Abstract
Obesity is a risk factor for arterial and venous thromboembolism. However, it is not known whether obesity mediates risk through shared mechanisms. In a population-based cohort, we aimed to compare the impact of obesity measures on risk of venous thromboembolism (VTE) and myocardial infarction (MI), and explore how obesity-related atherosclerotic risk factors influenced these relationships. Measures of body composition including body mass index , waist circumference (WC), hip circumference (HC), waist-hip ratio (WHR) and waist-to-height ratio (WHtR) were registered in 6,708 subjects aged 25–84 years, who participated in the Tromsø Study (1994–1995). Incident VTE- and MI-events were registered until January 1, 2011. There were 288 VTEs and 925 MIs during a median of 15.7 years of follow-up. All obesity measures were related to risk of VTE. In linear models, WC showed the highest risk estimates in both genders. In categorized models (highest versus lowest quintile), WC showed highest risk in men (HR 3.59; 95 % CI 1.82–7.06) and HC in women (HR 2.27; 95 % CI 1.54–4.92). Contrary, WHR and WHtR yielded the highest risk estimates for MI. The HR of MI (highest vs. lowest quintile) for WHR was 2.11 (95 % CI 1.59–2.81) in men and 1.62 (95 % CI 1.13–2.31) in women. The risk estimates for MI were substantially attenuated after adjustment for atherosclerotic risk factors, whereas the estimates for VTE remained unchanged. Our findings suggest that the impact of body fat distribution, and the causal pathway, differs for the association between obesity and arterial and venous thrombosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have shown an association between arterial (e.g. myocardial infarction and stroke) and venous (deep vein thrombosis and pulmonary embolism) thromboembolism during the last decade [1–4]. It has been suggested that this link may be caused by shared risk factors [5]. Obesity has been consistently associated with both arterial and venous thromboembolism (VTE) [6–11]. Epidemiological studies have reported a 1.5–2 fold increased risk of myocardial infarction (MI) [12, 13] and a twofold to threefold increased risk of VTE [9, 14–17] in obese subjects. However, it is not known whether obesity contributes to the risk of arterial and venous thrombosis through the same pathophysiological mechanism(s).
Obesity is a quite heterogeneous condition, and body fat distribution may have differential impact on the risk of diseases [18]. Anthropometric measures such as waist circumference (WC), waist to hip ratio (WHR) and waist to height ratio (WHtR) have been shown to predict risk of arterial cardiovascular disease more accurately than body mass index (BMI) [19, 20]. Visceral obesity appears to be essential for development of the metabolic syndrome, and is closely related to atherosclerotic risk factors such as hypertension, dyslipidaemia and hyperglycaemia [18, 21]. Targeted interventions to reduce atherosclerotic risk factors lowers the risk of arterial thrombosis [22], further supporting the concept that arterial thrombosis in obese subjects to a large extent is mediated by a concurrent rise in atherosclerotic risk factors.
We have previously shown that all measures of obesity are associated with risk of VTE [23], and reported that WC yielded the highest risk estimates, and identified most subjects at increased risk [23]. The mechanism by which obesity increases the risk of VTE is unclear, and the impact of co-existing atherosclerotic risk factors on this relationship has not been well studied. If atherosclerotic risk factors modify the association between obesity measures and risk of VTE, targeted interventions to reduce atherosclerotic risk factors may potentially prevent obesity-related VTE.
In the present study, we aimed to investigate whether the body fat distribution and cardiometabolic consequences of obesity had differential impact on risk of arterial and venous thrombosis. Calculating the risk of two outcomes within the same cohort would allow us to assess the influence of potentially shared risk factors. Therefore, we compared the impact of various obesity measures on the risk of MI and VTE in analyses with and without adjustment for atherosclerotic risk factors within a population-based cohort recruited from a general population.
