Abstract
The nematicidal effect of a formulated product containing extract from Quillaja saponaria was evaluated against the root-knot nematodes. The product QL Agri® 35 (QL) was tested to record the effect on second stage juveniles motility, egg hatch and also against field populations in greenhouse experiments contacted in three different locations of Greece. Convulsive movement of second stage juveniles of Meloidogyne incognita was recorded after exposure for 8 days at a series of doses, while the most paralyzed juveniles were counted at the dose of 8 mg l−1. There was also a gradual decrease in the number of juveniles emerging from egg masses of the same nematode species when the dose of Q. saponaria was increased from 0 to 8 mg l−1. In greenhouse experiments, the use of Q. saponaria could control root-knot nematodes and prevent nematodes increase in soil. The present study demonstrates that the use of Q. saponaria extract has the ability to control root-knot nematodes. Control given by Q. saponaria in field populations infecting cucumber was similar to that of cadusafos (Rugby®) and oxamyl (Vydate®) under the tested dosages and the specific conditions of the experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKN) attack a wide range of crops and cause devastating crop losses in tropical and sub-tropical agriculture (Sikora and Fernandez 2005). Crops cultivated in plastic houses in Greece, such as cucumber and tomato, suffer from RKN and after the withdrawal of methyl bromide (MB), several other control methods have been investigated as possible alternative solutions. Although the use of other chemicals, mainly soil fumigants and in a lesser extent non-fumigants (Giannakou and Karpouzas 2003; Giannakou et al. 2002; Giannakou and Anastasiadis 2005) have replaced the gap created by MB removal, much concern about their use has directed research to finding environmentally friendly methods of nematode control. There are many fungi which act as female and egg parasites to root-knot nematodes with the best known of them being different species of Pochonia and Paecilomyces. Also several rhizosphere bacteria, including Pseudomonas spp. (Ali et al. 2002; Siddiqui et al. 2000), Bacillus subtilis (Sikora 1988), and B. firmus (Giannakou et al. 2004) have been found to reduce nematode hatch or motility and the invasion of roots. However, control of nematodes by these biocontrol agents is inferior to that given by chemicals. Attention has also been paid on the use of some plant extracts which have been used as alternatives to chemical nematicides. Different parts of Azadirachta indica such as leaves, seeds or oil extracted from this plant species have been extensively used for control of plant parasitic nematodes. However, azadirachtin which is the secondary limonoid metabolite in the Indian neem tree (A. indica), has shown better results against different insect species but not against plant parasitic nematodes. Good nematicidal efficacy has been reported only when crude neem products were used for soil application (Javed et al. 2008). Lately, oil extracted from the fruits of Melia azedarach has shown very promising results against second stage juveniles of M. incognita (Ntalli et al. 2009).
The present study was conducted to investigate the possible use of a commercial product (QL Agri® 35) based on the plant extract from Quillaja saponaria which is an indigenous tree of the Andes region in Chile. In fact, this extract is a mixture of saponins, polyphenols, salts and sugars (San Martin and Magunacelaya 2005). Many plant origin saponins are known to be antimicrobial, to inhibit mould, to protect plants from insect attack and may be considered a part of the plant defence system (Francis 2002). Triterpene saponins from Q. saponaria were used to control insect and nematodes (D’ Addabbo et al. 2005). D’Addabbo et al. (2009) stated that nematode suppression in soil after the use of Medicago spp. amendment, is partly due to the high amounts of saponins present in plant material. Argentieri et al. (2008) reported that saponins from Medicago arborea, M. arabica and M. sativa possess nematicidal effect against Xiphinema index. The nematicidal effect of the commercial product based on saponins was studied in laboratory experiments using second stage juveniles and egg masses of Meloidogyne incognita. Also, because of the limited amount of published field experimentation, three experiments were conducted in commercial greenhouses in Greece.
