Abstract
Black rot of pineapple caused by the fungus Chalara paradoxa is considered an aggressive and difficult disease to control. The use of natural products has been studied with the goal to incorporate them into integrated pest management and reduce the use of agrochemicals. Plant extracts have shown satisfactory results in controlling postharvest diseases by providing fungitoxic action and inducing plant resistance. The aim of this study was to evaluate C. paradoxa severity by the application of plant extract of Mormodica charantia, determinate effect of the plant extract on post-harvest quality, and induction of enzymes linked to host resistance induction. The following treatments were studied: plant extract of bitter melon at concentrations of 10, 100, 500 and 1000 ppm, the commercial products acibenzolar-S–Methyl and fungicide (thiabendazole) in the dosages recommended by the manufacturers, and distilled water as the control, with five replications and two plants per plot. The extract of M. charantia reduced the severity of black rot caused by C. paradoxa, compared to the control treatment, showing similar efficacy to the treatments with commercial inductor Acibenzolar-S-methyl and the fungicide. The enzymatic analysis showed increase of the peroxidase by the use of the bitter melon extract along the evaluation period. The enzymes polyphenol oxidase and phenylalanine ammonia lyase showed greater activity by treatment with M. charantia extract at 500 ppm. It was observed that the plant extract did not affect most of the variables analyzed related to postharvest quality of treated fruits. The use of the extract reduced the fruit weight loss, being a positive aspect under the studied conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brazil produced in 2012 nearly 40 million tons of pineapple in a cultivated area of 2.44 million hectares (FAOSTAT 2012), behind only India and China. Postharvest diseases cause high levels of losses, leading to wasted tons of fruits each year (Andersen 2012; Gunders 2012). Among the major postharvest diseases, black rot caused by the fungus Chalara paradoxa L. C. paradoxa is considered one of the most aggressive pathogens that affect pineapple and is difficult to control. The characteristic symptoms are rapid browning and rotting of the fruit skin and pulp, preventing its sale and consumption (Ferrari 2009).
Commonly, postharvest diseases are treated with preventive pesticides. However, overuse of these products has caused great environmental impact and consumer concerns (PARA 2010; Tavella et al. 2011). The potential of bioactive substances present in natural products and their use as a tool in sustainable disease control has received increasing attention in recent years aimed at reducing the negative impacts of agrochemicals (Castro et al. 2004; Gomes 2011).
Research involving the use of plant extracts has gained importance in recent years. Among the plant extracts with antimicrobial activity, Mormordica charantia L. has shown promising results in controlling plant pathogens (Faria et al. 2009; Celoto et al. 2011; Costa et al. 2011). Bitter melon (also known as bitter gourd and bitter squash) belongs to the Curcubitaceae family. It is commonly found in tropical, subtropical, and temperate regions. Many species are cultivated food crops, others are known as weeds in some regions (Chakravarty 1990; Lorenzi 2000). In many countries, it is used for its medicinal properties (Giron et al. 1991; Lans and Brown 1998; Grover and Yadav 2004).
Recently, bitter melon has been used as a gene donor to transform plants for resistance to pathogens. Tobacco, cotton and rice containing chitinase gene have shown enhanced resistance to fungal diseases (Xiao et al. 2007).
One of the limiting factors to the use of plant extracts in the control of plant pathogens is the possibility of causing changes in fruit quality (Carnelossi et al. 2009; Cruz et al. 2011). Thus, the present investigation aimed to determinate the effectiveness of the use in vitro and pre-harvest of M. charantia extract to control black rot of pineapple caused by C. paradoxa and its effect on fruit quality, and to determinae the production level of enzymes linked to the induction of host resistance.
Material and methods
Leaves of M. charantia were collected in Areia, Paraiba, Brazil, dried in an oven at 40 ° C to obtain constant weight (≈72 h) and subsequently triturated in a knife mill and stored in polyethylene bags at room temperature (25 ° C ± 5) for 5 days. The extract was obtained by maceration in absolute ethanol at a ratio of 200 g of the ground foliar tissue to 1 l of ethanol and then placed on a rotary evaporator for 4 h.