Materials and methods
Study population
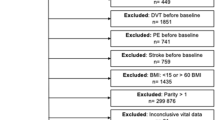
Participants were recruited from the fourth survey of the Tromsø Study, a single-center, prospective, population-based health study, with repeated health surveys of inhabitants in Tromsø, Norway. The fourth survey was carried out in the period 1994–1995 and consisted of two screening visits with an interval of 4–12 weeks. All women born in the period 1920–1944 and all men born in 1920–1939, and 5–10 % samples of subjects born 1940–1970, and 1910–1919, were invited to the more extensive second visit. A total of 7,965 subjects attended, yielding a response rate of 77 % in women and 74 % in men. The study was approved by the regional committee for medical research ethics and all participants gave their informed written consent to participate. Among the subjects who attended, 1,055 were women participating in a sub-study with limited evaluation data; these were excluded from our study. Subjects who did not give written consent to participate in medical research (n = 49), subjects with prior VTE (n = 26), subjects with prior MI (n = 401), subjects not officially registered as inhabitants of the municipality of Tromsø at the date of examination (n = 7), and subjects for whom data of BMI, body height, WC or HC were not available (n = 48) were excluded. Hence, a total of 6,379 subjects were included in our study and followed from the date of enrolment in 1994–1995 up to the end of the study period, January 1, 2011.
Measurements
Baseline information was collected by physical examination, non-fasting blood samples, and self-administered questionnaires. Anthropometric measures were performed with subjects wearing short-sleeved garments and no shoes. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). WC was measured in centimeters at the umbilical line. HC was measured in centimeters at the widest point at the hips. WHR ratios were calculated by dividing WC by HC. WHtR ratios were calculated by dividing WC by body height in centimeters. Information on self-reported diabetes and current smoking was collected from a self-administrated questionnaire. Blood pressure was recorded with an automatic device (Dinamap Vital Signs Monitor 1846, Critikon Inc.) by trained personnel. Participants rested for 2 min in a sitting position, and then three readings were taken on the upper right arm separated by 2-min intervals. The average of the last two readings was used in the analysis. Nonfasting blood samples were collected from an antecubital vein, serum prepared by centrifugation after 1 h respite at room temperature and analyzed at the Department of Clinical Chemistry, University Hospital of North Norway. Serum total cholesterol and triglycerides were analyzed by enzymatic colorimetric methods and commercially available kits (CHOD-PAP for cholesterol and GPO-PAP for triglycerides: Boeringer Mannheim, Germany). Serum HDL-cholesterol was measured after precipitation of lower-density lipoproteins with heparin and manganese chloride. Determination of glycosylated hemoglobin (HbA1c) in EDTA whole blood was based on an immunoturbidometric assay (UNIMATES, F. Hoffmann-La Roche AG). The HbA1c percent value was calculated from the HbA1c/Hb ratio.
Outcome assessment of venous thrombosis
Only symptomatic (e.g. fatal or required treatment) VTE events were included. All first events of VTE were identified by searching the hospital discharge diagnosis registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway (UNN) from date of enrolment in the Tromsø study (1994–1995) to January 1, 2011. All hospital care and relevant diagnostic radiology in the Tromsø municipality is provided exclusively by this hospital. The relevant discharge codes were ICD-9 codes 325, 415.1, 451, 452, 453, 671.3, 671.4, 671.9, for the period 1994–98, and ICD-10 codes I26, I80, I81, I82, I67.6, O22.3, O22.5, O87.1, O87.3 for the period 1999–2007. The hospital discharge diagnosis register included diagnoses from outpatient clinic visits and hospitalizations. An additional search through the computerized index of autopsy diagnoses was conducted, and cases diagnosed with VTE, either as a cause of death (part one of the death certificate), or as a significant condition (part two of the death certificate), were identified. We also searched the radiology database to identify potential cases of objectively confirmed VTE that may have been missed because of coding errors in the index of medical diagnoses. All relevant diagnostic procedures performed at the Department of Radiology, to diagnose VTE during the 13-year period, were systematically reviewed by trained personnel, and cases with objectively confirmed VTE were identified.
The medical records for each potential VTE case, derived from the hospital discharge diagnosis register, the autopsy register, or the radiology procedure register, were reviewed by trained personnel (i.e. research staff with medical background who were specifically trained in registration of VTE). A panel consisting of senior medical researchers validated the records in cases where there was doubt to whether the inclusion criteria were met. The personnel who recorded the events were blinded to the baseline variables. For subjects derived from the hospital discharge diagnosis register and the radiology procedure register, an episode of VTE was verified and recorded as a validated outcome when all four of the following criteria were fulfilled: (1) objectively confirmed by diagnostic procedures (compression ultrasonography, venography, spiral-CT, perfusion–ventilation scan, pulmonary angiography or autopsy); (2) the medical record indicated that a physician had made a diagnosis of DVT or PE; (3) signs and symptoms consistent with DVT or PE were present; (4) therapy with anticoagulants (heparin, warfarin, or a similar agent), thrombolytic agents or vascular surgery were required. For subjects derived from the autopsy registry, a VTE event was recorded as an outcome when the autopsy record indicated VTE as cause of death or as a significant condition.
