Abstract
The effectiveness of Quinone outside Inhibitor (QoI) fungicides against grape downy mildew in European vineyards has significantly decreased in the last decade. One nucleotide polymorphism, G143A in the cytochrome b gene of Plasmopara viticola, is involved in resistance to QoIs. Previous genetic examination on the mitochondrial genomes showed four major haplotypes (IR, IS, IIR, IIS) coexisting in European vineyards. A resistant allele (G143A) was present in IR and IIR haplotypes. The purpose of the present study was to estimate the diversity of the different mitochondrial haplotypes and their distribution in QoI-resistant populations before evaluating the potential cost of the resistant mutation G143A in P. viticola population. From 2000 to 2004, the frequencies of resistant isolates ranged from 0% to 23.25% with an average of 4.64 % among the populations examined. To evaluate the fitness of sensitive and resistant isolates, a comparison of different biological parameters including latent period, spore production and infection frequency was performed, enabling a fitness index (FI) to be determined. Resistant isolates exhibited greater infection frequency than sensitive isolates, whereas no significant difference was found in sporulation ability and latent period between sensitive and resistant isolates. To further investigate competitiveness among isolates, an assay including two resistant isolates in different proportion with a sensitive isolate was conducted on eight asexual growing cycles in the absence of a QoI fungicide. The competitiveness of resistant isolates varied according to their fitness parameters, suggesting that there is no noticeable cost of QoI resistance in controlled conditions in Plasmopara viticola.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Downy mildew caused by the oomycete Plasmopara viticola is a major grape disease worldwide. The pathogen is a native species from North America that was reported in Europe in 1878 in Coutras in (Bordeaux region) by Planchon (Galet 1977). Grapevine downy mildew (P. viticola) attacks all important cultivars for vine and table grape production. Depending on epidemic pressure and cultural practices, production losses of up 100% can occur. For effective control of the disease, various fungicides, including Quinone outside Inhibitor fungicides (QoIs), have been widely used. However, strong selection pressure following repeated QoI applications can result in the development of QoI-resistant populations which has limited the success of these fungicides in grapes and other crops (Gisi et al. 2002; Grasso et al. 2006). Thus, only a few years after the introduction of QoI fungicides in 1996, P. viticola-resistant populations were detected not only in French vineyards, (Magnien et al. 2003) but also in the vineyards of other European countries and the United States (Baudoin et al. 2008; Corio-Costet et al. 2008; Collina et al. 2005; Heaney et al. 2000; Sierotzki et al. 2005).
The QoI fungicides inhibit the cytochrome bc1 enzyme complex of the respiration chain in mitochondria (Jordan et al. 1999; Bartlett et al. 2002). Due to their site-specificity, QoI fungicides have been reported as being at high risk for encouraging the development of resistance in several target pathogens (Grasso et al. 2006). In most pathogens, two major mutations in the cytochrome b gene have been described (G143A, F129L) as being involved in resistance mechanisms. The mutation resulting in the replacement of glycine by alanine at amino acid codon position 143 (G143A) has been widely recognized (Gisi et al. 2002; Grasso et al. 2006).
To understand the mechanism leading to the evolution of fungicide resistance in a plant-pathogen system, Chen et al. (2007) conducted a phylogenetic analysis of a large mitochondrial DNA fragment including the cytochrome b gene (2.281 bp) across a wide range of P. viticola isolates. Four major haplotypes were detected belonging to two genetically distinct groups (I and II), in which the resistance allele carrying the G143A mutation can be present. This indicated at least two origins of fungicide resistance in the grapevine downy mildew population. In France, haplotype group I (IR and IS) and group II (IIR and IIS) reached a frequency of 75% and 25% respectively among populations tested, but their distribution may vary (Chen et al. 2007; Corio-Costet et al. 2008). For instance, in the Champagne (France) an average of 10% of haplotype group II was detected and the significance of the two mitochondrial haplotypes is unclear (Corio-Costet et al. 2006). As regards the resistant allele proportion IR and IIR within a population, the resistant haplotype IR ranged from 67.7% to 98.3% of resistant alleles depending on the location (Chen et al. 2007; Corio-Costet et al. 2006). The first objective of this work was to estimate the distribution of every haplotype in European P. viticola populations.
Although a biological test (Wong and Wilcox 2000; Genet et al. 1997) or molecular tools have been used to quantify the resistance alleles using real-time q-PCR, ARMS PCR or PCR, (Collina et al. 2005; Grasso et al. 2006; Toffolatti et al. 2007; Sirven and Beffa 2003) or to detect resistant haplotypes using the CAPS method, (Corio-Costet et al. 2006; Baudoin et al. 2008; Furuya et al. 2009) little is known about the fitness of QoI-resistant isolates. One study suggested that an artificial P. viticola population containing 5% of resistant isolates tended to increase QoI sensitivity (Genet et al. 2006). The second objective of our study was to try to understand how fungicide resistance develops by measuring the fitness of P. viticola iolates, without fungicide pressure. The fitness, or the selective value, of pathogens (Antonovics and Alexander 1989; Pringle and Taylor 2002) can be defined as the survival and reproductive success of an allele, individual or group, or as an organism’s ability to contribute to the subsequent gene pool (Vanderplank 1982), which is measured as a function of its ability to grow, reproduce and survive through repeated life cycles. It is estimated either by measuring components of fitness such as reproductive ability and pathogenicity, or by assessing the actual population after competing with each other on a susceptible host. Usually, the general ability of pathogens to accommodate resistance mutations without a harmful loss of fitness is largely unknown, but in early studies the appearance of fungicide resistance was not generally accompanied by an immediate fitness cost in the absence of fungicides (Anderson 2005). The rapidity of the appearance of QoI resistance and the behaviour of resistant isolates in populations could suggest a good fitness value of resistant isolates that is assessed here.
