Abstract
The extent of mycotoxin-induced responses in living roots of two maize (Zea mays L.) cultivars differing in their susceptibility to Fusarium was studied. Application of mycotoxin zearalenone (ZEN) or its derivates α-zearalenol (α-ZEL) and β-zearalenol (β-ZEL) caused a rapid depolarization of plasma membrane potential (EM). The extent of EM depolarization was dependent on the type of mycotoxin that have been used and showed concentration dependence. Interestingly, ZEN, but not its derivatives α-ZEL and β-ZEL, significantly decreased respiration of maize root cells. Electrolyte leakage increased with the duration of toxins treatment and was significantly higher in susceptible cv. Pavla than in resistant cv. Lucia. A strong superoxide dismutase insensitive nitro blue tetrazolium (NBT) reduction activity was identified in the root tips of control plants. This activity was rapidly inhibited by mycotoxin application in the meristematic/distal transition zones of roots in both cultivars examined. The level of recovery was a function of the mycotoxin concentration. Moreover, mycotoxin treatment also resulted in the onset and the progression of root cell death which was dependent on both, the type and concentration of mycotoxin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the hundreds of known mycotoxins produced by filamentous fungi, zearalenone (ZEN) is one of the most worldwide distributed mycotoxins (Scudamore and Patel 2000; Schollenderger et al. 2007). So far its effect has been studied on humans and animals. However, it was experimentally demonstrated that ZEN can be also toxic for plant cells causing a leakage of electrolytes and organic compounds, inhibition of plant cell membrane transport (Vianello and Macri 1978), inhibition of the oxidative phosphorylation (Vianello and Macri 1981), chromosome damage (Kumar and Sinha 1995) and disorders in photosynthesis and growth processes (Kosćielniak et al. 2009).
In contrast, several experimental studies clearly proved that ZEN is a key substance controlling plant development (Meng et al. 1992) and regulating the flowering process (Biesaga-Kosćielniak and Filek 2010) perhaps via activity similar to plant hormones (Biesaga-Kosćielniak 2001; Szechynska-Hebda et al. 2007). In fact, the exogenous application of ZEN in the culture of winter wheat isolated embryos strongly increased the percentage of generative plants (Biesaga-Kosćielniak 2001) and stimulated the growth of haploid wheat embryos formed following the pollination of wheat spikes with maize pollen (Biesaga-Kosćielniak et al. 2003). Moreover, ZEN, in combination with 6-benzylaminopurine, stimulated the regeneration of oilseed rape and, in combination with 2,4-dichlorophenoxyacetic acid, the regeneration of wheat (Biesaga-Kosćielniak et al. 2010). The ZEN-stimulated course of the photochemical reactions in PSII under salt stress and increased protection of the photosynthetic apparatus against the consequences of strong illumination have been demonstrated by Kosćielniak et al. (2011) in wheat and soybean seedlings.
However, unlike ZEN, any mention of the effect(s) of its derivates α-ZEL and β-ZEL on the cells of host plant roots is surprisingly missing in literature. To distinguish between the effects of ZEN and its derivates α-ZEL and β-ZEL on seedling roots of two maize cultivars differing in their susceptibility to Fusarium we investigated their effect on plasma membrane properties of root cells. Changes in membrane potential (EM) and ion leakage, root respiration, pattern of reactive oxygen species (ROS) production were related with the cell viability of root cells treated with the studied toxins.
Materials and methods
Plant material and growing conditions
For our investigations two maize cultivars provided by Zeainvent (Trnava, Slovakia) were used; tolerant (cv. Lucia) and susceptible (cv. Pavla) to Fusarium infection (Šrobárová et al. 2004). Seeds were surface-sterilized with sodium hypochlorite (1 % available chlorine) for 2 min and rinsed three times in sterile distilled water for 2 × 2 min. The seeds were germinated on a moistened filter paper in Petri dishes in the dark at 21 °C for 3 days.
Electrophysiological measurements
The measurements of EM of intact maize seedlings cortical root cells were performed using standard microelectrode technique described by Pavlovkin et al. (2006). After rinsing the roots with 0.5 mM CaSO4, the intact seedlings were mounted in a 4 cm3 volume plexiglass chamber and were perfused (10 cm3 min−1) constantly with a bathing solution containing 0.1 mM KCl, 1 mM CaCl2, with adjusted to pH 5.7 using 0.1 M HCl. The EM of both control and ZEN, α-ZEL or β-ZEL treated segments was then measured using micropipettes filled with 3 M KCl. The tip diameter was 0.5 μm, and tip potential −5 to −15 mV. A micromanipulator was used to insert micropipettes into single cortical maize roots cells located 3–5 mm behind the root tip. EM measurement was carried out at 22 °C.
