Abstract
Modeling metal sorption in soils is of great importance to predict the fate of heavy metals and to assess the actual risk driven from pollution. The present study focuses on adsorption of HM ions on two types of hydromorphic soils, including calcaric fluvisols loamic and calcaric fluvic arenosols. The individual and competitive adsorption behaviors of Cu and Zn on soils and soil constituents are evaluated comprehensively. It is established that the sorption processes were best described with the Langmuir model. The results suggest that the calcaric fluvic arenosols are more vulnerable to heavy metal input compared to fluvisols loamic. In all cases, Cu had a higher range of values of the adsorption process parameters relative to Zn. The Zn is likely to be the most critical environmental factor in such soils since it exhibited a decreased sorption under competitive conditions. The retention mechanisms of HM in hydromorphic soils are considered. Based on theoretical calculations of ion activity in soil solutions using solubility diagrams of Cu and Zn compounds, the possibility of precipitation of Cu hydroxide and Zn carbonate in the studied soils is shown. Direct physical methods of nondestructive testing (XAFS and XRD) are applied to experimentally prove the formation of these HM compounds on the surface of montmorillonite, the dominant mineral in hydromorphic soils, and calcite. Thus, the combination of both physicochemical methods and direct physical methods can provide a large amount of real information about the mechanisms of HM retain with solid phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HMs) are hazardous elements and well known to impose a threat to human health. Among HMs, Cu and Zn are the priority pollutants included in the US EPA list (Code of Federal Regulations 2019). As a result of growing ecological awareness about toxic HM globally, it has been recognized that there is a need for improving the protection of public health and the environment due to continuous exposure risks, even in small daily doses (Pirooty and Ghasemzadeh 2013). A high concentration of Cu can exert a negative influence on organisms, followed by destroying the cell metabolism and provoking immunotoxicity, embryotoxicity, and reproductive toxicity (Duan et al. 2016). Exposure to elevated levels of Zn can adversely affect the gastrointestinal, hematological, and respiratory systems. The high level of exposure can negatively alter the function of the cardiovascular and neurological systems in the humans (Radhakrishnan et al. 2016). High concentrations of Zn2+ ions are known to trigger cell death by apoptosis or necrosis in a variety of cell types in the brain (Nriagu 2019).
The excessive concentration of HM in soils can result from different sources. Fertilizers and agrochemicals products, mining-related industries, and waste incineration are among the contributing sources (Janoš et al. 2010). Adsorption is a major process responsible for the accumulation of HM. The study of the sorption capacity of soils relative to HM is important because it affects key ecological issues, such as prediction of the risk related to the bumping of metals in soil, remediation of polluted soils, and utilization of disposal (Vidal et al. 2009). In a broad sense, the sorption of HM includes diverse adsorption processes, e.g., the formation of extra- and intraspheric complexes and ion exchange, as well as surficial sedimentation and other mechanisms (McBride 1994). The sorption capacity of the soil is influenced by pH values and the presence of active adsorption centers in different soil components, such as carbonates, phosphates, organic matter, silicates, and (hydr)oxides (Sipos et al. 2008; Vidal et al. 2009; Fisher-Power et al. 2016). Clay minerals prevail in soil particles less than 0.002 mm in diameter. Since the negatively charged layered minerals dominate in the soil surface, they are characterized by a significant sorption capacity relative to HM (Plyaskina and Ladonin 2005). The adsorption and precipitation of Cu in soil carbonates play an important role in metal fixation because of the high dispersion of soil carbonates and the small solubility constant of carbonates of HM (Jinren and Weiling 2003; Burachevskaya et al. 2018). Assessment of the pollutants sorption by soil is usually based on laboratory experiments to quantitatively estimate the coefficient of metal distribution between the solid and liquid phases of soil (Vidal et al. 2009). By measuring the aqueous and adsorbed concentration of the contaminant at adsorption equilibrium, adsorption isotherms and related parameters can be derived (Mizutani et al. 2017). This simple approach can be expanded based on additional mechanisms, such as desorption, competition for adsorption sites in the case of polymetallic pollution, and heterogeneity of exchange sites in the solid phase of soil (Ding et al. 2009). The chemical and energetic heterogeneity in the surface of soil particles leads to irregular strength of the HM fixation and dependence of the interaction energy on the degree of the surface filling by the adsorbed cations and their individual properties (Saha et al. 2002).
Soil pollution in industrially developed regions takes place simultaneously with a wide range of chemicals (Linnik et al. 2019). However, the polyelemental character of contamination is rarely considered in the investigation due to mutual competition between cations for exchange sites, aggravated by specific properties of metals and heterogeneity of the adsorbent.
The formation of sediments of the low-soluble HM compounds is an important mechanism for the HM immobilization. Copper can be precipitated as Cu (OH)2, CuO, and Cu2CO3(OH)2; Zn, as ZnO, ZnCO3, and Zn5(OH)6(CO3)2 (Madrid and Diaz‐Barrientos 1992). Sophisticated analytical techniques such as X-ray powder diffraction (XRD), X-ray absorption near edge structure (XANES), and extended X-ray absorption fine structure (EXAFS) can be used to identify the HM-resistant compounds in soils (Nevidomskaya et al. 2016).
