Abstract
Verticillium spp. are soil-borne fungi containing many pathogenic species that affect a wide range of cultivated plants. In potatoes, Verticillium dahliae, V. nonalfalfae and V. albo-atrum cause Verticillium wilt or, together with other pathogens, are responsible for the occurrence of the potato early dying disease. Verticillium pathogens are notoriously difficult to control, and techniques proved to be efficient, such as chemical soil fumigation, are causing environmental concerns worldwide. Phosphites (Phi) represent a class of chemicals with a good environmental footprint, that are currently used to control oomycete pathogens, such as the potato late blight agent, Phytophtora infestans. To determine the potential inhibitory effects of Phi on Verticillium spp., isolates of V. dahliae and V. nonalfalfae were tested in vitro and the IC50 of Phi was found to vary between 60.9 and 481.9 μg/mL. The effects of Phi were further tested in field trials, carried for two years, using Phi-treated potato plants infected with a V. nonalfalfae isolate. Infection progression was assessed in both years by qPCR and was found to be significantly reduced in Phi-treated plants. The concentration of Phi in these plants, determined by high performance ion chromatography, was found to vary between 25.0 and 86.2 μg/mL in leaves and between 55.7 and 113.8 μg/mL in tubers. Even though the concentrations of Phi required to control Verticillium spp. were found to be relatively high compared to those needed to inhibit the development of oomycete pathogens, the results from this study indicate that Phi can limit the development of Verticillium spp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated potato (Solanum tuberosum) is the fourth most important food crop in the world and, with a production of over 388.2 million tons in 2017 (FAOstat data), represents the first non-grain food commodity (FAO 2018). Major difficulties in growing potato commercially are attributed to its susceptibility to various insect pests, such as the Colorado potato beetle (Leptinotarsa decemlineata) and to plant diseases such as late blight, caused by the oomycete Phytophthora infestans (Lobato et al. 2008; Haverkort et al. 2009). Nevertheless, many fungal pathogens can also cause substantial decrease in tuber yields. Among them are the soil-borne fungi Verticillium dahliae, V. nonalfalfae and V. albo-atrum that are causing Verticillium wilt. In heavily infested fields, Verticillium wilt causes early senescence of plants impacting tuber yields (Smith and Rowe 1984; Rowe et al. 1987; Powelson and Rowe 1993; Johnson and Dung 2010; El-Bebany et al. 2013a). Moreover, the combined action of these Verticillium species, and of other fungi, including species from Fusarium and Colletotrichum, root-lesion nematodes, and soft rot bacteria, induce symptoms described as the potato early dying disease (PED), causing very significant reductions in tuber yield and quality (Rowe et al. 1987; Powelson and Rowe 1993; Johnson and Dung 2010). For instance, the average yield in Prince Edward Island (PEI), a province that generates 22.3% of the total potato production in Canada (Statistics_Canada 2017), is around 33 tons/ha, which is below the national average, and significantly less than the yields of approximately 43 tons/ha recorded in the USA (Statistics_Canada 2012). The reduced potato yields in this province might be related to the ubiquitous presence of V. dahliae in the potato production areas (Celetti and Platt 1987; Mahuku and Platt 2002b; Borza et al. 2018). Propagules of V. dahliae, V. nonalfalfae and V. albo-atrum can persist in soil up to a decade, affecting a broad range of annual and perennial plants, including many species used as rotation crops (Rowe et al. 1987; Powelson and Rowe 1993; Agrios 2005; Larkin et al. 2011). Due to the fact that Verticillium isolates are genetically heterogeneous, this group of fungi represents one of the most challenging phytopathogens to control (Jiménez-Gasco et al. 2014; Inderbitzin and Subbarao 2014).
Pesticides are needed to ensure a good crop production, though many of these products have a negative impact on the environment. Pesticides, such as methyl bromide (Gamliel et al. 1997) and chloropicrin (Tsror et al. 2000), have been used to control different soil-borne pathogenic fungi by fumigation, including Verticillium spp., nematodes, insects, and weeds (Rowe and Powelson 2002; Agrios 2005; Bubici et al. 2019). However, these chemicals can be very toxic to humans and animals, and methyl bromide is also considered an ozone-depleting substance (Reimann et al. 2018). Consequently, their usage is restricted or even banned (e.g., methyl bromide) in many countries (Gareau 2017; Reimann et al. 2018), leaving producers with fewer alternative products to control Verticillium wilt in potatoes and other crops (Rowe and Powelson 2002; Ochiai et al. 2007; Davis et al. 2010; Larkin et al. 2011). Disease management strategies using biological control agents such as the antagonistic bacteria Serratia plymuthica and Paenibacillus polymyxa (Rybakova et al. 2016), crop rotation, bio-fumigation and green manures, and solarization (Johnson and Dung 2010) have failed to significantly reduce Verticillium soil inoculum and they are generally not cost effective. Therefore, currently, there are very few approaches that can be used to control Verticillium wilt in potatoes, while the incidence of Verticillium wilt is expected to increase in the future, due to global warming and decreased crop rotation time (Heale and Karapapa 1999; Siebold and Tiedemann 2012).
Phosphites (Phi, HPO32−/H2PO3−) are salts (anions) of phosphorous acid (H3PO3) (Guest and Grant 1991) that are effective in suppressing the development of various oomycetes species from the Phytiaceae and Peronosporaceae families in a large number of crops (Guest and Grant 1991; Thao and Yamakawa 2009; Borza et al. 2014; Gómez-Merino and Trejo-Téllez 2016; Liljeroth et al. 2016). Inhibitory effects of Phi have been reported in bacteria such as Erwinia amylovora (Aćimović et al. 2015), E. carotovora (Lobato et al. 2011) and Streptomyces scabies (Lobato et al. 2010) and on fungi such as Fusarium circinatum (Cerqueira et al. 2017), F. solani (Lobato et al. 2010; Lobato et al. 2011) and Rhizoctonia solani (Lobato et al. 2010). Phi-based fungicides are considered environmentally friendly because phosphites can be degraded to naturally occurring phosphates by soil microorganisms that harbor the enzyme phosphite dehydrogenase (Relyea et al. 2005).