For in vitro assay, treatments were composed by the bitter melon extract (E.M.S.C.) at concentrations of 10, 100, 500 and 1000 ppm, the plant activator acibenzolar-S-methyl (ASM) and the fungicide thiabendazole at the dosages recommended by the manufacturers, 750 ml/ha, and distilled water (control). The evaluation of the E.M.S.C. toxicity on C. paradoxa was performed by transferring 500 ml of each of the treatments described above to Petri dishes containing 20 ml of BDA and spreading over the culture medium surface. Then, a disk of 5 mm from a 3-day old pure fungal colony was deposited in the center of the plate. The mycelial growth was measured daily with a digital caliper rule, in two perpendicular, opposite directions. The total spore number and germinated spore number was determined with a spore suspension being obtained by adding 20 ml of ADE on the Petri plate and scraping the colony with soft bristle brush. The filtration of the suspension was made through a double layer of sterile gauze, determining the number of spores and germinated spores (1.0 × 104/ml) (Alfenas and Mafia 2007) in a Neubauer chamber. The work was performed at the Laboratory of Plant Pathology, Agricultural Science Center - CCA, Federal University of Paraíba - UFPB.
The experimental design was completely randomized with seven treatments, composed by four plant extract concentrations, plus the plant activator, the fungicide, and control (distilled water), with five replicates represented by two Petri dishes. The data were analyzed using the statistical program SISVAR and Sckott-Knott test at 5 % probability.
The pre-harvest experiment was carried out between September and November, 2012 in a commercial pineapple field located at the Mucambo Farm, county of Mari, Paraiba State (7 ° 2 ′60 ″ S and 35 ° 18′ 40 ″ W). The cultivar ‘ Perola ’ was used due to the fact of it being the most commonly grown in the region. The experimental design was a randomized block, using the same treatments described above for the in vitro assay, with five replicates represented by two plants per plot. The E.M.S.C. and ASM were applied in four sprays 15 days apart, with the first 45 days prior to harvest. The fungicide was sprayed weekly from the early stage of inflorescence until it was complete closed, totaling 4 applications, according to the manufacturer’s recommendation.
Fruits were harvested and transported to the Laboratory of Plant Pathology, where they were selected and used for in vivo experiments. To assess the severity of black rot, the fruits previously treated in the field (pre-harvest) were inoculated with C. paradoxa. A 2-mm hole was made in the middle region of the fruit, using a flamed punch, where a 5-mm disc, obtained from a 3-day old C. paradoxa culture in BDA, was deposited. The inoculated fruits were kept in a humid chamber, made of transparent polyethylene bags, for 24 h at 25 ± 1 °C, with light/dark cycle of 12 h. The assessment of the disease progression on the fruits was carried out for five consecutive days using a percentage scale notes (Brito et al. 2005), where 1 = 0 % affected area; 2 = 10 % of affected area; 3 = 20 % of affected area; 4 = 35 % of affected area; 5 = 50 % of affected area. The notes were used for determination of the area Under the Disease Progress Curve (AUDPC). At the end of the experiment, one fragment was removed from the middle region of each fruit for the enzymatic activity analysis.
The qualitative analysis of the fruits treated pre-harvest was carried out evaluating the loss of fruit weight; measuring the pH using a glass electrode potentiometer; total soluble solids, using a digital refractometer with automatic temperature compensation; total acidity, determined by titration with 0.1 N NaOH and expressed as percentage of citric acid; and fruit firmness, determined individually in two distinct points (between the loops) in the equatorial region of the inflorescence using a penetrometer (AOAC 1997). These analyzes were made on the first (DAY1) and last (DAY5) day of the experiment using a fragment removed from the fruits, except for the loss of fruit weight, which was performed in 24-h intervals for 4 consecutive days.
For the extraction of peroxidase, 3.0 g of fruit pulp were homogenized in 6 ml of extraction buffer 0.1 M phosphate pH 6.0. The resulting suspension was centrifuged for 15 min at 12,000 rpm, collecting the supernatant. The reactions for the determination of the peroxidase activity were prepared with the addition of 0.25 ml of the supernatant to the reaction medium containing 0.25 ml of 1.7 % guaiacol, 0.75 ml of 0.1 M phosphate buffer pH 6, and 0.25 ml of H2O2 at 1.8 %. The reactions were monitored with a spectrophotometer, observing the change in absorbance at the wavelength of 470 nm at 25 ° C, immediately after mixing. The activity was expressed in absorbance units (AU) min - 1 mg −1 protein.