The final objective of the study was to determine whether QoI resistance in P. viticola was associated with a resistance cost. For this, we performed fitness and competitiveness studies to investigate the potential of dispersal and the preservation of P. viticola isolates resistant to QoI fungicides. In particular, we assessed the following: i) the diversity of QoI haplotype in European countries: ii) the fitness of sensitive and resistant isolates of P. viticola, by comparing latent period, sporulation, infection frequency and competitiveness: and iii) the cost of QoI resistance in grapevine downy mildew.
Materials and methods
Plant material
All tests were performed with grapevine leaves of Cabernet Sauvignon cultivar. Grapevine plants (Vitis vinifera cv. Cabernet Sauvignon) were propagated from wood cuttings in a greenhouse. All tests were performed with grapevine leaves of 2-month-old plants (ten to 12 leaves) for experimentation on foliar discs.
P. viticola sampling and collection
A total of 1,366 downy mildew lesions on Vitis vinifera were collected between 2003 and 2004 or were donated by a Swiss colleague (D. Gobbin) for 2000 and 2001. Each sample from 2003 and 2004 came from a sparse plot on leaves collected between June and July during the growing season (Table 1).
For isolate collection, a total of 11 QoI-sensitive isolates and 12 QoI-resistant isolates were collected in 2003 at the beginning of the growing season on grapevine leaves in the Bordeaux vineyard in France (11 + 11) and one in the Freiburg vineyard in Germany (1) (Table 2). All monosporangial isolates were preserved on grapevine leaves at−20°C.
Fungicide sensitivity tests
To characterize the sensitivity of P. viticola isolates to QoI fungicides, single lesions were multiplied onto leaves of Vitis vinifera cv. Cabernet Sauvignon. Sporangia were collected and suspended in sterile water at 4°C. Inoculation was performed by depositing fifteen 10 μl droplets onto the abaxial face of each leaf. One day later the water droplets were removed by aspiration with a water pump. The leaves were incubated in Petri dishes under controlled conditions (22°C) with a 16 h light day (35 μm m−2 s−1) for 7 days. Freshly produced sporangia were harvested to inoculate the test units. Assays were described in Chen et al. (2007). Briefly, Cabernet Sauvignon leaf discs with abaxial surface exposed were prepared as described above. Excised discs were randomized and placed lower surface exposed on moist filter paper into Petri dishes, with eight repetitions for each concentration. Experiments were also duplicated independently. The QoI tested was famoxadone (IUPAC name: 3-anilino-5-methyl-5-(4-phenoxyphenyl)-1, 3-oxazolidine-2,4-dione) (WG 50%, Dupont de Nemours). Famoxadone was applied by spraying in water solution at desired concentrations. The solutions were applied using a hand sprayer in order to spray 10 μl per cm2. The fungicide test was repeated at least twice with a range of eight or ten concentrations adapted to the response of each isolate in order to obtain a dose-response curve. After using a discriminatory dose of 10 mg l−1, according to the sensitivity of each isolate, ranges of concentrations for resistant isolates (0, 50, 100, 200, 500, 800, 1,000, mg l−1) or for sensitive isolates (0, 0.02, 0.05, 0.08, 0.1, 0.15, 0.2, 0.5, 1, 2 mg l1) were prepared to determine the EC50 of isolates. After drying surfaces, the leaf discs were inoculated with three droplets of 10 μl per disc of inoculum at 20.000 sporangia per ml. Petri dishes were incubated for 7 days under the conditions described above. Seven days after inoculation, development of mildew on leaf discs was determined by assessing the proportion of sporulation per unit of leaf area (Genet et al. 1997). The average score for each fungicide concentration was converted to a percentage of inhibition by comparison with the untreated disks. The fungicide concentration inhibiting 50% of growth (EC50) discriminated two classes of isolates according to their sensitivity to famoxadone: famoxadone-sensitive isolates (EC50 and MIC <10 mg l−1) and highly resistant isolates (EC50 and MIC >1,000 mg l−1). Dose response curves for individual isolates were generated by plotting the relative growth values against log10 of the fungicide concentration used. The log10 effective dose to reduce growth by 50% (EC50) was calculated from the regression equation generated through the linear proportion of the sigmoid curve.
Measurements of aggressiveness components
The following fitness components were measured comparatively in sensitive and resistant isolates: latent period, the number of spores obtained per deposited spore after 7 days of growth, and the infection frequency, using methods similar to those described by Tooley et al. 1986 and Kadish and Cohen 1988. All the measurements were performed on three plates, each containing five discs of grapevine leaves inoculated as described above with three droplets of 15 μl of sporangial suspension at 2,500 spores per ml.
The latent period (LP) was estimated by observing sporangia appearance daily, as the mean period (hours) to obtain 50% of the total sporulation. Three plates containing five discs of leaf abaxial face on the top were inoculated with three droplets of 15 μl at 2,500 sporangia per ml, leading to 45 points per isolate per experiment. The experiments were repeated at least twice independently. The onset of sporulation was measured daily on a notation scale as described in Chen et al. 2007.
Spore production (Nt / Nt0) was assessed by washing five inoculated discs of each plate (3) in a vial containing 16 ml of Isoton II (saline buffer) supplemented with a drop of dispersant type IIIA, and the number of sporangia produced per plate containing five discs inoculated by three drops was determined (Nt) with a Multisizer 3 Coulter counter (Beckman Coulter, France, Villepinte). Experiments were repeated at least twice independently, giving six measures each corresponding to the sporulation obtained for 562 deposited sporangia (Nt0).