Several studies indicated that Phi-based fungicides are systemic fungicides, exerting their effects by two different modes of action (Smillie et al. 1989; Guest and Bompeix 1990; Guest and Grant 1991; Machinandiarena et al. 2012; Massoud et al. 2012; Stasikowski 2012; Lim et al. 2013; Wu et al. 2019). The direct mode of action entails an interaction between pathogens and Phi; in this case, in order to achieve good protection against oomycetes in planta, concentrations of Phi have to be higher than 100 μg/mL (Smillie et al. 1989; Guest and Grant 1991; Grant et al. 1992; Borza et al. 2014; Burra et al. 2014; Borza et al. 2017). In addition to the direct more of action, several studies showed that Phi act indirectly by priming the plant immune response. This additional protection explains the suppressive effects of Phi at rather low in planta concentrations (Smillie et al. 1989; Guest and Bompeix 1990; Guest and Grant 1991; Machinandiarena et al. 2012; Massoud et al. 2012; Stasikowski 2012; Lim et al. 2013)
Phi-based fungicides are recognized to be effective in controlling diseases caused by oomycete pathogens, and Phi’s inhibitory effects have been described in some bacterial and fungal species (Lobato et al. 2010; Aćimović et al. 2015; Gómez-Merino and Trejo-Téllez 2016; Cerqueira et al. 2017), however, their effects on Verticillium are largely unknown (Ribeiro Júnior et al. 2006; Mulè et al. 2002). The aim of this study was to assess the inhibitory effects of Phi on V. nonalfalfae and Verticillium dahliae, in in vitro and in vivo conditions. The in vitro inhibitory concentration of Phi was determined for several V. dahliae and V. nonalfalfae isolates; one of the isolates that was tested in vitro, V. nonalfalfae strain P1856 (Mahuku and Platt 2002a; Kasson et al. 2014), was selected for the subsequent two-year field trials. The in vitro data indicated that Phi can inhibit the growth of V. dahliae and V. nonalfalfae. Real-time quantitative PCR assessment of V. nonalfalfae abundance in potato plants revealed that the pathogen succeeded to establish itself in the plants; however, the growth of V. nonalfalfae was found to be significantly inhibited in Phi-treated potato plants, confirming the inhibitory action of Phi seen in the in vitro experiments. To better understand the efficacy of Phi, its concentration was determined in both leaves and tubers by high performance ion chromatography (HPIC). Overall, these results suggest that Phi can be used to limit the development of Verticillium pathogens in potatoes and, likely, in other crops.
Materials and methods
The in vitro effects of phosphite on V. dahliae and V.nonalfalfae strains
The Phi-based fungicide used in the experiments was Confine™ (Winfield Solutions, LLC, St. Paul, MN), which contains 45.8% (w/w) mono- and di- potassium salts of phosphorous acid and has a pH of approximately 5.8. To assess the effects of Phi, the V. dahliae and V.nonalfalfae isolates (Table 1) were grown on potato dextrose agar (PDA) supplemented with different concentrations of Confine™. Plugs of 5 mm in diameter were used to inoculate the PDA plates. The concentration of Phi in the medium was adjusted to 0.1, 0.5 and 1.0 mg/mL, and each treatment had four biological replicates. Plates were incubated at room temperature for 2 weeks, and the mycelial growth, estimated by measuring the diameter of the growth area, was recorded at 7 and at 14 days post-inoculation (DPI). The colony diameter (cm) from control and the three different concentrations of Phi, recorded on the 14th day, was used to calculate the half maximal inhibitory concentration (IC50) of Phi.
Experimental design of the field trials and application rates of ConfineTM
The field trials were performed for two successive years (Y1 and Y2) at the Agriculture and Agri-Food Canada, Harrington Research Station, in PEI, using the potato cultivar Shepody. The pathogen selected for inoculation was V. nonalfalfae strain 1856 (Kasson et al. 2014; Borza et al. 2019), which was originally described as V. albo-atrum group 1 (Mahuku and Platt 2002a). Field trials were conducted using a randomized complete block design (RCBD) with three treatments and a control (not inoculated with V. nonalfalfae, not treated with Confine™), each of which had four replicates. Each replicate consisted of a row of 10 plants, with 30 cm spacing between each seed tuber. In the first treatment, plants received four applications of 1% Confine™ during the growing season (T1, Confine™ - treated), while in the second treatment plants were inoculated with V. nonalfalfae strain 1856 at a concentration of 90,000 conidia/mL (T2, inoculated with V. nonalfalfae). Inoculation was performed 30 to 40 days after the planting of the potato seeds by applying 25 mL of inoculum on each side of the plant, in small holes (10 cm deep, 5 cm from the stem). Plants from the third treatment received four times 1% Confine™ during the growing season and were inoculated with V. nonalfalfae strain 1856 (T3, inoculated with V. nonalfalfae and Confine™ - treated) in the same conditions as in the second treatment.
Potato leaf and tuber sampling, extraction and analysis by high performance ion chromatography
Leaf samples were harvested for phosphite (Phi) and phosphate (Pi) quantification by high performance ion chromatography (HPIC). In the first year (Y1), Phi and Pi were determined at two time points, after three applications of Confine™ (Y1–1, August 9), and after four applications of Confine™ (Y1–2, August 26). In the second year (Y2), the two anions were assessed only once, after four applications of Confine™ (August 27). Sampling was done from control and treatments, with four replicates for each experimental condition, one from each block. Each replicate consisted of a pool of six leaves; each of these leaves was collected from different plants in the same block. All collected samples were immediately placed in a cooler with ice packs until transferred to -20 °C for storage. Samples from six tubers from different plants of the same replicate, were cut into small pieces, pooled and transferred into a 15-mL centrifuge tube, frozen in liquid nitrogen and stored at -80 °C until analysis. Four replicates were analyzed from each experimental condition (control and treatments) and sample type (leaves and tubers). Sample processing and analyses by HPIC were conducted as described by Borza et al. (2014). The concentrations of Phi and Pi were determined in the control (non-infected with V. nonalfalfae and not treated with Confine™) and in the treatments that received Confine™ applications, that is, in T1 (Confine™ - treated) and T3 (inoculated with V. nonalfalfae and Confine™ - treated). Since Confine™ was not applied on T2 (inoculated with V. nonalfalfae), this treatment was not analyzed by HPIC.