ZEN, α-ZEL and β-ZEL were dissolved in absolute ethanol and the stock solution (0.5 mg cm−3) was diluted to the final concentration (1, 10, 20, 40, 50 and 100 μg cm−3) with a bathing solution. This concentration range was used in all experiments because the threshold concentration was 1 μg cm−3 and lower concentrations did not show any impact on maize root cells.
Fusicoccin, (FC, Sigma, USA) a PM-H+-ATPase stimulator, was used (in 0.1 % ethanol at a final concentration of 30 μM) to monitor the functionality of the plasma membrane PM-H+ pump (Marrè 1979).
To establish anoxic conditions, the perfusion solution was saturated with N2 gas by flushing. The flow of the perfusion solution through the measuring chamber at 10 cm3 min−1 was sufficient to establish and to maintain anoxia (Pavlovkin et al. 1986).
Membrane permeability
Segments (5 mm long) of primary maize roots were aged in 0.5 mM CaSO4 solution for 2 h. After that, the solution was exchanged for a fresh one containing with various concentrations of ZEN, α-ZEL or β-ZEL and the conductivity was measured by conductivity meter OK-109-1 (Radelkis, Hungary). The values were related to the root fresh weight.
Respiration analyses
2.5 mm long segments cut from different zones of the maize primary roots were used for measurement of the total respiration rates (VT; nmol O2 g−1 DW s−1). Respiration was measured polarographically using an oxygen Clark-type electrode (YSI 5300, Yellow Springs Instrument, USA) at 25 °C. The root segments were sealed in a water-jacketed vessel containing 3 cm3 of fully aerated 10 mM Na-phosphate buffer (pH 6.8). In order to minimize the problems of nonlinear O2 depletion traces and to eliminate potential wound respiration, handling of roots was kept at a minimum and the uptake of O2 was measured immediately after excision from the intact root. Linear traces that indicated no wound-dependent increase in the respiration rate were used for the calculations.
ROS detection and quantification
Hydrogen peroxide production was assayed using the 3′,3′-diaminobenzidine (DAB) staining technique described previously (Repka 2002). Alternatively, hydrogen peroxide production in living root cells was assayed with confocal laser-scanning microscopy usinga 2′-7′-dichlorodihydrofluorescein diacetate (DCFDA, Invitrogen, USA) as a probe. Superoxide production was assayed using the nitro-blue tetrazolium (NBT) reduction assay as documented previously (Bielski et al. 1980). Quantification of superoxide radicals produced in plant tissues, based on the selective extraction of the formazan produced after NBT reduction was performed according to the published protocol (Grellet-Bournonville and Díaz-Ricci 2011). Root treatment with 2000 U cm−3 superoxide dismutase (SOD, EC 1.15.1.1) was performed in a KH2PO4 buffer (50 mM, pH7.5) at 23 C to destroy superoxide and determine whether NBT reduction is specifically caused by this ROS. NBT reduction was evaluated by dipping roots into 0.5 % (w/v) aqueous solution of NBT for 2 min at 23 C and block by fixation in 37 % formaldehyde for 4 min. The treatment with 50 mM KCN, 5 mM NaN3, 10 mM imidazole (IMI), or 25 mM diphenylene iodonium (DPI) in 0.1 % dimethyl sulfoxide was performed in the mycotoxins control solution for 30–60 min before evaluating the remaining NBT reduction activity. To verify the specificity of staining, control roots were incubated for 60 min in a water solution of an antioxidant propyl gallate (10 mM), before the NBT staining was performed.
Confocal laser-scanning microscopy
Propidium iodide (PI, Fluka, Switzerland) and fluorescein diacetate (FDA, Serva, Germany) were used to counter-stain the cell wall and nuclei of ruptured cells, and overall cell viability, respectively (Oh et al. 2010). Following treatment with various concentrations of mycotoxin, apical root segments of maize explants were sectioned 0.5 cm from the apex, stained 2 min in 10 μg cm−3 PI or 10 min in 5 μg cm−3 FDA, washed 2 min in distilled water and observed in the confocal microscope Olympus FV1000 (Olympus, Japan). Both dyes were excited at 488 nm and fluorescence was detected using 560–660 nm for PI or 505–550 nm barrier emission filters for FDA.
