Abstract
An experiment was conducted in open-top chambers (OTC) to study the effect of elevated CO2 (580 ± 20 μmol mol−1) on azoxystrobin degradation and soil microbial activities. Results indicated that elevated CO2 did not have any significant effect on the persistence of azoxystrobin in rice-planted soil. The half-life values for the azoxystrobin in rice soils were 20.3 days in control (rice grown at ambient CO2 outdoors), 19.3 days in rice grown under ambient CO2 atmosphere in OTC, and 17.5 days in rice grown under elevated CO2 atmosphere in OTC. Azoxystrobin acid was recovered as the only metabolite of azoxystrobin, but it did not accumulate in the soil/water and was further metabolized. Elevated CO2 enhanced soil microbial biomass (MBC) and alkaline phosphatase activity of soil. Compared with rice grown at ambient CO2 (both outdoors and in OTC), the soil MBC at elevated CO2 increased by twofold. Elevated CO2 did not affect dehydrogenase, fluorescein diacetate, and acid phosphatase activity. Azoxystrobin application to soils, both ambient and elevated CO2, inhibited alkaline phosphates activity, while no effect was observed on other enzymes. Slight increase (1.8–2 °C) in temperature inside OTC did not affect microbial parameters, as similar activities were recorded in rice grown outdoors and in OTC at ambient CO2. Higher MBC in soil at elevated CO2 could be attributed to increased carbon availability in the rhizosphere via plant metabolism and root secretion; however, it did not significantly increase azoxystrobin degradation, suggesting that pesticide degradation was not the result of soil MBC alone. Study suggested that increased CO2 levels following global warming might not adversely affect azoxystrobin degradation. However, global warming is a continuous and cumulative process, therefore, long-term studies are necessary to get more realistic assessment of global warming on fate of pesticide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming is projected to have significant impact on conditions affecting agriculture, including temperature and precipitation. During the last 12 years, the average rate of increase of CO2 has been 1.9 μL L−1 years−1 (Krull et al. 2005) and the Intergovernmental Panel on Climate Change (IPCC) has projected that, by 2050, the respective ranges for atmospheric carbon dioxide concentration will be 463–623 ppm (McCarthy et al. 2001). Accordingly, a consequence of this rise in CO2, a rise in earth’s average temperature from 2.0 °C to 4.5 °C (IPCC 2007) is predicted.
Plant protection has become a key component of modern intensive agriculture where high-yielding crop varieties are highly susceptible to disease and pest attack. Fate of xenobiotics has become crucial in defining the quality of our environment. Due to global warming, the change in soil microbial population and increase in temperature may have effects of the fate of plant protection chemicals that are applied to the soil or ultimately end up in the soil when foliar-sprayed.
Not much literature is available on the effect of climate change on pesticide fate. Reports based on modeling of the available data suggested that increase in temperature over last few decades has resulted in decrease in pesticides persistence (Williams et al. 1992; Bailey 2003; Bloomfield et al. 2006). Bailey (2003) estimated the change in persistence of autumn-applied isoproturon using real weather data for the period 1980–2001. The results suggested approximately 25 % decrease in the duration of weed control in the last 5 years and attributed this decline to increase in soil temperature.
Azoxystrobin [methyl (E)-2-{2-(6-(2-cyanophenoxy) pyrimidin-4-yloxy) phenyl}-3-methoxyacrylate] (Fig. 1), a strobilurin fungicide, is a broad-spectrum, systemic, and soil-applied fungicide. Azoxystrobin is moderately persistent in the soils with half-life of 54–135 days in aerobic soils and 36–45 days in anaerobic soils (Joseph 2000; Ghosh and Singh 2009; Singh and Singh 2010). Not much literature is available on effect of azoxystrobin on soil microbial activity. Bending et al. (2007) reported that azoxystrobin did not affect the microbial biomass in soils; however, fungicide significantly reduced dehydrogenase activity to varying extents in the low organic matter (OM)/biomass soil. However, Adetutu et al. (2008) suggested that azoxystrobin increased fungal diversity under light incubation while dark incubation reduced fungal diversity and bacterial diversity was unaffected.
