cd concentrations up to 25 mg/kg without showing any toxicity symptoms. This confirms the ability of this plant to establish and grow well in Cd-contaminated soil.
Many phytoremediation studies have shown the potential of weed species to remove heavy metals from contaminated environment. Weeds such as Poa annua (for Cu, As), Tephrosia purpurea (for Mn), Cannabis sativa (for Cr), Solanum nigrum (for Mn), Dissotis rotundifolia and Kyllinga erecta (for Pb), Calotropis procera (for Zn, Mn, Cd, Cu), Withania somnifera (for Cu, Mn, As), Eclipta alba (for Cu, Mn, As), Heliotropium ellipticum (for Cu, Mn, As), and Cannabis sativa, Solanum nigrum, and Rorippa globosa (for Cd) also showed good phytoremedial potential suggesting the use of these weedy plants for remediation of heavy metal-polluted soils (Varun et al. 2012; Girdhar et al. 2014; Lum et al. 2014).
Accumulation of particular type of metal is a selective process that may vary from plant to plant. The uptake of toxic metals, their translocations to plant parts, and the degree of tolerance to them are dependent on metal speciation and on the metabolism of the plants (Prasad 1999). Baker and Brooks (1989), Baker and Walker (1990) have all demonstrated selective heavy metal uptake in different species of Thlaspi. Most non-essential metals are likely excluded from plant uptake or are quickly immobilized in the plant (Fodor et al. 1998). This could be a possible reason for much higher uptake of Cd in the test plant. Moreover, many studies have demonstrated that Cd taken up by plants accumulates at higher concentration in the root than in the stem and leaves (Seregin and Ivanov 2001), including in hyperaccumulators (Küpper et al. 2000). Metal accumulation in root tissue can be accomplished either through deposition of the metal ions along the cell wall and/or inside the cell in the vacuoles (Salt and Kramer 2000). The sequestration of specific metal ions or metal-chelate complexes in the root cells is highly dependent on the metal ion in question. For example, Cd is generally found to be associated with cell walls outside the plasmalemma in the form of Cd precipitates and Cd crystals (Malone et al. 1974). Other mechanisms that plants have developed to cope with damage caused by Cd are related to some stress-signaling molecules, such as salicylic acid, jasmonic acid, nitric oxide, and ethylene. All these compounds were induced by Cd treatment, which suggests that they are involved in cell response to Cd toxicity (Rascio and Navari-Izzo 2011; Popova et al. 2012).
All the samples tested in the present investigation show greater accumulation of metal in the roots than the aboveground shoot, this could be attributed to the increased metal adsorption on the root surface, being facilitated by relatively less mobility of metals in the root zone (Hasan et al. 2007). As plant roots are in direct contact with metals in contaminated soil and must act as the conduit for transfer of metal to the stem and leaves, their response to the high metal concentration is important. The differences in root and shoot uptake can possibly be explained by the fact that one of the normal functions of root is to selectively acquire ions from the soil solution, whereas shoot tissue does not normally play this role. The results obtained in the present investigation are in conformity with the findings of Kadukova et al. (2004) and by other research groups (Lutts et al. 2004) which showed significant amount of metal accumulation in roots and shoots of plants as in this study, and together with the fact that there was no reduction in growth. The findings suggest that A. indicum could be a possible candidate for Cd as phytostabilizer.
Growth
Addition of Cd to soil inhibited growth of A. indicum in terms of shoot and root length. The highest value for root and shoot length among the treatments was observed at low concentrations of Cd only (Table 2). Cadmium is not an essential nutrient and at high concentration inhibits plant growth (Rascio and Navari-Izzo 2011). It has also been reported that even at relatively low concentrations, it alters plant metabolism (Van Assche and Clijesters 1990). Total biomass of plant was calculated and compared with their respective controls to assess the effect of Cd contamination on the overall growth of plant. All the treatments show less biomass than the control value at all sampling stages (Table 2). Biomass decreases with the increase in Cd concentration in the growth matrix with maximum value obtained in control (Cd 0 mg/kg) and the minimum value obtained in Cd 25-mg/kg treatment. Cd was found to inhibit growth in many plant species such as Lemna (Mohan and Hosetti 2006), Eichhornia (Zhu et al. 1999), and Spirodela (Banerjee and Sarker 1997). Cd was shown to interfere with the increased tissue permeability, hence the increase in toxicity, the increased cross linking of pectins in the middle lamella of cell wall which might inhibit cell expansion (Poschenrieder et al. 1989), and the direct and indirect effect on the growth hormone, auxin metabolisms, or auxin carriers.
