Abstract
In this study, the concentration of six metal(loid)s was examined in the fish Oreochromis aureus collected from El Comedero dam during a massive mortality event induced by a mine tailing spill. A major spill (~ 300,000 m3) of waste was released into the San Lorenzo River System following a rupture in the tailing dam of a mining plant in NW Mexico; consequently, the discharged material flowed into El Comedero dam. The accumulation of metal(oid)s in the tissues of O. aureus showed higher levels in the liver than in the guts and muscle. Concentrations in the liver were high (As, 1.1–1063; Cd, 8.9–392; Cu, 372–59,129; Hg, 0.46–19.79; Se, 8.7–748; and Zn, 116–820 μg g−1), revealing that these fish were exposed to high concentrations of these elements. The mortality of fish could have resulted from the combined effect of the six analyzed metal(loid)s, as well as other residues present in mine tailings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution from mining activities is one of the most common sources of highly toxic chemical substances in aquatic and terrestrial ecosystems (Mapenzi et al., 2020). The discharge of large quantities of materials occurs either directly from milling plants, or indirectly through accidental impoundment failures (Kossoff et al., 2014). The mining industry produces enormous volumes of waste, mainly tailings, which are often stored in impoundments behind dams. These can fail and have subsequent environmental, economic, and human health impacts (Kossoff et al., 2014). The chemical composition of tailings depends on the mineralogy of the ore body, the nature of the processing fluids, the efficiency of the extraction process, and the degree of weathering during storage in the impoundment. Metal(loid)s are present in tailings since no extraction process reaches 100% efficiency, and As, Cu, Cd, Hg, and Zn are generally present in high concentrations (Kossoff et al., 2014).
Tilapia is a model fish species commonly used as a bioindicator of water pollution due to its tolerance and availability in many contaminated sites (Chatterjee et al., 2016; Lin et al., 2005; Ndimele et al., 2017). This species is useful due to its capacity to accumulate metals, its sensitive response to pollutants, and its distribution in inland and estuarine waters in various parts of the world (Stickney, 2017). The tilapia is the fourth largest group of species in global aquaculture production (FAO, 2021). In Mexico, tilapia is the second most important aquaculture group with 53,000 t of production (FAO, 2021). Mexico ranks as the second most important country in aquaculture fisheries, with 116,000 t registered in 2018. Mexico is also the second largest international market for tilapia products, with ~ 228,000 t imported in 2018 (FAO, 2021). Despite the importance of freshwater fish as a protein source for local diets, pollution of metal(loid)s has been poorly documented.
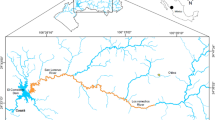
Mining in Mexico is a traditional economic activity, which is predominantly dedicated to the production of Cu, Zn, Ag, Fe, Pb, and Au. On the continental margin of the Gulf of California, numerous sites of mining interest were or are exploited (Páez-Osuna et al., 2017). The San Lorenzo basin, located in the Sinaloa and Durango states, is associated with about 16 mining sites. One important processing plant is located in the Santa María de Otáez region in Durango. Here, a tailing spill event occurred on January 21, 2013, when part of the processing plant’s tailing pond dike collapsed, and liberated ~ 300,000 m3 of wastes into Los Remedios River, which is associated with the main tributary of the upper San Lorenzo River and El Comedero (EC) dam (Páez-Osuna et al., 2015). The site where the failure occurred is located ~ 150 km from the upper San Lorenzo River where EC dam is situated (Fig. 1). On April 21, 2013, fishermen of EC dam reported a massive fish mortality event when they observed fish in poor condition with insufficient mobility. The case was investigated by collecting water samples (Páez-Osuna et al., 2015) and fish after the tailing spill. The objective of this study was to examine metal(loid) (As, Cd, Cu, Hg, Se, and Zn) concentrations in the muscle, liver, and guts of the blue tilapia O. aureus collected during the massive mortality event. The biota sediment accumulation factor was estimated to evaluate the potential toxicity of suspended sediments. Finally, speciation of the metal(loid)s dissolved in the waters was developed to identify the chemical forms hypothetically present during the mortality event. Therefore, the hypothesis involved is that O. aureus accumulates metal(loid)s in its tissues, mainly in the liver, which caused the subsequent mortality event by the ingestion of residues from the mine tailing spill.
Materials and methods
Study area, sampling, and chemical analyses of fishes
El Comedero dam is located (24°30ʹN; 106°45ʹW) in the southeastern Gulf of California and has a surface of ~ 9200 ha (Fig. 1). The water availability in the dam is permanent and the volume varies from 400 to 1900 Mm3. The basin of the upper San Lorenzo River drains into EC dam where depths can reach 70 m and surficial water temperature ranges from 21.9 °C in January to 31.2 °C in June. The production of fish exhibits variations that have been related to climate and overexploitation (Páez-Osuna et al., 2015). However, the reduced production in 2013 coincided with the tailing spill, so a relationship between mining spills and fish decline is plausible.