Verticillium identification and quantitation in stems and tubers from field trials
Sampling for V. nonalfalfae identification and quantitation was done in August, at the time points described in the previous section. Samples consisting of six stems collected from six different randomly-selected plants were pooled from each replicate. Stems, 2 to 4 cm long and maximum 1 cm in diameter, were cut from the lower part of the plant, i.e. approximately 10 cm from the ground, and placed in a Ziploc® plastic bag. After sampling, bags were stored at -20 °C. In the laboratory, slices of 100–200 mg from each stem were further cut into small pieces, mixed and placed in individual 1.5-mL tubes that were kept at -20 °C until used for DNA isolation. Tuber samples comprising the vascular system were cut from the stem end; further processing was performed identically to the procedure described for stems.
Field assessment of disease incidence and severity
Disease rating was done in both years (Y1 and Y2), at two time points towards the end of August, by estimating through visual inspection, the incidence of disease (% plants with symptoms) and disease severity (% disease symptoms on each plant) which included wilting, necrosis and chlorosis.
DNA isolation from stem and tuber samples
Small pieces of stem or tuber samples collected as described above, were ground in liquid nitrogen with a mortar and pestle. An amount of 100 mg of fine powder was used for DNA extraction using the GenJET™ plant genomic DNA purification kit (Fermentas Life Sci., Fisher Scientific, Toronto, ON). DNA was eluted in 100 μL of buffer and its concentration was determined using the Qubit® dsDNA Broad Range assay and a Qubit 2.0 fluorimeter (Life Technologies, ThermoFisher Scientific, Burlington, ON).
Real-time quantitative PCR
Identification and quantification of V. nonalfalfae and V. dahliae from plant samples was performed by real-time qPCR using the StepOnePlus Real-Time PCR system (Applied Biosystems, ThermoFisher Scientific, Burlington, ON). The qPCR primers used in the experiments are listed in the Supplementary materials (Table S1). The qPCR amplification using these primers, followed by gel electrophoresis, indicated that a single product of the correct size was obtained for each primer pair. In addition, following qPCR amplification, the melt-curve (0.3 °C from 55 to 95 °C) was determined to verify that the expected Tm and a single amplicon were obtained. The qPCR was performed in a 20 μL reaction volume using 2 μL of DNA (2 to 5 ng DNA), 1 μL 4 μM each of the forward and reverse primers and 10 μL 2X iTaq™ Universal SYBR® Green Supermix (BioRad, Mississauga, ON). Cycling parameters consisted of one denaturing cycle of 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Each biological sample had three technical replicates. The absolute quantification (standard curve) method was used to determine the amount of V. nonalfalfae and of V. dahliae in plant samples. The DNA standard for V. nonalfalfae was represented by the DNA isolated from V. nonalfalfae strain P1856 (Kasson et al. 2014; Mahuku and Platt 2002a), while the reference for V. dahliae was represented by the V. dahliae isolate Vs04–41 (Alkher et al. 2009; El-Bebany et al. 2013b). Quantification of V. nonalfalfae and V. dahliae was performed by comparing the data with triplicate DNA standards (five 1:5 serial dilutions covering the 0.2 ng to 0.32 pg range). The amount of DNA detected in plant samples (ng/g of plant tissue) was converted to number of cells/g of plant tissue by dividing the amount of DNA determined by qPCR to the estimated amount of DNA present in a V. dahliae cell (36.47 fg of DNA/genome) and V. nonalfalfae cell (35.54 fg of DNA/genome), as previously described (Borza et al. 2018; Borza et al. 2019). The rationale of this conversion was to provide a more meaningful, easy to understand estimate of the colonization level of potato-stem xylem by the mycelium and conidia of Verticillium ssp. The amount of DNA in the target samples and the amplification efficiency were determined automatically using StepOnePlus’ software. The identity of V. nonalfalfae and V. dahliae amplicons was also validated by DNA sequencing using the primers listed in supplementary data (Table S2).
Statistical analyses
Statistical analyses, ANOVA (One-Way and the General Linear Model) and T-test were performed with a 95% confidence interval using the software Minitab 17 (Minitab Inc., PA, USA). When significant effects of treatments were found in ANOVA, a multiple means comparison was carried out using Tukey’s analysis, with a 95% confidence interval.
Results
Effects of phosphites on the in vitro growth of V. dahliae and V. nonalfalfae strains
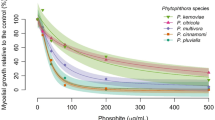
The in vitro testing of the effects of Phi on Verticillium isolates revealed that Phi can significantly suppress their development on the PDA medium, but the degree of inhibition varied greatly (Fig. 1). V. nonalfalfae strain P1856 was found to be the most sensitive, having the lowest IC50 (60.9 μg/mL), while V. dahliae strain Vs06–11 was found to be the most tolerant, having the highest IC50 (481.9 μg/mL) The IC50 values of the other two V. dahliae strains, Vs04–41 and Vs03–35, were 258.2 9 μg/mL and 479.7 9 μg/mL, respectively. The eight-fold difference in the IC50 of Phi among the four isolates indicated a significant natural variation within Verticillium with respect to their susceptibility to Phi.
Phosphite and phosphate contents of leaves and tubers
The content of Phi in the leaves and tubers showed some variation when trial data from the two successive years were compared from similar time points and tissue types (Table 2). Four applications of Confine™ resulted in much higher concentrations of Phi in leaves in the second growing season (Y2) in both treatments (T1 and T3) compared with the similar treatments and time point from the first year (Y1). The highest amount was 86.2 ± 10.9 μg Phi/g fresh tissue, determined in T3 from Y2, while the lowest was 25.0 ± 4.6 μg Phi/g fresh tissue, found in the same treatment, T3 from Y1. Interestingly, in Y1, a higher amount of Phi was detected in the first time point, in both treatments (T1 and T3) compared to the second time points from that year, even the second treatment received an additional application of Confine™ after the first sampling. In contrast with the situation observed in leaves, Phi concentration in tubers, in both T1 and T3 treatments, was found to be almost two-times higher in Y1 samples (113.8 ± 4.7 and 109.7 ± 16.4 μg Phi/g fresh tissue, respectively) compared to that of Y2 samples (61.9 ± 6.8 and 55.7 ± 15.3 μg Phi/g fresh tissue, respectively).