Outcome assessment of myocardial infarction
Adjudication of hospitalized and out-of hospital events was performed by an independent endpoint committee and based on data from hospital and out-of hospital journals, autopsy records, and death certificates. The national 11-digit identification number allowed linkage to national and local diagnosis registries. Cases of incident MI were identified by linkage to the discharge diagnosis registry at the UNN with search for ICD 9 codes 410–414 in the period 1994–98 and thereafter ICD 10 codes I20–I25. The hospital medical records were retrieved for case validation. Modified WHO MONICA/MORGAM [24] criteria for MI were used and included clinical symptoms and signs, findings in electrocardiograms (ECG), values of cardiac biomarkers and autopsy reports when applicable. Further, linkage to the National Causes of Death Registry at Statistics Norway allowed identification of fatal incident cases of MI that occurred as out-of-hospital deaths, including deaths that occurred outside of Tromsø, as well as information on all-cause mortality. Information from the death certificates was used to collect relevant information of the event from additional sources such as autopsy reports and records from nursing homes, ambulance services and general practitioners.
Statistical analyses
Follow-up time and risk estimates were calculated separately for VTE and MI. For each participant, person-years of follow-up were accrued from the date of enrolment in the Tromsø Study through the date the event of interest (e.g. VTE or MI) was diagnosed, the date the participant died or officially moved from the municipality of Tromsø, or up to the end of the study period January 1, 2011. During the follow-up period, 527 persons moved from the municipality of Tromsø and 1,756 persons died.
Statistical analyses were carried out using Stata version 13 (Statacorp LP). For BMI (kg/m2), overweight was defined as BMI 25–29.9 kg/m2 and obesity as BMI ≥ 30 kg/m2 in both genders [25]. For WC (cm), abdominal overweight was defined as ≥80 cm in women and ≥94 cm in men, and abdominal obesity was defined as ≥88 cm in women and ≥102 cm in men [25, 26]. For WHR (ratio), obesity was defined as ≥0.85 in women and ≥0.95 in men [27]. Gender specific Cox-proportional hazards regression models were used to estimate hazard ratios (HR) with 95 % confidence intervals (CI) for VTE and MI per 1 standard deviation (SD) increase in the anthropometric measurements, and to assess the risk of VTE and MI associated with established definitions of obesity. Analyses were performed in two adjustments models. Model 1 included age and smoking, whereas model 2 additionally included systolic blood pressure, total cholesterol, HDL cholesterol, triglycerides, HbA1C and self-reported diabetes mellitus In addition, Cox-proportional hazard regression models were used to calculate gender specific HR across quintiles (supplementary Table 1) of the different anthropometric measures. The proportional hazard assumption was verified by evaluating the parallelism between the curves of the log–log survivor function for different categories of the variables. Figures were created and processed in Graphpad Prism 5 (Graphpad Software Inc.) and Adobe Creative Suite 6 (Adobe Systems Software Ireland Ltd.).
Results
The mean baseline age was 61 years (range = 25–84, SD = 10.2) and 47.7 % (n = 3,043) were men. There were 925 incident MIs and 288 incident VTEs during a median follow-up of 15.7 years. Thirty-eight subjects developed both MI and VTE during follow-up. The overall crude incidence rates were 11.3 (95 % CI 10.6–12.1) per 1,000 person-years for MI, and 3.4 (95 % CI 3.0–3.8) per 1,000 person-years for VTE.
Table 1 shows the distribution of traditional atherosclerotic risk factors and anthropometric measures in men and women. Subjects with incident VTE during follow-up had higher BMI, WC, HC, WHR and WHtR than subjects with incident MI, whereas subjects with no event during follow-up had the lowest values. Baseline levels of traditional cardiovascular risk factors were higher among subjects with incident MI.