A set of 15 fish samples was collected in the section of the dam that receives the discharge of the upper San Lorenzo River 90 days after the tailing spill event on April 21, 2013. Fish were collected exactly where the massive mortality event occurred (gray area of Fig. 1). Special care was taken to choose those recently dead individuals to avoid working with decomposing tissues. Each specimen was measured, weighed, and dissected to separate the liver, guts, and a portion of the muscle. Separated tissues were lyophilized (72 h at − 52 °C and 80 × 10−3 mbar) and pulverized in a semiautomatic agate mortar. Acid digestion (5 mL of concentrated and purified nitric acid, Instra-analyzed J.T. Baker concentrated 69–70%) of duplicate aliquots (0.250 g of dry tissue) was carried out using Teflon vials with caps (Savillex) at 125 °C for 3 h (Bergés-Tiznado et al., 2015). Only livers were digested using 2 mL of H2O2 (30%) and 3 mL of concentrated HNO3. Analysis of As, Cd, Cu, Se, and Zn was carried out by atomic absorption spectrophotometry (AAS). Selenium and As were analyzed by AAS with a Zeeman correction background effect coupled to a graphite furnace oven (model AAnalyst 800, PerkinElmer, USA). A matrix modifier—a solution of Pd(HNO3)2 and Mg(NO3)2—was used in each sample atomization for both metalloids. Mercury was determined by AAS coupled to a cold vapor generator (model VGA110, Varian, USA). Samples were prepared by adding HNO3 (50%) and K2Cr2O7 (1%) before Hg analysis. To assess the accuracy of the employed procedure, certified reference material DOLT-4 (dogfish liver, NRC-CNRC, 2008) was analyzed. Concentrations of the analyzed elements were within the certified values (recoveries 91.6- 101.3%) and precision fluctuated from 2.3% for Cu to 8.8% for Zn. Blanks were analyzed to test for contamination using the same procedure.
Speciation of metal(loid)s in the water
Inorganic speciation of metal(loid)s was performed with the speciation program Visual MINTEQ version 3.1, considering physicochemical parameters, such as temperature, pH, dissolved oxygen (DO), electrical conductivity (ec), major ions, and the mean concentration determined for each element in the dissolved fraction of the waters of EC dam (Páez-Osuna et al., 2015). Water samples (n = 8) were collected simultaneously with the fish along the portion of the dam that receives the load of the upper San Lorenzo River (gray area in Fig. 1), where the massive mortality event occurred. Temperature and DO were measured in the waters using an oxygen meter (model DO200, YSI, Ohio, USA), while the pH and ec were measured with a pH meter (model HI 98,129, Hanna Instruments, Texas, USA). Calibrations of the instruments were performed using buffers (Orion 910,104, 910,110, Thermo Scientific) and a Hanna solution of 1413 μS cm−1 (HI 7031) at 25 °C. Concentrations of the major components were quantified according to standard methods (Online Resource).
The selenium: mercury ratio and the biota sediment accumulation factor
Selenium: mercury molar ratio was calculated from individual Se and Hg results of each tissue divided by the molecular weight of each element. The Se health benefit value (Se-HBV) for edible muscle was calculated according to Ralston et al. (2016): HBVSe = ([Se–Hg]/Se) × (Se + Hg). Selenium and Hg concentrations are given in nmol g−1 on a wet weight basis. The positive results indicate that Se exceeds Hg and it is beneficial to consumers, negative values mean the contrary (Ruelas-Inzunza et al., 2020). The magnitude of the value indicates Se surplus or deficit related to the theoretical consumption of the muscle of O. aureus. The biota sediment accumulation factor (BSAF) describes the bioaccumulation of metal(loid)s in the tissues of biota receptors. It also reflects the efficiency of metal(loid) accumulation in an organism and estimates the potential toxicity from sediment contaminants. BASF was calculated using the equation (Thomann et al., 1995): BASF = concentration of a chemical substance (metal(loid)s) in the organism/concentration of a chemical substance in sediments.
Risk assessment
The non-cancer risk assessments were calculated by comparing an estimate of exposure to a reference dose (RfD) for oral exposures (EPA, 2005) using the individual target hazard quotient (THQ) and the sum of THQs as the hazard index (HI): THQ = [EF × ED × FIR × C/RfD × BW × AT] × 10–3 and HI = ΣTHQ. EF is an exposure frequency of 365 days year–1, ED is a 70-year exposure period, C is the mean concentration of the element (mg kg–1), BW is the population body weight of 75, 65 and 20 kg for adult men, female, and children (3–5 years old), respectively, AT is the average exposure of 25,500 days, and FIR is the food ingestion rate under two different scenarios; in the first, a specific tilapia consumption of 15 g week–1 (2.2 g day–1) was considered, and the second was under an intake ration of 200 g week–1 (28.6 g day–1) equal to the total fish consumption rate per capita of Mexico in 2020 (SEMARNAT, 2021). There will be a risk if THQ or HI > 1; also, the RfD data for As, Cd, Hg, Se, and Zn were obtained from the IRIS Assessment Base (EPA, 2022). It is important to notice that the As average level was considered as inorganic As (Asi) and the total Hg average as methyl-Hg to be conservative about risks; also, Cu has not been evaluated. Finally, a safe intake was calculated according to the Provisional Tolerable Intake (PTI) per body weight (BW) set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). The data for each element were (WHO, 2022): Cd 25 μg kg−1 BWmonth−1; Cu 0.5 mg kg−1 BWday−1; Methyl-Hg 1.6 μg kg−1 BWweek−1; and Zn 0.3 mg kg−1 BWday−1. The PTI for As was withdrawn given the last data was considered no longer protective, with a best estimation exposure of 0.1–3 μg kg−1 BWday−1 for As. Thus, the lower limit range was used to evaluate the risk (0.1 μg kg−1 BWday−1); Se has no evaluation.
Statistical analysis
The results obtained from each variable were statistically analyzed by a Kruskal–Wallis nonparametric ANOVA followed by U Mann–Whitney multiple comparison test to compare molar ratios among tissues. Spearman rank correlations yielding an R statistic were used to determine associations among variables. Finally, mathematical models were applied to correlate element concentrations with the different variables (Zar, 2010).
Results and discussion
The tailing spill occurred ~ 150 km away from EC dam on January 21, 2013, and the massive fish mortality transpired ~ 90 days later. Considering the current, sinuosity, and topography of Los Remedios River (a tributary of the upper San Lorenzo River that flows into EC dam), the material spilled was probably transported in a period of ~ 35 days. This indicates that the first fragments of the spill arrived at EC dam on February 25, 2013. Subsequently, the transported material that accumulated at the entrance of the dam slowly increased the mine tailings volume in such a way that fish were exposed to acute toxicity, causing massive mortality in April 2013.
