Abstract
This article aims to draw an overview on the actual knowledge on bacteriostatic and bactericidal natural clays. Particular emphasis is given to the role of clay itself, the action of reduced metals located either in the structure of clay minerals or external to them as constituents of associate minerals, and the definition of the mechanisms of action based on the achievements found in all available studies being carried out so far. The term bactericidal is herein used when a clay or a clay mineral kill the bacteria, whereas the term bacteriostatic is used when those minerals stop bacteria growth and replication. The second part of this article deals with experimental studies on bactericidal natural clay, experience and perspective for the preparation of bactericidal natural clays, interesting on the authors perspective and experience for the preparation of pathogens safe both therapeutic and cosmetic natural mud/natural peloid, and better yet of both therapeutic 87oooand cosmetic peloid itself and designed and engineered peloid. The authors also show how to convert non-antimicrobial clay into antimicrobial one, opening the way in the field of pelotherapy to the preparation of sanitary safe peloids addressed, for instance, to the treatment of rheumatic disabilities, as well as to the preparation of antimicrobial peloids and, in particular, of dermatological ointments, all able to fight infectious skin disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the beginning of the present century, experience has shown that some types of clay can exhibit either bacteriostatic or bactericidal activity. Such action had rise high expectations when both public health and science are becoming more and more apprehensive and engaged relatively to the increasing resistance of bacteria to antibiotics, which are traditionally organic molecules, such as tetracycline and minocycline, which can inhibit the replication of bacteria DNA, of both Gram-positive and Gram-negative bacteria.
Clay is one of the oldest natural earth materials used by man for healing purposes in traditional medicine. Clays continues to be applied in modern life for the treatment of various topical and internal ailments (Carretero 2002; Carretero et al. 2006, 2013; Carretero and Pozo 2007; Veniale et al. 2007; Gomes and Silva 2007; Ferrell 2008; Rautureau et al. 2010, 2017; Young 2011; Gomes et al. 2013; Williams and Hillier 2014; Ghadiri et al. 2015; Moraes et al. 2017; Awad et al. 2017; Williams 2017; Gomes 2018; Viseras et al. 2019).
Brunet de Courssou (2002), Wilson (2003), Williams et al. (2004), Hu et al. (2005) and Tong et al. (2005) had published the first reports describing the antibacterial activity of some natural clays.
Microbial infection has been a constant presence throughout human history. Former human societies have used some natural compounds (e.g., garlic extracts) to heal health conditions actually recognized as being microbial infections. Within other natural antimicrobial agents, essential oils derived from plants, enzymes obtained from animal sources, and organic acids (e.g., sorbic, propionic and citric) deserve to be enhanced. In the case of essential oils, their activity could be attributed to the presence of hydrophilic functional groups, such as hydroxyl groups of phenolic components and/or the lipophilicity of some components. Usually, the compounds with phenolic groups as oils of clove, oregano, rosemary and vanillin are the most effective (Lucera et al. 2012).
Clay bactericidal character does not exist if clay is in the dry state. In fact the bactericidal action only exist in hydrated clay, for instance, in a suitable paste or poultice state. In addition, not every clay can exhibit bactericidal activity; in fact, only few clays can show such activity, although being only effective against specific bacteria.
The increasing resistance of bacteria to antibiotics is due to the worldwide misuse of them. A recent UK government report predicted that antimicrobial resistance is likely to overtake cancer as the leading cause of death over the next 30 years (O’Neill 2016).
As aforesaid, only few and certain specific clays exhibit bacteriostatic and bactericidal properties, for instance, clays showing in their compositions the clay minerals illite and smectite that bear ferrous iron in the respective structures, and clays bearing one or more than one of the following ferrous iron-rich associated phases, such as pyrite (FeS2), marcasite (FeS2) magnetite (FeFe2O4), pyrrhotite (Fe1−x S where x = 0–0.2) and goethite (FeO(OH)). These substances can inhibit microorganism’s growth acting as bacteriostatic agents or can disrupt bacteria cell membrane acting as bactericidal agents.
Also, besides reduced Fe, other reduced species of certain metals/ions, such as Ag, Cu, Zn and Au, when associated with certain clays or clay minerals, can act as bacteriostatic/bactericidal agents (Kostyniak et al. 2003; Sawai 2003; Top and Ulku 2004).
The clay-based natural or synthetic bactericidal compositions are used to the treatment of bacterially caused skin infections and skin diseases. According to McInnes (2012), chronic wound infections are a current and emerging clinical concern, particularly with the escalation in cases of diabetes and associated vascular impairment to extremities. Post-surgery wounds and ulcers acquired in Hospitals are also matter of concern. In addition, James et al. (2008), state that around 60% of chronic skin infections harbor bacterial biofilms that impede healing due to their intrinsic resistance to immune responses, antibiotics and disinfectants. The elimination of such bacterial biofilms must occur to make effective the application of antibiotics, and the previously mentioned bactericidal compositions clay-based, under the form of consistent pastes consisting of clay/water/reducing agent, could fight bacteria growth. Other suitable liquid or reducing agent could replace water, either enters in the clay composition as a chemical element of a fundamental clay mineral, or as a natural mineral component associated with the clay. The reducing agent could be a constituent, for instance, Fe2+ of the synthetic salt FeSO4.
Antimicrobial materials have the ability of inhibiting the growth or even killing certain types of microorganisms. The search for products having antimicrobial properties has gained particular importance in various applications, such as in raw materials for cosmetics and pharmaceuticals, hospital and veterinary products, and food manufacture and animal feeding, among others. One of these most interesting products is clay, either in natural state or after undergoing appropriate modifications, because experience points out for its effective role.
The clay antimicrobial activity does not exist if clay is in the dry state. In fact, only when clay is in the hydrated state, microbicidal action could exist. Such is an evidence of the fundamental role of the liquid phase associated with natural clay or to manipulated clay performed either in industry or in laboratory. Experience shows that in antimicrobial clay it is the liquid phase associated with the solid phase, either as it occurs in nature, or after undergoing appropriate modification and control in the laboratory, that is the major factor responsible for the bacteriostatic/bactericidal activity. The liquid phase bearing in solution de active agent (for instance, a reduced metal), and pH and Eh, are the determinant factors of the microbicidal action.
Many types of natural clay are unable to reduce bacterial populations, and they may either be innocuous or actually enhance bacterial growth. Otherwise, very few natural clays exhibit antimicrobial activity, and in some specific cases, they have to undergo laboratory treatments in order to get free and into solution the reducing agents that may contain.
Also, it has been found out that clay bactericidal character just is effective if it is in a reducing state and contains reducing agents, for example, reduced iron. Such microbicidal action has created high expectations when both public health and science are becoming more and more apprehensive and engaged relatively the increasing resistance of bacteria to antibiotics.
A significant number of scientific papers exist describing and investigating the mechanisms of action of the so-called “killer clays,” which can be a possible new answer to “superbug” infections. “Superbugs” are pathogens or disease caused by resistant microorganisms to multiple antibiotics, and such antibiotic resistance is presently a matter of major Public Health concern, since the resistance of the “superbug” to the action of multiple antibiotics has very negative consequences in Public Health (Diekema et al. 2004; Arias and Murray 2009). One example of “superbug” is the methicillin-resistant Staphylococcus aureus (MRSA). “This serious threat is no longer a prediction for the future” states a World Health Organization (WHO) report. “It’s happening right now in every region of the world and has the potential to affect anyone, of any age, in any country.”
According to a very recent report of WHO published in April 2019, the indiscriminate use of antibiotics and the diseases resistant to them are responsible for at least the death of 700,000 people each year, worldwide.
In Europe, a recent study carried out by the “European Centre for Disease Prevention and Control (ECDC)” report that every year 33,000 people die due to antibiotic-resistant bacteria, most of the deaths happening in Hospitals (the worst scenarios occurring in Italy, Greece, Romania, and Portugal). In the case of Portugal, the study estimates, in 2015, 24,021 infections and 1158 deaths. According to OCDE, up to 2050 the number of infections attributed to “superbug” could kill 2.4 millions of individuals in Europe, North America and Australia. In Portugal, 49,443 deaths are estimated.
The infections attributed to “superbug” are a real menace to modern Health Systems. However, such menace is not limited to Hospitals. The livestock industry has a central responsibility for the fact that antibiotics are becoming ineffective. Data show that 70% of animal farming in the European Union uses antibiotics (EU). For this reason, it is necessary to restrict the administration of antibiotics in the sector. Animals treated with antibiotics may end up being carriers of bacteria resistant to them. These antibiotics are transmitted to the plants through the manure used as fertilizer, and the produced foods—meat and vegetables—can pass to the humans.
The EU wants all antibiotics to be exclusively for human use, and there may be legislation preventing the use of antibiotics in a preventive way in animals for human consumption. Since 2006, the EU has banned the use of antibiotics to stimulate the growth of food industry animals.
In November 2018, the scientific journal “The Lancet Infectious Diseases” published the results of a study involving the twenty-eight EU countries plus Norway, Iceland and Liechtenstein, reporting the deaths and damage caused in people that make use of the Public Health Services of those countries due to “superbug.”
Data provided by the European Antimicrobian Resistance Surveillance-Net (EARS-Net), in 2015, assessed the contribution of infection types (bloodstream; urinary tract; respiratory tract; surgery sites; and other infections). The obtained results indicate 672,000 infections/year, which end in 33,110 deaths and in the loss of 875,000 days of healthy life due to incapacities.
Mortality and incapacity affect more babies less than 1 year old and people older than sixty-five. The worse situations occur in Italy, Greece, Romania and Portugal. In Portugal, the bacteria MRSA (Staphylococcus aureus) resistant to methicillin, Klebsiella pneumonia and Escherichia coli, the last two being resistant to various antibiotics of broad spectrum, such as quinolones, cefalosporins and carbapenems, are the most resistant to antibiotics.
The real menace of pathogens associated with human infection justifies the recent research being carried out with great expectation and apparent success of the “predatory therapy” that involves the use “in vivo” of innocuous “good bacteria,” specifically strains of Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus to kill “bad bacteria” of the Gram-negative group (Dashiff et al. 2011; Harini et al. 2013).