Hazard ratios for VTE and MI per 1 SD increase in the anthropometric measures are shown in Table 2. In analyses adjusted for age and smoking, all anthropometric measures were significantly associated with risk of VTE, with risk estimates ranging from 19 to 38 % increased risk per 1 SD. Similar results were found for risk of MI, except for hip circumference which showed a weaker association. WC exhibited the highest risk estimate for VTE in both genders (age-adjusted HR 1.28 (95 % CI 1.15–1.54) in women and 1.38 (95 % CI 1.17–1.63) in men), whereas WHtR exhibited the highest risk estimate for MI [age-adjusted HR 1.28 (95 % CI 1.16–1.42) for women and 1.29 (95 % CI 1.19–1.40) for men]. The risk estimates for MI were markedly lowered (9–19 % points) after adjustment for traditional cardiovascular risk factors, whereas the risk estimates for VTE remained unchanged or were slightly increased (Table 2).
Analyses evaluating risks according to established criteria for obesity are shown in Table 3. In men, 11, 21 and 27 % were obese according to the WHO cut-offs for BMI, WC and WHR, respectively. In women, the corresponding figures were 17, 37 and 29 %. Obesity, determined by established criteria, remained associated with VTE risk even after adjustment for atherosclerotic risk factors. BMI, WC and WHR cut-off levels were associated with increased risk of VTE in women, while only BMI and WC was associated VTE risk in men. In contrast, no associations were found between BMI or WC and future risk of MI after adjustment for atherosclerotic risk factors. WHR, however, was associated with a 26 % higher risk of MI in men even after adjustment (Table 3).
In quintile-based analyses (Fig. 1), the risk of VTE increased across higher quintiles of all obesity measures in both genders, and remained unchanged after adjustment for systolic blood pressure, total cholesterol, HDL cholesterol, triglycerides, HbA1C and diabetes mellitus. In women, hip circumference yielded the highest risk estimates for VTE, with adjusted HR of 2.27 (95 % CI 1.54–4.92). BMI, WC and WHR were associated with risk of MI, but the risk estimates were attenuated, and for BMI and WC no longer statistically significant, after adjustments for atherosclerotic risk factors. In men, WC yielded the highest risk estimates (HR upper vs. bottom quintile: 3.54, 95 % CI 1.82–7.06). Apparently, there was a threshold effect between the first and second quintile, with adjusted HR of 2.46 for the second quintile compared to the first. All anthropometric measures were associated with increased risk of MI in men, but hip circumference showed a weaker association than the other measures. The risk estimates for MI were substantially lowered (33–68 % points) after adjustment for atherosclerotic risk factors, while the risk estimates for VTE remained unchanged or were slightly increased. WHtR yielded similar HRs compared to WHR in both genders (data not shown).
Gender-specific hazard ratios with 95 % confidence intervals for venous thromboembolism (VTE) and myocardial infarction (MI) across quintiles of anthropometric measures. The Tromsø Study (1994-2011). The lowest quintile is used as reference. Intervals are shown in supplementary Table 1. BMI body mass index, WC waist circumference, HC hip circumference, WHR waist-hip ratio. a Women, adjusted for age and smoking. b Women, adjusted for age, smoking, systolic blood pressure, total cholesterol, HDL cholesterol, triglycerides, HbA1C and self-reported diabetes mellitus. c Men, adjusted for age and smoking. D Men, adjusted for age, smoking, systolic blood pressure, total cholesterol, HDL cholesterol, triglycerides, HbA1C and self-reported diabetes
Discussion
In the present study, obese individuals with high HC, a measure of subcutaneous fat [28], were at high risk of VTE, while a much weaker association was found between HC and risk of MI. In women, a high HC showed a protective effect against MI after multivariable adjustments, though the results were not statistically significant. Measures of abdominal obesity, which reflect visceral fat [28], were associated with high risk estimates for both MI and VTE. All risk estimates for MI were substantially reduced after adjustment for traditional atherosclerotic risk factors, whereas the risk estimates for VTE remained unchanged. Our findings suggest that body fat distribution is more important for risk of MI than VTE in obese subjects, and likewise, that the metabolic disturbances associated with obesity [18] show a different degree of association with the two disease entities.
In accordance with our findings, Glynn and co-workers showed that obesity, assessed by BMI, was a stronger predictor for VTE than for coronary heart disease in a cohort of male physicians [8]. However, as they did not investigate other anthropometric measures or adjust for covariates, our study is the first to directly compare the impact of these factors on the two outcomes.