Speciation of metal(loid)s
Water samples from EC dam, the pH varied from 5.06 to 8.51 (mean 7.34 ± 1.24), and conductivity from 131 to 197 µS cm−1 (170 ± 27 µS cm−1). The temperature registered during the sampling was 26.5 ± 1.6 °C. During the collection fish collection, the concentrations (mean ± SD) of the major components registered in the water samples were: Ca2+ 23.8 ± 4.2, Mg2+ 19.5 ± 15.3, Na+ 13.4 ± 3.2, K+ 3.0 ± 0.9, SO42− 14.1 ± 3.2, Cl− 6.9 ± 2.5, CO32− 9.8 ± 4.5, HCO3− 160 ± 68, and SiO2 12.0 ± 4.4 mg L−1. Concentrations of trace elements quantified in water samples from EC dam for the dissolved and suspended fraction were (Páez-Osuna et al., 2015): 7.10 ± 0.46 µg L−1 and 101 ± 10.0 µg g−1 for As, 0.314 ± 0.025 µg L−1 and 3.63 ± 0.50 µg g−1 for Cd, 6.01 ± 0.03 µg L−1 and 67.7 ± 3.8 µg g−1 for Cu, 0.109 ± 0.083 µg L−1 and 0.497 ± 0.023 µg g−1 for Hg, and 1068 ± 59 µg L−1 and 803 ± 64 µg g−1 for Zn, respectively. These dissolved concentrations were relatively low and below the upper limit value for drinking water established by the World Health Organization (UNEP, 2008): As (10 µg L−1), Cd (3.0 µg L−1), Cu (2000 µg L−1), Hg (1.0 µg L−1), and Zn (3000 µg L−1). In contrast, the suspended concentrations were relatively high. Particularly, the concentration of As, Zn, Cd, and Hg exceeded the probable effect level (PEL) established by the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life (CCME, 2001). The discussion of the comparison of these high levels with those from other regions is presented in Páez-Osuna et al. (2015).
In the dissolved fraction, metal(loid)s were found as free ions in negligible proportions (< 0.03%) except for Cd (6.3%), reducing the health risks of organisms. In natural waters, inorganic As can be found as As (V) and As (III), which are the most toxic chemical species for aquatic organisms (Osuna-Martínez et al., 2021). However, As was found entirely as As (V) in this study, mainly as HAsO42− (94.4%) and AsO43− (5.6%) (Fig. 2). The rest of the analyzed metals were found mainly as hydroxides and carbonates, e.g., CdCO3 (47.5%), Cu(OH)2 (49.1%), Hg(OH)2 (99.6%), and Zn(OH)2 (91.4%) (Fig. 2), which are considered chemical species of lower toxicity and bioavailable to biota.
Metal(loid)s in fish tissues
Concentrations of As, Cd, Cu, Hg, Se, and Zn in the liver, muscle, and guts are presented in Fig. 3. The sequence of the elements quantified in the muscle, liver, and guts was Zn > Se > Cu > As > Hg > Cd, Cu > Zn > As > Se > Cd > Hg, and As > Zn > Cu > Se > Cd > Hg, respectively. Metal(loid) concentrations exhibited great variability, particularly, in the liver and guts. Moreover, element concentrations in the liver were high, revealing that these fish were exposed to high levels of the six elements. The accumulation pattern of the six elements in the blue tilapia was consistently higher in the liver than in the guts and muscle except for As, with a higher concentration in the guts. This pattern agrees with previous studies in freshwater (Yap et al., 2015) and marine (Bergés-Tiznado et al., 2021; Páez-Osuna et al., 2017; Ruelas-Inzunza et al., 2011, 2020; Sujitha et al., 2019) fish species. Significant differences among the liver and the other two tissues for the same element were evident for Cu, Hg, Se, and Zn (Fig. 3). This pattern is certainly related to various types of fish organ exposure to a contaminated aquatic environment in the dam, as well as organ specificity in the uptake, storage, regulation, and excretion abilities (Bergés-Tiznado et al., 2015). The high accumulation of the six elements in the liver followed by the muscle is related to the main routes of capture and assimilation through diet and water, which is directly associated with metabolism and respiration (Ruelas-Inzunza et al., 2011). The liver’s capacity to accumulate these elements results from metallothionein activity, which interacts with such elements and reduce their toxicity (Yap et al., 2015). Other factors related to the high accumulation of metal(loid)s and metallothioneins in fish are the exposure time and the metal(loid) concentration (Mieiro et al., 2011). The liver of O. aureus is a highly active organ in the uptake, storage, and detoxification of metals, particularly for Cd, Cu, Hg, and Se. Therefore, this organ has been considered a potential biomonitor of metal pollution given its concentrations are proportional to those in the environment (Yap et al., 2015).
The guts and their content reflect the recent food uptake during the last hours before sampling. The O. aureus individuals collected during the massive mortality event exhibited high levels of the six elements, with As reaching the highest concentrations in the guts (Fig. 3). The low metal(loid) levels in the muscle reflect the low concentrations of binding proteins compared to the liver.
Once the metal(loid) is ingested, uptake occurs in the intestinal tissue through membranes via transporter proteins or/and ionic channels (Le Croizier et al., 2018). Thus, dietary accumulation initiates in the digestive tract. After entering the liver, metal(loid)s are released into the general blood circulation, reaching secondary accumulation organs such as the muscle. Metal(loid)s in fish are depurated mainly through urine in the kidney and bile excretion into the intestine before final elimination through feces (Le Croizier et al., 2018).
The high element concentrations found in the blue tilapia sampled after the tailing spill (90 days) showed great variability among individuals (Fig. 3). This could be explained by the different habitats within the dam and the upper San Lorenzo River, as well as the variable duration and concentration of metal(loid) exposure. Another relevant factor to consider is that the mine tailings transported from the spill point to the dam could be heterogeneous. Mine tailings are a mixture of minerals, fluids, washeries, and concentrators with a variable chemical composition (Kossoff et al., 2014).
