Abstract
Biocontrol agents and plant growth promoting microbes have emerged as promising tools for the management of plant diseases and in sustainable crop production. The present study was made to explore the potential metabolites of actinomycetes and endophytes of rice (Oryza sativa L.) and to assay the induced defence reactions in rice plants against bacterial blight (BB) of rice (Xanthomonas oryzae pv. oryzae). In-vitro studies revealed that few promising rhizospheric Streptomyces (S. fimicarius and S. laurentii) and endophytes (Pseudomonas putida and Metarhizium anisopliae) could suppress Xoo effectively in dual culture assay. P. putida + S. fimicarius + S. laurentii showed the highest (58.71%) inhibition of BB. Multiple growth promoting characteristics were assayed for these effective isolates. The effectiveness of the cell suspension (107 cfu/ml) of P. putida, M. anisopliae, S. fimicarius and S. laurentii in suppressing BB of rice was tested in planta by applying as seed treatment, root treatment, soil treatment and spray application. Lowest disease incidence was observed in plants treated with the combination of P. putida + S. fimicarius + S. laurentii (10.29%) as compared to other treatments. To understand the resistance mechanisms of rice plants against Xoo, few effective secondary metabolites were assayed where the total phenol content of the treated plants showed higher concentration (2.52%). Antibiotics were recorded at a higher peak of retention time, such as pyrisulfoxin B (12.26 min), APHE 4 (13.42 min), kanamycin C (22.22 min), nitracidomycin B (15.40 min), clavulanic acid (7.89 min), neothramycin A (16.22 min), nitracidomycin A (17.78 min) and furaquinocin E (24.74 min).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implementation of eco-friendly, cost effective and sustainable management practices are a requirement for management of one of the most devastating diseases like Bacterial blight (BB) of rice caused by Xanthomonas oryzae pv. oryzae (Xoo). To-date, various management strategies have been employed to reduce the losses caused by the pathogen but these approaches have shown only limited success. Total phenolic content in rice leaves pre-inoculated with Xoo significantly increases due to the application of different biocontrol agents (Gangwar and Sinha 2014). Biocontrol agents and Plant Growth Promoting Microbes (PGPM) are known to improve crop growth and helps induction of defence mechanisms through hypersensitive cell death or lignin accumulation in the cell walls (Nicholson and Hammerschmidt 1992).
Researchers have reported the use of species of the genus Streptomyces against plant pathogens including Xoo (Rizk et al. 2007; Park et al. 2011). A high percentage of antibiotics are derived from species of the genus Streptomyces, which enables them to compete with other microorganisms (de Lima Procopio et al. 2012). Hastuti et al. (2012) reported the use of Streptomyces sp. to reduce the intensity of the BB disease.
Endophytes have been used against a wide array of plant pathogens thereby enhancing plant growth and yield by means of several mechanisms. Endophytic strains help in enhancing plant growth by synthesizing phytohormones such as indole-3-acetic acid (IAA), cytokinins, gibberellins, production of siderophores, and solubilize insoluble inorganic phosphorus compounds in the soil (Dinic et al. 2014; Kumar et al. 2015).
The use of biocontrol agents and plant growth promoting microbes to manage plant diseases and improve plant growth is an emerging area of research. However, the success rate of some biological control agents under field conditions is limited. Hence, in this study, a new group of biocontrol agents have been evaluated for effective management of bacterial blight of rice. Moreover, beneficial microbes exhibit an extensive metabolic capability which enhances the defence mechanisms of plants and aids in suppression of plant pathogens. Hence, research on the secondary metabolites produced by any such microbes has also been carried out, which further aligns such processes with plant microbe interaction. Streptomyces spp. and endophytes are often under-explored for plant growth promoting activities as compared to other microbes. Studies conducted with rhizospheric Streptomyces and endophytes may reveal many unexplored facets of their biocontrol and plant growth promoting potential.
Our study aimed to develop a biocontrol strategy for the effective management of BB of rice using rhizospheric Streptomyces and endophytic microbes of the rice ecosystem, assessing secondary metabolites of these microbes for their role in the enhancement of defence related reactions in rice plants against BB. The main objectives of the present study were to: (i) isolate different rhizospheric Streptomyces and endophytic microbes of rice ecosystem; (ii) combine the effective rhizospheric Streptomyces and endophytic microbes for management of BB; (iii) detect plant growth promoting characteristics of promising isolates; and (iv)identify secondary metabolites produced by effective rhizospheric Streptomyces.
Materials and methods
Isolation of Xanthomonas oryzae pv. oryzae (Xoo), rhizospheric Streptomyces and endophytic microbes
Infected leaves showing typical symptoms of bacterial blight were collected from Regional Agricultural Research Station (RARS), Titabar and Assam Agricultural University, Jorhat for isolation of the pathogen Xoo using sucrose peptone agar media (g/L peptone 5, dipotassium phosphate 0.5, sucrose 20, magnesium sulfate heptahydrate 0.25, agar 20, pH 7.2–7.4) and modified Wakimoto’s media (g/L ferrous sulphate 0.05 calcium nitrate 0.5, sodium phosphate 0.82, peptone 5, sucrose 20, agar 17, pH 6.8–7.0). Sucrose peptone Agar (SPA) and modified Wakimoto’s media were the common culture media for isolation of Xoo.