Our findings of visceral obesity as an important predictor of MI are in agreement with previous findings in observational studies. In the INTERHEART study, a case–control study of 27,000 participants from 52 countries, WHR was the strongest predictor of MI, and subjects in the upper quintile had a 2.5 fold higher risk than those in the lower quintile [12]. The discriminatory power of WHR on risk of MI has been confirmed in two meta-analyses [13, 20], and several large studies, including the INTERHEART study, have reported lower risk of MI with increasing HC [12, 29, 30]. Cardiometabolic abnormalities associated with obesity are dependent on body fat distribution and accumulation of visceral adipose tissue, and experimental evidence supports a causal relation [18, 31–33]. In large cross-sectional studies, visceral fat, assessed by CT, was unfavourably associated with insulin resistance, serum lipid levels and blood pressure [34–36]. In contrast, subcutaneous adipose tissue, and the gluteofemoral fat depot in particular, exhibited a protective role against an unfavourable metabolic profile [37–40]. It has been suggested that gluteofemoral adipose tissue acts as a “metabolic sink”, by trapping excess fatty acids from the circulation [18]. Similar to our findings, previous cohort and case–control studies have shown that risk estimates for MI by anthropometric obesity measures were substantially lowered after adjustment for traditional atherosclerotic risk factors [12, 41, 42], supporting the concept that these risk factors partially mediates the risk of MI in obesity assuming that they were not present before obesity.
Few previous studies have investigated the impact of obesity measures on VTE risk. The Danish Diet, Cancer and Health Study followed 57,054 individuals for a median of 10 years, and reported that all anthropometric measures of obesity were associated with higher risk of VTE, with WC as the strongest predictor among men, and HC and BMI as the strongest among women [16]. In the Iowa Women’s Health Study [43] of 40,377 women aged ≥65, all anthropometric measures were associated with increased risk of VTE. Furthermore, both WC and HC (highest versus lowest tertile) were associated with more than 50 % higher risk of VTE after adjustment for BMI, which indicates that both WC and HC are important for risk assessment of VTE in women [43]. However, this may partly be due to the fact that peripheral and central obesity often coexist, as hip circumference is correlated with visceral fat [44]. In the Copenhagen City Heart Study [11], BMI and WHR were associated with increased risk of VTE in women, while WHR was only modestly associated with VTE in men. The majority of cohort studies found no association between traditional cardiovascular risk factors and risk of VTE [6–11]. Hence, it is not surprising that the association between obesity measures and risk of VTE was unaffected by adjustment for cardiovascular risk factors. Moreover, abdominal obesity was mandatory for the apparent association between the metabolic syndrome and risk of VTE, whereas none of the other components of the syndrome, alone or in cluster, were associated with VTE [10, 17, 45]. Thus, our findings further support the notion that the increased risk of VTE in obese subjects is not dependent of the cardiometabolic consequences of obesity, and that targeted reduction of atherosclerotic risk factors presumably will not be useful in preventing obesity-related VTE.
The underlying mechanism(s) for the strong associations between obesity measures and risk of VTE are not fully understood. The endocrine and paracrine function of adipose tissue has gained interest in the recent years. Obesity is associated with a state of chronic low-grade inflammation [46]. However, although studies have indicated an association between inflammation and coagulation [47], the role of inflammatory markers in the risk of VTE is unclear [48–50]. Obesity is associated with increased levels of procoagulant and hypofibrinolytic agents such as coagulation factor VIII, fibrinogen and plasminogen activator inhibitor 1, which may increase VTE risk [51]. However, in a study of postmenopausal women, there was an inverse association between HC and coagulation activity after adjustment for WC [52], which is in contrast to the strong association between HC and VTE. Adipokines, such as adiponectin, leptin and resistin, are physiologically active proteins secreted by adipose tissue that are involved in metabolic processes. In a small case–control study, leptin and resistin were associated with increased risk of chronic venous disease [53]. To our knowledge, no study has investigated the impact of adipokines on VTE risk. Obesity-induced stasis is another potential mechanism for the increased VTE risk, either alone or in combination with inflammation. Stasis is a well-recognized risk factor for VTE, and Willenberg et al. [54, 55] showed that abdominal obesity was accompanied by venous stasis caused by increased intra-abdominal pressure, and in a small study of 10 morbidly obese subjects, weight loss due to laparoscopic sleeve gastrectomy significantly improved blood flow of the femoral veins [56].