Photography and statistics
In all experiments at least five pictures from randomly selected roots were taken, and each experiment was repeated at least two times for each condition. Digital pictures were directly obtained with a Coolsnap CCD camera (RS Photometrics, USA). Only representative pictures are shown. All data were analyzed using a one-way analysis of variance (ANOVA) with P < 0.05 or 0.01. Means and standard deviation were calculated from three independent experiments (n = 10 apical root segments).
Results
Effects of ZEN and its derivatives on EM and electrolyte leakage
In order to detect immediate responses of root cells to ZEN, α-ZEL and β-ZEL, EM was recorded before and during toxins application (10, 20, 40, 50 and 100 μg cm−3) as well as after the removal of mycotoxins from the root media. All measurements were performed in outer cortical cells in a zone 3–5 mm behind the tips of intact primary roots. EM values of the outer cortical maize root cells varied between –119 and –148 mV with only negligible differences between cultivars. The initial EM values were −133 ± 6.5 mV, n = 14, for cv. Lucia and −135 ± 7 mV, n = 14, for cv. Pavla.
ZEN, α-ZEL and β-ZEL (50 μg cm−3) almost immediately induced membrane depolarization in root cells of both cultivars reaching the maximal depolarization values 10–60 min after the toxin application. The extent and rate of EM depolarization was dependent on the concentration of the particular toxin, and on the sensitivity of maize cultivars to Fusarium infection (Fig. 1). Complete or partial membrane repolarization was recorded in both cultivars treated by α-ZEL and β-ZEL. ZEN at all the concentrations applied (10, 50 and 100 μg cm−3) caused a rapid EM depolarization to the level of the diffusion potential (ED) which remained constant for a long time (Fig. 1a). It means that ZEN, especially in short-term experiments (up to 1 h), specifically influences only the active component of EM. The highest changes of EM were registered in root cells treated with ZEN, and the magnitude of EM depolarization decreased in the order ZEN > β-ZEL > α-ZEL in both cultivars. More expressive, and statistically significant EM depolarization was registered in susceptible cv. Pavla than in resistant cv. Lucia (Fig. 1b). The changes between the cultivars after α-ZEL and β-ZEL treatments were statistically significant. In contrast, we did not observe statistically significant differences between cultivars after treatment with ZEN (P = 0,05). Toxin specific responses in both cultivars were highly statistically significant (P = 0,001).
a Tracing of chart recordings showing the effects of 50 μg cm−3 α-ZEL, β-ZEL and ZEN on the membrane potential (EM) of outer cortex root cells; numbers at the traces denote recorded milivoltage. The time point of perfusion application of the toxin-containing medium is indicated by arrowheads. b Statistical evaluation of Δ EM differences between studied maize cultivars (denoted by asterisks) and toxins (depicted by small letters)
In order to detect the impact of ZEN and its derivates on PM-H+- ATPase functionality, we added the PM-H+-ATPase activator FC (30 μM) to the perfusion solution, when toxins-induced EM depolarization reached its bottom. Within 30 min FC induced a rapid increase of EM in root cells treated with all three concentrations of α-ZEL, β-ZEL (10, 50, 100 μg cm−3) and treated with 10 or 50 μg cm−3 of ZEN. The highest (100 μg cm−3) concentration of ZEN completely diminished the hyperpolarization effect of FC (data not shown). The results show that ZEN, α-ZEL and β-ZEL at concentrations lower than 100 μg cm−3 do not counteract FC-caused hyperpolarization.
Long-term treatment (up to 24 h) of maize roots with low (10 μg cm−3) concentration of ZEN and its derivates resulted in continuous depolarization of EM and ED in root cells of both cultivars (Fig. 2a). The differences in the extent of EM depolarization in root cells treated with ZEN, α-ZEL and β-ZEL were statistically significant between two maize cultivars.
ED was determined in order to distinguish between passive and active, i.e. energy-dependent components of EM by application of anoxia. Anoxia caused a rapid membrane depolarization to approximately –80 mV in root cells of both cultivars, the value considered to be ED. In ZEN-, α-ZEL- or β-ZEL-treated roots (10 μg cm−3), anoxia resulted in a significant ED decrease, which increased with the time of toxin treatment and was significantly lower in root cells of susceptible cv. Pavla (Fig. 2b).
The initial toxin-induced rapid EM depolarization did not correlate with the electrolyte leakage from treated maize roots within 2 h (Fig. 3). The increase in the membrane permeability and ion leakage started 2 h after toxin application reaching the maximum value after 24 h. However, during long-term toxin treatment, electrolyte leakage course followed the changes observed in EM and ED depolarization of root cells (Fig. 2a, b) and was significantly higher in susceptible cv. Pavla than cv. Lucia.