Materials and methods
Effect of Quillaja saponaria extract on J2 mobility
Eggs from Meloidogyne incognita reared on tomato (Solanum lycopersicum) were extracted with 1% sodium hypochlorite solution (Hussey and Barker 1973). Second-stage juveniles (J2s) were allowed to hatch in a modified Baermann funnel. All J2s hatching in the first 3 days were discarded and thereafter, J2s collected after 24 h were used in the experiments. The formulated product of Q. saponaria extract which is a mix of triterpenoid saponins, polyphenols, salts and sugars (San Martin and Magunacelaya 2005) was used at the doses of 0.5, 1, 2, 4 and 8 mg l−1. Clean tap water was served as the control. The recommended dose (2 mg l−1) was calculated assuming that: 1) no adsorption of the pesticide occurs in the soil colloids; 2) the moisture content of the soil is at field capacity, which for a sandy loam soil ranges between 15 and 24% (20% was assumed); and 3) pesticide residues are contained within the top 15 cm of the surface soil (Weber et al. 2000). According to these assumptions, the expected concentration of QL in soil solution, when the maximum recommended dose has been applied, was calculated to be 2 mg l−1. Subsequently, two higher and two lower concentrations of nematicide in the soil solution were selected for the experiment. One ml of tap water containing 500 J2s was pipetted onto a 6 cm Petri dish. The concentrations were made up in the Petri dishes by adding 9 ml of the nematicide solutions. Each treatment was replicated four times. One hundred and 60 juveniles were observed with the aid of an inverted microscope at 25 × magnification after 4 and 8 days and were ranked into three distinct categories: paralyzed, convulsive movement, and motile. However in two treatments fewer juveniles (145 and 152) were found in Petri dishes. The experiment was conducted twice and the results from each experiment are reported separately.
Effect of Quillaja saponaria extract on hatching of J2s
A population of Meloidogyne incognita originally obtained from tomato roots from greenhouse in Thessaloniki was reared on tomato cv. Beladonna and maintained in a temperature-controlled greenhouse. After 60 days, mature egg masses were hand picked from roots and placed in small plastic extracting trays made by 6 cm Petri dishes.
Solutions of QL (0.5, 1, 2, 4, and 8 mg l−1) were added to each extracting tray to cover egg masses. Egg masses were maintained for 10 and 20 days and then QL solutions were removed by washing them with tap water and placed in extracting trays filled with clean water. Extracting trays were covered to avoid loss of water and placed in incubator at 27οC. Hatching J2s were counted every 5 days, they were discarded and all trays were filled with fresh water. The number of J2s emerging over 6 weeks from the egg masses was counted and the experiment was terminated when J2s did not emerge any longer. The experiment was conducted twice and the results from each experiment are reported separately.
Effect of Quillaja saponaria extract on juvenile invasion
The efficacy of the QL was evaluated using tomato seedlings, cv Beladonna. Seedlings at the four-leaf stage grown in commercial potting soil in 50 cm3 plastic pots, were drenched with QL solution at the same doses from 0.5 to 8 mg l−1 to these previously used, for 2 weeks at 3-day intervals. One ml of tap water containing 200 J2s of M. incognita was pipetted onto each pot at the end of the 2 week drenching period. The plants were placed in a growth room at 27 ± 1°C and 25 days later were uprooted and stems were removed. Roots were carefully washed free of soil and boiled for 3 min in a solution of equal volumes of glycerol, lactic acid and distilled water plus 0.05% acid fuchsin. Roots were then washed in water and placed in vials containing equal volumes of glycerol and distilled water plus a few drops of lactic acid. Roots were chopped and two aliquots of one g each were taken. All developing stages of nematodes on the roots were counted using a stereoscopic microscope at 12.5 × magnification (Bridge et al. 1982). The experiment was conducted twice.