Background on bacteriostatic and bactericidal clays
Clay minerals as efficient bactericides
Specific clays and clay minerals have proved to be valuable in the treatment of bacterial diseases, including infections for which there are no effective antibiotics, such as Buruli ulcer disease caused by Mycobacterium ulcerans and multi-drug resistant infections.
The first indications of the bactericidal character of certain clays arose when the French humanitarian and philanthropist Line Brunet de Courssou who ran a Clinic in the Ivory Coast found that a certain type of clay of the so-called “French Green Clay” type imported from his native country was an efficient bactericide. Such type of clay being supplied by the French enterprises Argicur and Argiletz was very effective in combating Buruli ulcer that is a very common infectious disease in tropical regions—caused by the bacterium Mycobacterium ulcerans expressed by the so-called “flesh eating,” i.e., the necrosis of the skin tissues of both arms and legs. Mycobacterium ulcerans produces a lipid toxin, mycolactone, which destroys the fatty tissues under the skin (George et al. 1998, 2002).
The healing capacities of two apparently similar “French Green Clay” (Ref. CsAr02 and CsAg02) were assessed, but only the extended application of the clay CsAg02 caused tissue regeneration and wound healing. Williams et al. (2004) and Haydel et al. (2008) validated after the Brunet de Courssou observations.
Mycobacterium ulcerans is the third most common mycobacterial infection after tuberculosis and leprosy (Sizaire et al. 2006). MacCallum has isolated this bacterium in the Bairnsdale region (Australia). Albert Cook first described the disease, endemic in countries of Central and West Africa, in Buruli´s patients (Uganda). This disease causes skin loss, deformities and disabilities.
Earlier in France, Brunet de Courssou had the experience of the successful use of green clay (in paste form after being mixed with water) applied topically in the treatment of wounds and insect bites. Hence, Brunet de Courssou had imported the same type of clay for use in the Ivory Coast clinic.
In 2002, Brunet de Courssou used two clays, “iron-rich illite/smectite clays” known as “French Green Clays” mixed with water and margarine. The paste was applied topically to fifty patients, and the observed benefits were documented. The clay paste applied onto the ulcerated sites was removed, the wound cleansed, and the application renewed at least once daily.
The achieved results, presented in 2002 to a panel of Buruli ulcer specialists at the WHO, in the Geneva’s headquarters, impressed the experts that decided to require additional research in order to justify the curative effects before funding for further research.
In the meantime, Brunet Courssou dies, but his son Thierry continued the interest on this topic of research. Thierry and the geochemist Lynda Williams of Arizona State University applied for research support to the Clay Mineral Society. For this purpose, Lynda Williams counted with the collaboration of the microbiologist Shelley Haydel of the same Institution.
The bactericidal abilities of the above two clays were tested against five bacterial species, Mycobacterium marinum (very similar to Mycobacterium ulcerans) and antibiotic-resistant Staphylococcus aureus strains). The behavior of the two clays was different: one showed no effect on the bacteria, and in some trials even promoted its growth; the other inhibited completely the growth of bacteria and, to a significant extent, reduced the number of bacterial cells in cultural tests, behaving like the action of a broad-spectrum antibiotic. The investigations carried out by Lynda Williams and Shelley Haydel involving other scientists besides were published.
Williams et al. (2008) reported the results of detailed mineralogical and geochemical studies of the two previously mentioned clays in order to determine the factors justifying their curative abilities when applied topically as poultices to patients infected with Mycobacterium ulcerans. Although the authors suggest additional research, they favor a chemical mechanism for the bactericidal action of one of the clays (the pH and the oxidation state of the solution surrounding the nanometric clay particles would generate conditions that inhibit the viability of pathogenic bacteria) relatively to a physical mechanism (because the clay did not penetrate the cells of the bacteria).
Recent advances in clay research point out that some specific clays have microbicidal activity in particular for several species of pathogenic bacteria. However, the identification of their active components and action mechanisms still is an endeavor in progress (Williams et al. 2004; Ma’or et al. 2006; Haydel et al. 2008; Williams et al. 2008, 2009; Cunningham et al. 2010; Masurat et al. 2010; Williams and Haydel 2010; Williams et al. 2011; Otto and Haydel 2013a, b; Williams and Hillier 2014; Londono and Williams 2015; Behroozian et al. 2016; Morrison et al. 2016; Kalinowski et al. 2016; Panko et al. 2016; Williams 2017; Londono and Williams 2015; Londono et al. 2017; Svensson et al. 2017 and Wang et al. 2017).
For example, Williams et al. (2008) reported the results of detailed studies on the mineralogy and geochemistry of two clays in order to define the factors justifying their curative abilities when applied topically as poultices to patients infected with Mycobacterium ulcerans. Although more research was considered necessary, for the authors, the mechanism of bactericidal action of one of the clays would not be physical (because the clay did not penetrate the cells of the bacteria) but rather chemical (the pH and the oxidation state of the solution surrounding the manometric clay particles would generate conditions that inhibit the viability of pathogenic bacteria).
Also, Haydel et al. (2008) reported the results of tests performed on the evaluation of the bactericidal action of two iron-rich French clays used by Brunet Courssou in the treatment of Buruli ulcer patients on various Gram-negative and Gram-positive bacteria and, of course, on mycobacteria. The results demonstrate that the clay with reference CsAg02 had bactericidal activity against pathogenic E. coli, extended-spectrum b-lactamase (ESBL) E. coli, Pseudomonas aeruginosa, Mycobacterium marinum, and a combined bacteriostatic/bactericidal effect against S. aureus, penicillin-resistant S. aureus (PRSA), methicillin-resistant S. aureus and Mycobacterium smegmatis. However, it has also been demonstrated that other clay with reference CsAr02 having chemical and physical properties similar to those exhibited by CsAg02 is characterized by, either less antibacterial activity, or just bacteriostatic activity, relatively to the same strains. The authors had also showed that relevant changes in clay mineral structures due to dehydration, and at least partial dehydroxylation caused by heating at 550 °C, do not affect both antibacterial and bacteriostatic activities.
Zeev et al. (2006) studied a black mud from Dead Sea (sulfide-rich, low pH, hypersaline) and found mud antimicrobial properties against E. coli, S. aureus, C. albicans, and P. acnes. These worldwide-marketed mud packs are used for therapeutic (rheumatic disorders) and cosmetic purposes (mud masks to treat skin affections, particularly acne). For the authors, the antimicrobial activity may be owing to mud high salt concentration combined with its special ionic composition.
Bacteria communities are common in soils, and naturally in clays, and the bacterial diversity is higher for neutral pH than for acid or basic pH values (Flerer and Jackson 2006).
Mineralogical-identical clays can exhibit chemical variability, which correlates with variability in antibacterial activity. Actually, clay chemical composition appears to be more bactericidal effective than clay mineralogical composition. The bioavailability of metals to bacteria depends on the aqueous metal speciation in the clay poultice. The pH and oxidation state of the water added to the clay to make a poultice are important factors of the bactericidal action.
Williams et al. (2011) studied clays from deposits in other countries and established the link between bactericidal character and clays derived from the hydrothermal alteration of volcaniclastic materials, or rather pyroclastic ones. The “Oregon blue clay” from Grants Pass (Oregon) shows that illite–smectite (49.6% wt) and chlorite 3.1% are the dominant clay minerals and pyrite (8.2%) is the main non-clay mineral present. The study shows also that the bactericidal effect never occurs when the clay is in the dry state.
Geochemical and microbiological studies carried out on the “Oregon blue clay” showed their bactericidal effectiveness when hydrated and incubated with bacteria providing the total elimination of Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhimurium and also antibiotic-resistant extended-spectrum beta lactamase (ESBL) within 24 h for E. coli and Methicillin Resistant Staphylococcus aureus (MRSA).
Williams et al. (2011) link this effectiveness to chemical reactions enabling the disruption of a certain physiological function of the bacteria. According to the same authors, the clays considered bactericidal have in common the following properties, to which supplementary data provided by other authors are added:
-
1.
Green or blue color is common to all bactericidal natural clays;
-
2.
Bacterial natural clays are, as a rule, of volcanogenic hydrothermal origin, but the bactericidal character of blue/green clays of other origins had also been identified (Londono and Williams 2016; Londono et al. 2017);
-
3.
Bactericidal natural clays contain nanocrystals (< 200 nm) of illite and smectite able to provide high surface area and availability of reactive surfaces for biogeochemical interactions;
-
4.
Ferrous iron (Fe2+) and other transition metals, such as Co2+, Cu2+, Ni2+ and Zn2+ are present in all bactericidal natural clays;
-
5.
Bactericidal clays are soluble under extreme conditions of pH (< 4 or > 10) so that in low oxidation environments many metals are soluble; the oxidation of the transition metals increases the Eh of the suspension or pulp to values > 400 mV leading to the oxidation of bacteria; for pH values close to neutrality, ferrous iron and PTE’s are not soluble (Williams et al. 2008, 2011; Morrison et al. 2016); however, for Wang et al. (2017), clay bactericidal activity could be achieved at near pH = 6;
-
6.
Soluble Fe2+ is a critical antibacterial agent in natural bactericidal clays (Williams et al. 2011; Morrison et al. 2016);
-
7.
In dry environment, the bactericidal clays do not show antibacterial effect; the bactericidal mechanism involves always the presence of solutions and chemical reactions that affect cell membranes or metabolic functions;
-
8.
Clay leachate is more antibacterial effective than moist clay; clay leachate is initially antibacterial but lose its effect as oxidation affects the solution; the transition metals in leachate result, either from the solubility of the clay, or from the solubility of minerals associated therewith (for example, iron sulfides, as in the case of pyrite, FeS2);
-
9.
Clay serves to keep the system moist; the water is essential to keep in solution the potentially active reducing agent in order to provide its bioavailability and bio-reactivity;
-
10.
Previous clay autoclaving, even clay minerals dehydroxylation at high temperature happens by heating at 550 °C, does not affect antibacterial activity (Haydel et al. 2008), indicating that organic matter and hydroxyls are not associated with antibacterial activity.