Phosphates (Pi) concentration in leaves varied between 239.2 ± 14.6 and 474.5 ± 41.9 μg Pi/g fresh tissue (Table 2). Except for the first time point, no significant differences were determined between the control and treated samples (T1 and T3) when similar time points were compared, suggesting that the application of Confine™ did not influence Pi accumulation in leaves. Noticeable differences have been observed in T1 and T3, between the two time points from Y1. At the first time point, Pi concentrations in T1 and T3 were lower than in the second time point and for T1, this difference was statistically significant. However, the concentration of Pi in T1 was not statistically higher than that of T3 in any of the time points, indicating that the infection with V. nonalfalfae did not affect the uptake of Pi during Y1 and Y2 growth seasons. The concentration of Pi in the tubers ranged from 828.6 ± 41.3 to 1178.8 ± 38.6 μg Pi/g fresh tissue, more than twice than that of determined in leaves. Similarly, applications of Confine™ on leaves or the early infection with V. nonalfalfae of potato plants in T3 had no overall negative effect on the concentration of Pi in the tubers.
Amount of Verticillium estimated by qPCR
The level of infestation with V. nonalfalfae strain P1856 in the treated potato plants was evaluated by qPCR using DNA samples extracted from the potato stems. Two different target sequences were used for quantitation, the Inter Genic Spacer (IGS) ribosomal DNA sequences and the β-tubulin gene (Supplementary materials, Table S1). IGS are present in multiple copies in the genome of Verticillium species, including V. nonalfalfae, therefore, it allows the identification of lower amounts of target DNA as compared to β-tubulin, which is considered to be a single copy gene.
Estimates of the abundance of V. nonalfalfae in the potato plants, using IGS and β-tubulin provided similar results. V. nonalfalfae successfully colonized the plants that were artificially inoculated; that is, the plants from T3 and T4 (Table 3 and Table S3). The amount of V. nonalfalfae was reduced between 1.5 to 4 times in T4 compared to T3, in all time points and irrespective of the target sequences; most of these differences were statistically significant. These results suggest that Phi had inhibitory effects on in planta growth of V. nonalfalfae. Using IGS as target sequences, low amounts of V. nonalfalfae were found in a few tubers of Y2 samples (Table 3). The area in which the pathogen could be detected was restricted to the cortex area from the stem end of the tubers (data not shown).
As previous studies (Mahuku and Platt 2002b; Celetti and Platt 1987) and several surveys conducted by our laboratory (Borza et al. 2018; Borza et al. 2016; Borza et al. 2017; Wang-Pruski et al. 2016) indicated that V. dahliae is widely distributed in PEI, we investigated whether this species is present in the potato plants used in the Y1 and Y2 trials. The same samples used to quantify the level of infestation with V. nonalfalfae were used to quantify V. dahliae. As IGS provided a more sensitive method of detection and quantification, this sequence was targeted by the qPCR assay. Indeed, V. dahliae was found in all potato plants that were used in the Y1 and Y2 trials (Supplementary materials, Table S4), thought its abundance was lower than that of V. nonalfalfae from T2 and T3 (Table 3). Likely, the lower number of cells reflects the fact that the initial, natural inoculation of plants, was done by a lower number of V. dahliae propagules (Johnson and Dung 2010; Dung and Johnson 2012) as compared to the inoculum prepared using V. nonalfalfae strain P1856. This hypothesis is supported by the fact that in Y1, at the first sampling time point (August 9), the number of V. dahliae cells was much lower than at the second time point (August 26) (Supplementary data, Table S4). Noteworthy, using IGS as target sequences, V. dahliae was also detected in tubers from the Y2 trial (Supplementary materials, Table S4).
Species validation by sequencing
The presence of V. nonalfalfae strain P1856 in the potato plants used in the trials was verified by sequencing a fragment of the β-tubulin gene and another one from the IGS locus (Supplementary materials, Tables S1 and S2). Several samples from T2 and T3, from both years were sequenced along with the DNA of the original isolate of V. nonalfalfae strain P1856. Both β-tubulin and IGS sequences identified in plant samples were found to be identical to that of V. nonalfalfae strain P1856. The β-tubulin sequence identified in the plant samples had 100% identity with the β-tubulin present in the genome of V. nonalfalfae strain VnAa140/PSU140/NRRL 66861 (Kasson et al. 2019).
A fragment of the β-tubulin gene was used to validate the V. dahliae found in samples from the trials (Supplementary materials, Table S2). Indeed, sequence data confirmed that the β-tubulin sequence belongs to V. dahliae that having 100% identity, over 664 nucleotides, to many other V. dahliae sequences deposited in GenBank.
Disease rating
In both growth seasons, in late August, all plants exhibited limited disease symptoms including wilting, necrosis and chlorosis. Though all plants in the experiment displayed such symptoms, resulting in 100% disease incidence in control and treatments, disease severity was rather low in both years. In Y1, differences in disease severity between control and T1-T3 were not statistically different (data not shown). However, in Y2, a clear progression of disease from the first time point (August 21) to the second one (August 27) was observed (Fig. 2). At the first time point only T2, inoculated with V. nonalfalfae but not treated with Confine™, was statistically different from control, T1 and T2. At the second time point. There was a clear separation between V. nonalfalfae infested plants (T2 and T3) and uninfected plants (control and T2). This trend suggests that V. nonalfalfae strain P1856 is moderately virulent to the potato plants. The fact that T3 was found to be statistically different from T2 may reflect the fact that Phi had some inhibitory effects on both, V. nonalfalfae and the naturally occurring V. dahliae.
