Abstract
The soil-borne fungus Fusarium oxysporum can cause both Fusarium yellows and Fusarium root rot diseases with severe yield losses in cultivated sugar beet. These two diseases cause similar foliar symptoms but different root response and have been proposed to be caused by two distinct F. oxysporum formae speciales. Fusarium yellows, caused by F. oxysporum f. sp. betae, presents vascular discoloration, whereas Fusarium root rot, due to F. oxysporum f. sp. radicis-betae, appears as black rot visible on the root surface. The aim of this work was to study the host-pathogen interaction between sugar beet lines and isolates originally characterized as Fusarium oxysporum f. sp. betae. Eight susceptible sugar beet lines, selected by the USDA-ARS (US) and UNIPD (University of Padova, Italy) breeding programs, were inoculated with three different isolates of F. oxysporum f. sp. betae, the causal agent of Fusarium yellows, representing different genetic groups. All inoculated lines developed symptoms, but severity, expressed as area under the disease progress curve (AUDPC), differed significantly (P < 0.05) among lines. Two lines from UNIPD, 6 and 9, were the most susceptible to the disease, whereas the other lines showed similar levels. The three isolates of F. oxysporum f. sp. betae differed significantly (P < 0.05) in disease severity. Five weeks after inoculation the plants were harvested and roots examined. Surprisingly, severe root rot was observed in the susceptible UNIPD lines when inoculated with all three isolates, while this symptom was never observed in the USDA germplasm. The development of this disease symptom obviously depends on the plant genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium oxysporum Schlecht. is a ubiquitous soil-borne fungus that can induce vascular wilt or root rot on a wide range of plants, causing severe crop losses (Olivain and Alabouvette 1999; Kroes et al. 1998). This pathogen has a high level of host specificity and isolates have been divided into more than 120 different formae speciales (f. sp.) (Armstrong and Armstrong 1981). The two different formae speciales of Fusarium oxysporum that cause disease in sugar beet are: (i) F. oxysporum f. sp. betae, responsible for Fusarium yellows, characterized by interveinal yellowing on the leaves and vascular discoloration of the root, and (ii) F. oxysporum f. sp. radicis-betae which can cause Fusarium root rot (Hanson and Jacobsen 2009; Harveson 2009). The aim of this study was to test the hypothesis that development of root rot symptoms depends on host genotype. The reaction of different sugar beet germplasms to three F. oxysporum f. sp. betae isolates was examined to improve understanding of the plant response and to determine whether root rot observed in the field might be linked to a host component and not just to a fungal stain component. To achieve this aim, we compared the response of four US Fusarium-susceptible sugar beet germplasm lines and four Fusarium-susceptible sugar beet lines from the University of Padova (Italy).
Materials and methods
Eight susceptible sugar beet lines were grown in a greenhouse and examined. These included four Italian lines from the Department of Agronomy, Food, Natural resources, Animals and Environment (DAFNAE- University of Padova, Italy), named 6, 7, 9 and 18, and four USDA-ARS varieties, FC716 (Panella et al. 1995), C869 (Lewellen 2004), EL51 (Halloin et al. 2000) and SP7322 (Coe and Hogaboam 1971). Seeds were soaked in 0.5% sodium hypochlorite for 20 min, rinsed with sterile water and incubated overnight (with shaking at 150 rpm and 27 °C) in 50 mL of 0.3% hydrogen peroxide to enhance uniformity of plant germination (McGrath et al. 2000). Seeds were coated with Metalaxyl 0.93% (Syngenta, Basel, Switzerland) to control damping-off by Pythium species and sown in a previously sterilized peat-based potting mix (Sure-Mix, Michigan Grower Products, Galesburg, MI) in 13-cm-square plastic pots. Ten days after sowing, three seedlings per pot were transplanted into plastic pots with sterilized Sure-Mix to ensure uniform size and no evidence of disease. Plants were fertilized with slow-release Osmocote 14–14-14 (Everris International, Netherlands) added to each pot after transplanting. The plants were grown in the greenhouse (16 h light cycle, 24 °C) and were watered when soil was dry on the surface.