The rhizospheric soil samples for the isolation of Streptomyces were collected from rice fields of different locations of Jorhat and Lakhimpur districts of Assam using the modified protocol suggested by Maleki et al. (2013). The soil samples were collected from 10 to 15 cm depth. 5 g of soil sample was transferred into 45 ml sterile distilled water, shaken for 30 mins and serially diluted up to five times. 100 μl of 10−4 dilution was used for inoculation on Ken Knight media (g/L- dextrose 1, monopotassium dihydrogen phosphate 0.1, sodium nitrate 0.1, potassium chloride 0.1, magnesium sulphate 0.1, agar 15, pH 7.0–7.2). Then the plates were incubated for 3 days at 28 °C.
Endophytes were isolated from the healthy leaf, stem and root samples of rice plants collected from Jorhat and Majuli districts of Assam using the modified protocol suggested by McInroy and Kloepper (1995). Samples of 2 g each were weighed and cut into 2-3 cm pieces, surface disinfected with 70% alcohol, 1% sodium hypochlorite, and rinsed three times with sterile water. A 0.1 ml aliquot was taken from the final wash and transferred to a sterile petri plate which served as the sterility check. If any growth was observed on the sterility check, the samples were discarded. Each sample was then triturated using 9 ml of 0.02 M potassium phosphate buffer (pH 7.0). The triturate was serially diluted in potassium phosphate buffer solution and plated on potato dextrose agar media (g/L peeled potato 200, dextrose 20, agar 20, pH 6.0), rose Bengal agar media (g/L dextrose 10, papaic digest of soybean meal 5, monopotassium phosphate 1, magnesium sulfate 0.5, chloramphenicol 0.1, rose Bengal 0.05, agar 15, pH 7.0–7.2), nutrient agar media (g/L beef extract 1, peptone 2.5, glucose 1.25, agar 10, pH 6.9–7) and Kings B media (g/L protease peptone 20, dipotassium phosphate (anhydrous) 1.5, magnesium sulfate heptahydrate 1.5, glycerol 15, agar 20, pH 6.8). Potato dextrose agar and rose Bengal Agar media were used for the isolation of fungal endophytes, whereas nutrient agar and Kings B media were used for isolation of bacterial endophytes. McInroy and Kloepper (1995) isolated endophytic bacteria from surface-disinfested stems and roots of cotton and sweet corn.
In-vitro screening of effective rhizospheric Streptomyces and endophytes against Xoo
Antimicrobial activity of rhizospheric Streptomyces and endophytes were carried out using a modified dual culture assay method (Aspiras and Cruz 1985) using NA. The diameter of the inhibition zones was measured after 24 h and 48 h of incubation. Compatibility among the effective isolates was also tested in vitro using NA as basal media.
Characterization of effective rhizospheric Streptomyces and endophytes
For morphological and cultural characterization, colony characters were recorded and the following biochemical tests were done for bacteria: gram staining, levan production, oxidase test, nitrate reduction test, gelatin liquefaction test, Simmons’ citrate agar test, starch hydrolysis, catalase test, KOH test, indole production and arginine dihydrolase test. Colour and growth of the colony were recorded as the cultural character of the endophytic fungal pathogen. Morphological characters including size and shape of the conidia and the presence of septa were recorded.
The genomic bacterial DNA was isolated following the modified method of Cardinal et al. (1997). The fungal genomic DNA was done following the modified method of Al-Samarrai and Schmid (2000).
In-vitro screening of effective rhizospheric Streptomyces and endophytes for their plant growth promoting activities
Production of HCN by the effective rhizospheric Streptomyces and endophytes were determined by the standard protocol described by Bakker and Schipper (1987). Indole Acetic Acid production and production of siderophore were observed as described by Bric et al. (1991) and Schwyn and Neilands (1987) respectively. The assay of phosphate solubilizing activity of the effective rhizospheric Streptomyces and endophytes was determined on Pikovskaya agar as described by Pikovskaya 1948. Zinc solubilization was determined by standard protocol (Fasim et al. 2002). Potassium solubilization was observed by inoculating the effective strains on the Aleksandrov agar plates (Etesami et al. 2017).
Liquid chromatography-mass spectrometry (LC-MS) analysis of rhizospheric Streptomyces
The effective rhizospheric Streptomyces were grown in an appropriate culture media which was filtered and mixed with an equal volume of methanol and kept at 28 ± 1 °C overnight. The cultures were further centrifuged and the supernatant obtained was dried under vacuum. Dried powders were further dissolved in 2 ml of HPLC grade methanol which was passed through a 0.22 μm syringe filter. Each sample (20 μl) was loaded into a UPLC system (Waters, USA) and passed through C18 column with a gradient elution containing acetonitrile (Solvent A), 0.1% formic acid 95:5 H2O: ACN (Solvent B). Detection of the fractions was done using an EST-QTOF-Mass Spectrometer attached to the UPLC system in a positive ionization mode.