Being on the way of the chemical elements flow into water bodies, floodplain soils act as barriers and accumulators of not only vital elements but also pollutants while performing a protective function in valley landscapes. A feature of the soils of river floodplains is their dependence on frequently changing environmental conditions associated with the features of the relief, hydrological conditions, the dynamics of alluvial deposition, the variable nature of moisture, and the composition of vegetation. For this reason, the floodplain soils are in particular interest at the study of the HM absorption mechanisms. The complex process of soil formation, its high dynamism, and the specificity of water nutrition are responsible for a poorly study of the absorption capacity of floodplain soils relative to HM.
The aim of the present paper is to study mechanisms of Cu2+ and Zn2+ adsorption by the main types of hydromorphic soils in the Rostov region (Russia).
Materials and methods
Soil sampling
The objects of study were the hydromorphic soils of the Lower Don basin (Rostov region, Russia). The soils were classified as meadow heavy loamy soils (calcaric fluvisols loamic) on alluvial deposits and alluvial sandy soils (calcaric fluvic arenosols). Soils of both types were selected in the areas without anthropogenic impact, located in the floodplain of the Seversky Donets River, a large right-bank tributary of the Don. The absence of high concentrations of HM, including Zn and Cu (Table 1) in the natural soils, was shown by our previous studies (Konstantinova et al. 2020).
The calcaric fluvisols are located in the central part of a floodplain meadow on the left bank of the Seversky Donets River (N 48°21′06.67″, E 40°14′09.57). The calcaric arenosols are located at the edge of the floodplain on the right bank of the Seversky Donets River (N 48°20′12.91″, E 40°14′10.89″). The soil samples were taken from the surface layer (0–20 cm), dried at room temperature, ground and sieved (1 mm mesh) to remove residual, such as visible stones and plants, and then stored at 4 °C until further use. The studied soils differ from each other according to physicochemical parameters (Table 1).
The mineralogical composition of the clay fraction in hydromorphic soils is represented by mixed-layer mica–smectite formations with a high content of smectite packets (59–80%), hydromica (17–26%), kaolinite and chlorite (5–12%). An X-ray diffractometry using DRON-7 diffractometer in Cu-Kα-filtered radiation is used to determine the mineralogical composition of the clay fraction of soils with a size less than 1 μm. The soil particle size distribution analysis was performed according to the pipette method with pyrophosphate procedure of soil preparation (ISO 13,317–2 2001). Soil properties were determined using standard methods: pH of soil suspension in water by ISO 10,390 (2005); total organic carbon (TOC) content by sulfochromic oxidation, ISO 14,235 (1998); carbonates content by a Scheibler apparatus, ISO 10,693(1995) , and exchangeable cations by hexamminecobalt trichloride solution, ISO 23,470(2011) . The total content of Zn in the soils was determined by X-ray fluorescent (XRF) scanning spectrometer SPECTROSCAN MAKC-GV.
Heavy metal adsorption procedure
Air-dried aliquots (5 g) of the studied soil samples were flushed with 50 ml of metal solutions (Cu(NO3)2 and Zn(NO3)2) with the following Cu2+ and Zn2+ concentrations (mM L–1): 0.05, 0.08, 0.1, 0.3, 0.5, 0.8, and 1.0. The metal solutions were prepared from Cu(NO3)2 × 3H2O and Zn(NO3)2 × 6H2O salts of the ‘chemically pure grade.’ The HMs were added separately or simultaneously. The resulting suspensions were stirred for 1 h and then left in the calm state for 24 h. This period was sufficient for the adsorption to reach equilibrium. The time required for the system to reach equilibrium was in good agreement with the results of our kinetic adsorption experiments (Pinskii et al. 2010). The studies were carried out with a change in the ion strength from 0.0036 to 0.87 mol/L. The pH values were measured by potentiometry of the equilibrium solutions. Then, the suspension was centrifuged and filtered. The metal content in filtrate was determined by the atomic absorption spectrophotometer with electrothermic atomization and polarization Zeeman effect correction for the nonselective adsorption using an MGA-915MD spectrometer (Lumex Scientific-Production Company, St. Petersburg, Russia). The amount of metal adsorbed by the solid phase was found by the eliminating of the added amount from the measured concentration in the equilibrium solution (Du et al. 1999):
where Cad is the amount of metal adsorbed by a unit sorbent mass, mM kg–1; Ci is the initial metal concentration in solution, mM L–1; Cs is the equilibrium metal concentration in solution, mM L–1; V is the solution volume, ml; and m is the air-dry sorbent mass, g. The obtained data were used to draw the Cs–Cad plot, where Cs is the equilibrium concentration of metal added to the solution, mM L−1; and Cad is the specific content of metal in the sorbent phase, mM kg–1. The multi-solute competitive sorption experiments were conducted in the same manner as in the single-solute sorption experiments. The corresponding sorption isotherms for each metal were quantitatively described by parameters through fitting the experimental data to the Langmuir isotherm described by the following equations, respectively:
where Cad is the amount of adsorbed cations; C∞ is the maximum metal adsorption, mM kg–1; Ce is the equilibrium metal concentration, mM L–1; and KL is the Langmuir constant, L mM–1.