Metal(loid)s and body size
The O. aureus specimens exhibited variable sizes. Total length ranged from 22.2 to 41.3 cm (with a mean ± SE of 32.5 ± 6.1 cm), while body weight ranged from 200 to 840 g (523 ± 60 g). Thus, the tilapias collected during the mortality event were mainly pre-adults and adults. Table S1 (online resource) shows the correlation values of element levels in the three analyzed tissues, including weight and total length. Significant negative correlations (p < 0.05) were found for As concentrations in the muscle between both total length (R = − 0.60) and weight (R = − 0.53). No other associations were found between the rest of the elements in the muscle (p > 0.05). Instead, total length and weight were positively related to levels of As (R = 0.57), Cu (R = 0.57), Hg (R = 0.61), and Se (R = 0.61) in the liver (Table S1). The correlation values of the six metal(loid)s observed in the guts were not significant (p > 0.05).
The relationship between size and metal accumulation in aquatic biota is well known (Phillips, 1980). However, the explanation of this phenomenon for each element remains scarcely understood. The effect of size may be a function of one or several age-related parameters such as differences between the surface/volume ratio, or metabolic and feeding rates of larger (older) and smaller (younger) individuals (Phillips, 1980). This has been associated with feeding habit differences between older and younger individuals (Páez-Osuna et al., 1995). Nevertheless, an evident accumulation tendency of As, Cu, Hg, and Se was observed in the livers of larger organisms.
Selenium health benefit value (HBVSe) and the selenium-to-mercury ratio
The HBVSe in the muscle, liver, and guts were positive (Table S2 online resource). The results are certainly surprising since these fishes were exposed to the mining material transported from the spill site and could hypothetically be used for human consumption. However, ingestion is out of consideration as a result of the elements accumulated in the tissues. The Se:Hg molar ratio in the muscle, liver, and guts was > 1 (Table S2), indicating that Se is incorporated in selenoproteins (Bergés-Tiznado et al., 2015). Owing to the high affinity between Hg and Se, the formation of an Hg-Se complex has been suggested as the responsible mechanism for the protective effect of Se (Ralston et al., 2016). The variation of the Se:Hg molar ratio with size was negatively correlated (p < 0.05) with total length (R = − 0.62) and weight (R = − 0.64) (Fig. S1 Online resource). These associations between Se and Hg and the molar ratios in the tissues suggest that there was enough Se to counter the toxicity action of Hg during the mortality event.
Metal(loid)s in muscle, food safety guidelines, and risk assessment
There are various criteria to discern acceptable or adverse levels in the context of human health by consuming the edible fraction of fish. The muscle is generally the focus since it is the main support of the human diet. The local human population consumes the fillet of tilapia produced in Mexico, which was 116,000 t in 2018, with an average consumption of 3.08 kg per capita (FAO, 2021). Therefore, it is important to generate information, given most of the tilapia fisheries in NW Mexico occur in areas influenced by mining.
The Mexican (DOF, 2011) and international (Nauen, 1983) legal Cd levels are 0.5 µg g−1 wet weight (ww) in muscle, and no individual had concentrations above the limit (Fig. 3). Mean concentrations of As and Hg in muscle were below the maximum permissible limit (MPL) considered in the Mexican legislation (DOF, 2011) for fish and seafood (As: 80 µg g−1 ww and Hg as CH3Hg, 1.0 µg g−1 ww). Copper, Zn, and Se are not considered in the Mexican norm. Nonetheless, countries such as Australia and India established an MPL of 10 µg g−1 ww for Cu, and 40 and 150 µg g−1 ww for Zn in New Zealand and Australia, respectively. However, no specimen exhibited levels in the muscle above these limits (Fig. 3). Regarding Se, all specimens were above the threshold (0.3 µg g−1 ww) for fish and fish products established in Chile, but 60% of the muscle samples were below the limit of New Zealand (2.0 µg g−1 ww) for any type of foodstuff (Nauen, 1983).
Fish collected from a mortality event are not suitable for human consumption. However, this study evidenced that the element concentrations in the edible portion were low and below the MPL with the partial exception of Se (Fig. 3), generated from individual toxicological protocols for each metal(loid). The calculated risk assessments were different for both proposed scenarios (Table 1). There was no risk of adverse health effects in a consumption of 15 g of tilapia muscle in a week (all THQ and HI < 1). However, a portion of 200 g of muscle consumed weekly could be negative for children, presenting an HI > 1; this intake did not represent a risk for men and women. Additionally, the removal of the liver and guts to avoid risks is recommended in cases where O. aureus appears healthy but with suspected metal(loid) pollution.
If the PTI of each element is considered to estimate a safe weekly ration, the portions could be exaggerated for the essential elements Cu and Zn (from 17.5 kg in children to 1094 kg in adult men). For a non-essential risk scenario of Cd and Hg, children must consume 2.5 and 4.5 kg, women 8.2 and 14.6 kg, and men 9.5 kg and 16.8 kg of muscle. Finally, the safe intake of blue tilapia muscle proposed in this study would be 100, 325, and 375 g a week for children, women, and men, respectively, to avoid risks of adverse health effects by Asi and other metal exposure.