For the extraction of polyphenol oxidase (PPO), 3.0 g of fruit pulp were homogenized in 6 ml of extraction buffer 0.1 M sodium acetate pH 5.0. The resulting suspension was centrifuged for 15 min at 12,000 rpm, collecting the supernatant. The reactions for the determination of PPO activity were prepared with the addition of 0.5 ml of the supernatant to the reaction medium containing 0.25 ml of catechol-O-methyl 0.6 M and 0.75 ml of 0.1 M phosphate buffer pH 6 8. The solution was incubated for 15 min at 40 ° C and the reaction stopped by adding 2 ml 2 N perchloric acid. The reactions were monitored with a spectrophotometer, observing the change in absorbance at the wavelength of 395 nm at 25 ° C immediately after removal from the incubator. The PPO activity was expressed in absorbance units (AU) min- 1.mg −1 protein.
For the extraction of phenylalanine ammonia-lyase (PAL), 3.0 g of fruit pulp were homogenized in 6 ml of extraction buffer 0.1 M sodium acetate pH 5.0. The resulting suspension was centrifuged for 15 min at 12,000 rpm, collecting the supernatant. For the determination of PAL activity, 0.5 ml of the supernatant were transferred to test tubes and aliquots of the reaction medium containing 1.5 ml of TRIS- buffer 0.01 M EDTA pH 8.8, 0.5 ml of phenylalanine (30 μM) and 0.5 ml of distilled water, were added. After incubation in a water bath at 40 ° C for 1 h, the reaction was stopped with 2 ml of 5 M hydrochloric acid and the spectrophotometric readings made at 290 nm 25 ° C. The results were expressed in absorbance units (AU) min- 1 mg−1 protein. The “blank ” was prepared using 1.5 ml of 0.01 M Tris buffer pH 8.8, 0.5 ml enzyme extract and 1.0 ml of distilled water for each treatment tested. Analyses were performed in triplicate. The determinations of protein in the extracts were made by Bradford method (Bradford 1976) using Bovine Serum Albumin (BSA) as standard. The method of Bradford is a technique for total protein determination using the dye “Coomassie brilliant blue” BG-250. This method is based on the interaction between the dye BG-250 and protein macromolecules containing basic or aromatic amino acid side chains. In reaction pH, the interaction between the high molecular weight protein and BG-250 dye causes equilibrium displacement of dye to the anionic form that absorbs strongly at 595 nm.
Results
The E.M.S.C. exhibited no significant toxicity to the fungus C. paradoxa. There was no significantly inhibition of mycelial growth or sporulation, when E.M.S.C. was used in vitro, although statistically different from the control treatment with distilled water (Table 1). The E.M.S.C. effect on the fungal growth was observed at concentrations of 100, 500, and 1000 ppm. All E.M.S.C. concentrations evaluated reduced the spore germination.
The fungicide thiabendazole provided a lower rate of mycelial growth, lower levels of sporulation and spore germination (Table 1). These results confirm the fungicide efficiency, which is commonly used by pineapple producers in Paraiba brazilian state for postharvest decay control. The acibenzolar-S-methyl inducer did not inhibit fungal growth or sporulation compared to the control treatment, reducing only the level of spore germination (Table 1).
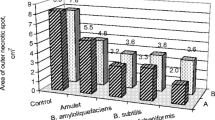
Pre-harvest application of E.M.S.C. did not affected black rot development on pineapple fruits inoculated with C. paradoxa. There was no significant difference among the treatments, suggesting the ineffectiveness of preventive application of E.M.S.C. when the fruits are infected after harvesting. Similarly, the fungicide and the plant activator were ineffective (Fig. 1). The treatment effect was evaluated up to the fifth day, the time at which the fruits of some treatments reached an advanced decay stage.
Area under disease progress curve (AUDPC) and percentage of protection (%) in pineapplefruits ‘Perola’ subjected to pretreatment with Mormordica charantia extract (EMSC), Acibenzolar-S-methyl (ASM), fungicide thiabendazole and control (distilled water). Means followed by different letters are different at the 5 % level of probability by Skott-Knott test
The plant extract used in this study decreased the severity caused by C. paradoxa, compared to the control. Furthermore, no significant difference was observed between the plant extract, the fungicide and the inducer. Therefore, the extract exhibited efficacy similar to the commonly used conventional treatments (Fig. 1).
The pre-harvest treatments with E.M.S.C., ASM, and fungicide produced significant increases in the activity of peroxidase, polyphenol oxidase, and phenylalanine ammonia lyase at the 1st (DAY1) and 5th day (DAY5) of evaluation.