Chlorophyll content
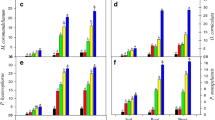
Photosynthesis is perhaps the most basic aspect in plant metabolism assessment related to growth and survival in adverse conditions. Metals like Cd, Pb, Zn, Cr, etc., when present in high concentration in soil, show potential toxic effects on overall growth and metabolism of plants (Agrawal and Sharma 2006). The data regarding chlorophyll content in leaves of A. indicum is presented in Fig. 1. It is clearly evident from the figure that chlorophyll content of leaves was influenced by the Cd treatments applied. Application of Cd decreases chl “a”, chl “b”, as well as total chlorophyll content in leaves with the increasing metal concentrations up to 60 DAT in all the treatments. After 60 DAT, chlorophyll content in all treatments stabilized and the values were nearly equal to control showing the adaptability of the plant to the Cd contamination. Chl “a” varied from 0.66–1.27 mg g−1 f.wt.; chl “b” varied from 0.76–1.41 mg g−1 f.wt.; and total chlorophyll varied from 1.42–2.68 mg g−1 f.wt. at 30 DAT to 1.18–1.33, 1.20–1.41, and 2.44–2.69 mg g−1 f.wt., respectively, at 90 DAT. Decrease in the total chlorophyll content has been well documented under heavy metal stress and may reflect the level of photosynthetic activity in plants (Ouzounidou 1995; Panda and Choudhary 2005; Jiang et al. 2007). Heavy metals are reported to inhibit chlorophyll biosynthesis, particularly by inhibiting 2-aminolevulinic acid hydrogenase and protochlorophyllide reductase (Ouzounidou 1995; Miyadate et al. 2011). Cd may interfere with different steps of Calvin cycle, resulting in the inhibition of photosynthetic substances and in poisoning of the cell cytoplasm (Pahlasson 1989). Similar results were reported by Phetsombat et al. 2006; Pandey et al. 2007.
Proline content
The plants exposed to heavy metals seem to induce accumulation of free proline (Talanova et al. 2000; Hayat et al. 2012). Proline accumulation, accepted as an indicator of environmental stress, is also considered to have important protective roles (Schat et al. 1997). The data regarding proline accumulation in A. indicum is presented in Fig. 2. At all the testing days, all treatments showed higher levels of proline in comparison to the control. The value of proline accumulation increases with the increased in the concentration of Cd in soil. Proline content varied from 0.43–0.93 μg/g f.wt. (at 30 DAT); 0.27–0.49 μg/g (at 60 DAT), and 0.08–0.23 μg/g (at 90 DAT). Proline plays important roles in osmoregulation, protection of enzymes, stabilization of the machinery of protein synthesis, regulation of cytosolic acidity, and scavenging of free radicals. It has been proposed by many workers that proline accumulates in plants to counteract stress-induced effects. In the present investigation also, all treatments showed higher levels of proline accumulation as compared to control. Application of Cd in soil appeared to increase the proline values up to 35 times that of the control. Thus, the data in the present clearly indicates that increase in proline content is related to increase in metal stress. Similar findings were reported by other workers also in Oryza sativa (Roy et al. 1992), sunflower (Kastori et al. 1992), and Brassica juncea (Singh and Tewari 2003).