The efficacy of Quillaja saponaria extract against root-knot nematodes in greenhouse experiments
Experiment 1
The first experiment was conducted at Vasilika Thessaloniki, in a commercial greenhouse for a 4 month period, from late June until late October. The experimental area was 840 m2 which was divided into 28 equal plots of 30 m2 each with four replicates per treatment. There were six treatments and one untreated control. Chemicals were applied in the corresponding plot as their commercial formulations as following: Q. saponaria extract 350 g l−1 as a single recommended dose of 1 l (QL1), 2 l (QL2) and 3 l (QL3) per 1,000 m2 respectively (QL Agri® 35; Natural Response S.A., Desert King, Chile), Q. saponaria extract 350 g l−1 at the dosage of 2 l per 1,000 m2 applied three times at 20-day intervals (QL4), oxamyl 247 g l−1 SL at the dose of 1.5 l per 1,000 m2 (Vydate; DuPont, USA) and cadusafos 100 g l−1 EC at the dose of 4 l per 1,000 m2 (Rugby; FMC Corporation, USA).
Prior to any chemical application, soil sampling was done to record the level of nematode infestation in soil in each plot. A metal soil sampler was used (2.5 cm × 10 cm) and the sampling depth was 0–30 cm. The first soil sampling (initial population) was done on 27th June. Twenty soil samples (200 g each), were randomly collected from each plot. All samples from each plot were placed in one polyethylene bag which was tightly closed to maintain soil moisture. Next day the soil of each plot was used for J2 isolation using the modified Baermann funnel technique. All chemicals were applied 1 day after soil sampling. Prior to chemical application, greenhouse soil was irrigated and cultivated with an L-bladed rotary cultivator. All chemicals were applied through the drip irrigation system. Just before the beginning of the chemical application, soil was watered for 15 min and chemicals were applied for a 20 min period. After that, all plots were irrigated for another 30 min. A total amount of 3 l per drip was used. Self rooted cucumber seedlings (cv. “Palmera”) were transplanted 1 day later.
The second soil sampling was conducted 2 months after transplanting (mid-season) of seedlings. Soil sampling procedures and analysis for nematodes were identical with those described previously.
The third soil sampling was done 2 months after the second sampling at the day of uprooting. Soil sampling and extraction procedure were identical as those described previously. Also, at the time of soil sampling, roots were randomly collected at 10–20 cm depth from 10 plants from each plot using a garden trowel. Roots from each plant were chopped and two aliquots of 1 g each were taken. All developing stages of nematodes on the roots were counted with the aid of a stereoscopic microscope at 35 × magnification (Bridge et al. 1982).
Experiment 2
The same procedure as described above was followed for the second experiment conducted in a commercial greenhouse at Kiparissia, Peloponnesus from early August until middle November. The experimental area was 600 m2 which was divided into 28 equal plots of 20 m2 each. Soil samplings, chemical applications and assessments were identical as described previously. The first soil sampling was conducted on 8th August while seedlings transplanting was done next day. The second soil sampling was conducted 45 days later and the third soil sampling, root sampling and uprooting of plants were done 50 days after the second soil sampling. In this study the susceptible cucumber cultivar “Palmera” was used grafted on Cucurbita maxima × Cucurbita moschata seedlings (common name “Power”), which is tolerant to root-knot nematodes, since it produces a longer and denser root system than the self-rooted hybrids.
Experiment 3
The third experiment was conducted in a commercial greenhouse at Marathonas, Attica. The first soil sampling was done on 31st March and seedlings transplanting on 7th April. Each plot was 10 m2 and each treatment was replicated four times. Soil samplings and chemical applications were identical as described in the first experiment. In this study the susceptible cucumber cultivar “Palmera” was used as self-rooted plants.
There were five treatments and one untreated control. Chemicals were applied in the corresponding plots at their commercial formulations of Q. saponaria extract 350 g l−1, and oxamyl 100 ml l−1 SL. All treatments as well as dosages and application times are presented at Table 1. Q. saponaria extract was used as a new test item while it was compared to oxamyl since its use is a common practice in Greek greenhouses. The efficacy of chemicals was assessed by counting the numbers of J2s in soil samples at 7 and 56 days after transplanting and numbers of nematodes per g of root 42 and 73 days after transplanting. The experiment was terminated on 20th June. The assessments of nematode population in soil and roots were identical with that described previously.