The activity of the enzyme peroxidase at treatments with E.M.S.C. at 100 and 500 and 1000 ppm exhibited significantly difference at DAY1 and DAY5 characterized by a gradual increasing of activity during the evaluation period of 5 days and consequent acceleration in fruit senescence after fungus inoculation. The peroxidase activity showed difference in treatments with ASM when DAY1 and DAY5 were compared. The fungicide showed no significant differences between assessments of peroxidase in postharvest fruits in the 1st and 5th day with and without fruit inoculation (Table 2).
The treatments with the M. charantia extract at 500 ppm and 1000 ppm had higher percentages of protection as well as higher amounts of enzymes such as polyphenol oxidase and phenylalanine ammonia liase.
The amount of peroxidase is not linked to the amount of hydrogen peroxide, because hydrogen peroxide acts as an activator at the time of reaction (conversion of guaiacol in tetraguaiacol) and the conversion speed is determining the peroxidase.
The enzyme polyphenol oxidase (PPO) was most active in non-inoculated fruits treated with E.M.S.C. at 500 ppm and assessed at the 5th day (DAY5), except the treatment 10 ppm at the 5th DAI. The PPO activity was inhibited in inoculated fruits treated with E.M.S.C. at 500 and 1000 ppm (Table 3). It suggests the fruit required higher enzyme activity as a result of host defense reactions triggered by the fungus inoculation and subsequent PPO inhibition.
The PAL showed the highest activity with the treatment E.M.S.C. at 500 ppm, assessed at DAY5, though not statistically different from the fruit inoculated with the fungus at the 5th DAI with the same extract concentration. The PAL activity on inoculated fruits was similar in all other E.M.S.C. concentrations and assessment time (Table 4). Pineapple fruits pre-harvest treated with E.M.S.C., Acibenzolar-S-methyl, and fungicide were analyzed at harvest and the subsequently five days, evaluating the loss of weight, total acidity, total soluble solids, pH, and firmness. Only the variable fruit firmness differed significantly from the other treatments, demonstrating greater firmness with the fungicide treatment. The other variables showed no significant differences (data not shown), led to the conclusion that the plant extract had no effect on most the variables in relation to fruit quality.
Treatment with E.M.S.C. positively influenced weight loss of pineapple fruit at all concentrations (10, 100, 500, and 1000 ppm), exhibiting smaller percentages of weight loss (Fig. 2). Treatment with S-methyl-Acibenzolar had the second highest weight loss of the fruit, peaking on the second day at 4 %, just below those untreated fruits (distilled water control), which had the highest weight loss at DAY5, reaching 7 % loss (Fig. 2).
When used at the concentrations evaluated in this study, M. charantia extract showed no significant toxicity or inhibited the growth of the fungus C. paradoxa, either in vitro or pre-harvest, although statistically different from the control (distilled water). The pre-harvest use of M. charantia extract did not affect fruit quality with respect to pH, total soluble solids, titratable acidity, fruit firmness, and produced less fruit weight loss.
The pre-harvest use of extract (M. charantia) exhibited not negative effect on titratable acidity, soluble solids, pH and fruit firmness and, produced smaller percentages of fruit weight loss. The use of extract caused significant increases in the activity of peroxidase, polyphenol oxidase, and phenylalanine ammonia lyase, demonstrating its potential use in increasing the host resistance to pathogens.
Discussion
Celoto et al. (2011) evaluated the use of methanol and aqueous extracts of M. charantia applied on banana fruits (Musa spp.), observing up to 80 % inhibition in the development of lesions caused by Colletrotrichum musae when applied two days prior to fungus inoculation.
The acceleration of senescence in pineapple fruits can be explained by the peroxidase enzyme participation in the biosynthesis of the plant hormone ethylene, known as the maturation hormone, and oxidation of phenolic compounds, which accumulate in response to infection (Taiz and Zeiger 2009).
The PAL activitity is related to the resistance of plants to pathogens, notably through its involvement in the first step of phenylpropanoids synthesis, with participation of phenylalanine and its conversion into acid-transcinnamic, resulting in compounds such as phytoalexins, phenols, and lignin, which provides greater resistance to plant cell wall (Nakazawa et al. 2001).
The results observed in these experiments agree those obtained by Win et al. (2007), who reported no negative effects on the skin color, firmness and soluble solids of banana fruit by post-harvest use of cinnamon (Cinnamomum zeylanicum) plant extract. Similarly, Carnelossi et al. (2009) observed no significant differences in pH and soluble solids values of papaya fruits (Carica papaya) treated with essential oils. On the other hand, Cruz et al. (2011) observed changes in post-harvest quality of mango fruits (Mangifera indica) treated with oils and extracts of Moringa oleifera, translated into slower ripening and consequently greater post-harvest viability.