In planta evaluation for management of BB of rice
The study was conducted in 30 cm diameter plastic pots, each containing 3 kg of clayey loam soil. The two rhizospheric Streptomyces and endophytic microbes showing highest inhibition (%) against Xoo were further screened for suppression of BB in pot-grown rice plants (cv. TN1) under green shade net conditions by applying as seed treatment (100 seeds/20 ml of solution), root treatment (100 seedlings/100 ml of solution), soil treatment (100 ml /plant) and spray application (2% solution). Per cent disease incidence (PDI) and per cent disease reduction were recorded to study the effect of rhizospheric Streptomyces and endophytic microbes. Disease severity percentage was calculated based on the IRRI standard evaluation system for rice (IRRI 2002). Total phenol content in rice plants treated with rhizospheric Streptomyces and endophytic microbes was estimated using the Folin-ciocalteu method (Bray and Thorpe 1954).
Statistical analysis
Statistical analysis of data was made using Analysis of Variance (ANOVA). The data per cent values were transformed by the angular transformation. Critical differences were calculated to compare different treatments.
Results
Characterization of Xoo, rhizospheric Streptomyces and endophytes
The bacterial blight pathogen (Xoo) appeared as light yellow, circular, convex, opaque with a smooth surface on modified Wakimoto’s and SPA media. Morphologically the bacterium was rod shaped and gram negative. Positive results were obtained for KOH test, citrate utilization, gelatin liquefaction, catalase test, levan production test and negative reaction to starch hydrolysis, nitrate reduction, oxidase test, indole production and arginine dihydrolase test. Field Emission Scanning Electron Microscope (FESEM) also revealed the bacterium to be rod shaped. Pathogenicity test of Xoo revealed typical disease symptoms of the inoculated plants within 10 days.
Sixteen rhizospheric Streptomyces were isolated from soil samples of rice fields which were assigned with unique identification codes such as S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15 and S16.
Twenty-six endophytic microbes were isolated from healthy leaves, stems and root samples of rice plants collected from different locations. Altogether 20 numbers of bacterial endophytes were isolated which were assigned with unique identification codes such as EB1, EB2, EB3, EB4, EB5, EB6, EB7, EB8, EB9, EB10, EB11, EB12, EB13, EB14, EB15, EB16, EB17, EB18, EB19, and EB20. Similarly, six fungal endophytes were isolated which were assigned with identification codes such as EF1, EF2, EF3, EF4, EF5 and EF6.
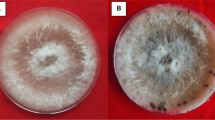
The inhibition zones produced (mm) by the effective rhizospheric Streptomyces sp. and endophytes against Xoo in dual culture assay were recorded (Table 1). Among the rhizospheric Streptomyces strains, S15 showed an inhibition zone (28 mm diam.) followed by S2 (24.54 mm diam.). Among the endophytes, EB8 showed the maximum inhibition zone (38.97 mm diam) followed by EF2 (24.9 mm diam). The compatibility test between effective rhizospheric Streptomyces and endophytic microbes revealed that EB8 + EF2, S2 + S15, EB8 + S2, EB8 + S15, EB8 + S2 + S15, EF2 + S2, EF2 + S15 and EF2 + S2 + S15 were found compatible in their reactions. The treatment combinations which showed positive results were again tested against Xoo which revealed that a combination of rhizospheric Streptomyces (S2 and S15) and endophytic bacterium (EB8) could inhibit the BB pathogen by up to 58.71%. The effective rhizospheric Streptomyces strains S2 and S15, as well as endophytic microbes EB8 and EF2, were further characterised.
The results of Gram staining revealed that rhizospheric Streptomyces S2 and S15 were gram positive, bearing long filamentous strands. The colony of S2 appeared grey, while the colony of S15 appeared dusty pink. S2 showed positive reaction in starch hydrolysis test, oxidase test, catalase test, gelatin liquefaction; whereas S15 showed positive responses for five biochemical tests namely, starch hydrolysis test, catalase test, gelatin liquefaction test, oxidase test and indole production. Moreover, scanning electron microscopy of S2 and S15 revealed that the bacterial cells were filamentous. The endophytic bacterium EB8 was gram negative and positive for the gelatin liquefaction test, catalase test, oxidase test, citrate utilization test, nitrate reduction test, arginine dihydrolase test, KOH test and levan production test. It produced a greenish yellow colony on King’s B medium which had smooth edges and convex shape. Scanning electron microscopy results also revealed the bacterium to be rod shaped.
The 16S rRNA gene was amplified from the bacterial genomic DNA using bacterial universal primers 27F and 1492R. For the fungal endophyte, the genomic DNA was subjected to PCR-amplification of the ITS region of the rDNA using ITS1 and ITS4 primers. Agarose gel (1.2%) electrophoresis showed a clean amplification of ~1400 bp fragments for rhizospheric Streptomyces S2 and S15 as well as endophytic bacterium EB8. PCR products were outsourced for sequencing and sequenced data were compared against the National Centre for Biotechnology Information using BLAST® (Basic Local Alignment Search Tool) programme (Altschul et al. 1990). The phylogenetic analysis revealed that rhizospheric S2 showed the highest homology with Streptomyces fimicarius (Fig. 1), whereas the rhizospheric S15 showed the highest homology with Streptomyces laurentii (Fig. 2). Moreover, endophytic bacterium EB8 showed the highest homology with Pseudomonas putida (Fig. 3).
