Abstract
The aim of this study was to detect the presence and determine the residue levels of DDT, lindane, endrin and polychlorinated biphenyls (PCBs) in the liver of wild boars from the area of West Pomerania, NW Poland; to determine the activity of glutathione S-transferase (GST) as a biomarker of biological response and to assess the toxicological risk for consumers of the wild boar offal. The presence of pesticide residues and PCBs was found in all examined liver samples. The highest concentration was observed for endrin, and then, the descending order was PCBs >DDTs >lindane >dl-PCBs. The mean hepatic concentrations of endrin, PCBs, DDTs and lindane were 117.28, 78.59, 67.95 and 7.24 ng/g lipid weight, respectively. Among the dioxin-like PCB congeners, 118 and 156 were dominant in liver samples. The mean toxic equivalent (TEQ) level calculated for dl-PCBs was 2.10 ± 1.11 pg WHO-PCB-TEQ/g. There was a statistically significant (p < 0.05) negative correlation between the concentration of lindane, DDTs and PCBs (as a sum of indicator congeners) in the liver and in the activity of GST. However, GST activities showed no significant correlation with any of the dl-PCBs. In five boar liver samples, the levels of certain organochlorine compounds exceeded the maximum residue levels (MRLs). In one sample, the MRLs were exceeded simultaneously for PCBs, endrin and DDTs and in another one—for endrin and DDTs. In the remaining three samples, only PCB levels were exceeded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of the natural environment with organochlorines is the main source of these substances in the food chain of animals and humans. Their toxic properties, persistence, high bioaccumulation and harmful health effects have all created the need to monitor the levels of residues of these compounds in the environment.
Organochlorine compounds, such as polychlorinated biphenyls (PCBs) and pesticides (OCPs), are typical persistent organic pollutants widespread in terrestrial and aquatic ecosystems. Their presence are directly related to their common use in the past—PCBs in the industry and OCPs in agriculture. Their presence in the environment are a direct result of purposeful and direct application, e.g. pesticides, or uncontrolled processes, such as leaks or evaporation. PCBs used to be applied as carriers for pesticides and antibacterial compounds, which also contributed to the dispersal of these compounds in agricultural and forest ecosystems (Falandysz 1999). Currently, PCB’s emission occurs mainly during the improper disposal of materials, substances and equipment containing PCBs and then their accidental release. This accidental and undesirable production of certain PCB congeners may occur during methane fermentation of municipal waste and combustion at temperatures lower than necessary for their thermal decomposition (Breivik et al. 2002; Rolecki 2002).
Contaminated soil is a significant source of PCB and OCP re-emission to the atmosphere due to its high retention capacity (Růžičková et al. 2008). As shown in the research by Jaward et al. (2004) on the levels of various persistent organic pollutants (POPs) in the air in Europe, the trends of PCBs contamination were linked to urbanised source areas, and higher concentrations of organochlorine pesticides, DDT and γ-HCH (lindane) were reported in the region in which they were produced or used in large amounts. Additionally, in some areas of Europe, fresh p,p′-DDT signals were observed, which may be due to the fact that DDT is still being used to control malaria vectors (Bouwman et al. 2011). Due to their high vapour pressure, most organochlorine compounds reach the atmosphere relatively easy from the soil surface and may be transported over great distances with air currents. This leads to the contamination of soil in areas where these compounds have not been used for many years (Wania and Mackay 1996). Numerous studies indicate that PCBs and OCPs are still being detected in soils used for agricultural purposes, forests and urban areas (Holoubek et al. 2007; Kumar et al. 2009).
Wild boar (Sus scrofa) seems to be the best indicator of environmental contamination, due to their unique foraging method. Its importance as a bioindicator is high, especially in relation to xenobiotics from the soil which increase their concentration along the food chain. Boars obtain food mostly by digging large amounts of soil. It is a typical omnivore, and its diet not only consists mainly of plants, including roots and bulbs but also earthworms and insect larvae and pupae. Wild boars also eat imagoes, small mammals, frogs, eggs and chicks of birds nesting on the ground and carrion. They are increasingly common in urban areas, where they feed on garbage and in landfills (Baubet et al. 2004; Genov 1981).