Infection frequency (IF) was defined as the proportion of inoculated leaf disks on which lesions developed. Observations were quantified as the number of inoculation points with a suspension of 2,500 spores per mL giving sporulating lesions 7 days after inoculation. Three plates containing five leaf discs were inoculated by three drops of 15 μl per disc. Experiments were repeated twice independently.
From these different parameters, a composite fitness index (FI) was calculated for each isolate using the formula \( {\hbox{AI}} = {\hbox{Ln }}({{\hbox{N}}_{\rm{t}}}/{{\hbox{N}}_{{{\rm{t}}0}}} \times {\hbox{IF}} \times {1}/{\hbox{LP}}) \) derived from Montarry et al. 2008.
Competitiveness test
The relative competitiveness of two pairs of resistant/sensitive isolates MIC128-COU15 (R1-S1) and PAU32-COU15 (R2-S1) was compared at three initial ratios of spore concentrations, 20:80, 50:50, and 80:20. Spore suspensions of each isolate were prepared as described previously. Mixed inocula were generated by thoroughly mixing appropriate volumes of sporangia to obtain the desired R:S ratios on the basis of 40.000 spores per ml total in the different mixtures. Inoculation was carried out with a mixed spore suspension in the same way as described for infection tests. Four plates containing five discs were inoculated for each ratio and every 7 days, four new plates with five discs were inoculated again with the spores obtained at 40,000 spores per ml. Then, a biological test with famoxadone at 100 mg l−1 was performed as described above in fungicide sensitivity test section and a reference curve was established to evaluate the ratio of resistant isolates in the spore mixtures obtained after each asexual cycle. To confirm those ratios, a quantitative PCR assay was also performed to quantify the presence of resistant alleles from spore suspensions used to inoculate the new leaf discs at each cycle. Two independent experiments were performed for 8 weeks.
Statistical analysis
Data from two experiments were pooled together according to homogeneity of variance using the ANOVA procedure in Systat software (Systat 11. software, 2004, USA) to determine homogeneity of variance among repeated experiments. The values or groups were compared using the Tukey t test with P < 0.05 for q-PCR assay or were compared using Fisher’s LSD test with P < 0.05 for the bioassay. All data for each fitness component were subjected to an analysis of variance (ANOVA). To analyze frequency haplotype distribution, a chi2 test was performed.
DNA extraction
P. viticola lesions were frozen. After freeze-drying overnight, DNA was extracted from each oil spot or spore suspension (containing infected grapevine leaf tissue and sporangia) according to the standard CTAB-phenol/chloroform method described by (Zolan et Pukkila 1986) with a slight modification (phenol-chloroform was replaced with isoamylic alcohol/chloroform). After extraction, DNA was precipitated with isopropanol, the pellet was washed with 70% ethanol and resuspended in 50 μl of sterile water.
Allele-specific restriction
The molecular method used to differentiate sensitive or resistant isolates was the amplification of the allele specific to the Cytochrome b gene of P. viticola (accession numbers DQ459464, DQ459466, DQ459467, DQ459469) exhibiting single nucleotide polymorphisms between sensitive and resistant isolates or between haplotype I or II (Chen et al. 2007). A CAPS analysis of cyctochrome b gene fragments was performed using the primers COB 279F and COB 865R (Table 3) under amplification conditions according to the method described by Baudoin et al. (2008) and Corio-Costet et al. (2006). Polymerase chain reaction (PCR) was performed in a 15 μl volume containing 1 μl of DNA diluted three-fold, 1.5 μl of 10x PCR buffer (Eurogentec), 1.5 mM MgCl2, 0.13 μM of each dNTP, 0.13 μM of each primer (COB 279 F and CB 865R), 0.2 U (3.33 nkat)Taq silverstar DNA (Eurogentec). The PCR program was a first denaturation step of 4 min at 96°C, followed by 36 cycles of 45 s at 96°C, 50 s at 50°C, 1 min at 72°C. This DNA fragment was cut with the restriction enzymes Sat1 and Hinf1, with the recognition sequence 5′-GCNGC-3′ and 5′–GANTC-3′. Cleaving reactions were performed in 10 μl volume with 1.2 μl of PCR product, 1 μl of 10X- buffer Tango (MBI fermentas), 1 U (16.67 nkat) of Sat1 and 1U (16.67 nkat) of Hinf1 enzymes (MBI Fermentas) at a temperature of 37°C and an incubation period of 2 h. Restriction fragments were visualized on 2% agarose after staining with ethidium bromide. Sat1 enzyme was used to detect the resistant mutation and to cut the amplified fragment of 605 bp, generating two fragments of 147 and 458 pb each. Hinf1 enzyme was used to determine haplotypes and to cut the fragment, generating two fragments of 222 and 383 pb.