Assessment of phytoremedial potential of plant
The BAC, BCF, and TF values (Table 3) are some of the key help to identify the suitability of plants for phytoextraction and phytostabilization by explaining the accumulation characteristics and translocation behaviors of metals in plants. Higher TF values indicate more allocation of absorbed metal to aboveground biomass. However, in the present study, TF values indicate that none of the sample allocated Cd in greater proportion to the aboveground biomass in A. indicum. In plants with low TF, there is less translocation of metals to the aboveground portions which may be due to immobilization of metals in roots by vacuole sequestration or cell wall binding, thereby preventing interaction with high molecular weight compounds in the plant cell cytoplasm (Salt et al. 1995). According to Mendez and Maier (2008), a plant suitable for phytostabilization should have BCF >1 and TF <1. All plant samples exhibited BCF value greater than 1 at all the concentrations. The plant partitioned a major part of metal in the root itself. High BCF and low TF values were observed in all the plant samples establishing A. indicum as a good phytostabilizer for Cd.
Conclusion
A. indicum was found to tolerate Cd concentrations up to 25 mg/kg which confirms the ability of this plant to establish and grow well in Cd-contaminated soil and accumulate substantial amount of Cd in root even when they are present in low concentrations in the soil. Thus, this plant species provides scope for decontamination of Cd-polluted soils. Significant metal accumulation along with decrease in biomass and chlorophyll and proline content suggest that the plant is able to tolerate Cd metal ions. However, it should still be tested in the field under metal-contaminated conditions. Further work is needed to obtain information about the putative heavy metal tolerance mechanisms of this species. Considering the accumulation ability, high BCF and low TF values establish A. indicum as a potential candidate plant for phytoremediation as a Cd phytostabilizer. Thus, phytoremediation employing indigenous weed species like A. indicum can be an ecologically viable option for sustainable and cost-effective management.
References
Adriano, D. (2001). Cadmium. In: (Eds.), Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, 2nd edition (pp. 264–314). New York-Berlin-Heidelberg: Springer-Verlag.
Agrawal, V., & Sharma, K. (2006). Phytotoxic effects of Cu, Zn, Cd and Pb on in vitro regeneration and concomitant protein changes in Holarrhena antidysentrica. Biologia Plantarum, 50, 307–310.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
Baker, A. J. M., & Walker, P. I. (1990). Ecophysiology of metal uptake by tolerant plants. In A. J. Shaw (Ed.), Heavy metal tolerance in plants evolutionary aspects (pp. 155–178). CRC Press: Boca Raton, FL.
Banerjee, G., & Sarker, S. (1997). The role of Salvinia rotundifolia in scavenging aquatic Pb (II) pollution: a case study. Bioprocess Engineering, 17, 295–260.
Bates, L. S., Waldren, R. D., & Teare, T. D. (1973). Rapid determination of free proline for water stress studies. Plant and soil, 39, 205–207.
D’Souza, R., Varun, M., Masih, J., & Paul, M. S. (2010). Identification of Calotropis procera L. as a potential phytoaccumulator of heavy metals from contaminated soils in urban North Central India. Journal of Hazardous Material, 184, 457–464.
Fodor, F., Cseh, E., Varga, A., & Zaray, G. (1998). Lead uptake, distribution and remobilization in cucumber. Journal of Plant Nutrition, 21, 1363–1373.
Girdhar, M., Sharma, N. R., Rehman, H., Kumar, A., & Mohan, A. (2014). Comparative assessment for hyperaccumulatory and phytoremediation capability of three wild weeds. Biotechnology, 4(6), 579–589. 3.
Hasan, S. H., Talat, M., & Rai, S. (2007). Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichhornia crassipes). Bioresource Technology, 98, 918–928.
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments. Plant Signaling and Behavior, 7(11), 1456–1466.
Igwe, J. C., & Abia, A. A. (2006). A bio-separation process for removing heavy metals from waste water using biosorbents. African Journal of Biotechnology, 5, 1167–1179.
Ji, P., Song, Y., Sun, T., Liu, Y., Cao, X., Xu, D., Yang, X., & McRae, T. (2011). In-situ cadmium phytoremediation using Solanum nigrum L.: the bio-accumulation characteristics trail. International Journal of Phytoremediation, 13, 1014–1023.
Jiang, H. M., Yang, J. C., & Zhang, J. F. (2007). Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environmental pollution, 147, 750–756.