Soil microorganisms and soil enzymes play important roles in the soil fertility and are the indicators of soil quality. Drigo et al. (2008), who reviewed the effect of elevated CO2 on soil microbial parameters and activities in rhizosphere of upland crops, showed mixed response on microbial biomass (MBC); some suggested increase in MBC (de Graff et al. 2006; Carney et al. 2007; Li et al. 2010) while others suggested no effect (Janus et al. 2005; Bazot et al. 2006; Lesaulnier et al. 2008). However, studies on the effect of elevated CO2 in rice crop have suggested that MBC was significantly higher in rice grown in elevated CO2 environment than in the rice maintained at ambient CO2 (Hoque et al. 2001; Inubushi et al. 2001, 2011). Soil enzyme activities are “sensors” of soil microbial functioning and soil physicochemical conditions. The impact of elevated CO2 on soil enzyme activities is not much studied, and available information suggests that specific enzyme activities are directly and/or indirectly affected by elevated CO2 (Kang et al. 2005, Henry et al. 2005; Das et al. 2011).
Absolutely no real-time study is available on the fate of pesticides under elevated CO2 atmosphere. Rice is an important C3 crop, and azoxystrobin is recommended in rice cultivation for the control of sheath blight and powdery mildew diseases. Therefore, this paper reports the effect of elevated atmospheric CO2 (580 ± 20 μmol mol−1) on the degradation of azoxystrobin in rice-planted (Oryza sativa L.) soil during kharif season of 2010 in open-top chambers (OTCs) under Indian tropical environment. Effect of elevated atmospheric CO2 and azoxystrobin on microbial activities, viz., MBC, dehydrogenase, fluorescein diacetate (FDA), and acid and alkaline phosphatase, was also studied.
Materials and methods
Soil
A sandy loam soil used in the present study was collected from the experimental farm of the Indian Agricultural Research Institute, New Delhi. Soil was collected from the surface at 0–15 cm depth and was used for filling pots without drying. Physicochemical characteristics of the soil determined by standard methods were pH 7.9 measured at 1:2.5 soil-to-water ratio (Jackson 1967); organic carbon, 0.3, by Walkley and Black method (Jackson 1967); and mechanical fractions (%) sand, 54.4; silt, 23.3; and clay, 22.3 employing the Bouyoucos hygrometer method (Black 1965).
Chemicals
An analytical grade azoxystrobin (96.2 % purity) was supplied by the Rallis India Ltd. The solvents and other reagents used were of analytical grade and were purchased locally from Merck Specialties Private Ltd, Mumbai, India.
Experimental setup
The present study was carried out in the OTCs of size 1.8 and 1.6 m located in the experimental farm of the Indian Agricultural Research Institute, New Delhi, as described by Pal et al. (2004). To maintain elevated levels of CO2 in OTC at 580 ± 20 ppm at crop canopy level, continuous injection of pure CO2 into OTC was carried out where it was mixed with air from air compressor before entering into the chamber. The air sample from the middle of the chamber was drawn periodically into a CO2 sensor (NDIR, make Topak, USA), and the set level of CO2 was maintained with the help of solenoid valves, Program Logic Control, and Supervisory Control and Data Acquisition (SCADA) winlog software (Make SELCO Italy). Carbon dioxide data logging, control, and operation were performed using a computer through DOIP (digital input and output module) on a real-time basis.
Five-week-old rice seedlings (var Pusa 44) were transplanted in plastic pots (20 × 20 cm) having 3.75 kg of farm soil (20 % moisture content). Three seedlings were planted in each pot, and a total of 90 pots were maintained. Pots were divided into three groups of 30 pots each. After 2 weeks, when the plant established well, pots of one set were transferred to OTC having elevated CO2 concentration (580 ± 20 ppm), one set to OTC at ambient CO2 concentration (~390 ppm) while third set was kept in the open. After 23 days of shifting the plant in OTC, azoxystrobin was applied to each pot at recommended dose (375 g a.i. ha−1) that corresponded to 0.86 mg per pot. Acetone solution (0.5 mL) of azoxystrobin was mixed with 500 mL water, and water was directly applied to the individual pot by drenching the soil. Among each set, 15 pots were treated with azoxystrobin while 15 pots were maintained as untreated control. Pots were maintained under flooded conditions, and water lost was supplemented daily. Top dressing of nitrogenous fertilizers was done by applying urea at 2 g per pot. Pots were removed at regular intervals for extraction of azoxystrobin residues. The meteorological observatory of the Indian Agricultural Research Institute, New Delhi, recorded the weather parameters.