Comparison with other regions
Concentrations of metal(loid)s in the blue tilapia quantified in this study were compared with those reported in tilapias from other areas (Tables 2, 3). The highest levels of the six elements are generally found in the liver and the lowest in the muscle, which is a pattern observed for a wide spectrum of fish species. It is noticeable that Cd in the muscle (0.28 ± 0.06 µg g−1 dw) of O. aureus from this study exhibited similar concentrations to those previously found in most regions where mining and agriculture pollution have been reported in tilapias (Table 2). Interestingly, the Cd found in our study and most studies from other regions is 3–5 times more elevated than the reported for the control in experiments with O. aureus (Allen, 1995). In this study, Cd exhibited the highest levels (52.0 ± 24.6 µg g−1 dw) in the liver compared to most regions, but lower than those reported in the tilapia of Lhasa, Tibet (China) (Jiang et al., 2014). Cadmium in the liver of O. aureus was ~ 267 times higher in the present study compared to the baseline (0.19 µg g−1 dw, Allen, 1995).
Copper in the muscle (1.48 ± 0.41 µg g−1 dw) was comparable to most studies, except for Jiang et al. (2014) in the Mozambique tilapia O. mossambicus, Ndimele et al. (2017) in the Nile tilapia O. niloticus from the Owo and Etegbin River (Nigeria) impacted by industrial activities, and O. niloticus from three dams in Sonora (Mexico) (Martínez-Durazo et al., 2021) impacted by mining activities. Conversely, the highest concentration of Cu in the liver (8758 ± 3692 µg g−1 dw) corresponded to O. aureus of the present study. The Zn concentrations in the muscle (14.7 ± 1.0 µg g−1) were comparable to or lower than most studies (Table 2). The Singida tilapia O. esculentus from Rukwa lake (Tanzania) (Mapenzi et al., 2020), and O. niloticus from Yaounde lake (Cameroon) (Léopold et al., 2015) exhibited higher levels than O. aureus from this study. In contrast, O. aureus from this study showed the highest Zn levels in the liver (220 ± 50 µg g−1 dw) compared to other studies.
Although the information on As is limited, it is evident that O. aureus showed intermediate concentrations in the muscle (0.82 ± 0.14 µg g−1 dw) compared to those reported in most regions of the world (Table 3). The highest levels reported in the muscle correspond to O. mossambicus from farms on the west coast of Taiwan (Ling et al., 2013), which are influenced by industry, agriculture, and groundwater with As. Arsenic exhibited high levels in the liver (200 ± 75 µg g−1 dw); however, they were lower than those registered in O. mossambicus reared in farms on Lhasa, Tibet (China) (Jiang et al., 2014). Selenium in the muscle (10.7 ± 0.4 µg g−1 dw) and liver (152 ± 46 µg g−1) of O. aureus showed higher levels compared to the limited number of studies (Table 3). Mercury in both muscle and liver exhibited a variable concentration between species and regions. However, Hg levels of O. aureus were intermediate in the present study (Table 3). The muscle and liver of O. aureus had levels 12-times higher in this study compared to the Hg baseline (0.31 µg g−1 dw; Allen, 1994).
From this robust contrasting (Tables 2, 3), it is possible to generalize that O. aureus collected during the mortality event in EC dam showed the highest levels of Cu and Se in the liver, and the second highest concentrations of As, Cd, Hg, and Zn in the same organ. Conversely, Cd, Cu, Zn, Hg, and As in the muscle showed similar or lower levels compared to most studies reported in the same and other species of tilapia. The limited number of studies does not allow a suitable comparison for Se. However, this confirms the great regulation capacity of elevated concentrations of the six metal(loid)s through the liver of O. aureus, in which concentrations were very high.
The biota sediment accumulation factor (BSAF)
Blue tilapia consume a heterogeneous diet with a wide range of natural foods such as benthic organisms, plankton, detritus, and decomposing organic matter (Stickney, 2017). Tilapias inhabit the bottom layer of the water column where they feed by digging through sediment in search of food. Therefore, high element concentrations found in the present study could be associated with the ingestion of detritus including mine tailings, together with other natural food items. Subsequent (2 days) to the sampling of fish, a set of eight surface water samples was collected in the upper San Lorenzo River and EC dam (Páez-Osuna et al., 2015). The results indicated that sediment-suspended concentrations were high and most of the samples exceeded the probable effect level (PEL) for the protection of aquatic life (CCME, 2001). The BSAF was estimated using sediment-suspended and liver concentrations given this organ quantitatively reflects environmental levels (Yap et al., 2015). BASF values were 71.2–134.7 for Cu; 8.3–16.8 for Cd; 3.5–7.6 for Hg; 1.5–2.1 for As; and 0.12–0.30 for Zn. The BASF values > 2 indicate that O. aureus is a macro-concentrator of Cu, Cd, Hg, and partially As. But BASF values < 1 indicate that this fish deconcentrates Zn (Thomann et al., 1995). Metal(loid)s present in the suspended particles enriched with tailing material occurred as multiple mixtures, whose concentrations were relatively high. The effect of metal(loid) mixtures on aquatic biota depend on the concentrations, type, the number of metal(loid)s involved, the animal taxa, exposure time (Arreguin Rebolledo et al., 2021), and speciation. In most metal mixture experimental studies, synergistic effects have been observed in aquatic organisms (Arreguin Rebolledo et al., 2021; Frías-Espericueta et al., 2008; and references therein). During the massive mortality of this study, O. aureus was exposed to a wide mixture of at least six metal(loid)s, probably under the synergistic effect of As, Cd, Cu, Zn, and the antagonistic effect caused by the excess of Se on Hg.
Conclusions
From the results of the present study, the toxicity associated with the high concentrations of the metal(loid)s can be partially responsible for the massive mortality of fish in EC dam. However, it is important to consider that other toxic elements could have been present and contributed to the massive mortality. In addition, suspended sediment during torrential rains can also cause acute fish mortality (Swinkels et al., 2014). Finally, although less likely, the effect of metal(loid)s could have first caused a depression of the fish’s immune system, which was then affected by a disease.