Kadukova, J., Papadontonakis, N., Naxakis, G., & Kalogerakis, N. (2004). Lead accumulation by the salt-tolerant plant Atriplex halimus. In C. Moutzouris, C. Christodoulatos, D. Dermatas, A. Koutsospyros, C. Skanavis, & A. Stamou (Eds.), Proceedings of the International Conference on Protection and Restoration of the Environment VII June 28–July 1. Greece: Mykonos.
Kastori, R., Petrovic, M., & Petrovic, N. (1992). Effect of excess lead, cadmium, copper and zinc on water relations in sunflower. Journal of Plant Nutrition, 15, 2427–2439.
Küpper, H., Lombi, E., Zhao, F. J., & McGrath, S. P. (2000). Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta, 212, 75–84.
Lasat, M. M. (2002). Phytoextraction of toxic metals: a review of biological mechanisms. Journal of Environmental Quality, 31, 109–120.
Lum, A. F., Ngwa, E. S., Chikoye, D., & Suh, C. E. (2014). Phytoremediation potential of weeds in heavy metal contaminated soils of the Bassa Industrial Zone of Douala, Cameroon. International Journal of Phytoremediation, 16(3), 302–319.
Lutts, S., Lefère, I., Delpéré, C., Kivits, S., Dechamps, C., Robledo, A., & Correal, E. (2004). Heavy metal accumulation by the halophyte species Mediterranean saltbush. Journal of Environmental Quality, 33, 1271–1279.
Malavolta, E. (1994). Fertilizantes e seu impacto ambiental: micronutrientes e metais pesados—mitos, mistificação e fatos. Petroquímica, São Paulo.p 153.
Malone, C., Koeppe, D. E., & Miller, R. J. (1974). Localization of lead accumulated by corn plants. Plant Physiology, 53, 388–394.
Mendez, M. O., & Maier, R. M. (2008). Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environment Health Perspective, 116(3), 278–283.
Miretzky, P., Saralegui, A., & Fernandez, C. A. (2004). Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere, 57(8), 997–1005.
Miyadate, H., Adachi, S., Hiraizumi, A., Tezuka, K., Nakazawa, N., Kawamoto, T., Katou, K., Kodama, I., Sakurai, K., Takahashi, H., Satoh-Nagasawa, N., Watanabe, A., Fujimura, T., & Akagi, H. (2011). OsHMA3, a P18-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytologist, 189, 190–199.
Mohan, B. S., & Hosetti, B. B. (2006). Phytotoxicity of cadmium on the physiological dynamics of Salvinia natans L. grown in macrophyte ponds. Journal of Environmental Biology, 27, 701–704.
Ouzounidou, G. (1995). Cu-ions mediated changes in growth, chlorophyll and other ion contents in a Cu tolerant Koeleria splendens. Biologia Plantarum, 37, 71–79.
Pahlasson, A. M. B. (1989). Toxicity of heavy metals (Zn, Cd, Cu, Pb) to vascular plants. Water Air and Soil Pollution, 47, 278–319.
Panda, S. K., & Choudhary, S. (2005). Chromium stress in plants. Brazilian Journal of Plant Physiology, 17(1), 19–102.
Pandey, S., Gupta, K., & Mukherjee, A. K. (2007). Impact of cadmium and lead on Catharanthus roseus—a phytoremediation study. Journal of Environmental Biology, 28, 655–662.
Peles, J. D., Brewer, S. R., & Barrett, G. W. (1998). Heavy metal accumulation by old-field plant species during recovery of sludge-treated ecosystems. American Midland Naturalist, 140, 245–251.
Phetsombat, S., Kruatrachue, M., Pokethitiyook, P., & Upatham, S. (2006). Toxicity and bioaccumulation of cadmium and lead in Salvinia cucullata. Journal of Environmental Biology, 27(4), 645–652.
Popova, L. P., Maslenkova, L. T., Ivanova, A. P., & Stoinova, Z. (2012). Role of salicylic acid in alleviating heavy metal stress. In P. Ahmad & M. N. V. Prasad (Eds.), Environmental adaptations and stress tolerance of plants in the era of climate change (pp. 441–466). New York: Springer.