Statistical Analysis
The data obtained from the dose-exposure experiment of J2s, were subjected to χ2 test of significance, while those of egg masses, soil and root sampling were subjected to analysis of variance. Treatments means were compared using the Tukey’s HSD test. Statistical analysis in all cases was done using SAS statistical package (SAS 1995).
Results
Effect of Quillaja saponaria extract on J2 mobility
In the control treatment 12.5% of J2s were paralyzed and 5% showed convulsive movement while the other 82.5% were motile (Table 2) after exposure for 8 days. There was an increase in the number of J2s showing convulsive movement when the dose of QL was increased to 2 and 4 mg l−1. However significantly more paralyzed J2s were counted only when the QL dose was increased to 8 mg l−1. At concentrations of 1, 2 and 4 mg l−1 more than 25% of J2s were paralyzed after exposure for 4 days, while at concentration of 8 mg l−1 83.7% of J2s were paralyzed. Similar results were obtained in the second experiment, where the most non-motile J2s (P < 0.001) were recorded after exposure for either 4 or 8 days to QL at the dose of 8 mg l−1.
Effect of Quillaja saponaria extract on hatching of J2s
There was no significant difference in the number of nematodes hatching from the untreated egg masses and those treated with 0.5, 1 and 2 mg l−1 of QL after exposure for 10 days in both experiments (Table 3). Significantly fewer nematodes were hatched from egg masses treated with 4 and 8 mg l−1 resulting in about 50% fewer nematodes compared to the control treatment. A gradual decrease in the number of J2s emerging from egg masses was recorded when the dose of QL was increased from 0 to 8 mg l−1 after 20 days exposure of egg masses. However, significant differences were recorded only after increase of the dose from 2 to 4 mg l−1 for both experiments after 10 days exposure. Also, significant differences were recorded after increase of the dose from 1 to 2 and 1 to 4 mg l−1 for the first and second experiment respectively after 20 days exposure (Table 2).
Effect of Quillaja saponaria extract on juvenile invasion
There was no significant difference in the number of nematodes invading tomato roots after they have been drenched with QL solution for 2 weeks in 3-day intervals at both experiments. These ranged from 10.6 to 23.1 nematodes per g of root (data not shown).
The efficacy of Quillaja saponaria extract against root-knot nematodes in greenhouse experiments
Experiment 1
Fewer nematodes were recorded in soil treated with QL extract compared to the control treatment (Table 4). However different doses or applications of QL resulted in different decreases of nematode populations in soil. At mid-season, significantly fewer nematodes were recorded from plots treated with QL 3 compared to the control, which was not statistically significant from oxamyl (Vydate) and cadusafos (Rugby) treatments. The lowest number of nematodes was recorded at cadusafos treatment followed by oxamyl and QL 3 treatments. At harvest, except for the QL 1 treatment, all the other QL treatments were significantly different to the control. Specifically at QL 3 and 4, an almost 50% decrease in nematode numbers was recorded. The latter treatments were statistically similar to cadusafos and oxamyl treatments. Fewer nematodes were recorded in the roots at all treatments compared to the control but only the QL 3 treatment was significantly different to the control (Table 5).
Experiment 2
Plots treated with either QL or chemical nematicides had significantly fewer nematodes in soil than the control plot based on the soil sampling at mid-season (Table 4). Statistically similar results were obtained between all QL treatments and the chemical ones. The lowest number was recorded with the treatment by oxamyl followed by the QL 2. Final population densities at harvest were lower at the chemical treatments followed by the QL 2. The population level at all the other QL treatments, except that of QL 4, was significantly lower to that recorded at control. Numbers of nematodes in roots were not significant different at all treatments compared to the control (Table 5).