Disease severity rating of the potato plants in the second year (Y2). The rating was done by estimating % disease symptoms which included wilting, necrosis and chlorosis. C, Control. T1, Confine™-treated. T2, infected with V. nonalfalfae strain P1856. T3, infected with V. nonalfalfae strain P1856 and Confine™ - treated. Y2–1, second year, first time point (August 21). Y2–2, second year, second time point (August 27)
Tuber yield
Total tuber market weight in Y1 suggested that the infection with V. nonalfalfae strain P1856 reduced the yield (Fig. 3). However, this trend was not again observed in Y2 when differences between control and treatments were minimal. The treatment with Confine™ had no clear positive or negative effects on the yield in either Y1 or Y2. In Y1 some reduction in the total tuber market weight was observed in T1 as compared to control, while no differences could be determined between T2 and T3. In Y2 the yield was slightly improved in T1 as compared to control and again, no differences between T2 and T3 could be determined.
Discussion
The suppressive effects of Phi on oomycete growth are well known (Guest and Grant 1991; Thao and Yamakawa 2009; Gómez-Merino and Trejo-Téllez 2016; Trejo-Téllez and Gómez-Merino 2018). However, its effects on bacteria and fungi have been less studied, likely due to the fact that a few study showed that its efficiency is not as high as against oomycetes (Lobato et al. 2010; Cerqueira et al. 2017). Previous in vitro studies indicated that the concentrations of Phi in excess of 1000 μg/mL are needed to suppress the growth of pathogens such as S. scabies (Lobato et al. 2010), F. circinatum (Cerqueira et al. 2017), F. solani (Lobato et al. 2010; Lobato et al. 2011) and R. solani (Lobato et al. 2010). In vitro data obtained in this study for V. nonalfalfae and V. dahliae strains indicate significant differences in the susceptibility to Phi, with an IC50 ranging from 60.9 to 481.9 μg/mL, concentrations that are somewhat lower than those found in other aforementioned bacteria and fungi. The rather large range of tolerance to Phi of Verticillium taxa needs to be further explored to determine whether it represents a trait that is isolate-specific or species-specific. Nevertheless, as suggested by Lobato et al. (2010), because of the rather high concentrations required to obtain significant inhibition in fungi, Phi’s effects can be best described as fungistatic and not fungicidal.
To further explore the possibility that Phi acts as an inhibitor of Verticillium spp., field experiments were run in two successive years. Phi concentrations in leaves and tubers showed large variations in Y1 and Y2, comparable to the range observed in previous experiments and field trials (Borza et al. 2014; Borza et al. 2017). Most importantly, substantial amounts of Phi were found in tubers, especially in Y1, indicating that Phi translocation was not affected by the presence of V. nonalfalfae and V. dahliae in the vascular system. During tuber maturation, nutrients with minerals are increasingly stored in tubers (tubers function as a sink for nutrients). The differences between the two years in the concentrations of Phi in leaves and tubers could be explained by an earlier maturation in Y1, as compared to Y2. However, we cannot exclude the possibility that other biotic and abiotic factors influenced the trend observed for Phi and Pi. No negative effects were observed, after multiple applications of Confine™, on the Pi concentration in leaves and tubers. Also, the early inoculation with V. nonalfalfae of plants in T3 had no obvious effects on Pi uptake from soil and translocation in the plant as differences observed in the concentration of Pi in the leaves and tubers of control, T1 and T3 plants, were minimal.
V. nonalfalfae strain P1856, which was used to inoculate the plants, was moderately affected by Phi, while the naturally-occurring V. dahliae was less influenced by Phi, similarly to what was found in the in vitro experiments. All V. dahliae strains were more tolerant to high concentrations of Phi (IC50, 258.2–497.7 μg Phi/mL) than the V. nonalfalfae strain P1856 (IC50, 60.9 μg Phi/mL). Concentrations of Phi determined in planta (in leaves and tubers) were close to the IC50 of V. nonalfalfae strain P1856 and this can explain its reduced abundance in T3 as compared to T2 determined by qPCR.
Some of the tubers tested in Y2 were found to be infected either by V. nonalfalfae or by V. dahliae or, in a few instances, by both pathogens. The qPCR methods and the sampling procedure proved to be sensitive enough to document this process. Overall, in the tuber samples, V. dahliae prevailed over V. nonalfalfae. Noteworthy, V. nonalfalfae strain P1856 and the naturally occurring V. dahliae were not mutually exclusive in either leaf or tuber samples. Whether the difference in the ability to colonize the vascular system of potato plants represents a strain-specific or a species-specific trait needs to be elucidated by further studies.
Data from field experiments suggests that V. nonalfalfae strain P1856 is moderately aggressive to potato cultivar Shepody, as it succeeded to colonize all plants that were inoculated, but disease severity (wilting, necrosis and chlorosis) was low, and no PED disease could be observed in the second half of August, towards the end of the growing season, in any of the two years in which field trials were conducted. The same observation applies to V. dahliae which colonized the potato plants from a naturally occurring inoculum. No strong vascular discoloration was observed in the tubers analysed though, as mentioned before, a large proportion of tubers have been found to be infected by one or by both Verticillium pathogens.
Previous papers documented the presence of pathogenic V. albo-atrum and V. dahliae and of non-pathogenic V. tricorpus in potato plants from PEI (Mahuku and Platt 2002a; Mahuku and Platt 2002b; Robinson et al. 2006). Recent studies showed that the former V. albo-atrum group 1 actually comprises two species, described as V. nonalfalfae and V. alfalfae, while V. albo-atrum group 2 represents the “true” V. albo-atrum (Inderbitzin et al. 2011; Inderbitzin and Subbarao 2014). The V. nonalfalfae strain P1856 used in our trials was isolated from potatoes in Manitoba and was initially reported as V. albo-atrum group 1 (Mahuku and Platt 2002a). Recent surveys of several fields carried out by our laboratory revealed that in PEI, the incidence of V. nonalfalfae and of V. albo-atrum is much lower than that of V. dahliae (Wang-Pruski et al. 2016; Borza et al. 2016; Borza et al. 2018). The fact that V. dalhiae was found to infect, late in the season, the potato plants used in this study was, therefore, not that surprising, as the experimental fields in Harrington Research Station were not fumigated prior to the trials.