Poschenrieder, C., Gunes, B., & Barcelo, J. (1989). Influence of cadmium on water relation, stomatal resistance, and abscisic acid content in expanding bean leaves. Plant Physiology, 90, 1365–1371.
Prasad, M. N. V. (1999). Metallothioneins and metal binding complexes in plants. In M. N. V. Prasad & J. Hagermeyer (Eds.), Heavy metal stress in plants: from molecules to ecosystems (pp. 51–72). Berlin: Springer Verlag.
Rascio, N., & Navari-Izzo, F. (2011). Heavy metal hyperaccumulating plants: how and why do they do it, and what makes them so interesting? Plant Science, 180, 169–181.
Roy, D., Bhumnia, A., Basu, N., & Banerjee, S. K. (1992). Effect of NaCl salinity on metabolism of proline in salt sensitive and salt-resistant cultivars of rice. Biologia Plantarum, 34, 159–162.
Salt, D. E., & Kramer, U. (2000). Mechanisms of metal hyperaccumulation in plants. In I. Raskin & B. D. Ensley (Eds.), Phytoremediation of toxic metals: using plants to clean-up the environment (pp. 231–246). New York: John Wiley & Sons, Inc.
Salt, D. E., Prince, R. C., Pickering, I. J., & Raskin, I. (1995). Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology, 109, 1427–1433.
Schat, H., Sharma, S. S., & Vooijs, R. (1997). Heavy metal-induced accumulation of free proline in a metal-tolerant and a non-tolerant ecotype of Silene vulgaris. Physiology of Plant, 101(3), 477–482.
Seregin, T. V., & Ivanov, V. B. (2001). Physiological aspects of toxin action of cadmium and lead on high plants. Plant Physiology, 48, 606–630.
Shi, G., Liu, C., Cui, M., Ma, Y., & Cai, Q. (2012). Cadmium tolerance and bioaccumulation of 18 hemp accessions. Applied Biochemistry and Biotechnology, 168(1), 163–173.
Singh, P. K., & Tewari, R. K. (2003). Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. Journal of Environmental Biology, 24, 107–112.
Talanova, V. V., Titov, A. F., & Boeva, N. P. (2000). Effect of increasing concentrations of lead and cadmium on cucumber seedlings. Biologia Plantarum, 43, 441–444.
Uraguchi, S., Watanabe, I., Yoshitomi, A., Kiyono, M., & Kuno, K. (2006). Characteristics of cadmium accumulation and tolerance in novel Cd-accumulating crops, Avena strigosa and Crotalaria juncea. Journal of Experimental Botany, 57(12), 2955–2965.
Van Assche, F., & Clijesters, H. (1990). Effects of metals on enzyme activity in plants. Plant Cell Environment, 13, 195–206.
Varun, M., D’Souza, R., Kumar, D., & Paul, M. S. (2011). Bioassay as monitoring system for lead phytoremediation through Crinum asiaticum L. Environmental Monitoring and Assessment, 178, 373–381.
Varun, M., D’Souza, R., Pratas, J., & Paul, M. S. (2012). Metal contamination of soils and plants associated with the glass industry in North Central India: prospects of phytoremediation. Environmental Science and Pollution Research, 19(1), 269–281.
Wei, S., & Twardowska, I. (2013). Main rhizosphere characteristics of the Cd hyperaccumulator Rorippa globosa (Turcz.) Thell. Plant Soil, 372(1–2), 669–681.
Zhu, Y. L., Zayed, A. M., Qian, J. H., Souza, M., & Terry, N. (1999). Phytoaccumulation of trace elements by wetland plants: water hyacinth. Journal of Environmental Quality, 28, 339–344.
Acknowledgments
We gratefully acknowledge the University Grants Commission for providing financial support by sanctioning the Post Doctoral Fellowship No. F./PDFSS201415SCUTT8854.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varun, M., Jaggi, D., D’Souza, R. et al. Abutilon indicum L.: a prospective weed for phytoremediation. Environ Monit Assess 187, 527 (2015). https://doi.org/10.1007/s10661-015-4748-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4748-3