Experiment 3
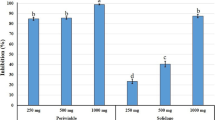
Low numbers of J2s in the soil were recorded at mid-season at all treatments (Fig. 1). The lowest mid-season nematode population was recorded in the plots treated with oxamyl prior to the transplanting and three times with QL Agri (Treatment 3) followed by those treated only with oxamyl twice (Treatment 6). Low numbers of J2s in soil were recorded at control at the middle of the cropping season. This could be explained by the fact that most of the J2s had invaded roots by the time soil had been sampled. However, nematode densities in soil were not significantly different in all treatments and control. Significant differences were recorded between soil populations at the end of the cropping season (Fig. 1). Zero levels of J2s in soil were recorded in the plots treated with oxamyl in Treatments 2 and 6. All treatments gave significantly lower nematode numbers per root compared to the control treatment. The lowest numbers of nematodes per g of root were recorded at oxamyl (Treatment 2) at 42 days after transplanting followed by the plots treated with oxamyl and QL Agri (Treatment 3). Seventy three days after transplanting, a significant reduction of nematodes in root samples was recorded between all treatments and the control plots. Zero level of nematodes was recorded in the plots treated with oxamyl and QL Agri (Treatment 3) (Fig. 2).
Numbers of second stage juveniles of Meloidogyne spp. in 200 g of soil prior to transplanting (initial), 42 (middle) and 73 (final) days after transplanting respectively. All treatments are referred to the dosages and application schedule shown in Table 1. Error bars represent the standard deviation of mean (n = 4)
Numbers of nematodes Meloidogyne spp per g of root 42 and 73 days after transplanting. All treatments are referred to the dosages and application schedule shown in Table 1. Error bars represent the standard deviation of mean (n = 4)
Discussion
The evidence presented in this study demonstrates that the use of Q. saponaria extract has an ability to control root-knot nematodes. Although it is not always the case, it has been shown that QL could be as effective as some well known commercial nematicides such as Rugby and Vydate. Our results are in agreement with those reported by San Martin and Magunacelaya (2005) in which an aqueous extract of Q. saponaria controlled plant-parasitic nematodes. A percentage of 94 and 98% of paralyzed J2s in the first and second experiment respectively is a potential efficacy from a control point of view. However the above efficacy was recorded in laboratory experiments where it is easier for a nematicide to come in contact and affect second stage juveniles. The most critical point of the motility study is that the dose which has shown the above results is higher than the recommended one, which is 2 mg l−1.
A gradual decrease of juveniles hatched from egg masses, submerged for different periods in extract solutions, was recorded with increasing dose and time of exposure. However this decrease was more obvious after increasing dose to 4 and 8 mg l−1 and after doubling the exposure time from 10 to 20 days. A decrease of 86 and 83% was recorded after exposure for 20 days in the first and second experiment respectively. Even when chemical nematicides were applied in doses two or four times those recommended, several J2 survived inside eggs and hatched when egg masses were transferred to clean water (Giannakou et al. 2005). This could be due to the action of gelatinous matrix, since Majtaheldi et al. (1991) reported that there was no significant reduction in the number of hatched J2s when egg masses of Meloidogyne chitwoodi and M. hapla were maintained in different doses of ethoprop for 4 days, while free eggs exposed to the same chemical were much more sensitive.
It seems that QL has no systemic effect on the roots to prevent nematode invasion since there is no a reduction in the nematode numbers entered roots after root ball was drenched with QL solution for 2 weeks at 3-day intervals.
In greenhouse experiment 1 only the chemical treatments (Vydate and Rugby) reduced J2s populations in soil significantly to all the other treatments and control, except the QL treatment (3 l) at mid-season. However at harvest, plots treated with QL (3 l and 2 l applied 3 times) reduced the J2 population in soil, at levels statistically not significant compared to the chemical treatments, but significant to the control. Although the number of nematodes per g of root was reduced in every treatment compared to the control, only the treatment in which QL was applied at the dosage of 3 l gave results significantly lower than all the other treatments.