Smectitic clays rich in montmorillonite are the most used in the preparation of antibacterial materials. This use is due to the unique and relevant properties of this clay mineral: (a) nanometric particle size; (b) high specific surface area (both external and internal); (c) high sorption capacity; (d) negative surface electric charge; (e) high cation exchange capacity of both inorganic and organic cations; (f) high catalytic activity; and (g) low or null toxicity.
Also it was recognized that certain metallic elements, such as Ag, Cd, Cr, Cu, Pb, Sn and Zn, and also the alkylammonium cations, especially those of quaternary amines, used as both skin antiseptics and disinfectants have antibacterial properties. Metal-montmorillonite and alkylammonium-montmorillonite complexes can easily be prepared. In the literature, it is possible to find some examples of the antibacterial properties in such compositions. Some authors tested with success the antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis (Ye et al. 2003; Tong et al. 2005; Hu and Xia 2006). Also, Morones et al. (2005) showed the bactericidal effect of Ag nanoparticles.
Within the inorganic bacteriostatic and bactericidal agents, some of them deserve particular emphasis: (a) metals/ions (Ag, Au, Cu and Zn); (b) metal oxides (CuO, FeO, MgO, TiO2 and ZnO); (c) certain clays. These substances can inhibit microorganism’s growth acting as bacteriostatic agents or by disrupting bacteria, cell membranes can act as bactericidal agents.
Magana and Quintana (2008) demonstrated the antibacterial activity of montmorillonite modified or loaded with Ag+ by ion exchange against E. coli. Metallic ion exchange montmorillonite, as is the case of Ag+-montmorillonite dispersed in water that can attract and adsorb the negatively charged bacteria, hence facilitating and promoting the antibacterial activity.
Williams et al. (2011) performed experiments involving the addition to E. coli cultures to aqueous leachate of the bactericidal clay (EDTA-oxalate solution extracted the surface fixed metals). The comparison between the results of chemical analyses of the E. coli population killed with aqueous leachate and the results of chemical analyses of E. coli from the control population showed that both Fe and P intracellular concentrations are lower in the control population. The P incorporation by the bacterium cells supports the idea of the regulatory role of polyphosphate or phospholipids in the control of Fe2+.
The Fenton reaction can cause degradation of critical cell components, but it shown that extracellular processes do not cause the death of cells. On the contrary, Fe2+ overcome the regulatory proteins on the outside of the membranes and oxidized as soon as it enters the cells it precipitates as Fe3+ producing lethal OH− radicals. This interpretation explains the bactericidal action of the Fe2+ bearing clay.
Several authors demonstrated that the primary in vitro antibacterial mechanism of action of clays is much dependent upon soluble metal ions, which are desorbed from the surfaces of clay mineral particles when hydrated (Cunningham et al. 2010; Otto and Haydel 2013a, b; Otto et al. 2014). Cunningham et al. (2010) consider that the effectiveness of the antibacterial activity depends much on the low pH environment required for metal ions speciation and bioavailability. Therefore, the antibacterial activity of the used clay-based products may be infeasible in topical applications because they can cause damage to tissues, since the pH of the treated tissues is significantly higher than the pH of the clay-based paste or clay-based suspension. Such conditions justify the reason why the authors propose in vivo studies.
Parolo et al. (2011) showed that Ag+ and quaternary ammonium surfactant-modified montmorillonite samples used as skin antiseptics and disinfectants exhibit suitable inhibition properties over E. coli growth, whereas samples of natural montmorillonite do not exhibit antibacterial activity.
Otto and Haydel (2013a, b) in a research paper entitled “Exchangeable ions are responsible for the in vitro antibacterial properties of natural clay mixtures” presented the results of four natural clay samples collected at the same geologic site. These samples exhibit in vitro antibacterial activity against a broad spectrum of bacterial pathogens, in particular Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA). Irregularly interstratified illite-montmorillonite, montmorillonite and kaolinite were the clay mineral species identified. The raw clay and the clay/water leachates present some metals such as Co, Cu, Fe, Ni and Zn. In the leachates, the metals fixed on clay mineral surfaces would be released and acting as microbicides. In addition, these leachates could be supplemented with chloride salts of Co, Cu, Fe, Ni and Zn. The authors have analyzed the leachates or extracts of clay/water mixtures obtained in acidic conditions and concluded that: (a) Cu2+, Fe2+ and Zn2+ concentrations in solution were favored by low pH (3–4.5) and responsible for the bactericidal activity; and (b) the killing activity could not be solely attributed to pH.
Morrison et al. (2013) studied the mineralogical variables that control the antibacterial activity of “Oregon blue clay” in the case of pathogens Escherichia coli and Staphylococcus epidermidis after incubated with clays collected either in both reduced and oxidized zones of the hydrothermal deposit. Based on XRD and elemental analyses, the authors concluded that only the clay samples from the reduced zones (blue zones), particularly in sites where mixed-layered illite–smectite (sometimes called rectorite in case of regular inter-stratification) and pyrite are present could exhibit bactericidal character. Clay samples from the oxidized zones (red or yellow zones) do not show bactericidal activity. However, the clay samples from white zones, i.e., without pyrite, show bactericidal activity, but not so effective when compared with samples from the blue zones. Using transmission electron microscopy (TEM), the authors could follow the behavior of the bacteria cell along the incubation process, and they could found that no cell lysis did occur. The experimental work indicates that antibacterial effectiveness correlates with high Fe2+, Fe3+ and Al3+ concentrations in the clay aqueous leachates. All antibacterial clay samples contain Fe2+, buffered solutions to pH (2.5–3.1), and oxidizing Eh (630–706 mV) conditions.
Gaskell and Hamilton (2014) elaborated an interesting overview showing the manipulation clay minerals properties to facilitate the treatment of infected wounds. The authors present evidences of the antimicrobial and healing effects of some natural clay minerals as well as a range of modifications including metal-ion exchange, the formation of clay-drug composites and the development of various polymer–clay systems.
Londono and Williams (2015) evaluated the antibacterial action of the lacustrine clay from the Colombian Amazon (AMZ) enriched in smectite and halloysite clay minerals. In this study, the authors compared the chemical composition of both Escherichia coli and AMZ clay after their inter-reaction and concluded that E. coli had adsorbed and increased the contents of the metals Al, Cu, Fe, Ni, P and Zn, mainly Al3+, extracted from clay dissolution under acidic (pH = 4.5) conditions. The authors considered that Al3+ whose ionic radius is significantly smaller than that of Fe2+ could replace Ca2+ and Mg2+ in the bacteria membrane. Also, due to Al3+ chemical affinity with phosphate ligands it could modify lipid–protein interactions when it is bound to phospholipids (Garcidueñas-Pina and Cervantes 1995). Al3+ could also interfere with the membrane electrical potential inhibiting membrane transport proteins (Xu et al. 2012). In acidic conditions, the displacement of membrane cations by H+ could compromise membrane integrity leading to cytoplasm leakage.
Morrison et al. (2016) showed that in the clay deposit located in Oregon Cascade Mountains, near Crater Lake (USA), marketed by OMT (Oregon Mineral Technologies), the deposit presents along the fault zone several clay blue zones derived from volcanogenic hydrothermal alteration of andesite porphyry and volcaniclastic rocks. This clay is able to destroy a broad range of human bacterial pathogens, including antibiotic-resistant strains, through the synergistic actions of Fe and Al. Aluminum appears to enhance Fe toxicity promoting changes and damage on the structure of bacterial membranes increasing their permeability and oxidation (Zatta et al. 2002).
Morrison et al. (2017) disclosed detailed geological, mineralogical, geochemical and microbiological data of OMT clay deposit. The authors studied and discussed the correlations between mineralogy and chemistry and the antibacterial activity of specific clays from the studied deposit. The bactericidal activity was only associated with pyrite-bearing clays characterized by low pH (< 4.2) and Eh > 600 mV. These factors determine, after clay rehydration in de-ionized water, the driving of mineral dissolution and metal hydrolysis to produce Fe2+ and Al3+ and hydroxyl radicals, which are essential to kill bacteria. Both pH and Eh values of hydrated clay are important for stabilizing the aqueous reactants. However, pyrite-bearing clays with pH values within 4.2–4.7 and Eh values > 400 mV, only promotes bacteria growth inhibition.
Caflisch et al. (2018) demonstrated that the OMT blue clay exhibited bactericidal activity against a significant range of human pathogens in both biofilm and planktonic states.
Wang et al. (2017) made an attempt to understand and explain the action mechanism of antibacterial clays bearing reduced agents (particularly Fe2+) in illite and smectite groups of clay minerals including illite, montmorillonite, nontronite and rectorite, the so-called reduced iron-containing clays (RIC). The authors have discussed the bactericidal activity against Pseudomonas aeruginosa and Escherichia coli and RIC. Soluble Fe2+ was been considered as a critical antibacterial agent in natural antibacterial clay minerals. The structural Fe2+, which produces lethal hydroxyl radicals (OH), at near neutral pH and other reactive oxygen species (ROS) upon its oxidation in air, are critical too. The importance of ROS in attacking cell membrane, intercellular penetration of soluble Fe2+ and its subsequent oxidation to produce OH that damage intracellular proteins was found.
Londono and Williams (2015), Londono et al. (2017) had find out an association between antibacterial action against Escherichia coli of AMZ clay and free Al, since only Al derived from the clay exceeded the minimum inhibitory concentrations for E. coli under acidic conditions. Besides Al, the clay chemical analysis has identified P, Cu, Fe, Mn and Zn. Ion imaging tests showed elevated Al levels in the bacterial membrane and high intracellular Fe levels, comparatively to those found on untreated controls.
Williams (2017) presented a very interesting review that updates all available information on antibacterial clays, aiming to understand the factors and mechanisms of action involved in the elimination of human pathogenic microorganisms. It has recognized that pH and Eh, i.e., the clay/water paste oxidation potential, are determining factors for the identification of clay antibacterial potential.