There are several reports of decreased weight loss in post-harvest fruit treated with different natural products. Souza et al. (2011) reported that mango treated with chitosan showed reduction in weight loss over time. Blum et al. (2008) evaluated the efficiency of carnauba (Copernicia prunifera) wax on postharvest treatment of persimmon (Diospyros kaki) and found reduction of weight loss. According to Chitarra and Chitarra (2005), weight losses of the order of 3 - 6 % are sufficient to cause a substantial decline in quality, though some products are still marketable up to 10 % loss. M. charantia extract has reduced the fruit weight loss in several plant species, considering an attribute that provides an increase in post-harvest fruit quality (unpublished data).
Conclusions
1. The M. charantia extract decreased the severity of black rot caused by C. paradoxa but did not influence the postharvest quality of fruits of pineapple in most of the variables analyzed.
2. The use of M. charantia extract had positive influence in reducing the weight loss of the fruits.
3. Analysis of peroxidase activity using M. charantia extract increased its activity. The enzymes polyphenol oxidase and phenylalanine ammonia lyase showed the highest activity values in the 500 ppm treatment, can be identified as the best dosage for integrated management of disease.
References
Alfenas A.C., Mafia, R.G. (2007). Métodos em Fitopatologia. Viçosa MG. Universidade Federal de Viçosa. Ed UFV.
Andersen. P.P. (2012) A situação alimentar futuro do mundo e o papel das doenças de plantas. Internacional food policy reseacherch institute. Washington, dc aps. net. Página visitada em 13 de outubro de 2013, a partir de: http:///www.apsnet.org/publications/ apsnetfeatures/Pages/World.
AOAC - Association of Official Analytical Chemists. (1997). Official methods of analysis of AOAC international (16th ed.). Gaitheersburg: AOAC.
Blum, J., Hoffmann, F. B., Ayub, R. A., Jung, D. L., & Malgarim, M. B. (2008). Uso de cera na conservação pós-colheita do caqui cv. giombo. Revista Brasileira de Fruticultura, Jaboticabal, SP, 30(3), 830–833.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Brito, N.M. de, Neves, C.M.L., Veras, V., Nascimento, L.C. do, Souto, F.M., Araújo, E., Nery, A.R. (2005). Controle pós-colheita de Thielaviopsis paradoxa em abacaxizeiro. In: SBPCFT (Sociedade Brasileira para o progresso da Ciência), 1., João Pessoa. Anais. João Pessoa: Editora, 2005. 1 CD-ROM.
Carnelossi, P. R., Schwan-Estrada, K. R. F., Cruz, M. E. S., Itako, A. T., & Mesquini, R. M. (2009). Óleos essenciais no controle pós-colheita de Colletotrichum gloeosporioides em mamão. Revista Brasileira de Plantas Medicinais, Botucatu, 11(4), 399–406.
Castro, H. G., Oliveira, L. O., Barbosa, L. C. A., Ferreira, F. A., Silva, D. J. H., Mosquim, P. R., & Nascimento, E. A. (2004). Teor e composição do óleo essencial de cinco acessos de Mentrasto. Quimica Nova, 27, 55–57.
Celoto, M. I. B., Papa, M. F. S., Sacramento, L. V. S., & Celoto, F. J. (2011). Atividade antifúngica de extratos de Momordica charantia L. sobre. Colletotrichum musae Revista Brasileira de Plantas Medicinais, Botucatu, 13(3), 337–341.
Chakravarty, H. L. (1990). Cucurbits of India and their role in the development of vegetable crops. In D. M. Bates, R. W. RW Robinson, & C. Jeffrey (Eds.), Biology and utilization of Cucurbitaceae (pp. 325–334). Ithaca: Cornell University Press.
Chitarra, M. I. F., & Chitarra, A. B. (2005). Pós-colheita de frutos e hortaliças: fisiologia e manuseio (p. 785). Lavras: UFLA.
Costa. J.G.M., Nascimento, E.M.M., Campos, A.R.,Rodrigues, F.F.G. (2011). Antibacterial activity of Momordica charantia (Curcubitaceae) extracts and fractions. Journal of Basic and Clinical Pharmacy. Disponível em: www.jbclinpharm.com.