Azoxystrobin extraction and analysis
At each sampling day, three azoxystrobin-treated and three untreated pots were removed from each group. Water was decanted from each pot and kept separately for azoxystrobin extraction and analysis. The rice plants were uprooted, soil separated from the roots, and then thoroughly mixed before taking sample for fungicide extraction.
Azoxystrobin from soil and water samples was extracted according to the method described by Ghosh and Singh (2009). Fifty grams soil (oven dry basis) or water sample (50 mL) was extracted using ethyl acetate (50 + 30 + 20 mL); ethyl acetate fraction from three extractions was pooled, dried over anhydrous Na2SO4, and evaporated to dryness at room temperature. The azoxystrobin residues were redissolved in 10 mL of acetone and were quantified for azoxystrobin using gas chromatography (Hewlett Packard, Model 5890) equipped with a Ni63 electron capture detector and fitted with HP-1 column [10 m (l) × 0.50 mm (i.d.) × 2.53 μm film thickness]. The operating conditions were oven temperature, 270 °C; injector temperature, 300 °C; detector temperature, 300 °C; carrier gas (nitrogen) flow rate, 45 mL min−1. Recovery of azoxystrobin from soil and water at 0.1 and 1 μg mL−1 levels was 88.6 % and 89.5 %, respectively (soil) and 90.3 % and 91.2 %, respectively (water). The limit of detection for azoxystrobin was 0.05 μg mL−1.
Samples (soil and water extracts) were analyzed for azoxystrobin metabolites using Hewlett Packard high-performance liquid chromatography (HPLC) as described by Ghosh and Singh (2009).
Microbial activity
MBC and enzymes were estimated in the soil samples at each sampling day. MBC was estimated by chloroform fumigation extraction method (Vance et al. 1987). Dehydrogenase activity was estimated by monitoring the rate of production of triphenyl formazan (TPF) from triphenyl tetrazolium chloride (Casida et al. 1964). The FDA hydrolysis assay was carried out following the method of Green et al. (2006). The alkaline and acid phosphatase activities of soil were determined by following methods described by Tabatabai and Bremner (1969).
Results and discussion
Azoxystrobin degradation
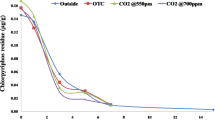
Figure 2 represents the azoxystrobin recovered from the rice-planted soil under different conditions, viz., control (ambient CO2 outdoors), ambient CO2 in OTC, and elevated CO2 in OTC. Results indicated that azoxystrobin persisted till the 50th day in soils of all the three treatments. There was not much difference in the amounts of azoxystrobin recovered from the rice-planted soils incubated under different conditions. At 50th day, 13 % of initially applied azoxystrobin was recovered from the soil maintained under elevated CO2, while the amount recovered from the treatments under ambient CO2 in OTC and outdoors were 16 % and 19 %, respectively. No azoxystrobin was recovered from the standing water samples after tenth day, and amounts recovered on tenth day were very low (0.3 % to 0.9 % of the initially applied azoxystrobin), suggesting that fungicide was fairly sorbed in the soils.
Dissipation data from all of three treatments fitted well to the first-order kinetic equation—log(C/C o) = −K obs t, where, C o is the initial concentration of azoxystrobin (in milligrams per kilogram), C is its concentration (in milligrams per kilogram) after time t (days), and K obs is the rate constant of the dissipation. The half-life (t 1/2) values for azoxystrobin were calculated using following:
The half-life values for the azoxystrobin in the different treatments were 20.3 days in rice grown outdoors, 19.3 days in rice grown in OTC under ambient CO2, and 17.5 days in rice grown in OTC under elevated CO2 (Table 1). These results suggested that azoxystrobin dissipated at slightly faster rate in rice grown under elevated CO2 atmosphere than in rice grown under ambient CO2 atmosphere, both in OTC and outdoors, but this difference was not statistically significant.
Half-life values of azoxystrobin in rice soils observed in this study are similar to the results earlier reported by Joseph (1999) who recorded a half-life of 14 days for azoxystrobin under field conditions. However, Gajbhiye et al. (2011) reported a much shorter half-life of 7.5, 7.9, and 8.1 days for azoxystrobin in grape field soils of Karnataka, Maharashtra, and Tamil Nadu, respectively.