The bactericidal character of certain clays was also recognized by Behroozian et al. (2016), after investigating the antibacterial activity of “Kisameet clay” occurring in a glacial clay deposit of Quaternary age, located 450 km to northwest of Vancouver—British Columbia, Canada. This clay shows a dark-greenish-gray color, which becomes light gray when dried.
The ESKAPE group of pathogenic bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species) identified in Kisameet clay is responsible for the majority of hospital infections caused by bacteria which are becoming increasingly resistant to antibiotics. Curiously, the name ESKAPE, composed of the initials of the names of the mentioned bacteria, is homophone of the English term “escape” that means to escape to the activity of all the antimicrobial agents available. For hundreds of years and empirically, the excellent “Kisameet clay” therapeutic properties were appointed in the treatment of diseases, such as ulcerative colitis, duodenal ulcer, arthritis, phlebitis and burns (Hauser 1950, 1952). In vitro experiments performed by Behroozian et al. (2016) showed that the antimicrobial activity of Kisameet clay is clearly significant in the case of the ESKAPE group bacteria.
Svensson et al. (2017), who previously had shown the effective activity of “Kisameet clay” against a variety of antibiotic-resistant bacteria, had studied the geochemistry and the microbiome of this clay. Surprisingly, the authors found in the clay's microbiome the presence of a great diversity of resident bacteria that could act as bio-control agents or as producers of novel bioactive compounds.
In fact, “Kisameet clay” being relatively rich in Fe presents a surprising number of different bacterial species represented by the following phyla: Proteobacteria (the most represented), Bacteroidetes, Actinobacteria, Acidobacteria and Firmicutes. Based on these studies, the possibility that particular bacterial species of the resident bacterial communities could contribute, through the genetic interaction between microbes, to their inhibitory properties should not be discarded.
Panko et al. (2016) investigated the physicochemical and microbiological properties of Black Sea mud/peloid. The authors found out that the mud/peloid microbiocenosis has the ability for self-purification and regeneration of its composition. The high content of nitrifying and iron-oxidizing aerobic bacteria justifies the antimicrobial effect for E. coli incubated in the mud suspension with an infective dose of 106 CFU/cm3.
Adusumilli and Haydel (2016) evaluated the in vitro antibacterial activity of a panel of clay mixtures and their derivative leachates against M. ulcerans. The authors also assessed the in vivo efficacy of topically applied hydrated clays on Buruli ulcer progression in mice infected with M. ulcerans. The research had revealed that specific clays exhibit in vitro bactericidal activity against Gram-negative and Gram-positive pathogens, and exhibit in vitro bactericidal activity against M. ulcerans. The results point out that hydrated clay poultices may offer a complementary and integrative strategy for topically treating Buruli ulcer disease.
Recently, Zarate-Reyes et al. (2017a, b) reported the compositional factors associated with the antimicrobial activity of a clayey mineral (Fe2+-saponite) sampled in Brunnenberg (Germany). The authors showed that this clay was active against Gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa, Salmonella and Klebsiella pneumonia, but it was not active against Gram-positive bacteria. Unlike Gram-positive bacteria, Gram-negative bacteria have an external membrane reason why their cell walls are less porous and receptive to solutes, including antibiotics.
Clay minerals as efficient carriers of antibacterial metals and drugs
In the last decades, studies recognized that clay minerals act as efficient carriers of active drug molecules, aiming towards applications in the medical industry. In fact, clay minerals or modified clay minerals can be used as carriers of drugs including antibacterial drugs.
Very recently, Viseras et al. (2019) produced a review of recent research on the use of clay minerals in advanced skin drug delivery systems, which for the authors represent an attractive alternative to the drug oral pathway. Clay minerals due to their singular properties are ideal candidates for the development of intelligent skin drug delivery systems. The authors after a brief introduction of skin anatomy and physiology and biopharmaceutical features of drug penetration through the skin layers present a review the use of clay minerals in the skin-addressed pharmaceutical field. Clay minerals-bearing medicines clay may be administered on/into/through the skin.
Cerri et al. (2004) showed the use of zeolites in biomedical applications, and presented an example of the use of zeolite as carrier for antibiotics in anti-acne topical therapy.
Holešová et al. (2016) studied the antimicrobial activity of kaolinite and of kaolinite nanocomposites against bacteria strains of Staphylococcus aureus, Escherichia coli and Candida albicans, and the authors found out that particularly the nanocomposites carrying chlorhexidine dihydrochloride as an antimicrobial agent were very effective.
The development of suitable materials with the ability to inhibit the growth of microbes is one of the current topics of medical research. For instance, diseases that affect the oral cavity such as oral candidiasis and periodontal diseases are a significant public health problem. A solution might be offered by anchoring the drug to a suitable carrier (e.g., the clay mineral halloysite) that can provide transport to the specific place in the body, gradual release and hence side-effects suppression.
Organoclays are a large group of hybrid materials of various types based on clay minerals, essentially montmorillonite, modified with organic compounds. Most common organoclays contain cationic surfactants, usually the alkylammonium type. Quaternary alkylammonium cations, a group of organic compounds, are suitable for modifying the surface properties of clay minerals. Quaternary ammonium salts used in the industry as broad-range antimicrobial and disinfection also exhibit activity against resistant bacteria (Yuen et al. 2015).
Antibacterial activity of clay-ciprofloxacin composites against the common skin bacteria Staphylococcus epidermis and Propionibacterium acnes was demonstrated to be a potential delivery system for ciprofloxacin molecules aimed at designing novel wound dressings (Hamilton et al. 2014).
Malachová et al. (2011) investigated the antibacterial and antifungal activities of nanocomposites based on montmorillonite (MMT) doped with the metals, such as: Ag-MMT, Cu-MMT and Zn-MMT. The tests were successfully against Escherichia coli, Pycnoporus cinnabarinus and Pleurotus ostreatus.
Aguzzi et al. (2014) presented a comprehensive and detailed study of the structure of montmorillonite–chitosan–silver sulfadiazine and the interactions involved, and Sandri et al. (2014) developed MMT/CS loaded with silver sulfadiazine (AgSD) as a dressing for cutaneous application against Pseudomonas aeruginosa; AgSD loaded nanocomposites improve antimicrobial properties of the drug.
Pourabolghasem et al. (2016) investigated the antibacterial activity of Cu-doped montmorillonite nanocomposites. The synthesis of Cu-clay nanocomposites was carried at 550 °C using a alkaline ion exchange processes in media containing copper sulfate. The antimicrobial effects of Cu-doped montmorillonite powders against pathogen bacterial strains of Escherichia coli and Staphylococcus aureus were tested in broth media. The results were positive with 99.98% mortality against E. coli, and 100% mortality against S. aureus. The montmorillonite alone, i.e., not Cu-doped, had no antibacterial activity.
Syafawani et al. (2016) had used Cu2+-doped kaolinite as the antibacterial agent against Gram-positive bacteria (S. aureus and E. faecalis) and Gram-negative bacteria (E. coli and P. aeruginosa). The authors found out that Cu2+-kaolinite, prepared by treatment with CuNO3·3H2O, was active and that becomes still more active after the modification of kaolinite with a cationic surfactant.
Ghorbanpour et al. (2017) made the characterization and evaluation of the bacterial activity of a synthetic Ag-nanoclay composite obtained by solid ion exchange. The tests showed clearly the antibacterial effects against Gram-negative Escherichia coli and Staphylococcus aureus.
Garshabi et al. (2017) used modified clay minerals to test the antibacterial properties of ZnO/nanoclay hybrids (montmorillonite immersed for some time into ZnCl at 350 °C and 450 °C) against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria. The obtained results suggest that this hybrid had a toxic effect on the bacteria, most probably associated with the photocatalytic mechanism of nanosize zincite, ZnO, which generates hydrogen peroxide causing the degradation of the bacteria membrane structure.
Jiao et al. (2017) produced an interesting article about preparation, characterization, antimicrobial and cytoxicity of Cu–Zn loaded montmorillonite.
Garshabi et al. (2017) used modified clay minerals to test the antibacterial properties of ZnO/nanoclay hybrids (montmorillonite immersed for some time into ZnCl at 350 °C and 450 °C) against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria. The obtained results suggest that this hybrid had a toxic effect on the bacteria, most probably associated with the photocatalytic mechanism of nanosize zincite (ZnO), which generates hydrogen peroxide causing the degradation of the bacteria membrane structure.
Bujdáková et al. (2018) tested the antibacterial properties and physicochemical characteristics of organoclays based on montmorillonite and quaternary alkylammonium and phosphonium cations, on the Gram-positive bacteria Staphylococcus aureus, and on the Gram-negative bacteria Escherichia coli. The antimicrobial effect of montmorillonite alone was negligible, but the organoclay integrating the dodecyltrimethylammonium cation had reduced the survival of both S. aureus and E. coli by over 93%.
It is possible to provide antibacterial properties to inert materials, such as clays, by treating them with metallic ions with bacteriostatic nature (Ag, Cu and Zn). In recent years, there has been much interest on the development of materials able to inhibit bacteria growth in everyday products, like paints, kitchenware, school and hospital tools, etc. The inorganic antibacterial materials, such as modified clays, show clearly advantages relatively to the traditionally used organic materials, for instance, in terms of chemical stability, thermal resistance, long lasting action and users’ safety.
Ghezzi et al. (2018) used halloysite nanotubes simply as the carrier of salicylic acid (SA), a crystalline organic acid reckoned as a key ingredient in a topical anti-acne product that is characterized by its bactericidal and antiseptic properties. Compared with the activity of free SA, the integrated system Hal/SA after being into contact with aqueous substrates provides enhanced antibacterial activity. Edraki and Zaarei (2018) have reported the antimicrobial activity of the hybrid composite Na-montmorillonite [Na-MMT/2-mercaptobenzimidazole (MBI)] against Staphylococcus epidermis and Escherichia coli. For the authors in this case, the clay mineral is just the vehicle in the interlayer spaces of the synthesized compound.