QoI real-time q-PCR
Genotyping of QoI-resistant mutations was performed with a real-time thermal iCycler iQ (BioRad, Hercules, CA, USA) using specific sets of primers and hybridization probes (Table 3). Primer sets qPV-L/qPV-R and qPV143R-L/qPV143RS-R (Table 3) were used to amplify preferentially one allele by matching the desired allele and mismatching the other allele at the 3′ end of the primers according to Sirven and Beffa (2003). Reverse primers were designed to have mismatches at the 3′ as described by Sirven and Beffa (2003). For each reaction, 1 μl of each primer at 10 nM and 7 μl of 2x-Mix blue SYBR green fluorescein Mix including Hot start DNA polymerase, DNTP and MgCl2 (Abgene, France) and 5 μl of DNA diluted three times were used in compliance with the manufacturer’s instructions. Assays (14 μl) were performed in ABgene PCR thermo-fast® 96, semi-skirted plates (Thermoscientific, France). Amplification and detection were performed in a real-time thermal iCycler iQ under the following conditions: one cycle at 94°C for 15 min, and 40 cycles at 94°C for 10 s, 60°C (primer set qPV-L/qPV-R) and 60°C (qPV143R-L/qPV143RS-R) for 10 s, and 72°C for 20 s. Increase in fluorescent emission signal from SYBR Green I (ΔRn) was recorded at 72°C without normalization of the signal. The iCycler™ Q software version 3.0a (BioRad) was used to analyze the data. For each sample, the amount of target DNA was quantified using appropriate calibration curves in which Ct was plotted against the amount of target DNA.
Results
Mitochondrial haplotype distribution in European vineyards
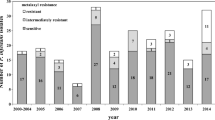
The survey of the geographical distribution of mitochondrial haplotypes from a panel of 1,366 P. viticola isolates collected between 2000 and 2004 in European vineyards was performed with an SNP typing assay. The frequency of the four main European haplotypes was estimated in four French, three German, one Greek, three Italian, one Romanian, three Portuguese and three Swiss populations (Table 1). Haplotype group I (IS and IR) predominated in all countries in a range from 77.44% to 97.02%, with an average of 84.33%. Haplotype group II (IIS and IIR) represented 2.98% to 22.56% depending on the country, with an average of 15.68%. With regard to QoI resistance, haplotype IR predominated, with levels depending on the country and locality in a range from 0% to 20.99% and an average of 4.14%. The resistant haplotype IIR was the most uncommon and was found only in two French vineyards in Bordeaux and Bourgogne. More resistant haplotypes were detected in 2003–2004 than in 2000–2001, with an average of 16.26% and 0% respectively.
QoI sensitivity and haplotype characteristics
Throughout the study, 11 sensitive isolates and 12 resistant isolates collected in 2003 were tested independently at least twice to evaluate their famoxadone sensitivity. EC50 values for resistant isolates were higher than 1,000 mg l−1 of famoxadone (Table 2). Sensitive isolates exhibited EC50 values ranging from 0.1 to 0.9 mg l−1, with a mean of 0.19 mg l−1. Seven sensitive isolates belonged to haplotype II and four belonged to haplotype I (COU15, COL07, MIC123 and EPE 09). By contrast, the resistant haplotype II was uncommon, while only three isolates (SAL19, PAU 32, COU 23) from the laboratory collection belonged to this haplotype. The level of QoI resistance between sensitive and resistant isolates expressed as the relative resistance factor varied in a range from 1111 to 25,000, with an average of 5263.
Latent period, sporangium production and infection frequency
Data on the fitness of 23 isolates inoculated separately onto grapevine leaf discs are given in Table 4. Three parameters (latent period, sporulation capacity and infection frequency) were recorded. A wide variation in fitness parameters was found in sensitive and resistant isolates. However, no difference was found for latent period between sensitive and resistant isolates (P = 0.523) (Table 4, Fig. 1). Sensitive isolates had a mean of 102.7 ± 1.57 h and resistant isolates needed 100.9 ± 1.32 h to reach their latent period. The range of latent period varied from 86.5 h to 140 h in sensitive isolates and from 89.5 to 139 h in resistant isolates.
Concerning spore production, no significant difference was observed between sensitive and resistant isolates (P = 0.137). Sensitive isolates produced an average of 704 ± 139 sporangia and resistant isolates produced 830 ± 171 sporangia per deposited sporangium (Table 4, Fig. 1). A wide variation was observed within the sensitive isolate group and the QoI-resistant group. Indeed, within a group some isolates produced 3.11- to 3.8-fold more sporangia than others in the resistant (PIC59/SAL19) and sensitive (BOM06/AVI01) groups, respectively. Sporangium size in sensitive and resistant isolates varied widely in a range from 8 to 33 μm in the sensitive and resistant groups, with an average of 18.47 μm ± 0.03 μm. We also observed a significant increase in resistant sporangium size from 3% to 4.6% in the absence or in the presence of famoxadone (data not shown).
As regards infection frequency (IF) in our experimental conditions, a significant difference was obtained (P = 0.02) between sensitive and resistant groups with 0.84 ± 0.03 of sensitive isolates and 0.94 ± 0.02 of resistant isolates succeeding in infecting leaves (Table 4, Fig. 1). The range of infection frequency varied from 0.42 to 0.97 in the sensitive group and from 0.68 to 1 in the resistant group.
From these different parameters, a composite fitness index (FI) was calculated for each isolate derived from FI of Tooley et al. (1986) and from the aggressiveness index of Montarry et al. (2008). The mean FI in the sensitive group was 1.75 ± 0.65 and 2.05 ± 0.45 in the resistant group, but no significant difference was observed (P = 0.147) (Table 4). The fitness index varied considerably depending on whether the isolates were sensitive or resistant (Fig. 2). A good correlation was obtained between spore production and the FI with a regression coefficient R2 equal to 0.86 (P < 0.001). The FI was then used to choose sensitive and resistant isolates for competitiveness tests having different fitness.
Competitive ability of resistant and sensitive isolates
The objective was to assess the competitive ability of resistant and sensitive isolates in mixed-isolate inoculations on leaf discs and to link these findings to the fitness parameters of isolates. The fitness index of the three isolates was different (Table 4, Fig. 2) with a sensitive isolate COU15 statistically intermediate (FI = 1.78, ab) between both resistant isolates PAU 32 (FI = 1.57, a) and MIC128 (FI = 2.15, b) (P = 0.015).