Ethyl acetate extracts from soil and water samples were analyzed for the formation of azoxystrobin metabolite using HPLC. HPLC chromatograms showed a peak other than the azoxystrobin at retention time of 2.57 min (Fig. 3). The figures given in Table 2 for azoxystrobin metabolite are the detector response (millivolts) for the metabolite. Metabolite was detected both in the soil and water samples, but not much difference was observed in the amounts formed in soil/water samples obtained from different treatments. Also, the amounts formed at different time intervals were nearly the same, suggesting that metabolite did not accumulate in soil/water and was further metabolized. HPLC-mass spectrometry analysis of leachate and soil samples indicated that this extra peak in HPLC chromatogram corresponds to a molecular ion peak at m/z 389 (M+) with base ion peak at m/z 344 (M+–COOH) and fragment ion peaks at m/z 372 (M+–OH); m/z 329 (M+–COOH, CH3); m/z 229 (MH +2 –C6H4CN, –COOH, CH3) and m/z 102 (C6H4CN+). The metabolite was tentatively characterized as azoxystrobin acid, which is formed by the hydrolysis of the ester moiety. These results are in line with the results obtained by previous workers, which suggested that azoxystrobin acid was recovered as the major metabolite of azoxystrobin degradation (Ghosh and Singh 2009; Singh and Singh 2010).
Effect on microbial parameters
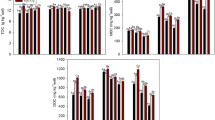
Table 3 represents the MBC in rice soils incubated under different environments. Results clearly indicated that MBC of soil samples incubated at elevated CO2 levels, both azoxystrobin-untreated and azoxystrobin-treated, was higher than the respective treatments maintained at ambient CO2, both outdoors and in OTC. Nearly two times increase in the soil MBC was observed in rice soil incubated at higher CO2 levels than both the controls. This increase could be attributed to more soil exudates in rice grown under elevated CO2 environment (Hill et al. 2007). There was no significant change in the MBC at different days of incubation. Earlier studies on effect of elevated CO2 in rice crop have suggested that microbial biomass carbon in rice grown in elevated CO2 environment was significantly higher than rice soil maintained at ambient CO2 (Hoque et al. 2001; Inubushi et al. 2001, 2011), and effect was cultivar-specific (Inubushi et al. 2001). However, Li et al. (2004) suggested that effect of elevated CO2 on soil biomass carbon was dependent on applied nitrogen (N). Elevated CO2 significantly increased microbial biomass carbon in the surface soil when N (90 kg ha−1) was in sufficient supply. Low N supplement (30 kg ha−1) limited the enhancement of root growth by elevated CO2, leading to diminished response of soil microbial biomass carbon to CO2 enrichment.
Azoxystrobin did not affect the MBC in any of the three treatments, and nearly the same amount of biomass carbon was observed in rice soils incubated without and with azoxystrobin, both under ambient and elevated CO2. Thus, when azoxystrobin is used at recommended dose, it did not affect MBC. These results are in line with the findings of Bending et al. (2006), who studied the effect of azoxystrobin in two soils varying in organic matter contents. However, Adetutu et al. (2008) suggested that azoxystrobin had some effect on fungal communities after 21 days (up to 84 days) of incubation in either light or dark soil microcosms. Light incubation increased fungal diversity while dark incubation reduced fungal diversity. Bacterial diversity was unaffected.
The dehydrogenase activity of the rice soils maintained under different CO2 environment is represented in Table 4. There was no difference in the dehydrogenase activity of soil maintained at ambient or elevated CO2, both azoxystrobin-untreated and azoxystrobin-treated, and values ranged between 1.71 and 2.13 μg TPF released g−1 of soil day−1. Thus, both elevated CO2 and azoxystrobin, alone or in combination, had no effect on the soil dehydrogenase activity. Earlier, Inubushi et al. (2010) studied dehydrogenase activity in rice soils that was earlier subjected at ambient and elevated CO2 and temperature (2 °C) and reported that there was no significant difference in the dehydrogenase activity of these soils. However, in a laboratory incubation study, Das et al. (2011) reported that dehydrogenase activity in four rice soils incubated at elevated CO2 concentrations significantly increased, and the increase was CO2 concentration-dependent with maximum increase observed at 600 μmol mol−1 CO2. Bending et al. (2007) reported that azoxystrobin application to soil had mixed effect on the dehydrogenase activity, and effect was dependent on the OM/microbial biomass status of the soils. Azoxystrobin inhibited dehydrogenase activity in low OM/biomass (biomass, 139.4 mg C kg−1 dw soil) soil while no effect was observed in high OM/biomass soil (biomass, 622.6 mg C kg−1 dw soil). In our study, at the time of azoxystrobin application, the biomass carbon of the soil was more than 560.4 μg C g−1 soil. Probably, this might be the reason that we did not observe any effect on dehydrogenase activity following azoxystrobin application.