It is important to highlight the limitations related to the sampling of this study, i.e., the difficulty of determining the cause of death at sublethal concentrations of metal(loids) when sampling after an event, particularly where water and suspended sediment metalloid concentrations may be variable and not fully representative of what the fish have been exposed to (overall, what would be an ideal post-spill sampling strategy). Conversely, the results of this study reveal that the tilapia is an effective biomonitor of water and sediment contamination during mine spill events, particularly via their liver concentrations.
Future research is needed to confirm the toxicity of different and multiple mixtures of metal(loid)s present in mine tailings to establish if the effects of such elements are synergetic or antagonist, or simply additive. Moreover, it is important to establish the chemical forms of the toxic elements present in the mine tailings to investigate effective remediation measures when mining spills occur.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdel-Moneim, A. M., Essawy, A. E., El-Din, N. K. B., & El-Naggar, N. M. (2016). Biochemical and histopathological changes in liver of the Nile tilapia from Egyptian polluted lakes. Toxicololy and Industrial Health, 32, 457–467.
Adeogun, A. O., Ibor, O. R., Omiwole, R., Chukwuka, A. V., Adewale, A. H., Kumuyi, O., & Arukwe, A. (2020). Sex-differences in physiological and oxidative stress responses and heavy metals burden in the black jaw tilapia, Sarotherodon melanotheron from a tropical freshwater dam (Nigeria). Comparative Biochemistry and Physiology Part C, 229, 108676.
Allen, P. (1994). Distribution of mercury in the soft tissues of the blue tilapia Oreochromis aureus (Staindachner) after acute exposure to mercury (II) chloride. Bulletin of Environmental Contamination and Toxicololgy, 53, 675–683.
Allen, P. (1995). Chronic accumulation of cadmium in the edible tissues of Orechromis aureus (Steindachner): Modification by mercury and lead. Archives of Environmental Contamination and Toxicology, 29, 8–14.
Arreguin Rebolledo, U., Páez-Osuna, F., & Fernández, R. (2021). Single and mixture toxicity of As, Cd, Cr, Cu, Fe, Hg, Ni, Pb, and Zn to the rotifer Proales similis under different salinities. Environmental Pollution, 271, 116357.
Authman, M. M. N., Abbas, H. H., & Abbas, W. T. (2013). Assessment of metal status in drainage canal water and their bioaccumulation in Oreochromis niloticus fish in relation to human health. Environmental and Monitoring Assesment, 185, 891–907.
Bergés-Tiznado, M. E., Márquez-Farías, F., Lara-Mendoza, R. E., Torres-Rojas, Y. E., Galván-Magaña, F., Bojórquez-Leyva, H., & Páez-Osuna, F. (2015). Mercury and selenium in muscle and target organs of scalloped Hammerhead sharks Sphyrna lewini of the SE Gulf of California: Dietary intake, molar ratios, loads, and human health risks. Archives of Environmental Contamination and Toxicology, 69, 440–452.
Bergés-Tiznado, M. E., Márquez-Farías, F., Osuna-Martínez, C. C., & Páez-Osuna, F. (2021). Arsenic in the top predators sailfish (Istiophorus platypterus) and dolphinfish (Coryphaena hippurus) off the southeastern Gulf of California. Environmental Geochemistry and Health, 43, 3441–3455.
CCME. (2001). Canadian Council of Ministers of the Environment. Canadian sediment quality guidelines for the protection of aquatic life. http://www.ccme.ca/sourcetotap/wqi.html. Accessed Dec 2021.
Chatterjee, S., Datta, S., Das, T. K., Veer, V., Mishra, D., Chakraborty, B., Datta, S., Mukhopadhyay, S. K., & Gupta, D. K. (2016). Metal accumulation and metallothionein induction in Oreochromis niloticus grown in wastewater fed fishponds. Ecological Engineering, 90, 405–416.
DOF. (2011). Norma Oficial Mexicana NOM-242-SSA1–2009, Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. Secretaría de Salud. 128 p. (in Spanish).
Dsikowitzky, L., Mengesha, M., Dadebo, E., Veiga de Carvalho, C. E., & Sindern, S. (2013). Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environmental Monitoring and Assessment, 185, 3117–3131.
El Mahmoud-Hamed, M. S., Montesdeoca-Esponda, S., Santana-Del-Pino, A., Zamel, M. L., Brahim, M., Tfeil, H., Santana-Rodriguez, J. J., Sidoumou, Z., & Ahmed-Kankou, M. (2019). Distribution and health risk assessment of cadmium, lead, and mercury in freshwater fish from the right bank of Senegal River in Mauritania. Environmental Monitoring and Assessment, 191, 493.
EPA. (2005). Human Health Risk Assessment Protocol. Chapter 7: Characterizing Risk and Hazard. https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap7.pdf. Accessed 17 Aug 2022.
EPA. (2022). Integrated Risk Information System (IRIS). https://www.epa.gov/iris. Accessed 17 Aug 2022.
FAO. (2021). Tilapia aquaculture in Mexico: assessment with a focus on social and economic performance. NFIA/C1219. FAO Fisheries and Aquaculture Circular. Food and Agriculture Organization of the United Nations.
Frías-Espericueta, M. G., Abad-Rosales, S., Nevárez-Velázquez, A. C., Osuna-López, I., Páez-Osuna, F., Lozano-Olvera, R., & Voltolina, D. (2008). Histological effects of a combination of heavy metal son Pacific White shrimp Litopenaeus vannamei juveniles. Aquatic Toxicology, 89, 152–157.
Frías-Espericueta, M. G., Quintero-Alvarez, J. M., Osuna-López, J. I., Sánchez-Gaxiola, C. M., López-López, G., Izaguirre-Fierro, G., & Voltolina, D. (2010). Metal contents of four commercial fish species of NW Mexico. Bulletin of Environmental Contamination and Toxicology, 85, 334–338.
Gymah, E., Akoto, O., Mensah, J. K., & Bortey-Sam, N. (2018). Bioaccumulation factors and multivariate analysis of heavy metals of three edible fish species from the Barekese reservoir in Kumasi Ghana. Environmental Monitoring and Assessment, 190, 553.