Very recently, Antonelli and Donelli (2018) investigated the effects of therapeutic mud on microorganisms of skin microbiome. The authors suggest that mud has several properties that may explain their action on skin microbiome with different effects on commensal and pathogenic microorganisms. More information about skin microbiome and of mud effects can be found in papers produced by the following authors: Gao et al. (2008), Grice et al. (2009), Cho and Blaser (2012), Belkaide and Segre (2014) and Belkaide and Hand (2014).
Other microbicidal minerals
Not only some clays and clay minerals can show microbicidal activity. For instance, some specific Fe-bearing minerals, such as pyrite, FeS2, proved to be highly reactive in water sanitation both in the form of slurries or leachates (Friedlander et al. 2015). However, the authors have demonstrated that pyrite leachate is more effective than pyrite slurry for the bacteria Escherichia coli.
Ferrous iron in solution drives the bacterial elimination and a steady production of ROS (Reactive Oxygen Species). In addition, pyrite oxidation by acid (H2SO4) treatment produces OH radicals. For the authors, ROS may disrupt the bacteria cell membrane, while Fe2+ in solution infiltrates and overwhelms the cell. Anyway, the experiments proved the importance of chemical interaction relatively to physical interaction in bacterial elimination. The chemical drives resulting from pyrite dissolution are: Fe2+ (aq.), ROS and acidity.
Regarding acidic pyrite dissolution at pH = 3, the experiments carried out by Friedlander et al. (2015) and Cunningham et al. (2010) point out that pH is not the driving factor of pyrite’s bactericidal properties.
Worldwide diarrheal diseases are the second most common cause of death for children under 5 years old. These diseases are attributed to pathogenic contamination of drinking water by Rotavirus, Cryptosporidium, Enterotoxigenic, Escherichia coli and Shigella.
Water disinfection is the removal, deactivation or killing of pathogenic microorganisms existing in water. Clays and modified clay minerals are good adsorbents for bacteria removal in water, reason why they are used in wastewater treatment and environmental bioremediation due to their large surface areas and surface charge (Yuan et al. 2015). The use of modified clay–polymer composites for removal of bacteria existing in water has been extensively discussed (Unuabonah and Taubert 2014).
Unuabonah and Taubert (2014), Unuabonah et al. 2017a, b) have shown the bacteriostatic character of clay composites.
Very recently in USA, two patents had been applied, one entitled “Synthetic antibacterial clay compositions and method of using same—Ref. US2013/0004544A1,” and the other “Antibacterial clay compositions for use as a topical ointment—Ref. US2018/0021374A1.” The scope of the first patent by Metge et al. (2013) considers the use of synthetic bactericidal compositions having clay like properties and the definition of a methodology to use the compositions to topical treatment of bacterial skin infections and skin diseases. The scope of the second invention applied by Tuba (2018) is also the treatment of bacterially caused skin infections and skin diseases, being the pH a determinant factor for rendering the clay bactericidal. When natural clays reveal antibacterial properties these are attributed to reducing agents, as a rule Fe2+ making part, either of clay minerals crystallochemical composition (particularly of smectite-rich clay minerals), or of external compounds, natural or synthetic.
The inventions discuss the preparation of pastes by mixing clay or clay minerals, natural or synthetic, with water and reducing agents, such as pyrite (FeS2) and its polymorph marcasite (FeS2), pyrrhotite (Fe1−x S, where x = 0–0.2), melanterite (FeSO4·7H2O), as well as the synthetic salt FeSO4.
Photo-Jones et al. (2015) studied and confirmed the antibacterial properties of Samian’s earth (SEGI) occurring in the Greek island Samos (Aegean Sea), whose pharmacological action had already been recognized by Dioscorides (In: “Matter of Medical Matter,” book V, 172), by Galenus (In: De Simplicium Medicamentorum) and by Pliny (In: Historia Naturalis, book XXXV). Dioscorides stated the existence of two varieties of SEGI, the variety called collyrion used as a medicament for eye affections, and the variety called aster used in the laundry sweep.
Geological, mineralogical and chemical studies conducted by Photo-Jones et al. (2015) have identified that colemanite, Ca2B8O11·5H2O and ulexite NaCaB5O9·8H2O, both boron minerals, and the boron element could occur in clay at concentrations up to a few thousand mg kg−1. The same authors tested preparations in which cultures of Staphylococcus aureus and Pseudomonas aeruginosa were added to the clay and concluded that the bactericidal effect identified for the two pathogenic microorganisms resulted from the presence and action of boron (B) in the form of boric acid, B(OH)3, rather than from the nature of the clay.
The antibacterial activity of metal-zeolite compositions had been demonstrated by Feng et al. (2000), Top and Ulku (2004), Jung et al. (2008), De la Rosa-Gomez et al. (2008), Ferreira et al. (2012), Lemire et al. (2013) and Rossainz-Castro et al. (2016).
Bui et al. (2016) demonstrated that bentonite from the Tam Bo deposit in Vietnam, after modification with Ag (silver) nanoparticles, can effectively inhibit the growth of opportunistic pathogens, such as Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus and Candida albicans.
Morrison et al. (2017) after having studied the antibacterial activity of several types of clay, (pyrite-bearing, non-sulfide-bearing and red oxidized clays) from Cascade Mountains, Oregon (USA), had found the greatest antibacterial activity for pyrite bearing-clays comparatively to non-sulfide-bearing white clays and had not found antibacterial activity for the red oxidized clays.
Milenkovic et al. (2017) demonstrated the antibacterial activity on Escherichia coli isolates using two zeolites, clinoptilolite (natural) and zeolite A (synthetic), enriched in Cu2+, Zn2+ and Ag1+ ions, and three distinct water media (peptone water, commercial spring water, lake water). The authors concluded that: (a) the antibacterial activity was linked to the metal type and not to the zeolite type; (b) the order for the antibacterial activity was Ag > Cu > Zn; (c) 100% antibacterial was achieved using Ag–zeolites; and (d) the antibacterial activity was not attributed to cytoplasmic membrane damage.
This powerful antibacterial activity of Ag–zeolites had been already demonstrated by Hrenovic et al. (2012, 2013), and by Demirci et al. (2014). The porous structure of zeolites enables metal cations to move freely, and this seems to be responsible for their bactericidal activity (Kwakye-Awuah et al. 2008).
Zarate-Reyes et al. ((2017a, b)) had shown the bacteriostatic character of brucite [Mg (OH)2], mineral in which Fe can replace a small part of Mg, on Escherichia coli.
Experimental studies on bacteriostatic and bactericidal clay
Authors’ interest on bacteriostatic/bactericidal clays was rise on the course of their studies and experiences on peloids. In terms of general composition, there are similarities between the clay/water systems used for bacteriostatic/bactericidal purposes, and the clay/water systems called peloids used in therapeutic/cosmetic topical applications within the medical field of peloidotherapy or pelotherapy, and practiced inside the balnearies and other facilities of Thermal Centres and Spas (Gomes et al. 2013; Gomes 2015, 2018).
In both the aforesaid systems, it is assumed that the liquid phase and not the solid phase is the major responsible factor for the medicinal/cosmetic properties, justifying the fact of peloids being studied and applied within the scope of the scientific field Medical Hydrology, particularly in the treatment of rheumatic diseases, such as osteoarthritis and low back pain.
A quite significant number of articles have been produced in recent years on peloids characterization, function and application (Delfino et al. 2003; Dreno 2004; François et al. 2005; Costantino and Lampa 2005; Costantino 2006; Veniale et al. 2007; Bellometti et al. 2007; Evcik et al. 2007; Fioravanti et al. 2011; Espejo-Antúnez et al. 2013; Maraver 2013, 2017; Gomes et al. 2013; Baschini et al. 2010, 2014; Fioravanti et al. 2014; Roques 2015; Gomes 2015; Gomes et al. 2015; Fioravanti and Chelesschi 2015; Centini et al. 2015; Rautureau et al. 2017; Maraver 2017; Gomes 2018).
Peloids are semisolid systems in which the solid component, generally consists of clay, and the liquid component consists of mineral water from spring sources, or from deep settled boreholes, seawater or salt-lake water. In the solid component, its particle size distribution and particle shape, essentially determine peloids relevant physical properties: (a) viscosity; (b) adhesiveness; (c) abrasiveness; (d) spreading; (e) heat capacity and specific heat; and (f) cooling rate. In the liquid component, its chemical constituents and respective concentration essentially determine peloids relevant chemical properties. Experience has shown that it is the interaction of the liquid component with the skin, the great responsible for peloids therapeutic interest.
As a rule, peloids are applied directly onto the skin, reason why they should be sanitary safe, both chemically and microbiologically. For cosmetic peloids, the EU has fixed the qualitative and quantitative guidelines for their sanitary safety against pathogenic microorganisms (European Standard EN ISO 17516:2014 Cosmetics-Microbiology-Microbiological Limit). However, the same do not happen so far for therapeutic or medical peloids, which are particularly used in balneological treatments of rheumatic disabilities.
Naturally, it would be rather important to have available medical peloids free of pathogens and even, in particular cases, bactericidal peloids. The authors have carried out studies on both natural and modified clay systems interesting on the authors’ perspective for the preparation of pathogens safe therapeutic and cosmetic designed and engineered peloid.
Extemporaneous peloids based on simple and chemically/microbiologically controlled composition were prepared mixing clays of well known mineralogical and chemical compositions with distilled and sterilized water or medicinal thermal water. The experimental work being carried out so far by the authors has as major objectives the development of bacteriological safe peloids as well as bactericidal peloids and bactericidal ointments able to treat particular infectious skin disorders.
Two types of natural clay were assayed: one a very white commercial Portuguese kaolin with reference A-130 is essentially composed of structurally disordered kaolinite and do not bears reduced transition metals as is the case of Fe common in kaolins, the kaolin being used in white ceramics production. Other very green Portuguese clay, with reference A-Campos, similar to the aforementioned “French green clays” in terms of mineral and chemical composition, is essentially composed of illite and montmorillonite, clay minerals which bear reduced Fe in their crystallochemical structures; the clay has been used for construction ceramics (mainly brick and roof tile) production.