Fusarium oxysporum f. sp. betae isolates used in this work, Fob220a, Fob13 and Fob216c, represent two of the known genetic groups (Hill et al. 2011); Fob220a belongs to clade B and is reported as a highly virulent isolate, Fob13 and Fob216c belong to the same genetic group (clade A) and have been previously shown as moderately virulent (Hanson et al. 2009) with all of them isolated in the US. A hyphae plug from the actively growing edge of a colony grown on potato dextrose agar (PDA, Sigma-Aldrich, St Louis, MO) was transferred to a flask containing V8 juice media. Flasks were incubated in the dark for 7 days using an incubator shaker (150 rpm, 27 °C) to produce conidia. 5-week-old plants were inoculated using a root-dip inoculation method (Hanson and Hill 2004) with a conidial suspension containing 4 × 104 conidia per ml. Briefly, for each variety the plants were gently removed from the soil and the roots rinsed with tap water. Twelve plants per treatment were randomly chosen and the roots were submerged in the spore suspension of one fungal isolate for 8 min, with the suspension shaken every minute. As a control, twelve plants were soaked in sterile water. Plants were replanted in the pots with three plants each pot, four pots per treatment. Each pot represents an independent experimental unit. A week after inoculation all damaged or dead leaves were removed to avoid confusion with transplanting damage. The response of these lines to F. oxysporum isolates was evaluated under controlled environmental conditions. Starting two weeks after inoculation, individual plants were rated weekly for foliar Fusarium yellows symptoms for 4 weeks using a modified 0–5 rating scale (Hanson and Hill 2004), where 0 was healthy plant and 5 was entire plant dead. Plants were harvested 5 weeks after inoculation by removing them from the pots. Roots were washed under running tap water to remove the attached potting mix. The roots were examined for surface rot symptoms and cut open for vascular symptom examination. Root symptoms were measured using a visual disease assessment scale from 0 to 5 for each plant, with 0 = no apparent root symptoms, 1 = discoloration 2 = discoloration with rot on root tip, 3 = less than 50% of root surface showing rot, 4 = more than 50% of root surface with rot, 5 = root completely rotted. For each variety, two randomly selected roots for each treatment were cut into pieces and placed onto PDA after surface disinfestation in 0.5% sodium hypochlorite for 30 s to confirm the presence of Fusarium isolates. The area under the disease progress curve (AUDPC) was calculated for each plant for the 4 weeks of rating using the formula proposed by Shanner and Finner (1977) and the mean AUDPC was determined for each isolate. Experiments were arranged in a completely randomized design with four replications in each treatment and repeated twice. AUDPC was subjected to analysis of variance (ANOVA) and comparison of genotype means was done with Tukey’s HSD test P = 0.05. The package used for analysis was Statistica 12.0 (StatSoft Inc. Tulsa, OK).
Results and discussion
The three isolates used in this study were previously described as Fusarium oxysporum f. sp. betae, the causal agent of Fusarium yellows in sugar beet (Hanson et al. 2009). Evaluation of the leaf symptoms allowed us to demonstrate that all three isolates used in the greenhouse experiment were pathogenic on the germplasm tested, developing typical Fusarium yellows foliar symptoms in all the sugar beet lines used.
Table 1 shows the results of two-way analysis of variance (ANOVA) revealing significant effects (P < 0.01) of germplasm (G), isolate (I) and their interaction (G × I). The isolates caused significant differences in disease severity in some of the eight germplasm tested (Table 2). This result is similar to those obtained in other studies (Hanson and Hill 2004; Hanson et al. 2009) where the three isolates were examined showing varying levels of virulence. In our experiment, Fob220a was more virulent than the other two isolates on all the inoculated genotypes, whereas Fob13 caused less disease symptoms and Fob216 showed the same level of virulence in all the tested genotypes (Table 2). All plants inoculated with sterile water as a control remained symptomless. Fungi re-isolated from symptomatic root tissue samples of each inoculated sugar beet line were identified as F. oxysporum, while no Fusarium isolates were observed from the control plants.
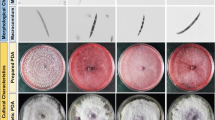
Visual examination of the root symptoms was performed on 10-week-old plants at 5 weeks after inoculation. All plants were harvested and roots were cut open and visually compared for symptoms. This allowed a clear distinction to be made between symptomatic and healthy plants and to identify the different susceptible responses. The UNIPD Italian lines 6 and 9 were the most susceptible to the disease, whereas the other lines showed similar levels of disease. All affected plants showed internal vascular discoloration, which occurred on taproots of both the USDA and UNIPD lines. Interestingly, although all the tested US lines and the Italian lines 7 and 18 developed the same intensity of foliar symptoms, such as wilting and yellowing, external root rot was observed only in the Italian roots, and these root rot symptoms were present in all the Italian accessions inoculated with each fungal isolate (Figure 1). Isolate Fob220a caused rot in more than 50% of the UNIPD plants and less than 10% in the USDA plants, with the latter showing only internal rot. In UNIPD lines, the rot usually started from the root tip and developed as a black discoloration in infected parts. In the most severe host-pathogen interactions, roots of the Italian lines were necrotic and visually destroyed. A positive and significant (P < 0.05) correlation was found between the foliar rating and root symptoms and the Pearson’s correlation coefficient for these variables was 0.811. This is a surprising result because in the past root rot has never been observed on beets inoculated with F. oxysporum f. sp. betae and the isolates causing root rot were demonstrated to belong to a distinct forma specialis (Martyn et al. 1989; Harveson and Rush 1997; Harveson and Rush 1998). It was therefore reported that two distinct pathogenic special forms induced the two different root symptoms (Harveson and Rush 1997), similarly to what has been observed in the tomato system (Rowe 1980).