Like dehydrogenase activity, no effect of elevated CO2 and azoxystrobin or combination was observed on FDA activity in rice soils (Table 5). However, Das et al. (2011) reported that FDA activity increased significantly following CO2 enrichment with mean increase of 41.9 % in the four soil types used in the study.
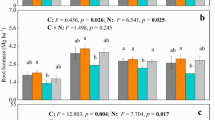
Phosphatases are involved in transformation of organic and inorganic phosphorous compounds in soil, and these activities are important factors in maintaining and controlling the rate of P cycling through soils. Phosphatase activity, both acidic and alkaline, of the soils in different treatments is shown in Table 6. Results suggested that alkaline phosphatase activity of the soils was more than the acidic phosphatase activity, as soil used in this study was slightly alkaline in nature (Das et al. 2011). Elevated CO2 had no effect on acidic phosphatase activity, and values ranged between 6.42 and 9.06 μg PNP released g−1 of soil h−1 in rice soil incubated under elevated and ambient CO2. However, there was a slight increase in the alkaline phosphatase activity in rice soil maintained at elevated CO2. Azoxystrobin did not affect the acidic phosphatase activity, but alkaline phosphatase activity was slightly inhibited, and effect was visible till 40th day. Earlier studies suggested increased phosphatase activity in soils under elevated CO2 (Kang et al. 2005; Das et al. 2011).
Temperature inside the OTC was nearly 2 °C higher than the outside temperature. Results indicated that the the higher temperature in OTC did not affect the soil biomass carbon and enzyme activity, as nearly similar results were obtained for various microbial parameters studied in rice grown outdoors and rice grown in OTC maintained at ambient CO2. Probably, temperature was not high enough to affect the soil microbial activities as Das et al. (2011) reported that significant change in soil microbial parameters was observed when temperature was increased by 10 °C.
Hypothesis before starting this experiment was that elevated CO2 inside the OTC will result in the increase in photosynthesis in rice, and this will result in better shoot/root growth and higher soil microbial activity than the rice grown at ambient CO2. Also, higher temperature in OTC may result in increased volatilization losses and faster azoxystrobin degradation in soil. Thus, combination of both parameters (higher temperature and higher microbial activity) may result in faster degradation of azoxystrobin in rice grown under elevated CO2 environment. However, results of this study suggested that increase in the atmospheric temperature in OTC did not have a significant effect on the persistence of azoxystrobin. Earlier, there was no real-time study available on the degradation of pesticides under elevated CO2 atmosphere or higher atmospheric temperature environment. The studies conducted so far on the effect of climate change on pesticide persistence/degradation are the modeling studies performed using data available over the last few decades. It is estimated that climate change would accelerate pesticide degradation due to increase in the temperature (Bailey 2003; Bloomfield et al. 2006; Boxall et al. 2009).
During the study, the rice plants were exposed to the elevated CO2 atmosphere for total of 73 days, which included 23 days of exposure before application of azoxystrobin and 50 days during the study after azoxystrobin application. Photosynthesis in plants is dependent on light, thus average sunshine hours per day will significantly affect the photosynthesis in plants. It was observed that, during initial 23 days of elevated CO2 exposure (before azoxystrobin application), there were more number of cloudy days and on an average crop was exposed to only 2 h of bright sunshine per day. However, during 50 days of azoxystrobin persistence study, average sunshine hours were more than six. During cloudy weather conditions, the plant did not make much use of the excess CO2 provided to them due to limited sunshine. We observed no visible change in plant growth parameters like root growth and shoot growth in rice plants grown under different environment. Probably due to more cloudy days during the experiment, there may not be much difference in the photosynthesis in rice grown under elevated and ambient CO2 atmospheres. Although MBC of soil maintained at elevated CO2 was nearly twice of MBC of control soils, it did not lead to significant increase in azoxystrobin degradation. Pesticide degradation is not the only direct factor of soil MBC as all soil microbes do not participate in pesticide degradation. Soil enzymes also play a significant effect on the pesticide degradation and may contribute towards pesticide degradation.