The Langmuir isotherm enables us to calculate the maximum adsorption capacity (C∞) of each metal on the solid phase and to quantify the metal affinity with soil (КL). The Langmuir model assumes monolayer coverage in a homogeneous adsorption mode occurred on a surface with binding sites having equal energies (Musso et al. 2014).
Adsorption mechanisms
The components of the mineral phases were used to study the mechanisms of HM immobilization in the soils. The montmorillonite sample and calcite were saturated with Cu2+ and Zn2+ ions using a saturated solution of metal nitrate for 1 week (Nevidomskaya et al. 2016). The calcite sample isolation from the studied fluvisols was carried out under MBS-10 binoculars in the magnification range from 5 to 32. The montmorillonite sample is a standard reference material (GSO 8694–2005).
X-ray powder diffraction
The X-ray diffraction based on synchrotron radiation was used for the analysis of the reference samples (Nevidomskaya et al. 2016). The structural accordance of montmorillonite mineral phase was compared to the diffraction patterns obtained at the Structural Materials Science beamline of the Kurchatov Center for Synchrotron Radiation (Chernyshov et al. 2009). A 1.7 T bend magnet of the Siberia-2 storage ring is the source of synchrotron radiation. The electron beam energy is 2.5 GeV; the average current is 120 mA.
Diffraction studies of monochromatic synchrotron X-ray radiation (λ = 0.68886 Å, Si monochromator) were performed in transmission geometry using a Fujifilm Imaging Plate two-coordinate detector at 0.68886 Å. X-ray diffraction patterns were recorded in integrated mode at 20 °C. The time of sample exposure was about 15 min. A silicon standard (NIST SRM 640C) was used for the angular calibration of the scale. Compared to the conventional X-ray diffractometry, the use of high-intensity monochromatic synchrotron radiation significantly improves the intensity and resolution of diffraction patterns once it is combined with a two-coordinate detector and a Si monochromator. The diffraction patterns were compared with the ICSD (Inorganic Crystal Structure Database) (https://icsd.products.fiz-karlsruhe.de/).
X-ray absorption spectroscopy
The XANES spectra at the K-edge of Zn (9659 eV) of the carbonate were obtained by spectrometer Rigaku R-XAS Looper in the fluorescence regime (Nevidomskaya et al. 2014). The zinc nitrate (Zn(NO3)2) was used as a reference compound. The XANES spectra first derivatives were calculated. This calculation is a means to obtain and identify detailed information on the state of Zn2+ ions in the studied sample (Nevidomskaya et al. 2014).
Statistical analysis
The SigmaPlot 12.5 software packages with a confidence coefficient of 0.95 were used for the statistical process. Each experimental isotherm point was fixed after three replications. For each sample analyzed through the X-ray absorption spectroscopy method, ten scans were collected and averaged to achieve a higher signal-to-noise ratio. For energy calibration, copper foils were used as standard samples. For statistical purposes, the 60-s exposure time was considered to collect the data for each point in the spectrum. For the X-ray powder diffraction analysis, each of the studied samples was examined in three replications to scrutinize the homogeneity of the fraction composition.
Results and discussion
Monoelement adsorption of HM
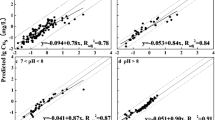
Figure 1 presents the isotherms of Cu2+ and Zn2+ adsorption by the calcaric fluvisols and calcaric fluvic arenosols during the separate input of metals from nitrate salt solutions. In all cases, adsorption of metals in the studied soils is described by Eq. (2) with a high degree of reliability (R2 = 0.99). Experimental data on Cu2+ and Zn2+ adsorption by the studied soils revealed some discrepancies in their adsorption capacity due to differences in chemical properties of these elements and between the soils themselves. Both soil types show a higher affinity to Cu2+ rather than Zn2+, as suggested by the shape of isotherms and their location near the y-axis. Isotherms of Cu2+ and Zn2+ adsorption by the fluvisols and arenosols have a typical Langmuir shape, indicating the metal adsorption by one mechanism or several mechanisms weakly distinguished from each other. The values of Langmuir parameters computed with the linearization of experimental isotherms are presented in Table 2.