The changes in the frequency of resistant isolates were related to their fitness parameters and their fitness index (Figs. 3 and 4). In one of the pairs used in this study R1-S1, after eight asexual cycles all ratios between sensitive and resistant isolates remained stable at 20%, 50% or 80% of R1 after quantification by q-PCR (Fig. 3). A slightly significant increase (P < 0.05) in 20% and 50% of initial ratios was observed at the eighth cycle with the biological quantification method.
Dynamic changes (means±SEM) in the frequency of QoI-resistant isolate sporangia in sporangial populations harvested from grapevine leaf discs inoculated with three mixtures of QoI-sensitive COU15 and QoI-resistant sporangia MIC128 in various proportions (20:80 (-■-), 50:50 (-□-) and 80:20 (--▲--) during eight asexual generations, assessed by bioassay or q-PCR method. The second sporulation cycle was initiated with sporangia resulting from the initial infection and subsequent sporulation cycles were conducted accordingly. Each point is mean of six replicates
Dynamic changes (means±SEM) in the frequency of QoI-resistant isolates sporangia in sporangial populations harvested from grapevine leaf disks inoculated with three mixtures of QoI-sensitive COU15 and QoI-resistant sporangia PAU 32 in various proportions (20:80 (-■-), 50:50 (-□-) and 80:20 (--▲--) during eight asexual generations, assessed by bioassay or q-PCR method. The second sporulation cycle was initiated with sporangia resulting from the initial infection and subsequent sporulation cycles were conducted accordingly. Each point is mean of six replicates
In the second pair (S1-R2), a decline in the frequency of R2 was observed after five cycles (Fig. 4) in all three initial ratios, indicating that the resistant isolate R2 was a weak competitor in comparison with its S1 partner. After five cycles, the proportion of resistant isolates decreased 20–25% compared to the initial ratio. With the q-PCR test, a significant and stronger decrease in the different ratios with R2 was observed from the third cycle, leading to a dramatic reduction during the eighth cycle, with a decrease of 5% (P = 0), 49.56% (P = 0.006) and 97.5% (P = 0.004) at the ratios 80:20, 50:50 and 20:80, respectively (Fig. 4).
Discussion
Diversity
According to previous results obtained in French vineyards (Chen et al. 2007; Corio-Costet et al. 2006, 2008), haplotype I is the most widespread haplotype in Europe, with a mean of 84.33% (IS-IR). Regarding the analyses of the 1,366 isolates obtained between 2000 and 2004, only 4.54% (IR-IIR) of isolates were QoI-resistant, with haplotype IR representing 91.19% of resistant haplotypes. By contrast, French vineyards were significantly more resistant during this period than other vineyards exhibiting few resistant isolates (0–2.94%) such as Greek, Italian, Swiss and Romanian vineyards. Haplotype IIR was rare, except in Bourgogne where it represented 21.2%. The role of the presence of haplotypes I and II in P. viticola population remains unclear.
Our results on 1,015 mildewed lesions showed that in French vineyards in 2003 and 2004, at the beginning of the growing season and before any selection by a fungicide, 76.75% of P. viticola isolates were still sensitive. However, according to Sierotzki et al. (2005), who showed a sharp decrease of QoI sensitivity in 2002 and 2003 in European vineyards, we found more resistant haplotypes in 2003–2004 than in 2000–2001, revealing an increase of the resistance during this period. Thus, the resistance frequencies are not the same everywhere and the differences found may depend on the pressure exerted by QoI fungicides and probably also on the population fitness in the absence of selection pressure.
Fitness parameters
The development and the spread of fungicide resistance depends on the fitness of resistant isolates, and knowledge of processes such as diversity, sex evolution and fungicide pressure is essential to try to prevent and/or to control the diseases better. For this, it is important to understand how fitness shapes the selection of individuals in nature under different selection pressure. From various sensitive or QoI resistant isolates belonging to different mitochondrial haplotypes, we measured fitness parameters without fungicide selection pressure. A major point should be made: resistant isolates are as fit as sensitive isolates, with regard to sporangia production, latent period and infection frequency in asexual cycles. Only infection frequency of resistant isolates was significantly higher than in sensitive isolates (P = 0.02). As the resistant isolates belonged essentially to haplotype I, it was possible that the difference was due in part to the belonging to the haplotype I group. A comparison between the fitness of haplotypes I and II inside sensitive group, or with the totality of isolates, including the resistant isolates, showed no significant difference in the spore production (P = 0.75 and 0.24), the latent period (P = 0.36 and 0.35), or the fitness index (P = 0.86 and 0.10). Only infection frequency was significantly more important for haplotype I (P = 0.03) and when QoI-resistant isolates were considered. As above, the significance of the infection frequency could well be related to the QoI resistance.
From this first study on the fitness parameters, we were thus able to conclude that the presence of G143A SNP in cytochrome b gene has no deleterious effect on growth parameters observed under controlled conditions and without selection pressure.
However, the overall fitness of one population relative to another would be difficult to predict solely on the basis of knowledge of fitness components. For this reason, the combination of different parameters would reasonably approximate overall fitness in calculating a composite fitness index. The fitness index described here, based on infection frequency, spore production and latent period enabled us to show that there was no significant difference between groups of haplotypes I and II and between sensitive and QoI resistant isolates. This index also revealed a good correlation with spore production suggesting that under our experimental conditions spore production could be a sufficient measure to assess the fitness of isolates. This FI based on growth parameters seems adequate to standard conditions, but one of its weaknesses is certainly that it does not take in account survival capacity under less favourable environmental conditions, or fitness potential of the sexual cycle (i.e. oospore production, overwintering).