The major part of variance was due to the toxin action and only minor part can be attributed to the cultivar (EM, ED, conductivity). A significant influence of cultivar was recorded after 8 h treatment (EM, ED). Toxin influence on the EM and ED was highly significant in all time points.
Effects of ZEN and its derivatives on root respiration
Treatment of maize root with ZEN (100 μg cm−3) for 30 min, resulted in decrease of root respiration, while α-ZEL and β-ZEL (100 μg cm−3) had only negligible effects (Fig. 4). The intensity of respiration decreased in the order ZEN > β-ZEL > α-ZEL and was higher in root segments of susceptible cv. Pavla, than in tolerant cv. Lucia.
Effect of ZEN and its derivatives on ROS production
Using DAB and DCFDA staining methods, no rapid H2O2 production was detected in control roots or in roots treated with mycotoxins at early time points (data not shown). DAB and/or DCFDA staining was detected in the root tip cells 12–18 h after the mycotoxin application indicating that H2O2 can be produced to a significant level only several hours after the application of ZEN or its derivates.
A high NBT reducing activity was found in the root tips of control plants of both maize cultivars studied (Fig. 5, CONTROL). The NBT reducing activity in roots was not associated with the production of superoxide since treatment of the root apex for up to 30 min with high levels of SOD (2000 U cm−3) was practically unable to prevent NBT reduction (Fig. 5, SOD). We suppose that another redox activity is involved in reducing NBT. Therefore, the other treatments were performed in untreated roots to use the proper control for comparison with mycotoxin exposure. Treatments with diphenylene iodonium (Fig. 5, DPI) and imidazole (Fig. 5, IMI) did not affect the level of NBT reduction activity, suggesting that a flavin containing NADPH oxidase-like enzyme is not involved in this redox activity. In contrast, we found that treatment of roots with NaN3 (Fig. 5, NaN3), or KCN (Fig. 5, KCN), rapidly abolished NBT reduction in both maize cultivars, demonstrating that an enzyme system rather than a chemical reaction is involved.
Effect of different inhibitors on NBT reduction activity in roots of maize seedlings. Roots were exposed to different inhibitor solutions containing 25 mM DPI (DPI), mM imidazole (IMI), 5 mM NaN3 (NaN3) or 50 mM KCN (KCN) for 30 min. NBT reduction was then performed by incubating roots in 0.5 % aqueous solution NBT for 2 min followed by fixation with 37 % formaldehyde for 4 min
Figure 6 and Table 1 show the effect of ZEN and its derivatives on the concentration-dependent decrease of NBT reducing activity in roots of studied maize cultivars. Pre-treatment of maize roots for 24 h with various concentration of ZEN (Fig. 6) substantially abolished NBT reduction in cv. Lucia at concentration higher than 10 μg cm−3 (ca 50 % decrease) and in cv. Pavla at concentration higher than 25 μg cm−3 (ca 35 % decrease).
Concentration-dependent effect of ZEN and its derivatives on NBT reduction activity in roots of maize seedlings. Roots were exposed for 24 h to different mycotoxins of the final concentrations indicated. NBT reduction was then performed by incubating roots in 0.5 % aqueous solution NBT for 2 min followed by fixation with 37 % formaldehyde for 4 min
On the contrary, a distinct pattern of NBT reduction was observed in roots treated with various concentrations of α-ZEL and β-ZEL (Fig. 6). In both maize cultivars, α-ZEL long-term treatment resulted in strong inhibition of NBT reduction which was strictly connected to meristematic zone (MZ) and distal transition zone (DTZ) at concentration 10 and 25 μg cm−3 in cv. Lucia and in cv. Pavla but at higher α-ZEL concentration (50 μg cm−3). Concentrations of α-ZEL higher than 25 μg cm−3 in cv. Lucia and higher than 50 μg cm−3 in cv. Pavla almost completely (98 %) abolished NBT reducing activity.
In long-term treated maize roots with β-ZEL the pattern of NBT reduction activity was similar to the pattern obtained in the cv. Lucia treated with ZEN but the intensity of NBT reduction was more pronounced (Fig. 6). β-ZEL substantially abolished NBT reduction also in cv. Pavla at concentration higher than 10 μg cm−3.
The kinetics of dose–response analysis of mycotoxin-induced decrease of NBT reducing activity was monitored in roots of both cultivars treated with ZEN at the concentration of 10 μg cm−3. In both cultivars, a first detectable signal of ZEN-induced NBT reducing activity inhibition appeared after 2 h in MZ (Fig. 7), persisted at least up to 8 h and NBT reduction recovered to a level of control (0 h) within 24 h. The same results were observed for α- and β-ZEL at the same concentration (data not shown).