The colony of endophytic fungus EF2 appeared white, turned green with conidia developing simultaneously. The hyphae appeared septate and branched. Aseptate, cylindrical to ovoid conidia formed in laterally adherent chains were observed. Conidia varied from 5.5 to 7.15 μm in length and 1.8 to 2.87 μm in width. For the fungal endophyte, the genomic DNA was subjected to PCR-amplification of the ITS region of the rDNA using ITS1 and ITS4 primers. Agarose gel (1.2%) electrophoresis showed a clean amplification of ~500 bp for endophytic fungus EF2. The phylogenetic analysis revealed that endophytic fungus EF2 showed the highest homology with Metarhizium anisopliae (Fig. 4).
Evaluation of different plant growth promoting activities of effective rhizospheric Streptomyces and endophytic microbes
Rhizospheric Streptomyces (S. fimicarius and S. laurentii) and endophytic P. putida could produce HCN, as the filter paper changed from yellow to brown. Moreover, rhizospheric Streptomyces fimicarius (2.42 μg/ml), S. laurentii (2.65 μg/ml) and endophytic P. putida (0.70 μg/ml) showed a positive result for IAA production due to the development of pink colour. The formation of clear zones around their colonies on Pikovskaya medium (PVK) indicated positive results for phosphorus solubilisation by rhizospheric S. laurentii (25.60 mm) and endophytic M. anisopliae (30.68 mm). Rhizospheric Streptomyces fimicarius (23.40 mm), S. laurentii (21.60 mm) and endophytic P. putida (40.20 mm), M. anisopliae (41.40 mm) showed positive results for zinc solubilisation due to the production of clear zones around their colonies when inoculated in ZSB medium. Rhizospheric S. fimicarius (19.60 mm), S. laurentii (26.20 mm) and endophytic P. putida (18.40 mm) produced siderophores as evidenced through the formation of a yellow halo. Endophytic P. putida (15.40 mm) could solubilize potassium as indicated by the formation of halo zone around their colonies when inoculated in Aleksandrov agar medium.
Detection of secondary metabolites produced by Streptomyces fimicarius and Streptomyces laurentii using liquid chromatography-mass spectrophotometry (LC-MS) analysis
The presence of various peaks that appeared in the chromatogram showed the presence of various active metabolites in methanol crude extract of S. fimicarius as shown in Fig. 5 and S. laurentii as shown in Fig. 6.
Five of the compounds in the S. fimicarius extract indicated by highest peaks were tentatively identified as pyrisulfoxin B with the peak at a retention time of 12.26 min, APHE 4 with the peak at a retention time of 13.42 min, kanamycin C with the peak at a retention time of 22.22 min, nitracidomycin B with the peak at a retention time of 15.40 min and clavulanic acid with the peak at a retention time of 7.89 min.
Four of the compounds in the S. laurentii extract indicated by highest peaks were tentatively identified as APHE 4 with the peak at a retention time of 13.42 min, neothramycin A with the peak at a retention time of 16.22 min, nitracidomycin A with the peak at a retention time of 17.78 min, and furaquinocin E with the peak at a retention time of 24.74 min.
Efficacy of the various combinations of rhizospheric Streptomyces and effective endophytic microbes in management of BB of rice
Selected rhizospheric Streptomyces and endophytic microbes were tested in pot-grown rice plants for effective management of BB of rice (var. TN1) by applying these as seed treatment, root treatment, soil treatment and spray application. Fourteen treatment combinations were tested in three replications, one maintained as uninoculated control and one as inoculated control. Data (Table 2) revealed that EB8 + S2 + S15 recorded the lowest disease incidence (10.29%) and disease severity of 9.73% respectively. Application of effective rhizospheric Streptomyces and endophytic microbes as different treatment combinations had a significant effect on rice yield and yield attributing characteristics. The highest yield (50.06 g per hill), tiller number per hill (21.66), shoot length (112.80 cm), number of panicles (20.66), panicle length (28.76 cm), panicle weight (29.16 g), shoot dry weight (63.76 g), root dry weight (46.54 g), root shoot ratio (0.73), root length (43.6 cm), test weight (25 g) were recorded in plants inoculated with P. putida + S. fimicarius + S. laurentii as compared to other treatments.
P. putida, M. anisopliae, S. fimicarius and S. laurentii were able to colonize the treated rice plants as they were recovered from the samples collected from inoculated treatments.
Estimation of phenols
Total phenolic content in rice leaves was determined using the Folin-Ciocalteau method. P. putida + S. fimicarius + S. laurentii recorded the highest phenol content of 2.52% as compared to other treatments. Total phenol content in rice leaves (Table 3) was estimated by adopting the Folin-Ciocalteau method. Hence, the difference in a significant level of phenol content was observed between inoculated control and all treatments.
Discussion
In our present study, 16 rhizospheric Streptomyces were isolated which were tested for their antagonistic potential against Xoo. Two isolates (S2 and S15) showed the highest inhibition zones, viz. 27.27 and 31.10%, respectively. Based on 16S rRNA sequence analysis, S2 showed maximum homology with Streptomyces fimicarius whereas S15 showed the highest homology with Streptomyces laurentii. Altogether, 26 endophytic microbes were isolated from healthy leaf, stem and root samples of rice. Two endophytes (EB8 and EF2) showed high zone of inhibition against Xoo in vitro, viz. 43.30% and 27.67% respectively. Based on 16S rRNA sequence analysis, endophytic bacterium EB8 showed maximum homology with Pseudomonas putida. Based on the 18S rRNA sequence analysis, endophytic fungus EF2 showed maximum homology with Metarhizium anisopliae.