This short duration study on the effect of elevated CO2 on degradation of azoxystrobin in rice soils suggested no significant effect of elevated CO2 on azoxystrobin degradation. Elevated CO2 increased microbial biomass carbon and alkaline phosphate activities. However, it is not advisable to draw a conclusion based on one season data, as climate change is a continuous process. Therefore, a long-term study under varying levels of CO2 is suggested to get the real effect of climate change on pesticide degradation.
References
Adetutu, E. M., Ball, A. S., & Osborn, A. M. (2008). Azoxystrobin and soil interactions: Degradation and impact on soil bacterial and fungal communities. Journal of Applied Microbiology, 105, 1777–1790.
Bailey, S. W. (2003). Climate change and decreasing herbicide persistence. Pest Management Science, 60, 158–162.
Bazot, S., Ulff, L., Blum, H., Nguyen, C., & Robin, C. (2006). Effects of elevated CO2 concentration on rhizodeposition from Lolium perrene grown on soil exposed to 9 years of CO2 enrichment. Soil Biology and Biochemistry, 38, 729–736.
Bending, G. D., Lincoln, S. D., & Edmondson, R. N. (2006). Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environmental Pollution, 139, 279–287.
Bending, G. D., Rodriguez-Cruz, M. S., & Lincoln, S. D. (2007). Fungicide impacts on microbial communities in soil with contrasting management histories. Chemosphere, 69, 82–88.
Black, C.A. (1965). Methods of soil analysis (part- 1 and 2) American Soc. Agron. Madison, Wisconsin, U.S.A.
Bloomfield, J. P., Williams, R. J., Gooddy, D. C., Cape, J. N., & Guha, P. (2006). Impact of climate change on the fate and behavior of pesticides in surface and ground water—A UK perspective. Science of the Total Environment, 369, 163–177.
Boxall, A. B. A., Hardy, A., Beulke, S., Boucard, T., Burgin, L., Falloon, P. D., et al. (2009). Climate change's effect on indirect exposure to agricultural pathogens/chemicals: Impacts of climate change on transport, fate, and exposure. Environmental Health Perspective, 117, 508–514.
Carney, K. M., Hungate, B. A., Drake, B. G., & Megonigal, J. P. (2007). Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proceedings of National Academy of Sciences USA, 104, 4990–4995.
Casida, L. E., Klein, D. A., & Santoro, T. (1964). Soil dehydrogenase activity. Soil Science, 98, 371–376.
Das, S., Bhattacharyya, P., & Adhya, T. K. (2011). Interaction effects of elevated CO2 and temperature on microbial biomass and enzyme activities in tropical rice soils. Environment Monitoring and Assessment, 182, 555–569.
De Graaff, M., Van Groenigen, K., & Six, J. (2006). Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta analysis. Global Change Biology, 12, 2077–2091.
Drigo, B., Kowalchuk, G. A., & van Veen, J. A. (2008). Climate change goes underground: Effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biology and Fertility of Soils, 44, 667–679.
Gajbhiye, V. T., Gupta, S., Mukherjee, I., Singh, S. B., Singh, N., Dureja, P., et al. (2011). Persistence of azoxystrobin in/on grapes and soil in different grapes growing areas of India. Bulletin of Environmental Contamination and Toxicology, 86, 90–94.
Ghosh, R. K., & Singh, N. (2009). Leaching behaviour of azoxystrobin in soil columns. Pest Management Science, 65, 1009–1014.
Green, V. S., Stott, D. E., & Diaek, M. (2006). Assay of fluorescene diacatae hydrolytic activity optimization of soil samples. Soil Biology and Biochemistry, 38, 693–701.
Henry, H. A. L., Juarez, J. D., Field, C. B., & Vitousek, P. M. (2005). Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Global Change Biology, 11, 1–8.
Hill, P. W., Marshall, C., Williams, G. G., Blum, H., Harmens, H., Jones, D. L., et al. (2007). The fate of photosynthetically fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. New Phytology, 173, 766–777.
Hoque, M. M., Inubushi, K., Miura, S., Kobayashi, K., Kim, H. Y., & Okada, M. (2001). Biological dinitrogen fixation and soil microbial biomass carbon as influenced by free-air carbon dioxide enrichment (FACE) at three levels of nitrogen fertilization in a paddy field. Biology and Fertility of Soils, 34, 453–459.