As with other studies on the fitness of oomycetes, such as Phytophthora nicotianae or P. infestans resistant to metalaxyl or mefenoxam (Hu et al. 2008; Kadish and Cohen 1988), QoI resistance did not show reduced fitness. However, it is possible that resistance cost might only become apparent under a specific set of environmental conditions which were not encountered in the experimental set-up, or that the associated antagonistic pleiotropy might not be detectable (Rosenberg 2001); or, yet again, that a fitness deficit linked to a resistance cost can be compensate for after numerous generations by different compensatory mechanisms (Anderson 2005; Cowen et al. 2001). One example of resistance cost due to environmental conditions was metalaxyl-resistant isolates of Plasmopara viticola that exhibited reduced fitness to grape leaves, but only at high temperature (Piganeau and Clerjeau 1985). A preliminary study performed under controlled conditions in our laboratory did not show differences between sensitive and resistant QoI isolates at 15°C or 28°C.
In connection with definite FI, we estimated competitiveness of sensitive and resistant pairs of isolates. The follow-up during 8 cycles of asexual reproduction showed that, in the absence of selection pressure, resistant isolates can compete with sensitive isolates in mixed inoculation depending on their fitness parameters. Thus, a resistant isolates with a lower FI (PAU32) than the sensitive isolates (COU 15) tends to disappear after eight cycles, while a resistant isolate (MIC 128) with a high FI preserves even tends to increase after eight cycles. These results were reinforced by other isolate pairs tested previously, where a resistant isolate with a higher FI (PIC59, FI = 2.75) than the sensitive isolate FEM103 (FI = 2.11) increased rapidly to reach 100% of resistant isolate in the whole mix after the fifth cycle. In the same way, another mix with PAU 32 (FI = 1.57) and a sensitive strain MIC 103 (FI = 1.77) also showed a decrease in the presence of PAU 32 from the fourth cycle (Abadie 2007).
Globally the competitiveness tests performed were coherent with the estimated parameters of the fitness and our FI, although imperfect, enabled us to obtain a good idea of the capacities of isolate survival under controlled conditions. In accordance with Kadish and Cohen (1988), we showed that the pattern of changes in frequency of resistant isolates could be largely dependent on fitness parameters in laboratory conditions. During the competitiveness assays, we used the bioassay or the q-PCR method, the curves of competitiveness (Figs. 3 and 4) showed some differences, although tendencies were similar. The bioassay seemed to facilitate the presence of resistant isolates (R1 and R2). One possible explanation could be that the method of q-PCR is strictly quantitative and applies directly to spores harvested after each asexual cycle, while the bioassay required an additional cycle in the presence of famoxadone, facilitating the growth of resistant isolates and potentially integrating others fitness parameters. Thus, the quantification of the percentage of resistant isolates based on reference curves in the presence of famoxadone in the bioassay could lead to a slight overestimation of the presence of resistant isolates compared to q-PCR carried out without fungicide pressure. However, the faster, easier q-PCR method is an adequate method to estimate the proportion of resistant isolates.
As with the analysis of fitness parameters, the competitiveness assays strengthened the idea that the presence of G143A mutation would have no deleterious effect on P. viticola under controlled conditions without fungicide selection pressure. As all resistant isolates used in this study have already been sequenced (Chen et al. 2007) and possessed only the G143A SNP, which is the most frequent, we would suggest that the G143A mutation does not induce a resistance cost in P. viticola. However, Fisher et al. (2004) have investigated the impact of the introduction of G143A SNP on bc complex activity of Saccharomyces cerevisiae and shown that it has little effect on the enzyme activity. In contrast, the association of the G143A with F129L, or other mutations, led to various deleterious effects on enzyme activity. It would be useful to assess the resistance cost of different mutations (i.e. G143A and F129L) in the laboratory, to understand why the F129L SNP was less frequent in P. viticola populations than in other pathogen populations.
The management of pesticides in the vineyard requires to a better understanding of how the resistance takes hold, spreads and is maintained. Important questions about the adaptation of QoI resistance are: how does QoI resistance develop in the vineyard? What is the probability of obtaining various combinations of SNPs, and what is the consequence on population fitness? In view of the importance of the development of QoI-resistance over the past decade, today these products are never applied alone and are little present in current formulations or, if they are, they are then associated with other fungicides. It would be interesting to follow the development of QoI resistance under various selection pressures and survey the development of the different SNPs in the vineyard and to study their fitness in laboratory conditions. Understanding the development of QoI resistance in the vineyard is not easy. Data obtained in vineyard in Brazil on a trial site exhibiting 100% frequency of G143A in P. viticola populations showed that after stopping QoI treatment for 3 years, the frequency returned to 1% in 2002, suggesting a multiannual instability of the resistance. In contrast, five subsequent applications of QoI fungicide sprays increased the frequency again to 100% within one season, immediately producing a resistant population (Sierotzki et al. 2008). In addition, a survey of the development of QoI resistance in different sites in French vineyards with few resistant isolates (less than 10%) showed the maintenance of QoI resistance in sites without QoI treatment during the growing season (Corio-Costet et al. 2006, 2008). These studies suggest that the resistant isolates possess rather good one fitness during the growing season (asexual cycle), but that the fitness of QoI resistant populations could be less good during the sexual phase and/or without selection pressure. Therefore, the contribution of sexual reproduction to global fitness might play an important role in maintaining QoI resistance. Since the cytochrome b gene is coded by the mitochondrial genome, it most likely depends on mitochondrial inheritance. After sexual reproduction, the level of resistant allele might decrease, depending on the mating type carrying the resistant allele, and/or the sexual fitness, and could explain contrasted results obtained in fields after sexual or asexual cycles.