Fluorescent pseudomonads can act as antagonists in the management of various plant diseases (Ganeshan and Manoj Kumar 2005). The mechanisms of biocontrol may include antibiosis, cyanide production, siderophore production, competition for space and nutrients, and activation of induced systemic resistance (Santoyo et al. 2012). Trejo et al. (1977) reported the production of pinkish blush on white aerial mycelium and light rose pigmentation by S. laurentii. The results of compatibility tests revealed that most of the antagonistic microbes (rhizospheric Streptomyces as well as endophytes) were compatible with each other except the combination P. putida, M. anisopliae, S. fimicarius and S. laurentii. The treatment combinations which showed positive results in compatibility tests were again tested in planta to determine their ability to inhibit Xoo as well as their plant growth promoting activity. Lowest disease incidence (10.29%) and disease severity (9.73%) were recorded in plants inoculated with P. putida, S. fimicarius and S. laurentii. Hence, the combination of treatments was found most effective compared to individual treatments in vivo which might be because of the multiple modes of action and synergistic effect of more number of organisms. Several researchers have also reported the combination of bio-agents into consortia was more effective as compared with a single agent (Larkin and Fravel 1998; Maketon et al. 2008; Bora et al. 2015; Bora et al. 2016). Consortia of three fungi, viz., T. harzianum, T. viride, and T. viride, one bacteria B. subtilis and one actinomycete, S. thermodiastaticus was found more effective against bacterial wilt of tomato under pot culture conditions (James and Mathew 2015). Four endophytes, namely Bacillus sp., B. subtilis, P. putida and Enterobacter sp., were found to be effective in inhibiting Xoo (Yousefi et al. 2018). Five Streptomyces isolates showed antagonistic activity against X. campestris pv. oryzae, the BB pathogen (Sripreechasak et al. 2014). Xiao et al. 2002 reported the ability of Streptomyces sp. to control Phytophthora root rots on alfalfa and soybean caused by Phytophthora medicaginis and P. sojae, respectively. El-Abyad et al. 1993 showed that 80% concentration of the culture filtrate of Streptomyces pulcher or Streptomyces canescens inhibited spore germination, mycelial growth and sporulation of Fusarium oxysporum f.sp. lycopersici, Verticillium alboatrum, and Alternaria solani causing major diseases of tomato. Hop et al. (2014) reported the ability of Streptomyces toxytricini VNO8-A-12 to reduce Xoo lesion lengths up to an extent of 38.3%, significantly reduced Xoo related yield loss up to 43.2%, and increased rice yield from 2.66% to 16.98% in the rice cultivar SSI and from 3.11% to 5.94% in the rice cultivar KD18. Law et al. (2017) showed that Streptomyces sp. exhibited inhibitory activity against the rice blast fungus, M. oryzae. Infected rice seedlings treated with Streptomyces sp. caused 88.3% reduction of rice blast disease. In the present study, the highest phenol content was observed in treatments comprising Pseudomonas putida, Streptomyces fimicarius and Streptomyces laurentii (2.52%). Gangwar and Sinha (2014) studied the total phenolic content in rice leaves pre-inoculated with Xoo. Maximum mean total phenolic content was observed in plants treated with four formulations of fluorescent pseudomonads and Trichoderma sp. followed by P. fluorescens and T. harzianum. Endophytic P. putida showed positive responses for HCN production, IAA production, siderophore production, phosphorus, potassium, and zinc solubilisation. Similar results were obtained for P. putida which could produce HCN, IAA and siderophores (Kumar et al. 2015), and solubilise phosphorus (Baliah et al. 2016), zinc (Bapiri et al. 2012) and potassium (Mursyida et al. 2015). Moreover, endophytic M. anisopliae showed positive responses for zinc and phosphorus solubilisation. Pandey et al. (2006) reported the ability of Metarhizium sp. to solubilise insoluble P to available forms. In the present study, rhizospheric Streptomyces (S. fimicarius and S. laurentii) showed positive responses in HCN production, IAA production, siderophore production and zinc solubilisation, whereas rhizospheric S. laurentii showed positive results for phosphorus solubilisation. These results confirmed the results obtained by Anwar et al. (2016), Thampi and Bhai (2017) for rhizospheric Streptomyces. The results suggested that increased growth of rice plants on the application of endophytic microbes (P. putida and M. anisopliae) and rhizospheric Streptomyces (S. fimicarius and S. laurentii) might be due to indole acetic acid, hydrogen cyanide, siderophore production, and zinc, phosphorus and potassium solubilisation.