Cruz, M.E.S., Jardinetti, V.A., Ryche, A.G.G., Simon, J.M., Cruz, M.J.S. (2011). Efeito de Moringa oleifera na qualidade de frutos de manga CV Tommy Atkins. Cadernos de Agroecologia, Cruz Alta- RS, v. 6, n.2.
Faostat - Food and Agriculture Organization of the United Nations Statistical. Faostat Database Prodstat. Disponível em: <http://faostat.fao.org/faostat/servlet/>. Acesso em: 15 jan. 2012.
Faria, F.A., Bueno, C.J., Stradioto Papa, M.F. (2009). Atividade fungitóxica de Momordica charantia L. no controle de Sclerotium rolfsii Sacc. Acta Sciece., Agronomica. Maringá, v. 31, n. 3, July./Sept.
Ferrari, J. T. (2009). Divulgação Técnica, Podridão negra do abacaxi. Biológico, São Paulo, 71(1), 49–51.
Giron, L. M., Freire, V., Alonzo, A., & Caceres, A. (1991). Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. Journal of Ethnopharmacology, 34, 173–187.
Gomes, E.C.S. (2011). Extrato de allamanda blanchetti na indução de fitoalexinas em sorgo e resistência em videira ‘superior seedless’ contra uncinula necator. Tese (Doutorado em Agronomia). UFPB Areia PB.
Grover, J. K., & Yadav, S. P. (2004). Pharmacological actions and potential uses of Momordica charantia: a review. Journal of Ethnopharmacology, 93, 123–132.
Gunders, D. (2012). Natural Resources Defense Council. Wasted: How America Is Losing Up to 40 Percent of Its Food from Farm to Fork to Landfill. BNRDC ISSUE PAPER. p.12-06.
Lans, C., & Brown, G. (1998). Observations on ethnoveterinary medicines in Trinidad and Tobago. Preventive Veterinary Medicine, 35, 125–142.
Lorenzi, H. (2000). Plantas daninhas do Brasil: terrestres, aquáticas, parasitas e tóxicas (3rd ed., p. 640). Nova Odessa: Istituto Plantarum.
Nakazawa, T., Komai, S., Tezuka, T., Hisatsune, C., Umemori, H., Semba, K., Mishina, M., Manabe, T., & Yamamoto, T. (2001). Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-d-aspartate receptor. The Journal of Biological Chemistry, 276, 693–69.
PARA - Programa de Análise de Resíduos de Agrotóxicos em Alimentos. Relatório de Atividades de 2010. Brasília. Gerência Geral de Toxicologia. ANVISA, 2010.
Souza, M.L, Morgado, C.M.A., Marques, K.M., Mattiuz, C.F.M., Mattiuz, B. (2011). Pós-colheita de mangas ‘tommy atkins’ recobertas com quitosana. Revista Brasileira de Fruticultura, Jaboticabal, SP, vol. esp., Pp. 337–343.
Taiz, L., & Zeiger, E. (2009). Fisiologia vegetal (4th ed., p. 819). Porto Alegre: Artmed.
Tavella, L.B., Silva, Í.N.. Fontes, L.O., Dias, Jairo, R.M., Silva, M.I.L. (2011). AGROPECUÁRIA CIENTÍFICA NO SEMI-ÁRIDO (ACSA). O uso de agrotóxicos na agricultura e suas consequências toxicológicas e ambientais. Patos. v. 07, n 02. p. 06–12. 2011. Página visitada em 13 de abril de 2014, a partir de:< http: www.cstr.ufcg.edu.br. Acesso em: 05 ago.
Win, N. K. K., Jitareerat, P., Kanlayanarat, S., & Sangchote, S. (2007). Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit. Postharvest Biology and Technology, 45(3), 333–340.
Xiao, Y. H., Li, X. B., Yang, X. Y., Luo, M., Hou, L., & Guo, S. H. (2007). Cloning and characterization of balsm pera class I chitinase gene (Mcchit 1) and its ectopic expressiong enhances fungal resistance in transgenic plants. Bioscience Biotechnology and Biochemistry, 71, 1211–1219.
Acknowledgments
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support made available for research and Laboratório de Biomassa CCA- UFPB by help us in experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, W.C.O., do Nascimento, L.C., Vieira, D.L. et al. Alternative control of Chalara paradoxa, causal agent of black rot of pineapple by plant extract of Mormodica charantia . Eur J Plant Pathol 142, 481–488 (2015). https://doi.org/10.1007/s10658-015-0627-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0627-6