A-130 kaolin was sampled in the Alvarães kaolin deposit located near to Viana do Castelo, in the northwest of Portugal. A-130 kaolin had been submitted to industrial processing, refining and beneficiation, in order to reduce grain size to less than 45 µm and concentrate kaolinite; its mineralogical composition determined by XRD analysis shows that the clay is mainly composed of kaolinite-D, i.e., disordered kaolinite making around 85% of the clay minerals total content, dioctahedral illite/mica making around 7%, and other minerals (mainly quartz) making about 8%. A-130 clay shows a specific surface area (SSA) of 30 m2 g−1 and cation exchange capacity (CEC)—18 meq/100 g.
A-Campos clay was sampled in the Clays of Aveiro-Ílhavo-Vagos geological formation (Upper Cretaceous), from an occurrence located near the center of Aveiro city (Portugal). The clay sample exhibits very sharp green color and is very finely grained (75% < 2 µm), plasticity index (PI) − 29, specific surface area (SSA)—95 m2 g−1, and cation exchange capacity (CEC)—60 meq/100 g. The XRD results show that A-Campos clay is mainly composed of dioctahedral and trioctahedral illite (70%) and smectite (20%). Kaolinite is a minor constituent (< 10% of the total clay minerals content).
Table 1 shows the chemical composition data corresponding to A-Campos and A-130 clays.
Two compositions or formulations were prepared based on each one of the two distinctive types of clay beforehand referred to and analyzed.
The first composition a sample of A-130 kaolin without reduced Fe was previously sterilized, and afterwards blended with distilled and sterilized water until a consistent paste had been obtained. Then, a solution of the synthetic salt, FeSO4, was added to the paste in order to incorporate in it Fe2+ as the reducing agent.
In the second composition, a sample of A-Campos clay was properly blended with distilled and sterilized water until a consistent paste had been obtained. Fe2+ assumed as being an effective reducing agent is a constituent of the structural octahedral sheets of both illite and montmorillonite clay minerals, and to get such Fe free and bioavailable acid activation was performed.
Very distinctive pH values (controlled along the compositions processing) were obtained in both studied compositions. As aforesaid very low (< 4) pH values could be by themselves bactericidal factors, acidic pH values being required conditions for the extraction of Fe2+ from the clay mineral structures (in the case of A-Campos clay), or from FeSO4 (in the case of A-130 clay), in order to make it bioavailable. In the preparation of both studied compositions, pH was controlled in order to be kept within the range 4–5.
Three bacteria strains: Pseudomonas aeruginosa 67p; Staphylococcus aureus ATCC 6538; Escherichia coli ATCC 25,922 were used in the tests carried out. For test control, one disk with com 5 µg of antibiotic (CIP, ciprofloxacin) was used. Test strains (0.5McFarland inoculum) were inoculated into 90 mm Petri dishes with Mueller–Hinton agar and in triplicate.
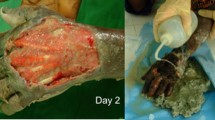
A sample of A-130 kaolin weighing 15 g was previously sterilized by heating at 200 °C during 1 h was mixed with bi-distilled and sterilized water in a 50/50 weight ratio. To the obtained paste, 0.15% of FeSO4 previously dissolved in water was added to the paste, and the Ref: CAUF was given to the composition. A sample of the paste weighing 50–80 mg and a sample of ciprofloxacin antibiotic with Ref: ANTIBIO afterwards placed in each of the disks. The plates wrapped in aluminum foil were incubated at 37 °C during 18 h. Figure 1 shows images relative to the evaluation of the antibacterial activity of the 3-component composition corresponding to Kaolin A-130.
The appearance of a zone without growth around the disk/sample is indicative of a positive action, i.e., material with antibacterial activity. The diameter of the zone of inhibition is related to the ability to inhibit, a larger diameter indicates superior antibacterial activity. Zones of inhibition were detected in the kaolin sample tested for all strains assayed. The diameter of these zones ranged from 19 to 29 mm. For the control sample (CIP), the obtained results were as expected, i.e., all strains showed susceptibility to ciprofloxacin.
Concerning A-Campos clay high bactericidal activity is shown for acid-activated samples, the acid treatment could turn free and bioavailable the structural Fe existing in illite and montmorillonite, whereas weak activity was shown for non acid-activated samples.
Assays were carried out too, and with equivalent results, substituting the bi-distilled and sterilized water by sulfurous sodium bicarbonate natural mineral–medicinal water (pH = 8.5) from the Portuguese São Pedro do Sul thermal spa.
As a conclusion of the overall experimental work being carried out, bacteriological safe and bactericidal peloids able to treat particular infectious skin disease can be developed using, either the 2-components paste of A-Campos clay, or the 3-components paste of A-130 kaolin. The authors consider also that clay can play a threefold role in clay-based bactericidal compositions:
-
1.
Clay is simply a vehicle or carrier of the bactericidal agent (metal), either just fixed in the ion form at the surfaces of the clay mineral particles taking advantage of the high specific surface area, electric charge and ion exchange capacity of the clay minerals, or as an associate component of the clay natural composition, for instance, reduced Fe bearing minerals, such as pyrite (FeS2), marcasite (FeS2), pyrrhotite (Fe1−x S) and arsenopyrite (FeAsS);
-
2.
Clay itself is an active bactericidal player in case of one or more than one of their constituent clay minerals bear in their crystallochemical structures reduced metals that could become free through acid-activation dissolution, pH control and eventual buffer being required; the pH of the clay composition or of the leachate conveniently should not be less than 4;
-
3.
Clay (natural, manipulated and modified, or synthetic), not containing reduced metals in its composition, therefore not exhibiting antibacterial properties, can acquire bacteriostatic or bactericidal properties if a reducing agent, natural or synthetic, is added to the clay and is properly blended with it.
Any one of the three cases referred to the antibacterial activity can be exhibited, either by the compositions themselves, or just by their leachates.
Clay with reference A-130 is clearly an example of bactericidal activity classified into the type 3 referred to, whereas clay with reference A-Campos is clearly an example of bactericidal activity classified into the type 2 referred to.
The white color of A-130 kaolin and other relevant physical properties are determinant in case of preparation of clay-based bacteriological safe and bactericidal ointments addressed to the healing of wound infections of the skin (Gomes et al. 2019).
Studies go on considering the effect of different variables such as pH, Eh and reducing agent nature. Particularly minerals bearing transition metals other than Fe, for instance: Cu associated with Fe in chalcopyrite (CuFeS2), Cu in covellite (CuS), and Zn in sphalerite (ZnS)], incorporated into the compositions, either in solution, or in slurry prepared with clay (bentonite and kaolin)/water (distilled and mineral–medicinal) mixtures, are considered. The activity of the aqueous leachates of the corresponding compositions will be studied too. The lifetime of clay-based topical ointments, i.e., the time along which the ointment being used maintains the bactericidal activity, is another research target.
Conclusions
This work was intended to be an attempt to get an overview, in a synthetic way, of the short history involving bacteriostatic and bactericidal clays. The main target was the understanding and explanation of clay intrinsic or extrinsic crystallochemical endowments and limitations, always on the basis of experimental work needed to identify and characterize the factors or agents determining and conditioning those properties, and to identify the bactericidal mechanisms of action.
Within the intervening agents and variables it was found out significance of the reducing agents, either naturally co-existing with the clay, or if being absent they adequately could be added to the clay.
Clay is an active player in clay-based compositions showing bacteriostatic/bactericidal activity because:
-
1.
it bears the antibacterial reducing agent (for instance, Fe under the form of pyrite (FeS2) as an associate component in the natural clay occurrence;
-
2.
it bears the antibacterial reducing agent in the structures of their constituent clay minerals (for instance, illite and/or montmorillonite), only requiring its previous release by partial dissolution;
-
3.
is a simple vehicle or carrier of the antibacterial reducing agent external to the clay, for instance, FeSO4 that has been adequately added in aqueous solution and properly mixed with the clay;
-
4.
clay minerals structural changes induced by heating, even if dehydration leads do the partial collapse of their structures, do not affect both clay bactericidal and bacteriostatic activities; such could be confirmed with kaolin A-130 that in the authors experiments had been previously sterilized by heating at 200 °C during 1 h.
This work was also intended to be an attempt to develop formulations of designed and engineered peloids (a variety of extemporaneous peloids) based on clay of simple, sterile and controllable composition, modified to exhibit bactericidal activity, and to which a particular type of mineral–medicinal water had been added, mixed and homogenized. As a paste or poultice or yet as a mask the obtained peloid conveniently exhibiting human skin friendly pH (easily buffered if necessary) could be used in Thermal Centres and Spas, for instance, in the treatment of special cases of infectious skin disorders.
Also, the frequently sanitary suspicious natural peloids and peloids themselves currently used in peloidotherapy or pelotherapy could be replaced by pathogens free bacteriostatic/bactericidal peloids in the treatment, for instance, of musculoskeletal disabilities, such as osteoarthritis and low back pain.
References
Adusumilli, S., & Haydel, S. E. (2016). In vitro antibacterial activity and in vivo efficacy of hydrated clays on Mycobacterium ulcerans growth. BMC Complementary and Alternative Medicine, 16(40), 1–9. https://doi.org/10.1186/s12906-016-1020-5.
Aguzzi, C., Sandri, G., Bonferoni, C., Cerezo, P., Rossi, S., Ferrari, F., et al. (2014). Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloids and Surfaces B: Biointerfaces, 113, 152–157.
Antonelli, M., & Donelli, D. (2018). Effects of balneotherapy and spa therapy on levels of cortisol as a stress biomarker: A systematic review. International Journal of Biometeorology. https://doi.org/10.1007/s00484-018-1599-y.
Arias, C. A., & Murray, B. E. (2009). Antibiotic-resistant bugs in the 21st century: A clinical super-challenge. The New England Journal of Medicine, 360, 439–443.
Awad, M., López-Galindo, A., Setti, M., & El-Rahmany, Viseras C. (2017). Kaolinite in pharmaceuticals and biomedicine. International Journal of Pharmaceutics, 533, 34–48.