The study on secondary metabolites detected five compounds in the S. fimicarius extract, pyrisulfoxin B, APHE 4, kanamycin C, nitracidomycin B, and clavulanic acid; whereas there were four compounds in the S. laurentii extract, APHE 4, neothramycin A, nitracidomycin A, and furaquinocin E. Hence, APHE 4 was produced by both S. fimicarius and S. laurentii. Lucas et al. (2012) mentioned the KNApSAcK database which reported the production of pyrisulfoxin B by S. californicus BS-75, APHE 4 by S. aurantiogriseus NPO-101, kanamycin C by S. kanamyceticus, nitracidomycin B and nitracidomycin A by S. viridochromogenes SANK 60784, neothramycin A by Streptomyces No. MC916-C4 and furaquinocin E by Streptomyces sp. KO-3988. Neto et al. (2005) isolated a secondary metabolite, clavulanic acid, from S. clavuligerus. Kanamycin is an aminoglycoside antibiotic which binds to the bacterial 30S ribosomal subunit causing misreading of t-RNA which leads to the inhibition of bacterial pathogens (Kotra et al. 2000). Nitracidomycin A and B can induce the spheroplast formation of gram-negative bacteria (Takeuchi et al. 1989). Clavulanic acid (CA) produced by Streptomyces sp. is an inhibitor of β-lactamases enzymes that induces the hydrolysis of β-lactam antibiotics. Neothramycin A has higher antimicrobial activity than neothramycin B (Takeuchi et al. 1976). A number of these antimicrobial compounds produced by S. fimicarius and S. laurentii might lead to direct inhibition of Xoo.
Research on rhizospheric Streptomyces and endophytic microbes on the development of novel biocontrol agents and plant growth promoting characteristics is still an emerging area that requires further investigation. The step by step isolation of rhizospheric Streptomyces and endophytes, their antagonistic effect against Xoo, along with the features of plant growth promoting activity and assessment of secondary metabolites of rhizospheric Streptomyces, can make a better formulation to achieve maximum yield of rice plants. The role of the fungal endophyte M. anisopliae in the suppression of Xoo and its plant growth promoting potential is a new area of research which needs further investigation. Moreover, the antagonistic effect of Streptomyces fimicarius and Streptomyces laurentii against Xoo, their plant growth promoting characteristics, and detection of secondary metabolites have not previously been identified. Further, the increased phenol content of rice plants upon the application of P. putida, M. anisopliae, S. fimicarius,and S. laurentii was not estimated in the earlier studies. In this study, a decrease in bacterial blight severity was observed with the increase of phenol content in rice leaves treated with the effective isolates (P. putida, M. anisopliae, S. fimicarius, S. laurentii).
This study highlights the great potential of rhizospheric Streptomyces (S. fimicarius and S. laurentii) and endophytes (P. putida and M. anisopliae) as biocontrol agents which possess multiple traits including plant disease suppression and plant growth promotion activity by producing indole acetic acid, hydrogen cyanide, siderophores, zinc, phosphorus and potassium solubilisation, increased phenol content in plants, and secondary metabolites production by S. fimicarius and S. laurentii. Thus the bioagents not only promote plant growth but also stimulate the defence pathways by enhancing host resistance against Xoo infection through production and activation of secondary metabolites and phenols related to ISR in rice. The present study thus showed the effects of P. putida, M. anisopliae, S. fimicarius and S. laurentii in rice plants challenged with Xoo when applied alone or in combination. Our findings indicated that rhizospheric Streptomyces (S. fimicarius and S. laurentii) and endophytes (P. putida and M. anisopliae) have immense potential to be developed as biocontrol agents for the effective management of bacterial blight of rice.
Conclusions
The study presents the characterization and effect of promising rhizospheric Streptomyces and endophytic microbes for the management of BB of rice (variety TN1). In vitro studies revealed that combination of endophytic P. putida, rhizospheric S. fimicarius and S. laurentii could inhibit the bacterial blight pathogen up to an extent of 58.71%. The combination P. putida + S. fimicarius + S. laurentii (EB8 + S2 + S15) was found most effective in vivo as the lowest disease incidence, disease severity and the highest phenol content was observed in plants treated with this combination. Indole acetic acid, hydrogen cyanide, siderophore production, zinc, phosphorus and potassium solubilisation by the effective isolates had profound activity in the plant growth promotion. Secondary metabolites produced by S. fimicarius and S. laurentii can be attributed to the antibacterial activity against Xoo. Based on the above results achieved in this study, rhizospheric Streptomyces (S. fimicarius and S. laurentii) and endophytic microbes (P. putida and M. anisopliae) have great potential to be developed as biocontrol agents for the effective management of bacterial blight of rice. P. putida, M. anisopliae, S. fimicarius and S. laurentii could effectively be used as biocontrol agents for Xoo suppression through host resistance and plant growth promotion.
References
Al-Samarrai, T. H., & Schmid, J. (2000). A simple method for extraction of fungal genomic DNA. Letters in Applied Microbiology, 30(1), 53–56.
Altschul, S., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic alignment search tools. Journal of Molecular Biology, 215(3), 403–410.
Anwar, S., Ali, B., & Sajid, I. (2016). Screening of rhizospheric actinomycetes for various in vitro and in vivo plant growth promoting (PGP) traits and for agroactive compounds. Frontiers in Microbiology, 7, 13–34.
Aspiras, R.B., & Cruz, A.R. (1985). Potential biological control of bacterial wilt in tomato and potato with Bacillus polymyxa Fu 6 and Pseudomonas fluorescens. In : Bacterial wilt disease in Asia and South pacific. Persley, G.D. (ed.). ACIAR Proceedings, Sydney, 13, pp. 89–92.