In experiment 2 significantly lower numbers of J2s were recorded in all treatments compared to the control plots at mid-season and harvest. The level of J2s in soil was reduced to 70% in QL (2 l) and 84 and 73% in treatments with oxamyl and cadusafos respectively compared to the control. Although this result shows that QL is inferior to the chemical products, we must keep in mind that the mode of action of products like QL has not been completely clarified. Both chemicals used in the present study act as nematicides in the doses they have been used while QL could act as a nematostat. This could be concluded from the fact that the lower doses of QL did not change the motility level of J2s in the Petri dishes experiments, although a low dose of QL reduced the invasion of J2s in the greenhouse experiments. That may be explained by a possible anti-trophic or disorientation effect of QL to J2s. Based on our results this is only one possible explanation which should be further investigated with specific experiments. There were no significant differences in the number of nematodes per g of root at harvest. However in both greenhouse experiments, many plants in the control treatments were dead by the end of the cropping season due to the action of secondary invading soil fungi, which means that plants with a medium level of nematode infestation were left in the control plots.
In experiment 3 good control of nematodes was achieved, based on the results of J2s extracted from soil, with the application of QL applied at 2 l, one day prior to transplanting, and at the dose of 1 l, nine and 20 days after transplanting. Sufficient control was also achieved when the same product was applied at the dose of 2 l one day prior to and 20 days after transplanting. However, the best results were recorded by the use of oxamyl as the only product used in the cropping season or the combination of oxamyl and QL prior to transplanting, followed by two additional applications of QL after 9 and 20 days. Surprisingly low numbers of J2s were recorded in the control plots at midseason. This could be due to the fact that all nematodes entered root system since there was not any disorientation effect of J2s towards roots.
In general, we conclude that the use of Q. saponaria can control root-knot nematodes and prevent nematode increase in soil. Control given by QL can be similar to that of oxamyl and cadusafos under specific circumstances. However the use of chemical nematicides is a practice employed for many years in the field with good results concerning root-knot nematode control. Control of nematodes using products such as QL, although some times inferior to that given by the chemical products, is very useful from a farmer’s point of view since it can also be used throughout the cropping season without any residue problems in the fruits. Another reason for employment of products such as QL is that the phenomenon of nematicide biodegradation has become recently quite common in greenhouse soil (Karpouzas et al. 2004) which leads to low efficacy of chemical control, while there has not been so far any reported evidence of enhanced biodegradation in plant extracts.
References
Ali, N. A., Siddiqui, I. A., Shaucat, S. S., & Zaki, M. J. (2002). Nematicidal activity of some strains of Pseudomonas spp. Soil Biology and Biochemistry, 34, 1051–1058.
Argentieri, M. P., D’Addabbo, T., & Tava, A. (2008). Evaluation of nematicidal properties of saponins from Medicago spp. European Journal of Plant Pathology, 120, 189–197.
Bridge, J., Page, S., & Jordan, S. (1982). An improved method for staining nematodes in roots. Report of the Rothamsted Experimental Station for 1981, Part 1, 171 pp
D’Addabbo, T., Curto, G., Greco, P., DiSilvestro, D., Coiro, M. I., & Lamberti, F. (2005). Prove preliminari di Lotta contro nematodi galligeni mediante estradi Quillaja saponaria Molina. Nematologia Mediterranea, 33, 29–34.
D’Addabbo, T., Avato, P., Tava, A., Agostineli, A., Jurzysta, M., & Avato, P. (2009). Nematicidal potential of materials from Medicago spp. European Journal of Plant Pathology, 125, 39049.
Francis, G. (2002). The biological action of saponins in animal systems: a review. The British Journal of Nutrition, 88, 587–605.