In the area of West Pomerania (NW Poland), biomonitoring of organochlorine compounds (pesticides and PCBs) has mainly concerned the aquatic environment (Tomza et al. 2006; Tomza-Marciniak and Witczak 2010). However, studies on residues of these compounds in the tissues of terrestrial mammals (red deer, roe deer, fox) are few and limited mainly of PCBs (Tomza-Marciniak et al. 2011, 2012). Biomonitoring research using wild game is particularly important due to the information about the extent of environmental contamination and the potential risk for consumers of venison. With this in mind, the aim of this study was to (1) detect the presence and determine residue levels of DDT, lindane, endrin and PCBs in the liver of wild boars from the West Pomerania; (2) determine the activity of glutathione S-transferase (GST) as a biomarker of biological response and then (3) assess the toxicological risk for the consumers of wild boar offal.
Materials and methods
Sampling
The present research was conducted on wild boars of various ages and sexes (Table 1), killed during the hunting seasons in 2010–2011, in an area situated in West Pomerania, in north-western Poland (53°20′50′′ N, 14°52′14′′ E). The areas from which the animals originated were mostly forested areas. Agricultural land is 34 % of the area.
The liver samples were obtained from individual-shot specimens and from wild-game collection centres. The liver from each animal was excised and homogenized. All samples were frozen (−80 ºC) and stored in the laboratory until analysis.
Chemicals
Reagents used in the analysis were as follows: n-hexane, acetone, dichloromethane and sulphuric acid from Merck (KGaA, Germany) and anhydrous sodium sulphate, Tris–Cl buffer and ethyl ether from Chempur (Poland). Florisil, EDTA, 2,4-dinitrobenzene and reduced glutathione (GSH) were obtained from Sigma-Aldrich, and the standards for gas chromatography were obtained from AccuStandard, Inc. (New Haven, USA).
Extraction and clean-up procedure
The samples were analysed for lindane (γ-hexachlorocyclohexane), endrin, DDTs (and its metabolites), PCBs (sum of six indicator congeners, 28, 52, 101, 138, 153, 180) and dioxin-like congeners (dl-PCB), 77, 114, 118, 126, 156, 157 and 169. The samples were prepared for analysis according to a previously described method (Tomza-Marciniak et al. 2011) with several modifications. Subsamples of ∼25 g were ground with anhydrous sodium sulphate in a mortar until obtaining a loose homogenous substance. The extraction of organochlorine compounds was carried out together with lipids using n-hexane/acetone mixture (v/v, 1:2.5) and next, with n-hexane/ethyl ether mixture (v/v, 9:1). The extracts obtained were concentrated in a rotary vacuum evaporator at 50 °C up to about 2 mL and transferred quantitatively with n-hexane to 10 mL, dried and weighed in test tubes. In order to determine the percentage of lipids, dissolvent was evaporated on a water bath under vacuum pressure using a rotary evaporator, and the residue was dried at 80 °C to a solid mass. After determining the lipid mass, the content of the test tubes was dissolved in 2 mL n-hexane and purified by adding 8 mL concentrated sulphuric acid. After layer separation, the upper n-hexane layer was transferred to 8-cm3 LiChrolut® glass column filled with 1 g activated florisil. The column was eluted with n-hexane and dichloromethane. The eluate obtained was concentrated on a water bath under vacuum pressure using a rotary evaporator to 0.5 mL.
Chromatographic analyses
The extract was submitted to gas–liquid chromatographic separation using the method of capillary gas chromatography with mass spectrometry GC/MS (Clarus 600 GC/MS Perkin Elmer) and using ELITE-5MS column (60 m × 0.25 mm × 0.5 μm). Chromatographic analysis was performed under the following conditions: column oven programme, 140 °C (1 min) and increased by 10 °C/min to 200 °C (5 min), by 10 °C/min to 280 °C (5 min) and by 10 °C/min to 300 °C (15 min). The flow rate (splitless) was 1 cm3/min and injection volume, 5 μl.