It has been established that C∞(Cu) > C∞(Zn) and the value of C∞ for the fluvisols is always higher than for the calcaric fluvic arenosols. Langmuir constants (KL) characterizing the energy of interaction between the adsorbed cations and SAC are also always higher than for the fluvisols. One of the reasons responsible for a higher absorption capacity of the fluvisols (Loamic) is related to the high content of organic matter, clay, and silt, relative to the arenosols, in which the TOC content is just 0.9%. Discrepancies between KL values for the studied Cu2+ and Zn2+cations are 17–27 times higher depending on the soil type. Hence, the properties of the metal cation exert a significant influence on the energy of interaction with the soil particle surface (Jalali, Moharrami 2007; Pinskii et al. 2014; Huang et al. 2018). The metal hydrolysis properties affect the retention tendency in a particular way. In this context, Cu2+ has more tendency to be retained compared to Zn2+ (Saha et al. 2002; Agbenin and Olojo 2004). In the more alkaline pH range, the influence of the metal hydrolysis on metal adsorption becomes dominant (Jalali and Moharrami 2007). The high electronegativity of metal leads to its strong interaction with the soil particle surface (Pinskii et al. 2014). Electronegativity is an essential contributing factor to determine the level of preference in which the HM tends to chemisorb with the highest order: Cu > Ni > Co > Pb > Cd > Zn > Mg > Sr (McBride 1994).
The obtained Misono softness parameter predicts the metal affinity sequences or preferential retention by soil components (Misono et al. 1967). The softness parameter is an index to determine the affinity of a metal to form covalent bonds with colloids (Sposito 1989). This parameter considers both the ionic charge and the ionization potential (which is higher for Cu than Zn) (Agbenin and Olojo 2004). Thus, the preferential retention of Cu to Zn is caused by differences in electronegativity, first hydrolysis constants, and the softness parameter.
Variation of pH during the monoelement adsorption of HM
To estimate discrepancies in Cu2+ and Zn2+ adsorption by soils and elucidate possible mechanisms of the interaction of metals with them, we accomplished an experiment with the determination of pH values in the equilibrium and corresponding initial solutions.
The dissolution of salts Cu(NO3)2 and Zn(NO3)2 provokes variation of pH values in the initial solutions due to processes of hydrolysis (Fig. 2). With increasing solution concentration, the pH value in the initial Cu(NO3)2 solution decreases gradually from 6.2 to 3.9; in the Zn(NO3)2 solution, from 6.4 to 5.9. In general, the pH value in the initial solutions at different amounts of the adsorbed Cu is much lower relative to Zn. In the calcaric fluvisols, the pH value varies from 7.4 to 6.6 for Cu and 7.4 to 7.1 for Zn. In equilibrium solutions of the calcaric fluvic arenosols, the pH value decreases from 7.4 to 6.4 and from 7.4 to 6.8, for Cu and Zn, respectively.
The contact of initial solutions with soil leads to two effects: (1) abrupt change of pH because of the buffer capacity of soil related to its composition, composition of the added solution, and properties of the adsorbed cation; (2) subsequent gradual decrease in pH in equilibrium solutions with the increase in adsorbed HM cations. The nature of the dependence of pH from the adsorbed metal amount is governed mainly by the composition of the solid phase: the presence of carbonates and hydrogen, which can exchange with HM cations. Analogous reasons for the pH variation in solutions resulted from sorption processes in soils were described in Mesquita and Silva (2002). Certain influence can also be exerted by processes taking place in the liquid phase during its compositional change due to the adsorption of HM cations and the transition of other cations into the solution. If the amount of adsorbed HM is close to the maximum adsorption value, the discrepancy between pH values in the initial and final solutions can reach 2.5–3.0 units and persist for a long time (Davis and Leckie 1978).
Displacement of the exchangeable hydrogen during the adsorption of HM cations, probably, plays an important role in the acidification of equilibrium solutions. Its presence in soils is related, first of all, to weakly acid exchange centers in the solid phases (Pinskii et al. 2018). A decrease in pH values can also be related to the interaction of HM hydroxo complexes with the soil mineral particles, leading to the completion of octahedral layer and the expulsion of hydrogen ions into the solution (Ladonin 1997).
The addition of Cu2+ salts to the soil provoked a notable decrease in pH value in the equilibrium solution, relative to the addition of Zn2+ salt (Fig. 2). Copper can be absorbed more firmly by soils, and, probably, this metal has a greater capacity to displace the exchangeable hydrogen from the solid phase into the solution. Adsorption of Zn by the studied soil types is characterized not only by lesser values of parameters C∞ and KL, but also by least variations of pH values, probably, because of its lower capacity to complex formation. A positive correlation between the formation of hydroxo complexes of HMs and the decrease in pH values in the soil–solution system is reported in McBride (1989).
Competitive adsorption of heavy metals
Figure 3 presents the isotherms of Cu2+ and Zn2+ adsorption by the calcaric fluvisols and calcaric fluvic arenosols during their combined addition to the studied soil. As in the case of monoelement adsorption, the isotherms are described well by Eq. (2). However, peculiarities of the interaction are influenced considerably by the competition for sorption sites (Table 2). Adsorption of Cu2+ by soils is much more intense relative to Zn2+.