Baschini, M. T., Pettinari, G. R., Vallés, J. M., Aguzzi, C., Cerezo, P., López-Galindo, A., et al. (2010). Suitability of natural sulphur-rich muds from Copahue (Argentina) for use as semisolid health care products. Applied Clay Science, 49, 205–212.
Baschini, M. T., Piovano, E., López-Galindo, A., Dietrich, D., & Setti, M. (2014). Composición y propriedades de fangos (peloides), aguas y sales procedentes de lagunas y lagos salinos usados com fines terapêuticos y cosméticos. In H. A. Torres (Ed.), Peloteraia: Aplicaciones médicas y cosméticas de fangos termales (pp. 145–154). Panama City: Fundación Bibilis.
Behroozian, S., Svensson, S. L., & Davies, J. (2016). Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. American Society of Microbiology, mBios, 7(1), e01842–15.
Belkaide, Y., & Hand, T. (2014). Role of the microbiota in immunity and inflammation. Cell, 157(1), 121–141.
Belkaide, Y., & Segre, J. A. (2014). Dialogue between skin microbiota and immunity. Science, 346, 954–959.
Bellometti, S., Gallotti, C., Pacileo, G., Rota, A., & Tenconi, M. T. (2007). Evaluation of outcomes in spa-treated osteoarthrosic patients. Journal of Preventive Medicine and Hygiene, 48(1), 1–4.
Brunet de Courssou, L. (2002). 5th WHO advisory group meeting on Buruli ulcer. Geneva: Study Group Report on Buruli Ulcer Treatment with Clay.
Bui, Q. C., Nguen, H. C., Vesentsev, A. I., Buhanov, V. D., Sokolovsky, P. V., & Mihaylyukova, M. O. (2016). The antibacterial properties of modified bentonite deposit tam bo in Vietnam. Research Result: Pharmacology and Clinical Pharmacology, 2(3), 63–74.
Bujdáková, H., Bujdáková, V., Májekcvá-Kosčová, H., Gaálová, B., Bizovská, H., & Bohác, P. (2018). Antimicrobial activity of organoclays based on quaternary alkylammonium and alkylphosphonium surfactants and montmorillonite. Applied Clay Science, 158, 21–28.
Caflisch, K. M., Schmidt-Malan, S. M., Mandrekar, J. N., Karau, M. J., Nicklas, J. P., Williams, L. B., et al. (2018). Antibacterial activity of reduced iron clay against pathogenic bacteria associated with wound infections. International Journal of Antibacterial Agents, 52, 1–5.
Carretero, M. I. (2002). Clay minerals and their beneficial effects upon human health: A review. Applied Clay Science, 21, 155–163.
Carretero, M. I., Gomes, C. S. F., & Tateo, F. (2006). Clays and human health. Handbook of clay science. In F. Bergaya, B. K. G. Theng, & G. Lagaly (Eds.), Developments in clay science no 1 (pp. 717–741). Amsterdam: Elsevier.
Carretero, M. I., Gomes, C. S. F., & Tateo, F. (2013). Clays, drugs and human health. In F. Bergaya & G. Lagaly (Eds.), Handbook of clay science, second edition, Part B. Techniques and applications, Chapter 5.5 (pp. 711–764). Amsterdam: Elsevier.
Carretero, M. I., & Pozo, M. (2007). Mineralogía aplicada: Salud y medio ambiente. Madrid: Thomson.
Centini, M., Tredici, M. R., Biondi, N., Buonocore, A., Maffei Facino, R., & Anselmi, C. (2015). Thermal mud maturation: Organic matter and biological activity. International Journal Cosmet Science, 37, 339–347.
Cerri, G., De’Gennaro, M., Bonferoni, M. C., & Caramella, C. (2004). Zeolites in biomedical application: Zn-exchanged clinoptilolite-rich rock as active carrier for antibiotics in anti-acne topical therapy. Applied Clay Science, 27, 141–150.
Cho, I., & Blaser, M. J. (2012). The human microbiome at the interface of health and disease. Nature Reviews Genetics, 13, 260–270.
Costantino, M. (2006). Sulphur mud-bath treatment in osteoarthrosis: Therapeutic activity and efficiency on the quality of life. Clinical Therapeutics, 157, 525–529.
Costantino, M., & Lampa, E. (2005). Psoriasis and mud bath therapy: Clinical-experimental study. Clinical Therapeutics, 156, 145–149.
Cunningham, T. B., Koehl, J. L., Summers, J. S., & Haydel, S. E. (2010). pH-dependent metal ion toxicity influences of the antibacterial activity of two natural mineral mixtures. PLoS ONE, 5, e9456.
Dashiff, A., Junka, R. A., Libera, M., & Kadouri, D. E. (2011). Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. Journal of Applied Microbiology, 110(2), 431–444.
De la Rosa-Gomez, I., Olguín, M. T., Garcia-Sosa, I., Alcantara, D., & Rodriguez-Fuentes, G. (2008). Silver supported on natural Mexican zeolite as an antibacterial material. Micropor Mesopor Mater, 39, 431–444. https://doi.org/10.1016/S1387-1811(00)00217-1.
Delfino, M., Russ, N., Migliaccio, G., & Carraturo, N. (2003). Experimental study on efficacy of thermal muds of Ischia Island combined with balneotherapy in the treatment of psoriasis vulgaris with plaques. Clinica Terapeutica, 154(3), 167–171.
Demirci, S., Ustaoglu, Z., Yilmazer, G. A., Sahin, F., & Baç, N. (2014). Antimicrobial properties of zeolite-X, and zeolite-A ion exchanged with G, Cu, and Zn against a broad range of microorganisms. Applied Biochemistry and Biotechnology, 172, 1652–1662.
Diekema, D. J., BootsMiller, B. J., Vaughn, T. E., Woolson, R. F., & Yankey, J. W. (2004). Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clinical Infectious Diseases, 38, 78–85.
Dreno, B. (2004). Topical antibacterial therapy for acne vulgaris. Drugs, 64(21), 2389–2397.
Edraki, M., & Zaarei, D. (2018). Modification of montmorillonite clay with 2-mercaptobenzimidazole and investigation of their antimicrobial properties. Asian Journal of Green Chemistry, 2, 189–200.
Espejo-Antúnez, L., Cardero-Durán, M. A., Garrido-Ardila, E. M., Torres-Piles, S., & Caro-Puértolas, B. (2013). Clinical effectiveness of mud pack therapy in knee osteoarthritis. Rheumatology (Oxford), 52, 659–668.
Evcik, D., Kavuncu, V., Yeter, A., & Yigit, I. (2007). The efficacy of balneotherapy and mud-pack therapy in patients with knee osteoarthritis. Joint Bone Spine, 74, 60–65.
Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N., & Kim, J. O. (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Research, 52(4), 662–668.
Ferreira, L., Fonseca, A. M., Botelho, G., Almeida-Aguiar, C., & Neves, I. C. (2012). Antimicrobial activity of faujasite zeolites doped with silver. Micropor Mesopor Mater, 160, 126–132. https://doi.org/10.1016/j.micromeso.2012.05.006.
Ferrell, R. E. (2008). Medicinal clay and spiritual healing. Clays and Clay Minerals, 56, 751–760.
Fioravanti, A., Cantarini, L., Guidelli, G. M., & Galeazzi, M. (2011). Mechanisms of action of spa therapies in rheumatic diseases: What scientific evidence is there? Rheumatology International, 31(1), 1–8.
Fioravanti, A., & Chelesschi, S. (2015). Mechanisms of action of balneotherapy in rheumatic diseases. Balnea, 10, 43–56.
Fioravanti, A., Tenti, S., Gianitti, C., Fortunati, N. A., & Galeazzi, M. (2014). Short and long-term effects of mud-bath treatment on hand osteoarthritis: A randomized clinical trial. International Journal of Biometeorology, 58(1), 79–86.
Flerer, N., & Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences of the United States of America, 103, 626–631. https://doi.org/10.1073/pnas.0507535103.
François, G., Micollier, A., & Rouvie, I. (2005). Les Boues Thermales, Atelier Santé Environmental (p. 29). Rennes: ENSP (École Nationale de la Santé Publique).
Friedlander, L. R., Puri, N., Martin, A., Schoonen, A., & Karzai, A. W. (2015). The effect of pyrite on Escherichia coli in water: Proof-of-concept for the elimination of waterborne bacteria by reactive minerals. Journal of Water and Health, 13, 1. https://doi.org/10.2166/wh.2014.013.
Gao, Z., Tseng, C. H., Strober, B. E., Pei, Z., & Blaser, M. J. (2008). Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE, 3, e2719.
Garcidueñas-Pina, R., & Cervantes, C. (1995). Microbial interactions with aluminum. BioMetals, 9, 311–316.
Garshabi, N., Ghorbanpour, M., Nouri, A., & Loftiman, S. (2017). Preparation of zinc oxide-nanoclay hybrids by alkaline ion exchange method. Brazilian Journal of Chemical Engineering, 34(4), 1055–1063.
Gaskell, E. E., & Hamilton, A. R. (2014). Antimicrobial clay-based materials for wound care. Future Medicinal Chemistry, 6(6), 641–655. https://doi.org/10.4155/fmc.14.17.
George, K. M., Barker, L. P., Welty, D. M., & Small, P. L. C. (1998). Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infection and Immunity, 66(2), 587–593.
George, K. M., Chatterjee, D., Gunawardana, G., Welty, D., Hayman, J., Lee, R., et al. (2002). Mycolactone: A polyketide toxin from Mycobacterium ulcerans, required for virulence. Science, 283(5403), 854.
Ghadiri, M., Chrzanowski, W., & Rohanizadeh, R. (2015). Biomedical applications of cationic clay minerals. RSC (Royal Society of Chemistry) Advances, 5, 37. https://doi.org/10.1039/c4ra16945j.
Ghezzi, L., Spepi, A., Agnolucci, M., Cristani, C., Giovannetti, M., Tiné, M., et al. (2018). Kinetics of release and antibacterial activity of salicylic acid loaded into halloysite nanotubes. Applied Clay Science, 160, 88–94.