Giannakou, I. O., & Anastasiadis, I. (2005). Evaluation of chemical strategies as alternatives to methyl bromide for the control of root-knot nematodes in greenhouse cultivated crops. Crop Protection, 24, 499–506.
Giannakou, I. O., & Karpouzas, D. G. (2003). Evaluation of chemical and integrated strategies as alternatives to methyl bromide for the control of root-knot nematodes in Greece. Pest Management Science, 59, 883–892.
Giannakou, I. O., Sidiropoulos, A., & Prophetou-Athanasiadou, D. (2002). Chemical alternatives to methyl bromide for the control of root-knot nematodes. Applied Soil Ecology, 26, 69–79.
Giannakou, I. O., Karpouzas, D. G., & Prophetou-Athanasiadou, D. (2004). A novel non-chemical nematicide for the control of root-knot nematodes. Applied Soil Ecology, 26, 69–79.
Giannakou, I. O., Karpouzas, D. G., Anastasiadis, I., Tsiropoulos, N. G., & Georgiadou, A. (2005). Factors affecting the efficacy of non-fumigant nematicides to control root-knot nematodes. Pest Management Science, 61, 961–972.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp including a new technique. Plant Disease Reporter, 57, 1025–1028.
Javed, N., Gowen, S. R., El-Hassan, S. A., Inam-ul-Haq, M., Shahina, F., & Pembroke, B. (2008). Efficacy of neem (Azadirachta indica) formulations on biology of root-knot nematodes (Meloidogyne javanica) on tomato. Crop Protection, 27, 36–43.
Karpouzas, D. G., Hatziapostolou, P., Papadopoulou-Mourkidou, E., Giannakou, I. O., & Georgiadou, A. (2004). The enhanced biodegradation of fenamiphos in soils from previously treated sites and the effect of soil fumigants. Environmental Toxicology and Chemistry, 23(9), 2099–2107.
Majtaheldi, H., Santo, G. S., & Pinkerton, J. N. (1991). Efficacy of ethoprophos on Meloidogyne hapla and M. Chitwoodi and encanced biodegradation in soil. Journal of Nematology, 23, 372–379.
Ntalli, N. G., Menkisoglou-Spiroudi, U., Giannakou, I. O., & Prophetou-Athanasiadou, D. A. (2009). Efficacy evaluation of a neem (Azadirachta indica A. Juss) formulation against root-knot nematodes Meloidogyne incognita. Crop Protection, 28, 489–494.
San Martin, R., & Magunacelaya, J. C. (2005). Control of plant-parasitic nematodes with extracts of Quillaja saponaria. Nematology, 7, 577–585.
SAS Inst Inc. (1995). SAS/STAT software changes and enhancements through release 6.11. Cary: SAS Inst. Inc.
Siddiqui, L. A., i Ouresh, S. A., Sultana, V., Etheshamul-Haque, S., & Ghaffar, A. (2000). Biological control of root-knot disease complex of tomato. Plant and Soil, 227, 163–169.
Sikora, R. A. (1988). Interrelationship between plant health promoting rhizobacteria, plant parasitic nematodes and soil microorganisms. Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent, 53, 867–878.
Sikora, R. A., & Fernandez, E. (2005). Nematode parasites of vegetables. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (pp. 319–392). Wallingford: CABI Publishing.
Weber, J. B., Gail, G. W., Linker, H. M., Wilcut, J. W., Leidy, R. B., Senseman, S., et al. (2000). A proposal to standardize soil/solution herbicide distribution coefficients. Weed Science, 48, 75–88.
Acknowledgements
Special thanks are due to BASF Hellas for providing the formulated product of Q. saponaria. Technical work given by Miss Paraskevi Ntallia and Mr. Stefanos Kamaras is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giannakou, I.O. Efficacy of a formulated product containing Quillaja saponaria plant extracts for the control of root-knot nematodes. Eur J Plant Pathol 130, 587–596 (2011). https://doi.org/10.1007/s10658-011-9780-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9780-8