The presented data show a decrease in C∞ values for both cations. Moreover, the sum of C∞ (Cu) and C∞ (Zn) during the joint adsorption is equal within the error limit to mean C∞ values during the monoelement adsorption of Cu and Zn from each soil. The presence of both metal cations in the system is reflected mostly in the Zn adsorption, with C∞ decreasing 2.4–2.5 times. For example, the adsorption capacity of Kastanozems decreases drastically during the polyelement contamination: Pb is absorbed 5 times less, Cu 2 times less, and Zn 4 times less (Panin and Siromlya 2005). Hence, the two cations compete for the same sites. At the same time, KL values in the Langmuir equation during the polyelement adsorption increase, relative to the process of monoelement adsorption.
Thus, adsorption sites in the studied soils are heterogeneous in terms of energy. Under the influence of competition from other ions, ions of each metal interact ultimately with reaction centers characterized by the maximum affinity (Ladonin 1997; Pinskii et al. 2010). Under conditions of competition, Cu occupies a dominant position and interacts with adsorption centers characterized by the maximum affinity. As a result of the polyelement adsorption, the amount of each adsorbed metal will decrease, while the strength of its bond with the pedogenic adsorption complex will increase.
Processes of the sedimentation of HM ions in soil solutions
The formation of sediments of the low-solubility compounds can also influence the behavior of Cu and Zn cations in the studied systems. It is often challenging to separate adsorption from precipitation in the experiments. To this end, the sorption is used to confirm the declining trend of solute in the liquid phase, which is resulted from adsorption and sedimentation or polymerization process (Jinren and Weiling 2003). Based on the formation of aluminum orthophosphate, the possibility of the formation of sediments of the low-soluble compounds during adsorption was demonstrated by Sposito (1984). In this case, the sedimentation equation is identical to the Langmuir equation. Thus, both processes become experimentally indiscernible.
The possibility of the formation of the low-solubility Cu–Zn carbonate and hydroxide sediments was studied in the floodplain soils based on the solubility diagrams (Fig. 4). Figure 4 also shows logarithms of the concentrations of these metals in equilibrium solutions of the calcaric fluvisols and calcaric fluvic arenosols obtained during the study of the cations adsorption. Computations were accomplished using the data on constants of the stability of associated HM species and their solubility constants (Table 3).
Analysis of the presented data shows that the concentration of Cu2+ ions in equilibrium solutions during absorption by the calcaric fluvisols and calcaric fluvic arenosols lies on a straight line corresponding to the solubility of sediment Cu(OH)2, suggesting the possibility of the formation of a compound, in addition to its adsorption by hydromorphic soils. Data points of Zn2+ concentration are grouped slightly lower, relative to the more soluble ZnCO3. Obviously, carbonate can also form near pH = 7.3. In the field of higher and lower pH values, the formation of this sediment is less likely, because Zn concentration in the equilibrium solution is lower than the values needed for sedimentation.
According to Sparks (2001), the HM sorption by soil and soil components produces a new phase composed of sediment Me(OH)2 (low-solubility compound). In this case, the surficial precipitation is observed at ion concentrations and pH values much lower than those when the solution becomes saturated relative to the solid phase. A local pH heterogeneity related to protonation of the surface can be observed near the surface of mineral or organomineral particles of soil. The pH values near the surface can be much higher than in the solution volume (Kurochkina et al. 2014). This phenomenon also promotes sedimentation of the low-soluble HM hydroxides and carbonates.
The comparison of diffraction patterns for the layered silicate phases of montmorillonite is performed before and after modification with a saturated Cu(NO3)2 solution. The results showed appreciable changes in the diffraction pattern of the contaminated phases of montmorillonite compared to the original samples (Fig. 5). Additional diffraction peaks corresponding to a new crystalline phase appeared in the diffraction patterns of montmorillonite saturated with Cu2+ ions.
A peculiar feature of layered silicate phases is the chemical and energetic heterogeneity of their surface characterized by the presence of structural defects and different functional groups, which can act as active centers during metal adsorption (Minkina et al. 2018). These active centers on the surface of montmorillonite can include exchangeable cations, surface hydroxyl groups, and oxygen atoms of the tetrahedral lattice. Some active centers occur on the lateral faces of minerals formed during the splitting of minerals.
The saturation of phases of layered silicates with a Cu(NO3)2 solution at a constant pH of 3.9 increases the share of active acidic centers, which affects the proportions of acid–base active centers on the surface of mineral phases (Ponizovskii and Mironenko 2001). Hydrolysis processes, which shift the system equilibrium, also contribute. During the initial saturation period, Ca2+ cations are desorbed from the interlayer positions of layered silicate into the contacting solution.
X-ray diffraction data showed that Cu2+ ions are sorbed from the saturated solution by active centers on the internal surface of the lattice of dioctahedral aluminosilicates, and surface hydroxyls at the octahedrally coordinated aluminum atom play the main role (Furnare et al. 2005). X-ray diagnostics revealed that excess Cu2+ ions are removed from the system due to the formation and precipitation of coarsely crystalline Cu2(NO3)(OH)3 (Fig. 3).