Huang, Y. K., Lin, K. H., Chen, H. W., Chang, C. C., Liu, C. W., Yang, M. H., & Hsueh, Y. M. (2003). Arsenic species contents at aquaculture farm and in farmed mouthbreeder (Oreochromis mossambicus) in blackfoot disease hyperendemic areas. Food and Chemical Toxicology, 41, 1491–1500.
Izaguirre-Fierro, G., Páez-Osuna, F., & Osuna-López, J. I. (1992). Heavy metals in fishes from Culiacán valley, Sinaloa, Mexico. Ciencias Marinas, 18, 143–151.
Jiang, D., Hu, Z., Liu, F., Zhang, R., Duo, B., Fu, J., Cui, Y., & Li, M. (2014). Heavy metals levels in fish from aquaculture farms and risk assessment in Lhasa, Tibetan autonomous region of China. Ecotoxicology, 23, 577–583.
Kapia, S., Rao, B. K. R., & Sakulas, H. (2016). Assessment of heavy metal pollution risks in Yonki Reservoir environmental matrices affected by gold mining activity. Environmental Monitoring and Assessment, 188, 586.
Khallaf, E. A., Galal, M., & Authman, M. (2003). The biology of Oreochromis niloticus in a polluted canal. Ecotoxicology, 12, 405–416.
Kossoff, D., Dubbin, W. E., Alfredsson, M., Edwards, S. J., Macklin, M. G., & Hudson-Edwards, K. A. (2014). Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Applied Geochemitry, 51, 229–245.
Le Croizier, G., Lacroix, C., Artigaud, S., Le Floch, S., Raffray, J., Penicaud, V., Coquillé, V., Autier, J., Rouget, M. L., Le Bayon, N., Lae, R., & De Morais, L. T. (2018). Significance of metallothioneins in differential cadmium accumulation kinetics between two marine fish species. Environmental Pollution, 236, 462–476.
Léopold, E. N., Jung, M. C., & Emmanuel, E. G. (2015). Accumulation of metals in three fish species from the Yaounde Municipal Lake in Cameroon. Environmental Monitoring and Assessment, 187, 560.
Lin, T. S., Lin, C. S., & Chang, C. L. (2005). Trace elements in cultured tilapia (Oreochromis mossambicus): Results from a farm in Southern Taiwan. Bulletin of Environmental Contamination and Toxicology, 74, 308–313.
Ling, M., Wu, C., Yang, K., & Hsu, H. (2013). Differential accumulation of trace elements in ventral and dorsal muscle tissues in tilapia and milkfish with different feeding habits from the same cultured fishery pond. Ecotoxicology and Environmental Safety, 89, 222–230.
Mapenzi, L. L., Shimba, M. J., Moto, E. A., Maghembe, R. S., & Mmochi, A. J. (2020). Heavy metals bio-accumulation in tilapia and catfish species in Lake Rukwa ecosystem Tanzania. Journal of Geochemical Exploration, 208, 106413.
Martínez-Durazo, A., Cruz-Acevedo, E., Betancourt-Lozano, M., & Jara-Marini, M. E. (2021). Comparative assessment of metal bioaccumulation in Tilapia and Largemouth Bass from three dams of the Yaqui River. Biological Trace Element Research, 199, 3112–3125.
Mbewe, G., Mutondo, M., Maseka, K., & Sichilongo, K. (2016). Assessment of heavy metal pollution in sediments and Tilapia fish species in Kafue River of Zambia. Archives of Environmental Contamination and Toxicology, 71, 383–393.
Mieiro, C. L., Bervoets, L., Joosen, S., Blust, R., Duarte, A. C., Pereira, M. E., & Pacheco, M. (2011). Metallothioneins failed to reflect mercury external levels of exposure and bioaccumulation in marine fish – Considerations on tissue and species specific responses. Chemosphere, 85, 114–121.
Nauen, C. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular, 764, 102.
Ndimele, P. E., Pedro, M. O., Agboola, J. I., Chukwuka, K. S., & Ekwu, A. O. (2017). Heavy metal accumulation in organs of Oreochromis niloticus (Linnaeus, 1758) from industrial effluent-polluted aquatic ecosystem in Lagos, Nigeria. Environmental Monitoring and Assessment, 189, 255.
NRC-CNRC. (2008). DOLT-4, Dogfish liver Certified Reference Material for Trace Metals. Ottawa: National Research Council Canada—Conseil National de Recherches Canada, Ottawa
Okogwu, O. I., Nwonumara, G. N., & Okoh, F. A. (2019). Evaluating heavy metals pollution and exposure risk through the consumption of four commercially important fish species and water from Cross River ecosystem, Nigeria. Bulletin of Environmental Contamination and Toxicology, 102, 867–872.
Osuna-Martínez, C. C., Armienta, M. A., Bergés-Tiznado, M., & Páez-Osuna, F. (2021). Arsenic in waters, soils, sediments, and biota from Mexico: An environmental review. Science of the Total Environment, 752, 14062.
Páez-Osuna, F., Álvarez-Borrego, S., Ruiz-Fernández, A. C., García-Hernández, J., Jara-Marini, M., Bergés-Tiznado, M. E., Piñón-Gimate, A., Alonso-Rodríguez, R., Soto-Jiménez, M. F., Frías-Espericueta, M. G., Ruelas-Inzunza, J., Green-Ruiz, C., Osuna-Martínez, C. C., & Sánchez-Cabeza, J. A. (2017). Environmental status of the Gulf of California: A pollution review. Earth-Science Reviews, 166, 181–205.
Páez-Osuna, F., Bojórquez-Leyva, H., Bergés-Tiznado, M., Rubio-Hernández, O., Fierro-Sañudo, J. F., & Ramírez-Rochín, J. (2015). Heavy metals in waters and suspended sediments affected by a mine tailing spill in the upper San Lorenzo River, NW México. Bulletin of Environmental Contamination and Toxicology, 94, 583–588.