Bakker, A. W., & Schipper, B. (1987). Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biology and Biochemistry, 19, 451–457.
Baliah, N. T., Pandiarajan, G., & Kumar, B. M. (2016). Isolation, identification and characterization of phosphate solubilizing bacteria from different crop soils of Srivilliputtur Taluk, Virudhunagar District, Tamil Nadu. Tropical Ecology, 57(3), 465–474.
Bapiri, A., Asgharzadeh, A., Mujallali, H., Khavazi, K., & Pazira, E. (2012). Evaluation of zinc solubilization potential by different strains of fluorescent pseudomonads. J. Appl. Sci. Envt. Mngt. Journal of Applied Sciences and Environmental Management, 16(3), 295–298.
Bora, L. C., Kataki, L., Talukdar, K., Nath, B. C., & Sarkar, R. (2015). Molecular characterizations of microbial antagonists and development of bioformulations for management of bacterial wilt of Naga chilli (Capsicum chinense Jacq.) in Assam. Journal of Experimental Biology, 3(2), 109–122.
Bora, L. C., Kataki, L., Talukdar, K., & Khan, P. (2016). Yield enhancement and bacterial wilt suppression in Bhut Jolokia (Capsicum assamicum) using consortia of microbial antagonists. Biopesticides International, 12(2), 165–171.
Bray, H. G., & Thorpe, W. V. (1954). Analysis of phenolic compounds of interest in metabolism. Methods of Biochemical Analysis, 1, 27–52.
Bric, J. M., Bostock, R. M., & Silverstone, S. E. (1991). Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Applied and Environmental Microbiology, 57(2), 535–538.
Cardinal, M. J., Meghrous, J., Lacroix, C., & Simard, R. E. (1997). Isolation of Lactococcus lactis strain producing inhibitory activity against Listeria. Food Biotechnology, 11(2), 129–146.
de Lima Procopio, R. E., da Silva, I. R., Martins, M. K., de Azevedo, J. L., & de Araújo, J. M. (2012). Antibiotics produced by Streptomyces. The Brazilian Journal of Infectious Diseases, 16(5), 466–471.
Dinic, Z., Ugrinovic, M., Bosnic, P., Mijatovic, M., Zdravkovic, J., Miladinovic, M., & Jošic, D. (2014). Solubilization of inorganic phosphate by endophytic Pseudomonas sp. from French bean nodules. Ratarstvo i povrtarstvo, 51(2), 100–105.
El-Abyad, M. S., El-Sayed, M. A., El-Shanshoury, A. R., & El-Sabbagh, S. M. (1993). Towards the biological control of fungal and bacterial diseases of tomato using antagonistic Streptomyces spp. Plant and Soil, 149(2), 185–195.
Etesami, H., Emami, S., & Alikhani, H. A. (2017). Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. Journal of Soil Science and Plant Nutrition, 17(4), 897–911.
Fasim, F., Ahmed, N., Parsons, R., & Gadd, G. M. (2002). Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiology Letters, 213(1), 1–6.
Ganeshan, G., & Manoj Kumar, A. (2005). Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. Journal of Plant Interactions, 1(3), 123–134.
Gangwar, G. P., & Sinha, A. P. (2014). Effect of fungal and bacterial bioagent application on total phenolic content in rice leaves pre-inoculated with Xanthomonas oryzae pv. oryzae (Uyeda and Ishiyama) Dowson. Journal of Appied and Natural Science, 6(1), 254–257.
Hastuti, R. D., Lestari, Y., Suwanto, A., & Saraswati, R. (2012). Endophytic Streptomyces spp. as biocontrol agents of rice bacterial leaf blight pathogen (Xanthomonas oryzae pv. oryzae). HAYATI Journal of Biosciences, 19(4), 155–162.
Hop, D. V., Hoa, P. T. P., Quang, N. D., Ton, P. H., Ha, T. H., Hung, N. V., Van, N. T., Hai, T. V., Quy, N. T. K., Dao, N. T. A., & Thom, V. T. (2014). Biological control of Xanthomonas oryzae pv. Oryzae causing Rice bacterial blight disease by Streptomyces toxytricini VN08-A-12, isolated from soil and leaf-litter samples in Vietnam. Biocontrol Science, 19(3), 103–111.
IRRI. (2002). Standard Evaluation System for Rice (SES) (p. 56). International Rice Research Institute: Manila, Philippines.
James, D., & Mathew, K. S. (2015). Evaluation of endophytic microbial consortium for the management of bacterial wilt of tomato caused by Ralstonia solanacearum. Journal of Biological Control, 29(3), 148–156.
Kotra, L. P., Haddad, J., & Mobashery, S. (2000). Aminoglycosides: Perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrobial Agents and Chemotherapy, 44(12), 3249–3256.
Kumar, V., Kumar, A., Pandey, K. D., & Roy, B. K. (2015). Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Annals of Microbiology, 65(3), 1391–1399.
Larkin, R. P., & Fravel, D. R. (1998). Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Disease, 82(9), 1022–1028.
Law, J. W. F., Ser, H. L., Khan, T. M., Chuah, L. H., Pusparajah, P., Chan, K. G., et al. (2017). The potential of Streptomyces as biocontrol agents against the Rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Frontiers in Microbiology, 8, 3.