Tuber yields were very different in the two years. In Y1, a rather strong effect on tuber yield could be attributed to the infection with V. nonalfalfae, as the yields of T2 and T3 were much lower than that of control and T1. However, this trend was not observed in Y2. Confine™ treatment had no influence on tuber production as there were no differences between control and T1, and T2 and T3, respectively. Overall, these results reflect the well-known fact that tuber yield is influenced by a large number of biotic and abiotic factors of great complexity, also suggesting that Verticillium pathogens and Confine™ application had a rather limited effect on this quantitative parameter. These results obtained by using Confine™, a Phi-based fungicide, are similar to those recently reported in a study in which thiophanate-methyl, a benzimidazole compound, was evaluated for its efficacy in controlling V. dahliae development in potatoes (Bubici et al. 2019). In the current study, as well as in the field experiments reported by Bubici et al. (2019), treatments decreased plant colonization by delaying the development of Verticillium spp., but did not improve tuber yields.
The worldwide usage of Phi-based formulations, on a broad range of crops, is increasing every year, to control oomycete pathogens that are causing severe diseases such as blights and downy mildew, or as biostimulants and fertilizers (Thao and Yamakawa 2009; EFSA 2014; EFSA et al. 2018; Trejo-Téllez and Gómez-Merino 2018). This study demonstrated that the benefits of using these compounds extend beyond oomycetes pathogens, as they can limit the development of pathogenic Verticillium species, with potentially positive effects on crop health and development.
References
Aćimović, S. G., Zeng, Q., McGhee, G. C., Sundin, G. W., & Wise, J. C. (2015). Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Frontiers in Plant Science, 6, 16.
Agrios, G. N. (2005). Plant pathology (5th ed.). Cambridge: Elsevier Academic Press.
Alkher, H., El Hadrami, A., Adam, L. R., & Daayf, F. (2009). Cross-pathogenicity of Verticillium dahliae between potato and sunflower. European Journal of Plant Pathology, 124, 505–519.
Borza, T., Schofield, A., Sakthivel, G., Bergese, J., Gao, X., Rand, J., & Wang-Pruski, G. (2014). Ion chromatography analysis of phosphite uptake and translocation by potato plants: Dose-dependent uptake and inhibition of Phytophthora infestans development. Crop Protection, 56, 74–81.
Borza, T., Govindarajan, A., Gao, X., Peters, R., Ganga, Z., Rand, J., Beaton, B., Best, K., Pruski, K., & Wang-Pruski, G. (2016). Maritimes regional meeting, 2015/Réunion régionale des Maritimes, 2015. Detection and quantification of Verticillium dahliae and Verticillium albo-atrum in potato and strawberry plants. Canadian Journal of Plant Pathology, 38, 141–147.
Borza, T., Peters, R. D., Wu, Y., Schofield, A., Rand, J., Ganga, Z., Al-Mughrabi, K. I., Coffin, R. H., & Wang-Pruski, G. (2017). Phosphite uptake and distribution in potato tubers following foliar and postharvest applications of phosphite-based fungicides for late blight control. Annals of Applied Biology, 170, 127–139.
Borza, T., Beaton, B., Govindarajan, A., Gao, X., Liu, Y., Ganga, Z., & Wang-Pruski, G. (2018). Incidence and abundance of Verticillium dahliae in soil from various agricultural fields in Prince Edward Island, Canada. European Journal of Plant Pathology, 151, 825–830.
Borza, T., Govindarajan, A., Stephen, J., Best, K., Pruski, K., & Wang-Pruski, G. (2019). Verticillium dahliae and Verticillium nonalfalfae occurrence and abundance in several agricultural fields from Nova Scotia, Canada, assessed by real-time quantitative PCR. European Journal of Plant Pathology, 154, 1171–1177.
Bubici, G., Marsico, A. D., Gaber, L., & Tsror, L. (2019). Evaluation of thiophanate-methyl in controlling Verticillium wilt of potato and artichoke. Crop Protection, 119, 1–8.
Burra, D., Berkowitz, O., Hedley, P. E., Morris, J., Resjo, S., Levander, F., Liljeroth, E., Andreasson, E., & Alexandersson, E. (2014). Phosphite-induced changes of the transcriptome and secretome in Solanum tuberosum leading to resistance against Phytophthora infestans. BMC Plant Biology, 14, 254.
Celetti, M. J., & Platt, H. W. (1987). A new cause for an old disease: Verticillium dahliae found on Prince Edward Island. American Potato Journal, 64, 209–212.
Cerqueira, A., Alves, A., Berenguer, H., Correia, B., Gómez-Cadenas, A., Diez, J. J., Monteiro, P., & Pinto, G. (2017). Phosphite shifts physiological and hormonal profile of Monterey pine and delays Fusarium circinatum progression. Plant Physiology and Biochemistry, 114, 88–99.
Davis, J. R., Huisman, O. C., Everson, D. O., Nolte, P., Sorensen, L. H., & Schneider, A. T. (2010). Ecological relationships of Verticillium wilt suppression of potato by green manures. American Journal of Potato Research, 87, 315–326.
Dung, J. K., & Johnson, D. A. (2012). Roles of infected seed tubers and soilborne inoculum on Verticillium wilt of ‘russet Burbank’ potato. Plant Disease, 96, 379–383.
EFSA. (2014). Statement on the dietary risk assessment for proposed temporary maximum residue levels (t-MRLs) for fosetyl-Al in certain crops. EFSA Journal, 12, 3695.
EFSA, Brancato, A., Brocca, D., De Lentdecker, C., Erdos, Z., Ferreira, L., Greco, L., Jarrah, S., Kardassi, D., Leuschner, R., Lythgo, C., Medina, P., Miron, I., Molnar, T., Nougadere, A., Pedersen, R., Reich, H., Sacchi, A., Santos, M., Stanek, A., Sturma, J., Tarazona, J., Theobald, A., Vagenende, B., Verani, A., & Villamar-Bouza, L. (2018). Reasoned opinion on the modification of the existing maximum residue levels for fosetyl-Al in tree nuts, pome fruit, peach and potato. EFSA Journal, 16, 5161.