Kinetics of NBT reduction activity in the roots of two maize cultivars exposed to 10 μg cm−3 ZEN. Control roots were treated with distilled water. At the time points indicated, NBT reduction was performed by incubating roots in 0.5 % aqueous solution NBT for 2 min followed by fixation with 37 % formaldehyde for 4 min
Effect of ZEN and its derivatives on cell death progression
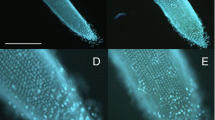
To examine the effect of ZEN and its derivatives on the cell death progression in maize roots, the apical root segments were treated with the increasing concentration of mycotoxins. After 24 h, the onset and the extent of cell death was determined by confocal laser-scanning microscopy using PI as a fluorescent marker. As shown in Fig. 8, there were reproducible cultivar-, concentration-, and mycotoxin-specific differences in the onset and the extent of the cell death. Treatment of the root segments with various concentrations of ZEN resulted in almost identical cell death responses in both maize cultivars. At 50 μg cm−3, PI stain was predominantly localised in nuclei of mycotoxin-treated cells, while at the higher concentration of the mycotoxin, cell death was denoted by extensive cell shrinkage (Fig. 8, top rows). Almost the same type of cell death response, in respect of the onset and the extent of cell death, was observed in apical root segments of cvs. Lucia and Pavla treated with various concentration of α-ZEL (Fig. 8, middle rows). In contrast, the most complex dynamics of the onset and the extent of cell death were documented in apical root segments treated with β-ZEL (Fig. 8, bottom rows). After 24 h, the exposure of cv. Lucia roots to 10 μg cm−3, PI strongly labelled nuclei of the cells and at the higher concentration of the toxin, cells died completely. It was also evident, that root cells of the cv. Pavla exhibited much higher sensitivity to β-ZEL because no viable cells in the root segments treated with 10 μg cm−3 were detected. The critical discrimination limit in the sensitivity of the both maize cultivars is apparently at 10 μg cm−3 β-ZEL.
Concentration-dependent effect of ZEN and its derivatives on cell death progression in two maize cultivars. The roots were treated for 24 h with the mycotoxins at the final concentrations indicated and then stained with propidium iodide. Representative pictures of five roots per treatment. Bar = 30 μm
Discussion
The plasma membrane is not only a barrier providing a selective transport of nutrients but it is also a target of toxic metabolites produced by fungi. Some authors reported that changes in protein or lipid composition of membranes caused by some Fusarium spp. toxins induce structural alterations in cell membrane properties (Lemmens et al. 1997; Marrè et al. 1993; Santini et al. 2008). The alterations of root plasma membrane properties by ZEN have been documented by Vianello and Macri (1978, 1981), but results about impact of α-ZEL and β-ZEL on plant roots are lacking.
In our present work we demonstrate some of temporal course of fast- and short-term changes of the plasma membrane immediately after exposition of root cells to elevated concentrations of ZEN, α-ZEL and β-ZEL in the culture medium. According to our results, ZEN-, α-ZEL- and β-ZEL-induced changes in root plasma membrane occur in the intervals of minutes to hours. These toxins in dependence on their concentrations induced fast changes of the membrane status. Basically they are represented by initial depolarization and subsequent repolarization in case of α-ZEL and β-ZEL but not in case of ZEN application. The magnitude of the two alternations in the EM was concentration-dependent and membranes in the depolarized state were able to repolarize in the presence of α-ZEL and β-ZEL. The ability to repolarize the plasma EM spontaneously seems to be a non-specific electrical reaction that indicates that α-ZEL and β-ZEL probably induced changes in the charge at the cytoplasmic face of the plasma membrane. If the changes in EM are induced by a certain stimulus, it may be considered as a primary signal leading to activation of a relevant intercellular signalling cascade. For different biotic and abiotic stresses, changes in the plasma transmembrane potential and related alterations in the ion fluxes at the plasma membrane are usually scored as the earliest cellular responses (Novacky 1980).