Comparison of the XANES spectra of the initial Zn (NO3)2 compound and the saturated Zn phase of carbonates revealed a shift (more than 1 eV) by the position of the main energy maximum, which means a change in the charge state as a result of electron transfer from Zn. Analysis of the K-edge XANES spectra of Zn adsorbed by soil carbonates revealed that the metal ions substitute Ca at octahedral sites of calcite (Fig. 6). This observation is followed by 1 s → 4p electron transition, and metal ions coordinate with carbonate ions as ligands to make up absorption complexes on the surface of the mineral calcite, first of all, at the defect and fracture sites. The extended X-ray absorption fine structure spectroscopy was used to confirm Zn incorporation into calcite. Zn occupies the Ca position in octahedral coordination, with Zn–O distances only slightly larger than in pure ZnCO3 (Elzinga and Reeder 2002).
Conclusion
Regularities in the absorption of Zn and Cu by the main types of hydromorphic soils in southern Russia are studied. Using combination of physicochemical and direct physical methods provides detailed information about the nature of the soil components and possible interactions with HM. Isotherms of sorption by the calcaric fluvisols (loamic) demonstrate a high affinity of the soil particle surface to the studied metals, relative to calcaric fluvic arenosols. This is due to the higher content of organic matter, clay, and silt in the fluvisols, compared to the arenosols. Experiments with the sorption of Cu and Zn by the studied soils during their joint presence demonstrated that the soil particle surface includes reaction centers with different selectivity relative to metals. Under conditions of competition, Cu manifests a higher affinity to adsorption centers than Zn. Analysis of the solubility diagrams of metal compounds revealed a possibility of the formation of sediments of the low-solubility Cu hydroxides and Zn carbonates in the studied soils. Analysis of the XANES spectra and XRD identification confirmed the results based on simulations of the solubility diagram of metal compounds. In addition to ion exchange processes, the system is also marked by the formation of a Zn carbonate adsorbed on the surface of soil carbonates (calcite), and Cu ions are precipitated on the surface of the main layered minerals as the coarse crystalline phase Cu2(NO3)(OH)3. The obtained results imply that montmorillonite and calcite can be used as an efficient material for the retention of Zn and Cu in soils.
References
Agbenin, J. O., & Olojo, L. A. (2004). Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma, 119(1–2), 85–95. https://doi.org/10.1016/S0016-7061(03)00242-8
Burachevskaya, M. V., Minkina, T. M., Mandzhieva, S. S., Bauer, T. V., Chaplygin, V. A., Sushkova, S. N., et al. (2018). Comparing two methods of sequential fractionation in the study of copper compounds in Haplic chernozem under model experimental conditions. Journal of soils and sediments, 18(6), 2379–2386. https://doi.org/10.1007/s11368-017-1711-7
Chernyshov, A. A., Veligzhanin, A. A., & Zubavichus, Y. V. (2009). Structural materials science end-station at the kurchatov synchrotron radiation source: Recent instrumentation upgrades and experimental results. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 603(1–2), 95–98. https://doi.org/10.1016/j.nima.2008.12.167
Code of Federal Regulations (CFR) (Annual Edition) (2019). Title 40: Part 423, Appendix A - Protection of Environment. Accessed 1 July 2019.
Davis, J. A., & Leckie, J. O. (1978). Effect of adsorbed complexing ligands on trace metal uptake by hydrous oxides. Environmental science & technology, 12(12), 1309–1315. https://doi.org/10.1021/es60147a006
Ding, S. L., Sun, Y. Z., Yang, C. N., & Xu, B. H. (2009). Removal of copper from aqueous solutions by bentonites and the factors affecting it. Mining Science and Technology (China), 19(4), 489–492. https://doi.org/10.1016/S16745264(09)60091-0
Du, Q., Sun, Z., Forsling, W., & Tang, H. (1999). Complexations in illite-fulvic acid-Cu2+ systems. Water Research., 33(3), 693–706.
Duan, X., Xu, M., Zhou, Y., Yan, Z., Du, Y., Zhang, L., et al. (2016). Effects of soil properties on copper toxicity to earthworm Eisenia fetida in 15 Chinese soils. Chemosphere, 145, 185–192. https://doi.org/10.1016/j.chemosphere.2015.11.099
Elzinga, E. J., & Reeder, R. J. (2002). X-ray absorption spectroscopy study of Cu2+ and Zn2+ adsorption complexes at the calcite surface: Implications for site-specific metal incorporation preferences during calcite crystal growth. Geochimica et Cosmochimica Acta, 66, 3943–3954.