The main strengths of our study are the prospective design, long-term follow-up, large number of participants, and validated VTE and MI-events. All hospital-care in the region is exclusively provided by a single hospital, which facilitates the completeness of our outcome registries. Some limitations merit consideration. The out-of-hospital events may be more prone to misclassification due to less informative medical records. In addition, there is always a potential for unrecognized fatal events. Nondifferential misclassification, which leads to bias toward the null, is often pointed out as a potential limitation of cohort studies when exposure is self-reported or modifiable over time. When comparing the impact of body fat distribution on risk of MI and VTE within the same population, the degree of random misclassification of exposure and co-variates would be similar with regard to both outcomes. Even though our study design ensures equal distribution of unrecognized confounders, these may have differential impact on the outcomes. As body weight tend to increase with age [57], this probably leads to underestimation of associations for the anthropometric measures. Lastly, there will potentially be some degree of unmeasured confounding, such as the use of lipid lowering and antihypertensive drugs.
In conclusion, our findings suggest that the impact of body fat distribution, and the causal pathway, differs for the association between obesity and arterial and venous thrombosis. Further studies are warranted to shed light over the mechanisms for risk of VTE in obesity in order to tailor preventive actions in the future.
References
Eliasson A, Bergqvist D, Bjorck M, Acosta S, Sternby NH, Ogren M. Incidence and risk of venous thromboembolism in patients with verified arterial thrombosis: a population study based on 23,796 consecutive autopsies. J Thromb Haemost. 2006;4(9):1897–902.
Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348(15):1435–41.
Sorensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370(9601):1773–9.
Lind C, Flinterman LE, Enga KF, Severinsen MT, Kristensen SR, Braekkan SK, et al. Impact of Incident Venous Thromboembolism on Risk of Arterial Thrombotic Diseases. Circulation. 2013.
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102.
Braekkan SK, Hald EM, Mathiesen EB, Njolstad I, Wilsgaard T, Rosendaal FR, et al. Competing risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: the Tromso study. Arterioscler Thromb Vasc Biol. 2012;32(2):487–91.
Wattanakit K, Lutsey PL, Bell EJ, Gornik H, Cushman M, Heckbert SR, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost. 2012;108(9):508–15.
Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–82.
Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–9.
Quist-Paulsen P, Naess IA, Cannegieter SC, Romundstad PR, Christiansen SC, Rosendaal FR, et al. Arterial cardiovascular risk factors and venous thrombosis: results from a population-based, prospective study (the HUNT 2). Haematologica. 2010;95(1):119–25.
Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121(17):1896–903.
Yusuf S, Hawken S, Ôunpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case–control study. Lancet. 2005;366(9497):1640–9.
de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6.
Hansson PO, Eriksson H, Welin L, Svardsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913”. Arch Intern Med. 1999;159(16):1886–90.
Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139(2):289–96.
Severinsen MT, Kristensen SR, Johnsen SP, Dethlefsen C, Tjonneland A, Overvad K. Anthropometry, body fat, and venous thromboembolism: a Danish follow-up study. Circulation. 2009;120(19):1850–7.
Steffen LM, Cushman M, Peacock JM, Heckbert SR, Jacobs DR Jr, Rosamond WD, et al. Metabolic syndrome and risk of venous thromboembolism: longitudinal Investigation of Thromboembolism Etiology. J Thromb Haemost. 2009;7(5):746–51.
Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–86.
Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(9):680–7.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738.
Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the aha task force on risk reduction. Circulation. 1998;97(18):1876–87.
Borch KH, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, et al. Anthropometric measures of obesity and risk of venous thromboembolism: the Tromso study. Arterioscler Thromb Vasc Biol. 2010;30(1):121–7.
MORGAM Manual [cited 2014 January 22]. Available from. http://www.ktl.fi/publications/morgam/manual/followup/form22.htm.
Obesity: preventing and managing the global epidemic; report of a WHO consultation. Geneva: WHO; 2000. xii, 253s. p.
Lean MEJ, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311(6998):158–61.
Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311(6998):158–61.
Seidell JC, Pérusse L, Després J-P, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74(3):315–21.