The reaction of root cells to higher concentrations of ZEN-, α-ZEL- or β-ZEL was clearly cultivar-specific. The typical pattern was the initial depolarization and subsequent repolarization of EM in both maize cultivars. At the highest α-ZEL and β-ZEL concentrations (up to 50 μg cm−3), the lack of repolarization discriminated between the maize cultivars. The rate and magnitude of repolarization by the concentrations (50 μg cm−3) in susceptible cv. Pavla did not exceed 6 mV in contrast to tolerant cv. Lucia, where the magnitude of the repolarization was about 22 mV. However, the depolarization of the plasma membrane may be a consequence of the inhibition of the plasma membrane H+-ATPase activity, particularly at higher concentrations of toxins, as it was suggested for example for fusaric acid in tomato root cells (D’Alton and Etherton 1984). To characterize the immediate effect of ZEN and its derivates on the root PM-H+-ATPase, which is responsible for the active component of EM, we performed a set of experiments with FC. The results show that the function of the H+-ATPase is not directly influenced by the toxin treatment due to the fact that ZEN, α-ZEL, β-ZEL at concentrations lower than 100 μg cm−3 do not counteract the FC-caused hyperpolarization. The response may indicate independent sites or mode of action for alteration of H+-ATPase activity by toxins and FC.
These differences recorded in electrophysiological measurements were fully consistent with the conductometric analysis and anoxia experiments. The decrease in the EM (Fig. 2a) and ED (Fig. 2b) was accompanied by the loss of electrolytes from the root cells treated by ZEN, α-ZEL or β-ZEL (Fig. 3). The loss of solutes from plant tissues invaded by pathogenic microorganisms, or those treated with metabolic pathogen products, appears in several host pathogen systems earlier than some other detectable alterations (Pavlovkin et al. 1986; Santini et al. 2008).
Based on these results, we can conclude that ZEN, α-ZEL and β-ZEL in long-term experiments induce permanent changes of ED, in membrane permeability, and may be responsible for the typical visible symptoms of ZEN, α-ZEL and β-ZEL toxicity especially in susceptible cv. Pavla.
All measured parameters, including root EM, underlined the sensitivity of cv. Pavla and higher tolerance of cv. Lucia.
In order to characterise the impact of ZEN and its derivates on the energy metabolism of root cells, we analysed O2 uptake by excised root segments. The toxin treatment (30 min) of maize root resulted in case of ZEN in a significant decrease of root respiration representing ca. 50 % of that of the control. This inhibition could be caused by ZEN affecting the electron transport through the cytochrome pathway or oxidative phosphorylation. Experiments with isolated pea stem and maize root mitochondria showed that ZEN causes an inhibition of the oxidative phosphorylation, uncoupling and mitochondrial ATPase activity inhibition (Vianello and Macri 1978, 1981). On the other hand, no significant differences were registered in root segments treated with α-ZEL or β-ZEL in comparison to the control.
To understand the mechanisms by which mycotoxins induce their toxicity is important for prediction, prevention, and cure of mycotoxin-induced harmful effects on plants. Numerous studies have demonstrated that the response to pathogens generally involves an oxidative burst involving H2O2 or the free radical O2 – within the first 4 h of interaction (Hammond-Kosack and Jones 1996; Lamb and Dixon 1997; Wojtaszek 1997). Our results focused on ROS production in maize roots exposed to ZEN show that ZEN and its derivatives prevent, although to a different extent, a high NBT reducing activity found in the root tips of control plants. Pre-treatment of root tips with SOD and various pharmacological inhibitors clearly demonstrated that mycotoxin-induced inhibition of NBT reducing activity is SOD insensitive and depends on an enzyme system. However, it is important to mention that, at the lower (10 μg cm−3) mycotoxin concentration the ability to revert the inhibition of NBT reduction after 24 h of treatment was found in both maize cultivars. The NBT reducing activity that recovered after 24 h of ZEN exposure in both maize cultivars showed the same sensitivity to NaN3 and KCN than before mycotoxin treatment, and was still insensitive to SOD, IMI and DPI. This indicates that the enzyme involved in the recovery of NBT reduction activity is similar to the one observed before mycotoxin exposure. Since it has not been directly proved experimentally, this recovery process may be either associated with an increased expression of a mycotoxin-sensitive enzyme allowing a partial restoration of the activity or it may be due to the production of a new isoform of the enzyme or of an element involved in its regulation.