El-Bebany, A. F., Adam, L. R., & Daayf, F. (2013a). Differential accumulation of phenolic compounds in potato in response to weakly and highly aggressive isolates of Verticillium dahliae. Canadian Journal of Plant Pathology, 35, 232–240.
El-Bebany, A. F., Alkher, H., Adam, L. R., & Daayf, F. (2013b). Vegetative compatibility of Verticillium dahliae isolates from potato and sunflower using nitrate non-utilizing (nit) mutants and PCR-based approaches. Canandian Journal of Plant Pathology, 35, 1–9.
FAO. (2018). World food and agriculture - statistical pocketbook. Rome: Food and Agriculture Organization of the United Nations.
Gamliel, A., Grinstein, A., Peretz, Y., Klein, L., Nachmias, A., Tsror, L., Livescu, L., & Katan, J. (1997). Reduced dosage of methyl bromide for controlling Verticillium wilt of potato in experimental and commercial plots. Plant Disease, 81, 469–474.
Gareau, B. J. (2017). Sociology in global environmental governance? Neoliberalism, protectionism and the methyl bromide controversy in the Montreal protocol. Environments, 4, 73.
Gómez-Merino, F., & Trejo-Téllez, L. (2016). Conventional and novel uses of phosphite in horticulture: Potentialities and challenges. Italus Hortus, 23, 1–13.
Grant, B. R., Grant, J. H., & Harris, J. (1992). Inhibition of growth of Phytophthora infestans by phosphate and phosphonate in defined media. Experimental Mycology, 16, 240–244.
Guest, D., & Bompeix, G. (1990). The complex mode of action of phosphonates. Australasian Plant Pathology, 19, 113–115.
Guest, D., & Grant, B. (1991). The complex action of phosphonates as antifungal agents. Biological Reviews, 66, 159–187.
Haverkort, A. J., Struik, P. C., Visser, R. G. F., & Jacobsen, E. (2009). Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Research, 52, 249–264.
Heale, J. B., & Karapapa, V. K. (1999). The Verticillium threat to Canada’s major oilseed crop: Canola. Canadian Journal of Plant Pathology, 21, 1–7.
Inderbitzin, P., & Subbarao, K. V. (2014). Verticillium systematics and evolution: How confusion impedes Verticillium wilt management and how to resolve it. Phytopathology, 104, 564–574.
Inderbitzin, P., Bostock, R. M., Davis, R. M., Usami, T., Platt, H. W., & Subbarao, K. V. (2011). Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS One, 6, e28341.
Jiménez-Gasco, M. d. M., Malcolm, G. M., Berbegal, M., Armengol, J., & Jiménez-Díaz, R. M. (2014). Complex molecular relationship between vegetative compatibility groups (VCGs) in Verticillium dahliae: VCGs do not always align with clonal lineages. Phytopathology, 104, 650–659.
Johnson, D. A., & Dung, J. K. S. (2010). Verticillium wilt of potato – The pathogen, disease and management. Canadian Journal of Plant Pathology, 32, 58–67.
Kasson, M. T., Short, D. P., O'Neal, E. S., Subbarao, K. V., & Davis, D. D. (2014). Comparative pathogenicity, biocontrol efficacy, and multilocus sequence typing of Verticillium nonalfalfae from the invasive Ailanthus altissima and other hosts. Phytopathology, 104, 282–292.
Kasson, M. T., Kasson, L. R., Wickert, K. L., Davis, D. D., & Stajich, J. E. (2019). Genome sequence of a lethal vascular wilt fungus, Verticillium nonalfalfae, a biological control used against the invasive Ailanthus altissima. Microbiology Resource Announcements, 8, e01619–e01618.
Larkin, R. P., Honeycutt, C. W., & Olanya, O. M. (2011). Management of Verticillium wilt of potato with disease-suppressive green manures and as affected by previous cropping history. Plant Disease, 95, 568–576.
Liljeroth, E., Lankinen, Å., Wiik, L., Burra, D. D., Alexandersson, E., & Andreasson, E. (2016). Potassium phosphite combined with reduced doses of fungicides provides efficient protection against potato late blight in large-scale field trials. Crop Protection, 86, 42–55.
Lim, S., Borza, T., Peters, R. D., Coffin, R. H., Al-Mughrabi, K. I., Pinto, D. M., & Wang-Pruski, G. (2013). Proteomics analysis suggests broad functional changes in potato leaves triggered by phosphites and a complex indirect mode of action against Phytophthora infestans. Journal of Proteomics, 93, 207–223.
Lobato, M. C., Olivieri, F. P., González Altamiranda, E. A., Wolski, E. A., Daleo, G. R., Caldiz, D. O., & Andreu, A. B. (2008). Phosphite compounds reduce disease severity in potato seed tubers and foliage. European Journal of Plant Pathology, 122, 349–358.
Lobato, M. C., Olivieri, F. P., Daleo, G. R., & Andreu, A. B. (2010). Antimicrobial activity of phosphites against different potato pathogens. Journal of Plant Diseases and Protection, 3, 102–109.
Lobato, M. C., Machinandiarena, M. F., Tambascio, C., Dosio, G. A. A., Caldiz, D. O., Daleo, G. R., Andreu, A. B., & Olivieri, F. P. (2011). Effect of foliar applications of phosphite on post-harvest potato tubers. European Journal of Plant Pathology, 130, 155–163.
Machinandiarena, M. F., Lobato, M. C., Feldman, M. L., Daleo, G. R., & Andreu, A. B. (2012). Potassium phosphite primes defense responses in potato against Phytophthora infestans. Journal of Plant Physiology, 169, 1417–1424.
Mahuku, G. S., & Platt, H. W. (2002a). Molecular evidence that Verticillium albo-atrum grp 2 isolates are distinct from V. albo-atrum grp 1 and V. tricorpus. Molecular Plant Pathology, 3, 71–79.
Mahuku, G. S., & Platt, H. W. B. (2002b). Quantifying Verticillium dahliae in soils collected from potato fields using a competitive PCR assay. American Journal of Potato Research, 79, 107–117.