PCBs and pesticides were identified and quantified against three standard solutions (custom PCB standard and custom pesticide standard; AccuStandard, Inc., New Haven, USA). The accuracy of analyses was verified using the certified reference material (SRM 1946, National Institute of Standards & Technology, USA). Accuracy of methods applied was also checked by the addition of known amount of internal standard solution, Pesticides Surrogate Spike Mix, Supelco (Sigma-Aldrich). The concentration of organochlorine compounds ranged between: for PCBs—81 to 91 % and for OCPs—85 to 96 % of the reference values. Limit of detection for the analysed compounds was 0.02 ng/g. The quality of the analytical procedure was checked by analysis of blank samples. No cross-contamination was found. The toxic equivalents (TEQs) were calculated for dioxin-like PCBs (WHO-dlPCB-TEQ) using the toxic equivalency factor (TEF) values described by Van den Berg (2006). The concentration of the analysed compounds was converted to lipids.
Glutathione S-transferase activity assay
For the determination of GST activity, 1 g of liver was homogenized in a glass homogenizer in ten volumes of buffer (50 mM Tris–Cl buffer, pH 8.1, 1 mM PMSF, 2 mM EDTA). Homogenates were centrifuged for 15 min at 15,000 rpm at 4 °C. Supernatants were collected and stored at −80 °C until analysis.
Twenty microlitres of buffer sample diluted in assay (0.1 M phosphate buffer, 2 mM EDTA, pH 6.5) were transferred to 180 μl assay buffer with 1 mM reduced GSH and 2 mM 1-chloro-2,4-dinitrobenzene (CDNB). After 1-min lag time, increasing absorbance was monitored for 4 min at 15 s intervals at 25 °C. The activity of GST was reported as unit per milligramme of protein. One unit of GST-specific activity was defined as the amount of enzyme that catalyses the formation of 1 nmol of product per minute. Total protein was assayed by Bradford method with bovine serum albumin as a standard (Bradford 1976).
Statistical data analysis
Statistical analysis of the data was performed using STATISTICA software (Statsoft Inc., version 7.1 Statsoft). Prior to analyses, data were investigated to determine their distribution using the Shapiro–Wilks’ W test. Hepatic organochlorine compound concentrations and GST activity between sexes were compared by Mann–Whitney U test. Differences were considered as significant at a level of p < 0.05. The relationships between hepatic organochlorine compound concentrations and GST activity were calculated using Spearman’s correlation analysis.
Results and discussion
The data on organochlorine compounds in the liver of wild boars from the West Pomerania region are given in Table 2. The activities of GST are given for females, males and all specimens taken together in Fig. 1. The results of the analysis which examined the correlation between the concentration of organochlorine compounds in the liver and GST activity are shown in Table 3.
Hepatic concentrations of organochlorine pesticides and polychlorinated biphenyls
In our study, the presence of pesticides was detected in all liver samples. In comparison, in a study conducted in southern Italy (Naccari et al. 2004), livers of wild boars contained only DDT, only in 8 of the 54 samples tested, at concentrations lower than those obtained in our study. In another research, this time in northern Italy (Naso et al. 2004), livers of roe deer contained one of the DDT metabolites (p,p′-DDE) and lindane, and no endrin was found in any of the tested samples. In our study, endrin concentration was the highest among the examined pesticides and averaged 117.28 ng/g lipid weight (lw). Endrin is a cyclodiene pesticide which previously had been used as a foliar insecticide, mainly on field crops and as a rodenticide to control mice and voles (Smith 1991). In addition, endrin is produced as a result of isodrin transformation, another substance used in agriculture. Since this pesticide is rapidly metabolized by animals and poorly accumulated in the body (WHO 1992), it can be concluded that the presence in the examined livers was the result of current dietary intake of endrin alone or isodrin. The determined endrin residues in the livers of the wild boars were twice lower than in other studies in Western Pomerania, but in areas with landfills containing obsolete pesticides (Tomza-Marciniak 2013, in press).