Recent studies demonstrated that in animal cells ZEN induces ROS-mediated cell death (Yu et al. 2011). The present study shows that ZEN, α-ZEL or β-ZEL treatment reduces the viability of maize root cells in a dose- and mycotoxin-dependent manner, and induces cell death by to date unknown process. Many mechanisms have been proposed to explain the signalling pathways involved in ZEN-mediated cell death, in which mitochondria are considered to play a critical role (Ayed-Boussemaet et al. 2008; Chayma et al. 2009). This is in good accordance with earlier results describing the effect of ZEN (F-2) substance on isolated animal and plant mitochondria (Vianello and Macri 1981). Actually, recent studies revealed the ZEN-mediated loss of intracellular ATP levels triggers the release of proapoptotic molecules through mitochondrial alterations of Bcl-2/Bax protein levels and finally induces cell death (Yu et al. 2011). Unfortunately, to date there is no relevant information about the induction of cell death induced by ZEN derivatives, α-ZEL and β-ZEL. However, our data presented here clearly indicate that both ZEN derivatives possess the ability to trigger cell death. A very interesting finding from our study is that the cell death inducing capacity of β-ZEL was greater than that of ZEN and α-ZEL. So, in the future, it will be of the interest to investigate mycotoxin-induced progression of cell death to identify the triggers and signalling pathways leading to the overall mycotoxin phytotoxicity.
Based on our results we can conclude that maize roots exposed to ZEN and its derivates show some common early and late responses representing rapid EM changes, inhibition of O2 uptake, inhibition of oxidative burst involving O2 – radical especially in the meristematic/distal transition zones of roots, and stimulated progression of cell death.
This occurs when concentration of mycotoxins oversteps the limits of naturally occurring mycotoxin concentrations especially in case of ZEN which toxicity is considerable higher than the toxicity of its derivates.
References
Ayed-Boussema, I., Bouaziz, C., Rjiba, K., Valenti, K., Laporte, F., Bacha, H., et al. (2008). The mycotoxin Zearalenone induces apoptosis in human hepatocytes (HepG2) via p53-dependent mitochondrial signaling pathway. Toxicology in Vitro, 22, 1671–1680.
Bielsky, B. H. J., Shiue, G. G., & Bajuk, S. (1980). Reduction of nitro blue tetrazolium by CO2 − and O2 − radicals. Journal of Physical Chemistry, 84, 830–833.
Biesaga-Kosćielniak, J. (2001). Zearalenone as a new hypothetical regulator of plant growth and development (pp. 1–135). Krakow: Monograph of Institute of Plant Physiology, Polish Academy of Sciences.
Biesaga-Kosćielniak, J., & Filek, M. (2010). Occurrence and physiology of zearalenone as a new plant hormone. In E. Lichtfouse (Ed.), Sustainable agriculture reviews 3. Sociology, organic farming, climate change and soil science (pp. 419–435). Berlin: Springer.
Biesaga-Kosćielniak, J., Marcińska, I., Wędzony, M., & Kosćielniak, J. (2003). Effect of zearalenone treatment in the production of wheat haploids via maize pollination system. Plant Cell Reports, 21, 1035–1039.
Biesaga-Kosćielniak, J., Kosćielniak, J., & Janeczko, A. (2010). The impact of zearalenone and thidiazuron on indirect plant regeneration of oilseed rape and wheat. Acta Physiologiae Plantarum. doi:10.1007/s11738-010-0495-9.
Chayma, B., Cecile, M., Ossama, S. D., Salwa, A. E., Catherine, B., Christophe, L., et al. (2009). Fusarial toxin-induced toxicity in cultured cells and in isolated mitochondria involves PTPC-dependent activation of the mitochondrial pathway of apoptosis. Toxicological Sciences, 110, 363–375.
D’Alton, A., & Etherton, B. (1984). Effect of fusaric acid on tomato root hair membrane potentials and ATP levels. Plant Physiology, 74, 39–42.
Grellet-Bournonville, C. F., & Díaz-Ricci, J. C. (2011). Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochemical Analysis, 22, 268–271.
Hammond-Kosack, K. E., & Jones, J. D. G. (1996). Resistance gene-dependent plant defense responses. Plant Cell, 8, 1773–1791.
Kosćielniak, J., Biesaga-Kosćielniak, J., Janeczko, A., Filek, W., & Kalaji, H. M. (2009). Can the Gibberella zae toxin zearalenone affect the photosynthetic productivity and increase yield formation in spring wheat and soybean plants? Photosynthetica, 47, 586–594.
Kosćielniak, J., Ostrowska, A., Biesaga-Kosćielniak, J., Filek, W., Janeczko, A., Mohamed-Kalaji, H., et al. (2011). The effect of zearalenone on PSII photochemical activity and growth in wheat and soybean under salt (NaCl) stress. Acta Physiologiae Plantarum, 33, 2329–2338.
Kumar, N., & Sinha, K. K. (1995). Effect of zearalenone on some physiological and biochemical processes of gram and mustard seeds. In A. K. Roy & K. K. Sinha (Eds.), Recent advances in phytopathological researches (pp. 149–162). New Delhi: M.D. Publications PVT.