The possibilities of combating QoI resistance in P. viticola by mixing QoIs with other fungicides, or stopping the use of QoI fungicides for a few years in order to diminish resistance after each annual sexual cycle are currently solutions used by wine growers. The application of a QoI mixed with other fungicides does not seem to eliminate selection pressure, but delays it compared to the application of QoI fungicide alone (Toffolatti et al. 2007). From a practical point of view, care should be taken not to use QoI fungicides again on plots of land having presented QoI resistance during the growing season, or then to preserve products containing QoIs for rare or selective applications during very strong epidemic pressure, and by alternating treatments with fungicides possessing different mode of action.
In conclusion, the mitochondrial haplotype II is little present in P. viticola populations and does not seem to have repercussions on resistant populations. Resistance can be very variable according to the vineyard under consideration and the selection pressure exerted. During the experiment under controlled conditions, we did not detect a significant difference of fitness either between 23 resistant and sensitive isolates, or between haplotypes I and II. The composite fitness index used here may help us to classify isolates according to different phenotypic and genotypic traits (i.e. fungicide resistance, mating-type, agressiveness) and to have them compete according to several parameters of fitness, or used to carry out studies on the efficacy of alternative methods (i.g. elicitors, breeding, biological fight) on specific population or isolates. It would also be useful to estimate the survival potential of resistant isolates exhibiting single, double or triple resistance to fungicides (i.e. Qoi, CAA and/or phenylamide fungicides), already encountered in the vineyard; but that would require including parameters of overwintering, and of capacity of sexual reproduction, to be used under field conditions. A study of fitness of the resistant isolates during the sexual cycle would be necessary to complete this work and to acquire better understanding of QoI resistance evolution under various pressures of selection.
Abbreviations
- CAPS:
-
Cleaved Amplified Polymorphic Sequence
- FI :
-
fitness index
- QoI:
-
Quinone outside Inhibitor
- ARMS:
-
Amplification refractory mutation system
- SNPs:
-
Single Nucleotide Polymorphisms
References
Abadie, P. (2007). Adaptation du mildiou de la vigne à la pression fongicide: étude du fitness. MSc thesis of Genetic and Plant Development. Bordeaux University. pp. 20.
Anderson, J. B. (2005). Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nature Reviews Microbiolology, 7, 547–556.
Antonovics, J., & Alexander, H. M. (1989). The concept of fitness in plant-fungal pathogen systems. In W. E. Fry & K. J. Leonard (Eds.), Plant disease epidemiology (pp. 185–214). New-York: Mc Graw-Hill.
Bartlett, D. W., Clough, J. M., Godwin, J. R., Hall, A. A., Hamer, J., & Parr-Bobrzanski, B. (2002). The strobilurin fungicides. Pest Management Science, 58, 649–662.
Baudoin, A. I., Olaya, G., Delmotte, F., Colcol, J. F., & Sierotzki, H. (2008). QoI resistance of Plasmopara viticola and Erysiphe necator in the Mid-Atlantic United States. Plant Management Network. Plant Health Progress. doi:10.1094/PHP-2008-0211-02-RS.
Chen, W.-J., Delmotte, F., Richard Cervera, S., Douence, L., Greif, C., & Corio-Costet, M.-F. (2007). At least two origins of fungicide resistance in grapevine downy mildew populations. Applied Environmental Microbiology, 73, 5162–5172.
Collina, M., Landi, L., Guerinin, P., Branzanti, M. B., & Brunelli, A. (2005). QoI resistance of Plasmopara viticola in Italy: biological and quantitative real-Time PCR approaches. In H. W. Dehne, U. Gisi, K. H. Kuck, P. E. Russell, & H. Lyr (Eds.), Modern fungicides and antifungal compounds IV (pp. 81–88). Hampshire: BCPC.
Corio-Costet, M. -F., Delmotte, F., Martinez, F., Giresse, X., Raynal, M., Richart-Cervera, S., et al. (2006). Resistance of Plasmopara viticola to QoI fungicides: origin and diversity. Paper presented at the 8th Int. Conf on Pest and Diseases 2006, pp 612-620, AFPP Eds, CD-Rom.
Corio-Costet, M. -F., Martinez, F., Delmotte, F., Douence, L., Richart-Cervera, S., & Chen, W. -J. (2008). Resistance of Plasmopara viticola to QoI fungicides: Origin and diversity. In H. W. Dehne, U. Gisi, K. H. Kuck, P. E. Russell, & H. Lyr (Eds.), Modern fungicides and Antifungal compounds V (pp. 107–112). Brauschweig: DPG Selbstverlag.
Cowen, L. E., Kohn, L. M., & Anderson, J. B. (2001). Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. Journal of Bacteriology, 183, 2971–2978.
Fisher, N., Brown, A. C., Sexton, G., Cook, A., Windass, J., & Meunier, B. (2004). Modeling the Qo site of crop pathogens in Saccharomyces cerevisiae cytochrome b. European Journal of Biochemistry, 271, 2264–2271. doi:10.1111/j.1432-1033.2004.04169.x.
Furuya, S., Suzuki, S., Kobayashi, H., Saito, S., & Takayanagi, T. (2009). Rapid method for detecting resistance to QoI fungicides in Plasmopara viticola populations. Pest Management Science, 65, 840–843.