Lindane, the most toxic HCH isomer, appeared in the smallest amounts. Its concentration ranged from 0.94 to 20.42 ng/g lw, and the average was 7.24 ng/g lw. A similar level of this pesticide was found by Naso et al. (2004) in the liver of European roe deer from Italy. However, in wild boars in Japan, Hoshi et al. (1998) determined lindane at levels below the detection limit. Also, Naccari et al. (2004) did not detect this pesticide in wild boar liver or any other organ. Lindane, similar to endrin, is relatively rapidly metabolized and excreted from the body. Given the short half-life of this pesticide in the environment, it is believed that its presence in the body must be the result of the current wild boar exposure to this compound. Lindane has been widely used as an insecticide besides serving for soil amendment and wood. While the agricultural and forestry use of lindane in European countries was banned many years ago, it was used until recently as a measure against external parasites of humans and animals (Struciński 2009). Research by Dvorská et al. (2009) showed ongoing γ-HCH emission in some European countries, the greatest in northern France, southern and eastern Germany, the Czech Republic and Poland.
Among the organochlorine pesticides, DDT has the highest durability in the environment. In the animal body, it undergoes biotransformation to DDE, the major metabolite of DDT transformation. In our research, the share of this metabolite in total DDTs in the liver of the boar was the greatest, at over 64 % (Fig. 2). Also Hoshi et al. (1998), Hoekstra et al. (2003) and Naso et al. (2004) found that p,p′-DDE is the most frequent DDT in the liver and adipose tissue of wild terrestrial mammals, and in some cases, p,p′-DDE was the only DDT. Another metabolite with a relatively high share (23.2 %) was p,p′-DDD. This metabolite is formed in the soil environment under anaerobic conditions from p,p′-DDT and is taken by animals with their diet. Based on the ratio of the sum of p,p′-DDE and p,p′-DDD to p,p′-DDT, one can evaluate whether the presence of DDT is the result of the use of this pesticide in the past or whether it is due to the fresh input. The ratio showed that the area inhabited by the examined wild boars was not covered by a fresh input of DDT, and the presence of DDT in the analysed organs resulted from the past use of this insecticide. Our research shows that the content of DDTs in the liver of wild boars had a wide range, i.e. from 4.03 to 257.84 ng/g lw, with the mean concentration of 67.95 ng/g lw. Comparing the obtained data with the results of other authors, it was found that the concentration of DDTs in the liver of the wild boars in NW Poland was lower than in the liver of wolverine from Canada (Hoekstra et al. 2003) and European roe deer from Italy (Naso et al. 2004) but greater than in the liver of wild boars in southern Italy (Naccari et al. 2004).
Available literature shows that the presence of residual PCBs, including dl-PCBs, in the tissues of hunted animals is still being detected in many countries (Naso et al. 2004; Naccari et al. 2004; Suutari et al. 2012). However, reports on PCBs in the liver of wild boars in Poland and other European countries are scarce. Most papers usually concern the presence of these compounds in the adipose tissue of those animals (Zasadowski et al. 2003).
In our research, PCBs were detected in all the liver samples, and the mean concentration of PCBs (as a sum of six indicator congeners) was 78.59 ng/g lw. These results are considerably higher than in a study on wild boars living in southern Italy (Naccari et al. 2004) where in all examined boars, hepatic PCB concentrations were below the detection limit. A similar situation was observed by Shore et al. (2001) in the liver of wolves in Russia. Elevated PCB concentrations in domestic animals and wildlife were found in areas where PCBs used to be produced. In northern Italy, in Brescia, PCBs had been produced for more than 50 years (until 1984) (Turrio-Baldassarri et al. 2009). Accordingly, Naso et al. (2004) detected PCBs in 97 % of the liver and adipose tissue samples of roe deer from northern Italy, and the determined hepatic concentration was two times higher than in our study. Much higher PCB levels than in our paper were also observed by Kocan et al. (2001), who found a mean concentration of 103.2 ng/g in the meat of game animals in eastern Slovakia, where PCBs had been produced for 25 years.
PCB levels in the liver of wild boars in our study were higher than those observed in roe deer and deer living in the same area (Tomza-Marciniak et al. 2011). These differences result from the different fashion of foraging. Deer and roe deer are typical herbivores, whereas wild boar is omnivorous, obtaining food mainly through digging large amounts of soil. As indicated by literature data, the soils of West Pomerania have been observed to contain organochlorine compounds, including PCBs (Witczak and Abdel-Gawad 2012).