Páez-Osuna, F., Pérez-González, R., Izaguirre-Fierro, G., Zazueta-Padilla, H. M., & Flores-Campaña, L. M. (1995). Trace metal concentrations and their distribution in the lobster Panulirus inflatus (Bouvier, 1895) from the Mexican Pacific coast. Environmental Pollution, 90, 163–170.
Phillips, D. J. H. (1980). Quantitative aquatic biological indicators (p. 488). Applied Science Publishers Ltd.
Ralston, N. V. C., Ralston, C. R., & Raymond, L. J. (2016). Selenium health benefit values: Updated criteria for mercury risk assessments. Biological Trace Element Research, 171, 262–269.
Rosseland, B. O., Teien, H. C., Basnet, S., Borgstrøm, R., & Sharma, C. M. (2017). Trace elements and organochlorine pollutants in selected fish species from Lake Phewa. Nepal. Toxicological & Environmental Chemistry, 99, 390–401.
Ruelas-Inzunza, J., Amezcua, F., Coiraton, C., & Páez-Osuna, F. (2020). Cadmium, mercury, and selenium in muscle of the sacalloped hammerhead Sphyrna lewini from the tropical Eastern Pacific: Variation with age, molar ratios and human health risk. Chemosphere, 242, 125180.
Ruelas-Inzunza, J., Rojas-Ruiz, E., Spanopoulos-Hernández, M., & Barba-Quintero, G. (2015). Mercury in the blue tilapia Oreochromis aureus from a dam located in a mining region of NW Mexico: Seasonal variation and percentage weekly intake (PWI). Environmental Monitoring and Assessment, 187, 233.
Ruelas-Inzunza, J., Vega-Sánchez, B., Ramos-Osuna, M., & Páez-Osuna, F. (2011). Trophic transfer and dietary mineral intake of essential elements in thunus albacares and Katsuwonus pelamis from the Eastern Pacific. Biological Trace Element Research, 143, 231–239.
Sallam, K. I., Abd-Elghany, S. M., & Mohammed, M. A. (2019). Heavy metal residues in some fishes from Mazala lake, Egypt, and their health-risk assessment. Journal of Food Science, 84, 1957–1965.
SEMARNAT. (2021). Consulta Temática. Consumo Nacional Aparente por destino y especie. http://dgeiawf.semarnat.gob.mx:8080/ibi_apps/WFServlet?IBIF_ex=D2_PESCA03_02&IBIC_user=dgeia_mce&IBIC_pass=dgeia_mce&NOMBREANIO. Accessed 17 August 2022.
Stickney, R. R. (2017). Tilapia feeding habits and environmental tolerances. In P. W. Perschbacher & R. R. Stickney (Eds.), Tilapia in Intensive Co-culture (pp. 25–35). Wiley.
Sujitha, S. B., Jonathan, M. P., Aurioles-Gamboa, D., Campos Villegas, L. E., Bohórquez-Herrera, J., & Hernández-Camacho, C. J. (2019). Trace elements in marine organisms of Magdalena Bay, Pacific coast of Mexico: Bioaccumulation in a pristine environment. Environmental Geochemistry and Health, 41, 1075–1089.
Swinkels, L. H., Van de Ven, M. W. P. M., Stassen, M. J. M., Van der Velde, G., Lenders, H. J. R., & Smolders, A. J. P. (2014). Suspended sediment causes annual acute fish mortality in the Pilcomayo River (Bolivia). Hydrological Processes, 28, 8–15.
Thomann, R. V., Mahony, J. D., & Muller, R. (1995). Steady state model of biota-sediment accumulation factor for metals in two marine bivalves. Environmental Toxicological and Chemistry, 4, 989–998.
UNEP. (2008). Water quality for ecosystem and human health. 2nd edn. United Nations Environment Programme Global Environment Monitoring System/Water Programme, Burlington.
WHO. (2022). World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). https://apps.who.int/food-additives-contaminants-jecfa-database/. Accessed 17 Aug 2022.
Yap, C. K., Jusoh, A., Leong, W. J., Karami, A., & Ong, G. H. (2015). Potential human health risk assessment of heavy metals via the consumption of tilapia Oreochromis mossambicus collected from contaminated and uncontaminated ponds. Environmental Monitoring and Assessment, 187, 584.
Zar, J. (2010). Biostatistical analysis (5th ed.). Prentice Hall Pearson.
Acknowledgements
This work was carried out with the collaboration of the fishermen of El Comedero dam Jorge Zazueta, Miguel Urrea, and Pedro Murguía. Authors thank H. Bojórquez-Leyva for his assistance in the analytical work.
Funding
Work supported by the Dirección General de Asuntos del Personal Académico, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica and the Universidad Nacional Autónoma de México (DGAPA, PAPIIT, UNAM) Project IN203922 titled “Metales, metaloides y microplásticos en organismos de importancia ecológica y comercial de la eco-región del Golfo de California”.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. FPO conceived the ideas and designed the research; Formal analysis: MEBT, MGFL, GVC, JALC, SGAS, JFFS, JRR; Funding acquisition: FPO; Methodology: MEBT, MGFL, GVC, JALC, SGAS, JFFS, JRR; Investigation: FPO; Resources and Supervision: FPO; Writing – original draft: FPO; Writing – review & editing: FPO, MEBT.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Páez-Osuna, F., Bergés-Tiznado, M.E., Fregoso-López, M.G. et al. High accumulation of metals and metalloids in the liver of the blue tilapia (Oreochromis aureus) during a massive mortality event induced by a mine tailing spill. Environ Geochem Health 45, 3155–3169 (2023). https://doi.org/10.1007/s10653-022-01399-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01399-2