Heitmann BL, Frederiksen P, Lissner L. Hip circumference and cardiovascular morbidity and mortality in men and women. Obes Res. 2004;12(3):482–7.
Lissner L, Bjorkelund C, Heitmann BL, Seidell JC, Bengtsson C. Larger hip circumference independently predicts health and longevity in a Swedish female cohort. Obes Res. 2001;9(10):644–6.
Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13.
Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–16.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80.
Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26.
Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715–22.
Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring, Md). 2010;18(11):2191–8.
Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107(12):1626–31.
Yim J-E, Heshka S, Albu JB, Heymsfield S, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol. 2008;104(3):700–7.
Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–8.
Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34(6):949–59.
Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes. 2001;25(7):1047–56.
Rexrode KM, Carey VJ, Hennekens CH, et al. ABdominal adiposity and coronary heart disease in women. J Am Med Assoc. 1998;280(21):1843–8.
Lutsey PL, Virnig BA, Durham SB, Steffen LM, Hirsch AT, Jacobs DR Jr, et al. Correlates and consequences of venous thromboembolism: the Iowa Women’s Health Study. Am J Public Health. 2010;100(8):1506–13.
Kuk JL, Janiszewski PM, Ross R. Body mass index and hip and thigh circumferences are negatively associated with visceral adipose tissue after control for waist circumference. Am J Clin Nutr. 2007;85(6):1540–4.
Borch KH, BrÆKkan SK, Mathiesen EB, NjØLstad I, Wilsgaard T, Størmer J, et al. Abdominal obesity is essential for the risk of venous thromboembolism in the metabolic syndrome: the Tromsø study. J Thromb Haemost. 2009;7(5):739–45.
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45.
Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–704.
Hald EM, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Brox J, et al. High-sensitivity C-reactive protein is not a risk factor for venous thromboembolism: the Tromso study. Haematologica. 2011;96(8):1189–94.
Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30(8):1672–8.
Kamphuisen PW, Eikenboom JC, Vos HL, Pablo R, Sturk A, Bertina RM, et al. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb Haemost. 1999;81(5):680–3.
Braekkan SK, Siegerink B, Lijfering WM, Hansen JB, Cannegieter SC, Rosendaal FR. Role of obesity in the etiology of deep vein thrombosis and pulmonary embolism: current epidemiological insights. Semin Thromb Hemost. 2013;39(5):533–40.
Peverill RE, Teede HJ, Malan E, Kotsopoulos D, Smolich JJ, McGrath BP. Relationship of waist and hip circumference with coagulation and fibrinolysis in postmenopausal women. Clinical science (London, England: 1979). 2007;113(9):383–91.
Allison MA, Cushman M, Callas PW, Denenberg JO, Jensky NE, Criqui MH. Adipokines are associated with lower extremity venous disease: the San Diego population study. J Thromb Haemost. 2010;8(9):1912–8.
Willenberg T, Clemens R, Haegeli LM, Amann-Vesti B, Baumgartner I, Husmann M. The influence of abdominal pressure on lower extremity venous pressure and Hemodynamics: a human in-vivo model simulating the effect of abdominal obesity. Eur J Vasc Endovasc Surg. 2011;41(6):849–55.
Willenberg T, Schumacher A, Amann-Vesti B, Jacomella V, Thalhammer C, Diehm N, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664–8.
Wiewiora M, Piecuch J, Gluck M, Slowinska-Lozynska L, Sosada K. Shear stress and flow dynamics of the femoral vein among obese patients who qualify for bariatric surgery. Clin Hemorheol Microcirc. 2013;54(3):313–23.
Baum CL 2nd, Ruhm CJ. Age, socioeconomic status and obesity growth. J Health Econ. 2009;28(3):635–48.
Acknowledgments
K.G. Jebsen TREC is supported by an independent grant from the K.G. Jebsen Foundation. Lars D. Horvei receives a grant from the Northern Norway Regional Health Authority. The authors would like to thank Rod Wolstenholme for help with preparing the figures.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
The study was approved by the regional committee for medical research ethics and all participants gave their informed written consent to participate.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horvei, L.D., Brækkan, S.K., Mathiesen, E.B. et al. Obesity measures and risk of venous thromboembolism and myocardial infarction. Eur J Epidemiol 29, 821–830 (2014). https://doi.org/10.1007/s10654-014-9950-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9950-z