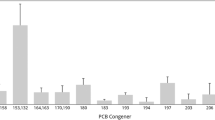
Among dioxin-like congeners, only PCB 77, 118 and 156 were present in each examined sample. The lack of the other congeners was observed only in a few samples. Generally, PCB 118 and 156, mono-ortho congeners, were dominant compounds in liver samples. Among non-ortho PCBs, the dominant congener was PCB 77 with a mean concentration of 32.41 pg/g lw. The concentration of PCB 126 was rather low, which given its high TEF (0.1) was reflected in WHO-dlPCB-TEQ (mean 2.10 pg/g lw) (Table 2).
Our previous studies carried out in the area of West Pomerania (Tomza-Marciniak et al. 2011) show that TEQ levels calculated for dl-PCBs in the liver of red deer and roe deer were a few times lower, 0.32 and 0.29 pg/g lipids, respectively. WHO-dlPCB-TEQ was much lower than reported by other authors in other parts of the world. For example, in Finland, Suutari et al. (2012) observed in reindeer hepatic WHO-dlPCB-TEQ was on average 12 pg/g lipids.
Statistical analysis showed that for most analysed organochlorine compounds, sex was not a significant factor for the observed concentrations of these compounds. Although in males, hepatic concentrations of PCBs and OCPs were higher than in females, but it was not statistically significant. Only PCB 77 showed a statistically significant difference (p < 0.014).
Some authors explain the sex-related differences in the levels of these compounds with the ability to eliminate part of the lipophilic contaminants accumulated in the liver during pregnancy and lactation. This was confirmed by research in which young individuals have greater PCB levels than adults (Suutari et al. 2012; Dip et al. 2003). The lack of statistical confirmation of such differences was also noted in a paper by Hoekstra et al. (2003), who explained the fact by a small size of the examined population which affected the statistical power of the conducted analyses.
Activities of glutathione S-transferase
GST is a phase II enzyme, involved in the cellular detoxification of xenobiotics and plays a fundamental role in the protection against toxic chemicals (Sheehan et al. 2001). According to Fitzpatrick et al. (1997), the GST is involved in the metabolism of organochlorine compounds. Several recent studies have shown that chronic exposure to organochlorine compounds may result in the inhibition of GST activity (Greco et al. 2010; Tian et al. 2012). Also in our study, we observed a statistically significant (p < 0.05) negative correlation between the concentration of lindane, DDTs and PCBs (as sum of indicator congeners) in the liver and the activity of GST. However, GST activities showed no significant correlation with any of dl-PCBs (Table 3).
In contrast, in some studies, no dependence was found between PCB and GST activity (Richardson et al. 2010), while in others, GST increased its activity along with the increasing exposure to PCB (Routti et al. 2008). Hamed et al. (2003) showed that induced GST activity indicates an adaptation of the organism to enhanced pollution stress.
No significant differences in GST activity were observed among sexes. In females, mean hepatic GST activity was 236.3 U/mg of protein and in males 260.9 U/mg of protein (Fig. 2) The activity of this enzyme had a very wide range, from 20 to 1,103.2 U/mg of protein.
Evaluation of toxicological risk to consumers
The health risk to animals and people resulting from exposure to organochlorine compounds is associated with their ability to induce metabolic, endocrine and reproductive disruptions (Safe 1994; Gregoraszczuk et al. 2004; Langer 2010). The significant abilities of PCBs and OCPs to bioaccumulate and induce toxic effects have created the need to monitor levels in their food.
The interpretation of PCB and OCP determinations is not easy, as both in Poland and the rest of the EU, there are no regulations to establish their highest permissible levels in edible offal or meat of hunted animals. The assessment of potential contamination of wild boar meat uses the norms established for farmed animals, as are presented in Table 4.
Our results showed that in five samples of wild boar livers, the determined concentrations of certain organochlorine compounds exceeded maximum residue levels (MRLs). In one sample, PCBs, endrin and DDTs exceeded MRLs at the same time while in another, it was concurrently endrin and DDTs. In the remaining three samples, only PCBs thresholds were exceeded. MRLs for PCBs were exceeded in four samples, for endrin in two samples and for DDTs in three samples.