Fisher-Power, L. M., Cheng, T., & Rastghalam, Z. S. (2016). Cu and Zn adsorption to a heterogeneous natural sediment: Influence of leached cations and natural organic matter. Chemosphere, 144, 1973–1979. https://doi.org/10.1016/j.chemosphere.2015.10.109
Furnare, L. J., Strawn, D. G., & Vailionis, A. (2005). Polarized XANES and EXAFS spectroscopic investigation into copper (II) complexes on vermiculite. Geochimica et Cosmochimica Acta, 69(22), 5219–5231. https://doi.org/10.1016/j.gca.2005.06.020
Huang, L., Jin, Q., Tandon, P., Li, A., Shan, A., & Du, J. (2018). High-resolution insight into the competitive adsorption of heavy metals on natural sediment by site energy distribution. Chemosphere, 197, 411–419. https://doi.org/10.1016/j.chemosphere.2018.01.056
ICSD database: https://icsd.products.fiz-karlsruhe.de/
ISO 10390 (2005). Soil Quality – Determination of pH.
ISO 10693 (1995). Soil Quality – Determination of Carbonate Content – Volumetric Method.
ISO 13317–2 (2001). Determination of Particle Size Distribution by Gravitational Liquid Sedimentation Methods – Part 2: Fixed Pipette Method.
ISO 14235 (1998). Soil Quality – Determination of Organic Carbon by Sulfochromic Oxidation.
ISO NF EN 23470 (2011). Soil Quality – Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations.
Jalali, M., & Moharrami, S. (2007). Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma, 140(1–2), 156–163. https://doi.org/10.1016/j.geoderma.2007.03.016
Janoš, P., Vávrová, J., Herzogová, L., & Pilařová, V. (2010). Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma, 159(3–4), 335–341. https://doi.org/10.1016/j.geoderma.2010.08.009
Jinren, N., & Weiling, S. (2003). Applicability of the Langmuir equation to copper sorption by loess with high carbonate content. In B. Kronvang (Ed.), The Interactions between Sediments and Water pp (pp. 259–263). Dordrecht: Springer.
Konstantinova, E., Burachevskaya, M., Mandzhieva, S., Bauer, T., Minkina, T., Chaplygin, V., et al. (2020). Geochemical transformation of soil cover and vegetation in a drained floodplain lake affected by long-term discharge of effluents from rayon industry plants, lower Don River Basin. Southern Russia: Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00683-3
Kurochkina, G. N., Pinskii, D. L., Haynos, M., Sokolowska, Z., & Tsesla, I. (2014). Electrokinetic properties of soil minerals and soils modified with polyelectrolytes. Eurasian soil science, 47(7), 699–706. https://doi.org/10.1134/S1064229314070084
Ladonin, D. V. (1997). Specific adsorption of copper and zinc by some soil minerals. Eurasian soil science, 30(12), 1326–1332.
Linnik, V. G., Minkina, T. M., Bauer, T. V., Saveliev, A. A., & Mandzhieva, S. S. (2019). Geochemical assessment and spatial analysis of heavy metals pollution around coal-fired power station. Environmental geochemistry and health. https://doi.org/10.1007/s10653-019-00361-z
Lur’e, Y. Y. (1979). Analytical chemistry handbookю Moscow: Khimia (in Russian).
Madrid, L., & Diaz-Barrientos, E. (1992). Influence of carbonate on the reaction of heavy metals in soils. Journal of Soil Science, 43(4), 709–721. https://doi.org/10.1111/j.1365-2389.1992.tb00170.x
McBride, M. B. (1989). Reactions controlling heavy metal solubility in soils. In B. A. Stewart (Ed.), Advances in soil science (pp. 1–56). New York: Springer.
McBride, M. B. (1994). Environmental Chemistry of Soils. New York: Oxford University Press.
Mesquita, M. E., & Silva, J. V. (2002). Preliminary study of pH effect in the application of Langmuir and Freundlich isotherms to Cu–Zn competitive adsorption. Geoderma, 106(3–4), 219–234. https://doi.org/10.1016/S00167061(01)00125-2
Minkina, T. M., Nevidomskaya, D. G., Shuvaeva, V. A., Soldatov, A. V., Tsitsuashvili, V. S., Zubavichus, Y. V., et al. (2018). Studying the transformation of Cu2+ ions in soils and mineral phases by the XRD, XAFS, and sequential fractionation methods. Journal of Geochemical Exploration., 184, 365–371. https://doi.org/10.1016/j.gexplo.2016.10.007
Misono, M., Ochiai, E. I., Saito, Y., & Yoneda, Y. (1967). A new dual parameter scale for the strength of Lewis acids and bases with the evaluation of their softness. Journal of Inorganic and Nuclear Chemistry, 29(11), 2685–2691. https://doi.org/10.1016/0022-1902(67)80006-X
Mizutani, K., Fisher-Power, L. M., Shi, Z., & Cheng, T. (2017). Cu and Zn adsorption to a terrestrial sediment: Influence of solid-to-solution ratio. Chemosphere, 175, 341–349. https://doi.org/10.1016/j.chemosphere.2017.02.069
Musso, T. B., Parolo, M. E., Pettinari, G., & Francisca, F. M. (2014). Cu(II) and Zn(II) adsorption capacity of three different clay liner materials. Journal of Environmental Management, 146, 50–58. https://doi.org/10.1016/j.jenvman.2014.07.026
Nevidomskaya, D., Minkina, T., Soldatov, A., Motuzova, G., & Podkovyrina, Yu. (2014). Usage of X-ray absorption spectroscopy and extractive fractionation in studies of the Cu (II) and Zn (II) ions in soils. Eurasian Soil Science., 3(4), 238–324.