Galet, P. (1977). Mildiou. In P. Galet (Ed.), Les maladies et les parasites de la vigne (pp. 89–222). Montpellier: Paysan du midi.
Genet, J.-L., Steva, H., Vincent, O., & Cazenave, C. (1997). A method for measuring the level of sensitivity of Plasmopara viticola populations to cymoxanil. EPPO bulletin, 27, 217–225.
Genet, J.-L., Jaworska, G., & Deparis, F. (2006). Effect of dose rate and mixture of fungicides on selection for QoI resistance in populations of Plasmopara viticola. Pest Management Science, 62, 188–194. doi:10-1002/ps.1146.
Gisi, U., Sierotzki, H., Cook, H., & McCaffery, A. (2002). Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Management Science, 58, 859–867.
Grasso, V., Palermo, S., Sierotzki, H., Garibaldi, A., & Gisi, U. (2006). Cytochrome b structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Management Science, 62, 465–472.
Heaney, S. P., Hall, A. A., Davies, S. A., & Olaya G. (2000). Resistance to fungicides in the QoI-STAR cross-resistance group: current perspectives. In Proc. Brighton Conf. Protec Conf. Pest and diseases (pp. 755–764). Farham: BCPC.
Hu, J. H., Hong, C. X., Stromberg, E. L., & Moorman, G. W. (2008). Mefenoxam sensitivity and fitness analysis of Phytophthora nicotianae isolates from nurseries in Virginia, USA. Plant Pathology, 57, 728–736. doi:10.111/j.1365-3059.2008.01831x.
Jordan, D. B., Livingston, R. S., Bisaha, J. J., Duncan, K. E., Pember, S. O., Picollelli, M. A., et al. (1999). Mode of action of famoxadone. Pesticide Science, 55, 105–108.
Kadish, D., & Cohen, Y. (1988). Competition between metalaxyl-sensitive and metalaxyl-resistant isolates of Phytophthora infestans in the absence of metalaxyl. Plant Pathology, 37, 558–564.
Magnien, C., Micoud, A., Glain, M., & Remuson, F. (2003). QoI resistance of downy mildew-monitoring and tests 2002. Paper presented at the 7th Int. Conf. on Pest and Diseases 2003, 8 pages. AFPP Eds, CD-Rom.
Montarry, J., Glais, R., Corbiere, R., & Andrivon, D. (2008). Adaptation to the most abundant host genotype in an agricultural plant-pathogen system-potato late blight. Journal of Evolution Biology, 21, 1397–1407. doi:10.1111/j.1420-9101-2008.01557.x.
Piganeau, B., & Clerjeau, M. (1985). Influence différentielle de la température sur la germination de sporocyste et la sporulation des souches de Plasmopara viticola sensible et résistantes aux phénylamides. In Fungicides for Crop Protection. BCPC monograph, 31, 327–330.
Pringle, A., & Taylor, J. W. (2002). The fitness of filamentous fungi. Trends in Microbiology, 10, 474–481.
Rosenberg, S. M. (2001). Evolving responsively: adaptive mutation. Nature Review Genetic, 2, 504–515.
Sierotzki, H., Kraus, N., Assemat, P., Stanger, C., Cleere, C., Windass, J., et al. (2005). Evolution of resistance to QoI fungicides in Plasmopara viticola populations in Europe. In H. W. Dehne, U. Gisi, K. H. Kuck, P. E. Russell, & H. Lyr (Eds.), Modern fungicides and Antifungal compounds IV (pp. 73–80). Hampshire: BCPC.
Sierotzki, H., Kraus, N., Pepin, S., Ferandes, N., & Gisi, H. (2008). Dynamics of QoI resistance in Plasmopara viticola. In H. W. Dehne, U. Gisi, K. H. Kuck, P. E. Russell, & H. Lyr (Eds.), Modern fungicides and antifungal compounds V (pp. 151–157). Brauschweig: DPG Selbstverlag.
Sirven, C., & Beffa, R. (2003). Resistance to fenamidone: monitoring by real-time quantitative PCR on Plasmopara viticola. Pflanzenschutz-Nachrichten Bayer, 56, 523–5332.
Toffolatti, S. T., Serrati, L., Sierotzki, H., Gisi, U., & Vercesi, A. (2007). Assessment of QoI resistance in Plasmopara viticola oospores. Pest Management Science, 63, 194–201.
Tooley, P. W., Sweigard, J. A., & Fry, W. E. (1986). Fitness and virulence of Phytophthora infestans from sexual and asexual populations. Phytopathology, 76, 1209–1212.
Vanderplank, J. E. (1982). Host-pathogen Interactions in plant disease (p. 27). New-York: Academic.
Wong, F. P., & Wilcox, W. F. (2000). Distribution of baseline sensitivities to azoxystrobin among isolates of Plasmopara viticola. Plant disease, 84, 275–281.
Zolan, M. E., & Pukkila, P. J. (1986). Inheritance of DNA methylation in Coprinus cinereus. Molecular Cell Biology, 6, 195–200.
Acknowledgements
We thank D. Gobbin and colleagues from IFV for their help in sampling of the collection and Aquitaine Region Government for their financial support. We thank S. Gambier, S. Richart-Cervera, and L. Douence for technical support. We thank J-L. Genet from Dupont Protection for the gift of famoxadone.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corio-Costet, MF., Dufour, MC., Cigna, J. et al. Diversity and fitness of Plasmopara viticola isolates resistant to QoI fungicides. Eur J Plant Pathol 129, 315–329 (2011). https://doi.org/10.1007/s10658-010-9711-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-010-9711-0