According to current regulations, the maximum level for the sum of dioxins, furans and dl-PCBs (expressed as PCDD/F-PCB-TEQ) in the liver of terrestrial animals and products derived thereof is 10 pg/g lipids. In our study, mean TEQ level calculated only for dl-PCBs was 2.10 ± 1.11 pg WHO-PCB-TEQ/g. Given that the contributions of PCBs to total TEQs observed in some reports ranged from 34 to 68 % (Suutari et al. 2009), it must be concluded that TEQs obtained were low and do not pose a risk to consumers.
Although in several livers we observed levels exceeding the maximum permissible for some compounds, a full assessment of the potential health risk should allow for the mean volume of wild boar meat consumption. Although the edible offal of wild boars and other hunted animals is valued and often consumed, the volume of consumption is much lower than for beef and pork offal. That is why it seems necessary to develop separate norms for the meat and offal of hunted animals.
The determination of organochlorine compounds in game animals not only reflects the degree of environmental contamination in which they live but also enables an assessment of toxicological risk for the consumers of venison. Literature data indicate that the levels of PCBs and OCPs observed in our study were higher than tissues of animals coming from non-contaminated areas but at the same time were lower than in contaminated areas. Therefore, there are no definitive premises to conclude that the examined area of West Pomerania is significantly contaminated with organochlorine compounds. Nevertheless, one should bear in mind that the meat and offal of hunted animals from the area may contain elevated concentrations of these compounds and hence may pose some risk for venison consumers.
References
Baubet, E., Bonenfant, C., & Brandy, S. (2004). Diet of the wild boar in the French Alps. Galemys, S16, 101–113.
Bouwman, H., van den Berg, H., & Kylin, H. (2011). DDT and malaria prevention: addressing the paradox. Environmental Health Perspectives, 119(6), 744–747.
Bradford, M. (1976). A rapid and sensitive assay of protein utilizing the principle of dye binding. Analytical Biochemistry, 72, 248–254.
Breivik, K., Sweetman, A., Pacyna, J. M., & Jones, K. C. (2002). Towards a global historical emission inventory for selected PCB congeners—a mass balance approach 1. Global production and consumption. The Science of the Total Environment, 290(1), 181–198.
Dip, R., Hegglin, D., Deplazes, P., Dafflon, O., Koch, H., & Naegeli, H. (2003). Age-and sex-dependent distribution of persistent organochlorine pollutants in urban foxes. Environmental Health Perspectives, 111(13), 1608–1612.
Dvorská, A., Lammel, G., & Holoubek, I. (2009). Recent trends of persistent organic pollutants in air in central Europe—Air monitoring in commination with air mass trajectory statistics as a tool to study the effectivity of regional chemical policy. Atmospheric Environment, 43, 1280–1287.
Falandysz, J. (1999). Polichlorinated biphenyls (PCBs) in an environment: chemistry, toxicity, analysis, concentrations and risk assessment. Gdańsk: Fundacja Rozwoju Uniwersytetu Gdańskiego.
Fitzpatrick, P. J., O’Halloran, J., Sheehan, D., & Walsh, A. R. (1997). Assessment of a glutathione S-transferase and related proteins in the gill and digestive gland of Mytilus edulis (L.), as potential organic pollution biomarkers. Biomarkers, 2, 51–56.
Genov, P. (1981). Food composition of wild boar in North-eastern and Western Poland. Acta Theriologica, 26, 185–205.
Greco, L., Serrano, R., Blanes, M., Serrano, E., & Capri, E. (2010). Bioaccumulation markers and biochemical responses in European sea bass (Dicentrarchus labrax) raised under different environmental conditions. Ecotoxicology and Environmental Safety, 73, 38–45.
Gregoraszczuk, E., Augustowska, K., & Ptak, A. (2004). Environmental xenoestrogens causes endocrine alterations associated with early fetal loss. Polish Journal of Endocrinology, 6, 819–824.
Hamed, R. R., Farid, N. M., Elowa, S. E., & Asdalla, A. M. (2003). Glutathione related enzyme levels of freshwater fish as bioindicators of pollution. Environmentalist, 23, 313–322.
Hoekstra, P. F., Braune, B. M., Wong, C. S., Williamson, M., Elkin, B., & Muir, D. C. (2003). Profile of persistent chlorinated contaminants, including selected chiral compounds, in wolverine (Gulo gulo) livers from the Canadian Arctic. Chemosphere, 53(5), 551–560.