Inubushi, K., Hoque, M., Miura, S., Kobayashi, K., Kim, H. Y., & Okada, M. (2001). Effect of free-air CO2 enrichment (FACE) on microbial biomass in paddy field soil. Soil Science and Plant Nutrition, 47, 737–745.
Inubushi, K., Mizuno, T., Lou, Y., Hasegawa, T., Lin, Y., Cheng, W., Kobayashi, K., Okada, M. (2010). Microbial biomass and activities in a Japanese paddy soil with differences in atmospheric CO2 enrichment, soil/water warming and rice cultivars. 19th World Congress of Soil Science, Soil Solutions for a Changing World, 1–6 August 2010, Brisbane, Australia.
Inubushi, K., Cheng, W., Mizuno, T., Lou, Y., Hasegawa, T., Sakai, H., et al. (2011). Microbial biomass carbon and methane oxidation influenced by rice cultivars and elevated CO2 in a Japanese paddy soil. European Journal of Soil Science, 62, 69–73.
IPCC. (2007). Climate Change 2007: The physical science basis, contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jackson, M. L. (1967). Soil chemical analysis. New Delhi: Prentice Hall Inc.
Janus, L., Angeloni, N., McCormack, J., Rier, S., Tuchman, N., & Kelly, J. (2005). Elevated atmospheric CO2 alters soil microbial communities associated with trembling aspen (Populus tremuloides) roots. Microbial Ecology, 50, 102–109.
Joseph, R.S.I. (2000) Metabolism and degradation of the fungicide azoxystrobin. In: Book of Abstracts of 219th ACS National Meeting, ACS: American Clinical Society National Meeting, San Francisco, CA, March 26–30, 2000, Washington DC.
Kang, H., Kim, S. W., Fenner, N., & Freeman, C. (2005). Shifts of soil enzyme activities in wetlands exposed to elevated CO2. Science of the Total Environment, 337, 207–212.
Krull, E. S., Skjemstad, J. O., Burrows, W. H., Bray, S. G., Wynn, J. G., Bol, R., et al. (2005). Recent vegetation changes in central Queensland, Australia: Evidence from δ13C and 14 C analyses of soil organic matter. Geoderma, 126, 241–259.
Lesaulnier, C., Papamichail, D., McCorkle, S., Ollivier, B., Skiena, S., Taghavi, S., et al. (2008). Elevated atmospheric affects soil microbial diversity associated with trembling aspen. Environmental Microbiology, 10, 926–941.
Li, X., Han, S., Guo, Z., Shao, D. & Xin, L. (2010) Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biology and Biochemistry, 42, 1101-1107.
Li, Z., Yagi, K., Sakai, H., & Kobayashi, K. (2004). Influence of elevated CO2 and nitrogen nutrition on rice plant growth, soil microbial biomass, dissolved organic carbon and dissolved CH4. Plant and Soil, 258, 81–90.
McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S. (2001). Climate change: Impacts adaptation and vulnerability. Third Assessment Report of Intergovernmental Panel for Climate Change, Cambridge University Press, Cambridge.
Pal, M., Karthikeyapandian, V., Jain, V., Srivastava, A. C., Raj, A., & Sengupta, U. K. (2004). Biomass production and nutritional levels of berseem (Trifolium alexandrium) grown under elevated CO2. Agriculture Ecosystem and Environment, 101, 31–38.
Singh, N., & Singh, S. B. (2010). Effect of moisture and compost on fate of azoxystrobin in soils. Journal of Environment Science and Health, B45, 676–681.
Tabatabai, M. A., & Bremner, J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry, 1, 301–307.
Vance, E. D., Broke, P. C., & Jenkinson, D. S. (1987). Microbial biomass measurements in forest soils: The use of chloroform fumigation–incubation method in strongly acidic soils. Soil Biology and Biochemistry, 19, 697–702.
Williams, J. R., Richardson, C. W., & Griggs, R. H. (1992). The weather factor: Incorporating weather variance into computer simulation. Weed Technology, 6, 731–735.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manna, S., Singh, N. & Singh, V.P. Effect of elevated CO2 on degradation of azoxystrobin and soil microbial activity in rice soil. Environ Monit Assess 185, 2951–2960 (2013). https://doi.org/10.1007/s10661-012-2763-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2763-1