Nevidomskaya, D. G., Minkina, T. M., Soldatov, A. V., Shuvaeva, V. A., Zubavichus, Ya. V., Podkovyrina , Yu.S. . (2016). Comprehensive study of Pb (II) speciation in soil by X-ray absorption spectroscopy (XANES and EXAFS) and sequential fractionation. Journal of Soils and Sediments, 16, 1183–1192. https://doi.org/10.1007/s11368-015-1198-z
Nriagu, J. (2019). Zinc toxicity in humans Encyclopedia of Environmental Health (2nd ed.). Amsterdam: Elsevier.
Panin, M. S., & Siromlya, T. I. (2005). Soil Chemistry-adsorption of copper by soils of the Irtysh river region. Semipalatinsk Oblast. Eurasian Soil Science, 38(4), 364–373.
Pinskii, D. L., Minkina, T. M., Bauer, T. V., Nevidomskaya, D. G., Mandzhieva, S. S., & Burachevskaya, M. V. (2018). Copper adsorption by chernozem soils and parent rocks in southern Russia. Geochemistry International, 56(3), 266–275. https://doi.org/10.1134/S0016702918030072
Pinskii, D. L., Minkina, T. M., & Gaponova, Y. I. (2010). Comparative analysis of mono-and polyelement adsorption of copper, lead, and zinc by an ordinary chernozem from nitrate and acetate solutions. Eurasian Soil Science, 43(7), 748–756. https://doi.org/10.1134/S1064229310070045
Pinskii, D. L., Minkina, T. M., Mandzhieva, S. S., Fedorov, Y. A., Bauer, T. V., & Nevidomskaya, D. G. (2014). Adsorption features of Cu (II), Pb (II), and Zn (II) by an ordinary chernozem from nitrate, chloride, acetate, and sulfate solutions. Eurasian Soil Science, 47(1), 10–17. https://doi.org/10.1134/S1064229313110069
Pirooty, S., & Ghasemzadeh, M. (2013). Toxic effects of Lead on different organs of the human body. KAUMS Journal (FEYZ), 16(7), 761–762.
Plyaskina, O. V., & Ladonin, D. V. (2005). Compounds of heavy metals in granulometric fractions of certain soil types. Moscow University Soil Science Bulletin, 4, 36–43. ((in Russian)).
Ponizovskii, A. A., & Mironenko, E. V. (2001). Mechanisms of lead (II) sorption in soils. Eurasian Soil Science, 34, 371–381.
Radhakrishnan, K., Sethuraman, L., Panjanathan, R., Natarajan, A., Solaiappan, V., & Thilagaraj, W. R. (2016). Biosorption of heavy metals from actual electroplating wastewater using encapsulated Moringa oleifera beads in fixed bed column. Desalination and Water Treatment, 57(8), 3572–3587. https://doi.org/10.1080/19443994.2014.985725
Saha, U. K., Taniguchi, S., & Sakurai, K. (2002). Simultaneous adsorption of cadmium, zinc, and lead on hydroxyaluminum-and hydroxyaluminosilicate-montmorillonite complexes. Soil Science Society of America Journal, 66(1), 117–128. https://doi.org/10.2136/sssaj2002.1170
Sillen, L. G., & Martell, A. E. (1970). Stability constants of metal-ion complexes. London: The Royal Society of Chemistry.
Sipos, P., Németh, T., Kis, V. K., & Mohai, I. (2008). Sorption of copper, zinc and lead on soil mineral phases. Chemosphere, 73(4), 461–469. https://doi.org/10.1016/j.chemosphere.2008.06.046
Sparks, D. L. (2001). Elucidating the fundamental chemistry of soils: past and recent achievements and future frontiers. Geoderma, 100(3–4), 303–319. https://doi.org/10.1016/S0016-7061(01)00026-X
Sposito, G. (1984). The surface chemistry of soils. New York: Oxford University Press.
Sposito, G. (1989). The chemistry of soils. New York: Oxford University Press.
Vidal, M., Santos, M. J., Abrão, T., Rodríguez, J., & Rigol, A. (2009). Modeling competitive metal sorption in a mineral soil. Geoderma, 149(3–4), 189–198. https://doi.org/10.1016/j.geoderma.2008.11.040
Acknowledgements
The Russian Foundation of Basic Research funded this investigation (Projects No. 19-34-60041 and 19-29-05265).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bauer, T.V., Pinskii, D.L., Minkina, T.M. et al. Application of XAFS and XRD methods for describing the copper and zinc adsorption characteristics in hydromorphic soils. Environ Geochem Health 44, 335–347 (2022). https://doi.org/10.1007/s10653-020-00773-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00773-2