In conclusion, this screening of different sugar beet lines infected with three F. oxysporum f. sp. betae isolates revealed that US and Italian germplasm showed variable response to fungal infection. Based on our results, all genotypes showed different levels of foliar disease symptoms and internal vascular discoloration, while external root rot was observed only on the Italian germplasm. To the best of our knowledge, this is the first report of Fusarium root rot induced in beet by Fusarium oxysporum f. sp. betae. Development of this symptom depends on the plant genotype and relative susceptibility. Indeed, the external root rot symptoms caused by the fungal strains isolated in US and used in this work have been observed only on the Italian beet lines. However, more studies are needed to understand the different external root rot symptoms and characterize any possible genetic link between breeding selection and resistance to Fusarium oxysporum f. sp. betae.
References
Armstrong, G. M., & Armstrong, J. K. (1981). Formae speciales and races of Fusarium oxysporum causing wilt diseases. In In Fusarium: Diseases, Biology and Taxonomy (pp. 391–399). University Park: Penn State University Press.
Coe, G. E., & Hogaboam, G. J. (1971). Registration of sugarbeet parental line SP 6322-0. Crop Science, 11, 947.
Halloin, J. M., Saunders, J. W., Theurer, J. C., & McGrath, J. M. (2000). Registration of EL51 sugarbeet germplasm. Crop Science, 40, 586.
Hanson, L. E., Hill, A. L., Jacobsen, B. J., & Panella, L. (2009). Response of sugarbeet lines to isolates of Fusarium oxysporum f. sp. betae from the United States. Journal of Sugar Beet Research, 46, 11–26.
Hanson, L. E., & Hill, A. L. (2004). Fusarium species causing Fusarium yellows of sugarbeet. Journal of Sugar Beet Research, 41, 163–178.
Hanson, L. E., & Jacobsen, B. J. (2009). Fusarium yellows. In R. M. Harveson, L. E. Hanson, & G. L. Hein (Eds.), Compendium of Beet Diseases and Pests (pp. 28–29). St. Paul: APS Press.
Harveson, R. M., & Rush, C. M. (1997). Genetic variation among Fusarium oxysporum isolates from sugar beet as determined by vegetative compatibility. Plant Disease, 81, 85–88.
Harveson, R. M., & Rush, C. M. (1998). Characterization of Fusarium root rot isolates from sugar beet by growth and virulence at different temperatures and irrigation regimes. Plant Disease, 82, 1039–1042.
Harveson, R. M. (2009). Fusarium root rot. In R. M. Harveson, L. E. Hanson, & G. L. Hein (Eds.), Compendium of Beet Diseases and Pests (pp. 30–31). St. Paul: APS Press.
Hill, A. L., Reeves, P. A., Larson, R. L., Fenwick, A. L., Hanson, L. E., & Panella, L. (2011). Genetic variability among isolates of Fusarium oxysporum from sugar beet. Plant Pathology, 60, 496–505.
Kroes, G. M. L. W., Baayen, R. P., & Lange, W. (1998). Histology of root rot of flax seedlings (Linum usitatissimum) infected by Fusarium oxysporum f. sp. lini. European Journal of Plant Pathology, 104, 725–736.
Lewellen, R. T. (2004). Registration of rhizomania resistant, monogerm populations C869 and C869CMS sugarbeet. Crop Science, 44, 357–358.
Martyn, R. D., Rush, C. M., Biles, C. L., & Baker, E. H. (1989). Etiology of a root rot disease of sugar beet in Texas. Plant Disease, 73, 879–884.
McGrath, J. M., Derrico, C. A., Morales, M., Copeland, L. O., & Christenson, D. R. (2000). Germination of sugar beet Beta vulgaris L. seed submerged in hydrogen peroxide and water as a means to discriminate cultivar and seed lot vigor. Seed Science and Technology, 28, 607–620.
Olivain, C., & Alabouvette, C. (1999). Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytologist, 141, 497–510.
Panella, L., Ruppel, E. G., & Hecker, R. J. (1995). Registration of four multigerm sugarbeet germplasm resistant to Rhizoctonia root rot: FC716, FC717, FC718, and FC719. Crop Science, 35, 291–292.
Rowe, R. C. (1980). Comparative pathogenicity and host ranges of Fusarium oxysporum isolates causing crown and root rot of greenhouse and field-grown tomatoes in North America and Japan. Phytopathology, 70, 1143–1148.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This research does not include any animal and/or human trials.
Ethical approval
The authors bear all the ethical responsibilities of this manuscript.
Rights and permissions
About this article
Cite this article
Hanson, L., De Lucchi, C., Stevanato, P. et al. Root rot symptoms in sugar beet lines caused by Fusarium oxysporum f. sp. betae . Eur J Plant Pathol 150, 589–593 (2018). https://doi.org/10.1007/s10658-017-1302-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1302-x