Massoud, K., Barchietto, T., Le Rudulier, T., Pallandre, L., Didierlaurent, L., Garmier, M., Ambard-Bretteville, F., Seng, J. M., & Saindrenan, P. (2012). Dissecting phosphite-induced priming in Arabidopsis infected with Hyaloperonospora arabidopsidis. Plant Physiology, 159, 286–298.
Mulè, R., Fodale, A. S., & Tucci, A. (2002). Control of olive Verticillium wilt by trunk injection with different doses of fosetyl-Al and Benomyl. Acta Horticulturae, 586, 761–764.
Ochiai, N., Crowe, F. J., Dick, R. P., & Powelson, M. L. (2007). Effects of green manure type and amendment rate on Verticillium wilt severity and yield of russet Burbank potato. Plant Disease, 91, 400–406.
Powelson, M. L., & Rowe, R. C. (1993). Biology and management of early dying of potatoes. Annual Review of Phytopathology, 31, 111–126.
Reimann, S., Elkins, J. W., Fraser, P. J., Hall, B. D., Kurylo, M. J., Mahieu, E., Montzka, S. A., Prinn, R. G., Rigby, M., Simmonds, P. G., & Weiss, R. F. (2018). Observing the atmospheric evolution of ozone-depleting substances. Comptes Rendus Geoscience, 350, 384–392.
Relyea, H. A., Vrtis, J. M., Woodyer, R., Rimkus, S. A., & van der Donk, W. A. (2005). Inhibition and pH dependence of phosphite dehydrogenase. Biochemistry, 44, 6640–6649.
Ribeiro Júnior, P. M., Resende, M. L. V. d., Pereira, R. B., Cavalcanti, F. R., Amaral, D. R., & Pádua, M. A. d. (2006). Fosfito de potássio na indução de resistência a Verticillium dahliae Kleb., em mudas de cacaueiro (Theobroma cacao L.). Ciência e Agrotecnologia, 30, 629–636.
Robinson, N., Platt, H. W. B., & Hale, L. (2006). Potato plant and tuber infection and soil colonization by Verticillium tricorpus and Verticillium albo-atrum "group 2". Canadian Journal of Plant Pathology, 28, 540–547.
Rowe, R. C., & Powelson, M. L. (2002). Potato early dying: Management challenges in a changing production environment. Plant Disease, 86, 1184–1193.
Rowe, R. C., Davis, J. R., Powelson, M. L., & D.I. R. (1987). Potato early dying: Causal agents and management strategies. Plant Disease, 71, 482–489.
Rybakova, D., Schmuck, M., Wetzlinger, U., Varo-Suarez, A., Murgu, O., Müller, H., & Berg, G. (2016). Kill or cure? The interaction between endophytic Paenibacillus and Serratia strains and the host plant is shaped by plant growth conditions. Plant and Soil, 405, 65–79.
Siebold, M., & Tiedemann, A. V. (2012). Potential effects of global warming on oilseed rape pathogens in northern Germany. Fungal Ecology, 5, 62–72.
Smillie, R., Grant, B. R., & Guest, D. (1989). The mode of action of phosphite: Evidence for both direct and indirect modes of action on three Phytophthora spp. in plants. Phytopathology, 79, 921–926.
Smith, V. L., & Rowe, R. C. (1984). Characteristics and distribution of propagules of Verticillium dahliae in Ohio potato field soils and assessment of two assay methods. Phytopathology, 74, 553–556.
Stasikowski P. M. (2012) Biochemical effects of phosphite on the phytopathogenicity of Phytophthora cinnamomi Rands. PhD, Murdoch University.
Statistics_Canada (2012) Catalogue no. 22–008-X, Canadian Potato Production [Online]. Available: http://www.statcan.gc.ca/pub/22-008-x/22-008-x2011002-eng.pdf [Accessed].
Statistics_Canada (2017) Prince Edward Island has the largest potato crop in Canada [Online]. Available: https://www150.statcan.gc.ca/n1/pub/95-640-x/2016001/article/14801-eng.htm [Accessed].
Thao, H. T. B., & Yamakawa, T. (2009). Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Science and Plant Nutrition, 55, 228–234.
Trejo-Téllez L., Gómez-Merino F. (2018) Phosphite as an inductor of adaptive responses to stress and stimulator of better plant performance. In: Biotic and Abiotic Stress Tolerance in Plants, pp. 203–238 Ed S. Vats. Singapore: Springer Nature.
Tsror, L. L., Erlich, O., Peretz-Alon, I., Cahlon, Y., Hadar, A., Cohen, Y., & Klein, L. (2000). Control of Verticillium dahliae prior to potato production by soil fumigation with chloropicrin. Acta Horticulturae, 532, 201–204.
Wang-Pruski, G., Borza, T., Govindarajan, A., Gao, X., Beaton, B., Best, K., Ganga, Z., & Pruski, K. (2016). Maritimes Regional Meeting, 2015/Réunion régionale des Maritimes, 2015. Detection and quantification of Verticillium dahliae in soil of potato and strawberry fields and its distribution in PEI and Nova Scotia. Canadian Journal of Plant Pathology, 38, 141–147.
Wu, L., Gao, X., Xia, F., Joshi, J., Borza, T., & Wang-Pruski, G. (2019). Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiological and Molecular Plant Pathology, 106, 49–56.
Acknowledgments
This work was supported by the Canadian Agri-Science Cluster for Horticulture grant PA2, Prince Edward Island Potato Board and The Agronomy Company of Canada. We thank Dr. Harold W. (Bud) Platt for providing the strains used in experiments, Dr. Jennie Rand and Heather Elliot (Acadia University) who performed the HPIC analyses. We also thank the personnel in Dr. Rick Peters’ lab for their help with the field experiments and disease rating.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was not submitted for publication to another journal. All authors listed have contributed to the work, have read the manuscript and declare that there are no potential conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Borza, T., Peters, R.D., Gao, X. et al. Effects of phosphite on the in vitro growth of Verticillium nonalfalfae and Verticillium dahliae and on their in vivo ability to infect potato plants. Eur J Plant Pathol 155, 1333–1344 (2019). https://doi.org/10.1007/s10658-019-01859-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01859-z