Holoubek, I., Klánová, J., Jarkovský, J., Kubík, V., & Helešic, J. (2007). Trends in background levels of persistent organic pollutants at Kosetice observatory, Czech Republic. Part II Aquatic and terrestrial environments 1996–2005. Journal of Environmental Monitoring, 9, 564–571.
Hoshi, H., Minamoto, N., Iwata, H., Shiraki, K., Tatsukawa, R., Tanabe, S., et al. (1998). Organochlorine pesticides and polychlorinated biphenyl congeners in wild terrestrial mammals and birds from Chubu region, Japan: interspecies comparison of the residue levels and compositions. Chemosphere, 36(15), 3211–3221.
Jaward, F., Farrar, N., Harner, T., Sweetman, A., & Jones, K. (2004). Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environmental Science and Technology, 38(1), 34–41.
Kocan, A., Petrik, J., Jursa, S., Chovancova, J., & Drobna, B. (2001). Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere, 43(4–7), 595–600.
Kumar, K., Priya, M., Sajwan, K., Kõlli, R., & Roots, O. (2009). Residues of persistent organic pollutants in Estonian soils (1964-2006). Estonian Journal of Earth Sciences, 58(2), 109–123.
Langer, P. (2010). The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Frontiers in Neuroendocrinology, 31(4), 497–518.
Naccari, F., Giofrè, F., Licata, P., Martino, D., Calò, M., & Parisi, N. (2004). Organochlorine pesticides and PCBs in wild boars from Calabria (Italy). Environmental Monitoring and Assessment, 96(1–3), 191–202.
Naso, B., Zaccaroni, A., Perrone, D., Ferrante, M., Severino, L., Stracciari, G., et al. (2004). Organochlorine pesticides and polychlorinated biphenyls in European roe deer Capreolus capreolus resident in a protected area in Northern Italy. The Science of the Total Environment, 328(1–3), 83–93.
Richardson, K. L., Lopez Castro, M., Gardne, S. C., & Schlenk, D. (2010). Polychlorinated biphenyls and biotransformation enzymes in three species of sea turtles from the Baja California Peninsula of Mexico. Archives of Environmental Contamination and Toxicology, 58(1), 183–193.
Rolecki, R. (2002). Toxicological characteristics of permanent organic pollutants and routes of human exposure Report GF-POL-INV-R15 under the GEF project GF/POL/01/004. http://ios.info.pl/gef/doc/GF-POL-INV-R15.PDF. Accessed 12.11.2002.
Routti, H., Letcher, R., Arukwe, A., van Bavel, B., Yoccoz, N., Chu, S., et al. (2008). Biotransformation of PCBs in relation to phase I and II xenobiotic-metabolizing enzyme activities in ringed seals (Phoca hispida) from Svalbard and the Baltic Sea. Environmental Science and Technology, 42(23), 8952–8958.
Růžičková, P., Klánová, J., Čupr, P., Lammel, G., & Holoubek, I. (2008). An assessment of air-soil exchange of polychlorinated biphenyls and organochlorine pesticides across Central and Southern Europe. Environmental Science and Technology, 42(1), 179–185.
Safe, S. (1994). Polychlorinated biphenyls (PCBs) environmental impact, biochemical and toxic responses, and implication for risk assessment. Critical Reviews in Toxicology, 24(2), 87–149.
Sheehan, D., Meade, G., Foley, V. M., & Dowd, C. A. (2001). Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochemical Journal, 360, 1–16.
Shore, R., Casulli, A., Bologov, V., Wienburg, C., Afsar, A., Toyne, P., et al. (2001). Organochlorine pesticide, polychlorinated biphenyls and heavy metal concetrations in wolves (Canis lapus L.) from north-west Russia. The Science of the Total Environment, 280(1–3), 45–54.
Smith, A. G. (1991). Chlorinated hydrocarbon insecticides. In W. J. Hayes Jr. & E. R. Laws Jr. (Eds.), Handbook of pesticide toxicology. Volume 2, Classes of pesticides (pp. 731–916). Toronto: Academic.
Struciński, P. (2009). 1,2,3,4,5,6-hexachlorocyclohexane. Podstawy i Metody Oceny Środowiska Pracy, 3(61), 75–126.