Lucas, X., Senger, C., Erxleben, A., Grüning, B. A., Döring, K., Mosch, J., Flemming, S., & Günther, S. (2012). StreptomeDB: A resource for natural compounds isolated from Streptomyces species. Nucleic Acids Research, 41(D1), D1130–D1136.
Maketon, M., Apisitsantikul, J., & Siriraweekul, C. (2008). Greenhouse evaluation of Bacillus subtilis AP-01 and Trichoderma harzianum AP-001 in controlling tobacco diseases. Brazilian Journal of Microbiology, 39(2), 296–300.
Maleki, H., Dehnad, A., Hanifian, S., & Khani, S. (2013). Isolation and molecular identification of Streptomyces spp. with antibacterial activity from northwest of Iran. BioImpacts: BI, 3(3), 129–134.
McInroy, J. A., & Kloepper, J. W. (1995). Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Canadian Journal of Microbiology, 41(10), 895–901.
Mursyida, E., Mubarik, N. R., & Tjahjoleksono, A. (2015). Selection and identification of phosphate-potassium solubilizing Bacteria from the area around the limestone Mining in Cirebon Quarry. Research Journal of Microbiology, 10(6), 270–279.
Neto, A. B., Hirata, D. B., Cassiano Filho, L. C. M., Bellão, C., Badino Júnior, A. C., & Hokka, C. O. (2005). A study on clavulanic acid production by Streptomyces clavuligerus in batch, fed-batch and continuous processes. Brazilian Journal of Chemical Engineering, 22(4), 557–563.
Nicholson, R. L., & Hammerschmidt, R. (1992). Phenolic compound and their role in disease resistance. Annual Review of Phytopathology, 30, 369–389.
Pandey, A., Trivedi, P., Kumar, B., & Palni, L. M. S. (2006). Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian central Himalaya. Current Microbiology, 53(2), 102–107.
Park, S. B., Lee, I. A., Suh, J. W., Kim, J. G., & Lee, C. H. (2011). Screening and identification of antimicrobial compounds from Streptomyces bottropensis suppressing rice bacterial blight. Journal of Microbiology and Biotechnology, 21(12), 1236–1242.
Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya, 17, 362–370.
Rizk, M., Abdel, R. T., & Metwally, H. (2007). Screening of antagonistic activity in different Streptomyces species against some pathogenic microorganisms. Journal of Biological Sciences, 7(8), 1418–1423.
Santoyo, G., Orozco-Mosqueda, M. D. C., & Govindappa, M. (2012). Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Science and Technology, 22(8), 855–872.
Schwyn, B., & Neilands, J. B. (1987). Universal assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 47–56.
Sripreechasak, P., Suwanborirux, K., & Tanasupawat, S. (2014). Characterization and antimicrobial activity of Streptomyces strains from soils in southern Thailand. Journal of Applied Pharmaceutical Science, 4(10), 024–031.
Takeuchi, T., Miyamoto, M., Ishizuka, M., Naganawa, H., Kondo, S., Hamada, M., & Umezawa, H. (1976). Neothramycins A and B, new antitumor antibiotics. The Journal of Antibiotics, 29, 93–96.
Takeuchi, M., Inukai, M., Enokita, R., Takatsu, T., Takamatsu, Y., Takahashi, S., & Haneishi, T. (1989). Nitracidomycins A and B, new enteromycin-group antibiotics. The Journal of Antibiotics, 42(2), 329–332.
Thampi, A., & Bhai, R. S. (2017). Rhizosphere actinobacteria for combating Phytophthora capsici and Sclerotium rolfsii, the major soil borne pathogens of black pepper (Piper nigrum L.). Biological Control, 109, 1–13.
Trejo, W. H., Dean, L. D., Pluscec, J., Meyers, E., & Brown, W. E. (1977). Streptomyces laurentii, a new species producing thiostrepton. The Journal of Antibiotics, 30(8), 639–643.
Xiao, K., Linkel, L. L., & Samac, D. A. (2002). Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biological Control, 23(3), 285–295.
Yousefi, H., Hassanzadeh, N., Behboudi, K., & Firouzjahi, F. B. (2018). Identification and determination of characteristics of endophytes from rice plants and their role in biocontrol of bacterial blight caused by Xanthomonas oryzae pv. oryzae. Hellenic Plant Protection Journal, 11(1), 19–33.
Acknowledgements
We thank Assam Agricultural University, Assam, India for rendering necessary facilities and support during the tenure of the investigation.
Funding
This work was funded by Directorate of Post Graduate Studies, Assam Agricultural University, Jorhat, Assam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All the authors have no conflict of interests in the research that was carried out.
Ethical approval
This article does not contain any work on human participants or animals conducted by the authors.
Informed consent
Informed consent was not relevant to this article since no information regarding individual author was involved in this study.
Additional information
Kakumoni Saikia is regarded as the first author
Rights and permissions
About this article
Cite this article
Saikia, K., Bora, L.C. Exploring actinomycetes and endophytes of rice ecosystem for induction of disease resistance against bacterial blight of rice. Eur J Plant Pathol 159, 67–79 (2021). https://doi.org/10.1007/s10658-020-02141-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02141-3