Lamb, C., & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 251–275.
Lemmens, M., Joseph, R., Schuhmacher, R., Grausgruber, H., Buerstmayr, H., Ruckenbauer, P., et al. (1997). Head blight (Fusarium spp.) on wheat: investigation on the relationship between disease symptoms and mycotoxin content. Cereal Research Communication, 25, 459–465.
Marrè, E. (1979). Fusicoccin a tool in plant physiology. Annual Review of Plant Physiology, 30, 273–288.
Marrè, M. T., Vergani, P., & Albergoni, F. C. (1993). Relationship between fusaric acid uptake and its binding to cell structures by leaves of Egeria densa and its toxic effects on membrane permeability and respiration. Physiological and Molecular Plant Pathology, 42, 141–157.
Meng, F. J., Han, Y. Z., Que, Y. M., & Wang, H. (1992). Zearalenone, a key substance controlling plant development. In C. M. Karssen, L. C. Van Loon, & D. Vreuggdennilcedes (Eds.), Advances in plant regulation (pp. 291–297). Dordrecht: Kluwer.
Novacky, A. (1980). Disease-related alteration in membrane function. In: R. M. Spanswick, W. J. Lucas, Dainty J. (Eds), Plant membrane transport. Current concept issues (pp. 369–380). Elsevier/North Holland Biomedical Press.
Oh, D., Lee, S. Y., Bressan, R. A., Yun, D., & Bohnert, H. J. (2010). Intracellular consequences of SOS1 deficiency during salt stress. Journal of Experimental Botany, 61, 1205–1213.
Pavlovkin, J., Novacky, A., & Ullrich-Eberius, C. I. (1986). Membrane potential changes during bacteria-induced hypersensitive reaction. Physiological and Molecular Plant Pathology, 28, 125–135.
Pavlovkin, J., Luxová, M., Mistríková, I., & Mistrík, I. (2006). Short- and long-term effects of cadmium on transmembrane electric potential (Em) in maize roots. Biologia, 61, 109–114.
Repka, V. (2002). Hydrogen peroxide generated via the octadecanoid pathway is neither necessary nor sufficient for methyl jasmonate-induced hypersensitive cell death in woody plants. Biologia Plantarum, 45, 105–115.
Santini, A., Šrobárová, A., Pavlovkin, J., Čiamporová, M., & Ritieni, A. (2008). Fusaproliferin effects on the photosystem in the cells of maize seedlings leaves. European Journal of Plant Pathology, 120, 363–371.
Schollenderger, M., Drochner, W., & Müller, H. M. (2007). Fusarium toxins of the scirpentriol subgroup: a review. Mycopathologia, 165, 101–108.
Scudamore, K. A., & Patel, S. (2000). Survey for aflatoxins, ochratoxin A, zearalenone and fumonisins in maize imported in to the United Kingdom. Food Aditives and Contaminants, 17, 407–416.
Šrobárová, A., Nadubinská, M., & Čiamporová, M. (2004). Relative efficacy of fusariotoxins on young maize plants. Cereal Research Communication, 2, 241–248.
Szechyńska-Hebda, M., Skrzypek, E., Dąbrowska, E., Biesaga-Kosćielniak, J., Filek, M., & Wędzony, M. (2007). The role of oxidative stress induced by growth regulators in the regeneration process of wheat. Acta Physiologiae Plantarum, 329, 327–337.
Vianello, M., & Macri, F. (1978). Inhibition of plant cell membrane transport phenomena induced by zearalenone. Planta, 143, 51–57.
Vianello, M., & Macri, F. (1981). Effect of zearalenone (F-2) on pea stem, maize root and rat liver mitochondria. Planta, 153, 443–446.
Wojtaszek, P. (1997). Oxidative burst: and early response to pathogen infection. Biochemistry Journal, 322, 681–692.
Yu, J.-Y., Zheng, Z.-H., Son, Y. O., Shi, X., Jang, J.-O., & Lee, J.-C. (2011). Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicology in Vitro, 25, 1654–1663.
Acknowledgments
This work was supported by VEGA, project No. 0005 for VR, JP, No 0023 for RF and No. 0022 for ML.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Repka, V., Fiala, R., Luxová, M. et al. Responses of maize root cells to zearalenone and its derivatives α-zearalenol and β-zearalenol. Eur J Plant Pathol 138, 787–797 (2014). https://doi.org/10.1007/s10658-013-0351-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0351-z