Suutari, A., Ruokojärvi, P., Hallikainen, A., & Laaksonen, S. (2009). Polychlorinated dibenzo-p-dioxins, dibenzofurans, and polychlorinated biphenyls in semi-domesticated reindeer (Rangifer tarandus tarandus) and wild moose (Alces alces) meat in Finland. Chemosphere, 75(5), 617–622.
Suutari, A., Hallikainen, A., Ruokojärvi, P., Kiviranta, H., Nieminen, M., & Laaksonen, S. (2012). Persistent organic pollutants in Finnish reindeer (Rangifer tarandus tarandus L.) and moose (Alces alces). Acta Veterinaria Scandinavica, 54(1), 11.
Tian, X., Song, E., Pi, R., Zhu, R., Liu, L., Ma, X., et al. (2012). Polychlorinated biphenyls and their different level metabolites as inhibitors of glutathione S-transferase isoenzymes. Chemico-Biological Interactions, 198(1–3), 1–8.
Tomza, A., Witczak, A., & Ciereszko, W. (2006). Uptake of polychlorinated biphenyl congeners in freshwater mussels Anodonta complanata from the lower Odra River. Polish Journal of Environmental Studies, 15(4), 603–608.
Tomza-Marciniak, A. (2013). Residues of organochlorine pesticides and PCBs in wild boars from an area which once contained a landfill of obsolete pesticides and other chemicals. Fresenius Environmental Bulletin (in press).
Tomza-Marciniak, A., & Witczak, A. (2010). Distribution of endocrine-disrupting pesticides in water and fish from the Oder River, Poland. Acta Ichthyologica et Piscatoria, 40(1), 1–9.
Tomza-Marciniak, A., Pilarczyk, B., Wieczorek-Dąbrowska, M., Bąkowska, M., Witczak, A., & Hendzel, D. (2011). Polychlorinated biphenyl (PCB) residues in European roe deer (Capreolus capreolus) and red deer (Cervus elaphus) from north-western Poland. Chemistry and Ecology, 27(6), 493–501.
Tomza-Marciniak, A., Pilarczyk, B., Bąkowska, M., Tylkowska, A., Marciniak, A., Ligocki, M., et al. (2012). Polychlorinated biphenyl (PCBs) residues in suburban red foxes (Vulpes vulpes) from West Pomerania region (Poland)—preliminary study. Polish Journal of Environmental Studies, 24(1), 193–199.
Turrio-Baldassarri, L., Alivernini, S., Carasi, S., Casella, M., Fuselli, S., Iacovella, N., et al. (2009). PCB, PCDD and PCDF contamination of food of animal origin as the effect of soil pollution and the cause of human exposure in Brescia. Chemosphere, 76(2), 278–285.
van den Berg, M., Birnbaum, L. S., Denison, M., De Vito, M., Farland, W. F. M., Fiedler, H., et al. (2006). The 2005 World Health Organization revaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Science, 93, 223–241.
Wania, F., & Mackay, D. (1996). Tracking the distribution of persistent organic pollutants. Environmental Science and Technology, 30, 390–396.
WHO. (1992). Endrin (Environmental Health Criteria 130). Geneva: World Health Organization.
Witczak, A., & Abdel-Gawad, H. (2012). Comparison of organochlorine pesticides and polychlorinated biphenyls residues in vegetables, grain and soil from organic and conventional farming in Poland. Journal of Environmental Science and Health Part B-Pesticides Food Contaminants and Agricultural Wastes, 47(4), 343–354.
Zasadowski, A., Wyszyńska, A., & Wysoki, A. (2003). Evaluation of the contamination degree of roe-deer with cadmium and polychlorinated biphenyls in Warmia and Mazury district. Polish Journal of Veterinary Science, 6, 93–97.
Acknowledgment
The authors would like to thank Ms Diana Hendzel for help in obtaining material for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomza-Marciniak, A., Marciniak, A., Pilarczyk, B. et al. Wild boar (Sus scrofa) as a bioindicator of organochlorine compound contamination in terrestrial ecosystems of West Pomerania Province, NW Poland. Environ Monit Assess 186, 229–238 (2014). https://doi.org/10.1007/s10661-013-3368-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3368-z