Ghorbanpour, M., Mazloumi, M., Nouri, A., & Iotfiman, S. (2017). Silver-doped nanoclay with antibacterial activity. Journal of Ultrafine Grained and Nanostructural Materials, 50(2), 124–131. https://doi.org/10.22059/JUFGNSM.2017.02.07.
Gomes, C. S. F. (2015). In pelotherapy what is more important, the peloid solid phase or the peloid liquid phase? Balnea, 10, 125–142.
Gomes, C. S. F. (2018). Healing and edible clays: A review of basic concepts, benefits and risks. Environmental Geochemistry and Health, 40, 1739–1765. https://doi.org/10.1007/s10653-016-9903-4.
Gomes, C. S. F., Carretero, M. I., Pozo, M., Maraver, F., Cantista, P., Armijo, F., et al. (2013). Peloids and pelotherapy: Historical evolution, classification and glossary. Applied Clay Science, 75–76, 28–38.
Gomes, C. S. F., Gomes, J. H., Tacão, M., Henriques, I., & Silva, E. F. (2019). Bactericidal clay to be used as topical ointment in skin infections. In STCV’19, international symposium on thermalism and quality of life ourense, Spain.
Gomes, C. S. F., & Silva, J. B. P. (2007). Minerals and clay minerals in medical geology. Applied Clay Science, 36, 4–21.
Gomes, C. S. F., Silva, J. B. P., & Gomes, J. H. C. (2015). Natural peloids versus designed and engineered peloids. Boletín de la Sociedad Española de Hidrología Médica, 30(1), 15–36.
Grice, E. A., Kong, H. H., Conlan, S., Deming, C. B., Davis, J., & Young, A. C. (2009). Topographical and temporal diversity of the human skin microbiome. Science, 324, 1190–1192.
Hauser, E. A. (1950). Canamin clay and its properties. Can Chem Process Ind, 34, 979.
Hauser, E. A. (1952). Kisameet Bay clay deposit. In: Problems of clay and laterite genesis, symposium at annual meeting of the American institute of mining and metallurgical engineers, St Louis, MO (pp. 178–190).
Haydel, S. E., Remenih, C. M., & Williams, L. B. (2008). Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. Journal of Antimicrobial Chemotherapy, 61, 353–361.
Holešová, S., Hundáková, M., & Pazdziora, E. (2016). Antibacterial kaolinite based nanocomposites. Procedia Materials Science, 12, 124–129.
Hrenovic, J., Milenkovic, J., Goic-Barisic, I., & Rajic, N. (2013). Antibacterial activity of containing natural zeolite against clinical isolates of Acinetobacter baumannii. Microporous and Mesoporous Materials, 169, 148–152.
Hrenovic, J., Milenkovic, J., Ivankovic, T., & Rajic, N. (2012). Antibacterial activity of heavy metal-loaded natural zeolite. Journal of Hazardous Materials, 30, 201–212.
Hu, C. H., & Xia, M. S. (2006). Adsorption and antibacterial effect of copper-exchanged montmorillonite on Escherichia coli K-88. Applied Clay Science, 31, 180–184.
Hu, C. H., Xu, Z. R., & Xia, M. S. (2005). Antibacterial effect of Cu2+-exchanged montmorillonite on Aeromonas hydrophila and discussion on its mechanism. Veterinary Microbiology, 109, 83–88.
James, G. A., Swogger, E., & Wolcott, R. (2008). Biofilms in chronic wounds. Wound Repair and Regeneration, 16, 37–44.
Jiao, L., Lin, F., Cao, S., Wang, C., Wu, H., Shu, M., et al. (2017). Preparation, characterization, antimicrobial and cytoxicity studies of copper-zinc loaded montmorillonite. Journal of Animal Science and Biotechnology, 8, 7.
Jung, W. K., Koo, H. C., Kim, K. W., Shin, S., Kim, S. H., & Park, Y. H. (2008). Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Applied and Environment Microbiology, 74, 2171–2178. https://doi.org/10.1128/AEM.02001.07.
Kalinowski, B. E., Bengtsson, A., Pedersen, K., Lilja, C., Sellin, P., et al. (2016). Threshold density for microbial sulphate reduction in bentonite. In Goldschmidt conference proceedings, Yokohama, Abstract no. 771.
Kostyniak, P., Constanzo, P. M., Syracuse, J., Giese, R. (2003). Antimicrobial activity of modified clay minerals. Abstract in clays and clay minerals annual meeting, Athens Ga.
Kwakye-Awuah, B., Williams, C., Kenward, M. A., & Radecka, I. (2008). Antimicrobial action and efficiency of silver-loaded zeolite X. Journal of Applied Microbiology, 104, 1516–1524.
Lemire, J. A., Harrison, J. J., & Turner, R. J. (2013). Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nature Reviews Microbiology, 11, 371–384.
Londono, S. C., Hartnett, H. H., & Williams, L. B. (2017). The antibacterial activity of aluminium in clay from Colombian Amazon. Environmental Science and Technology, 51, 2401–2408. https://doi.org/10.1021/acs.est.6b04670.
Londono, S. C., Williams, L. (2015). Evaluating the antibacterial action of a clay from the Colombian Amazon. In International applied geochemistry symposium, April 2015, 7 pp.
Londono, S. C., & Williams, L. B. (2016). Unraveling the antibacterial mode of action of a clay from Colombian Amazon. Environmental Geochemistry and Health, 38, 363–379.
Lucera, A., Costa, C., Conte, A., & Del Nobile, M. A. (2012). Food applications of natural antimicrobial compounds. Frontiers in Microbiology, 3, 287.
Ma’or, Z., Henis, Y., Alon, Y., Orlov, E., Sorensen, K. B., & Oren, A. (2006). Antimicrobial properties of Dead Sea black mineral mud. International Journal of Dermatology, 45(5), 504–511.
Magana, S. M., & Quintana, P. (2008). Antibacterial activity of montmorillonites modified with silver. Journal of Molecular Catalysis A: Chemical, 281, 192–199.
Malachová, K., Praus, P., Rybková, Z., & Kosák, O. (2011). Antibacterial and antifungal activities of silver, copper and zinc montmorillonite. Applied Clay Science, 53, 642–645.
Maraver, F. (2013). Mechanisms of action of pelotherapy: State of the art. In J. Nunes, C. Gomes, & J. Silva (Eds.), Livro de Actas do III Congresso Iberoamericano de Peloides (pp. 9–18). São Miguel, The Azores: Ponta Delgada.
Maraver, F. (2017). Investigación actual en peloterapia. Libro de Resúmenes del V Congreso Iberoamericano de Peloides (pp. 33–35). Badajoz: Balneario El Raposo.
Masurat, P., Ericksson, S., & Pedersen, K. (2010). Microbial sulphide production in compacted Wyoming bentonite MX-80 under in situ conditions relevant to a repository for high-level radioactive waste. Applied Clay Science, 47, 58–64.
McInnes, A. D. (2012). Diabetic foot disease in the United Kingdom: About time to put feet first. Journal of Foot and Ankle Research, 5, 26.
Metge, D. W., Williams, L., Eberl, D. D., Bium, A. E., & Harvey, B. W. (2013). Synthetic antibacterial clay compositions and method of using them. United States Patent US2013/004544A1.
Milenkovic, J., Hrenovic, J., Matijasevic, D., Niksic, M., & Rajic, N. (2017). Bactericidal activity of Cu-, Zn-, and Ag-containing zeolites toward Escherichia coli isolates. Environmental Science and Pollution Research, 24, 20273–20281.
Moraes, J. D. D., Bertolino, S. R. A., Cuffini, S. L., Ducart, D. F., Bretzke, P. E., & Leonardi, G. R. (2017). Clay minerals: Properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes. International Journal of Pharmaceutics, 534, 213–219.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramirez, J. T., et al. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16, 2346–2353.
Morrison, K. D., Misra, R., & Williams, L. B. (2016). Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Scientific Reports, 6, 19043. https://doi.org/10.1038/Srep19043.
Morrison, K. D., Underwood, J. C., Metge, D. W., Eberl, D. D., & Williams, L. B. (2013). Mineralogical variables that control the antibacterial effectiveness of a natural clay deposit. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-013-9585-0.
Morrison, K. D., Williams, S. N., & Williams, L. B. (2017). The anatomy of an antibacterial clay deposit: A new economic geology. Economic Geology, Bulletin of the Society of Economic Geologists, 112(7), 1551–1570.
O’Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations of the review on antimicrobial resistance. London: Department of Health and the Wellcome Trust.
Otto, C. C., & Haydel, S. E. (2013a). Exchangeable ions are responsible for the in vitro antibacterial properties of natural clay mixtures. PLoS ONE, 8(5), 1–9.
Otto, C. C., & Haydel, S. E. (2013b). Microbicidal clays: Composition, activity, mechanism of action, and therapeutic applications. In A. Méndez-Vilas (Ed.), Microbial pathogens and strategies for combating them: Science, technology and education (pp. 1169–1180). Mexico city: FORMATEX.
Otto, C. C., Koehl, J. L., Solanky, D., & Haydel, S. E. (2014). Metal ions, non metal-catalyzed oxidative stress, cause clay leachate antibacterial activity. PLoS ONE, 9, e115172.
Panko, A. V., Kovzun, I. G., Ulberg, Z. R., Oleinik, V. A., Nikipelova, E. M., et al. (2016). Colloid-chemical modification of peloids with nano-and microparticles of natural minerals and their practical use. In: Chapter 14: Nanophysics, nanophotonics, surface studies, and applications (pp. 163–177). Springer Proceedings in Physics 183.
Parolo, M. E., Fernández, L. G., Zajonkovsky, I., Sánchez, M. P., Baschini, M. (2011). Antibacterial activity of materials synthesized from clay minerals. In A. Méndez-Vilas (Ed.), Science against microbial pathogens: Communicating current research and technological advances (pp. 144–151).
Photo-Jones, E., Keane, C., Jones, A. X., Stamatakis, M., Robertson, P., Hall, A. J., et al. (2015). Testing Dioscorides’ medicinal clays for their antibacterial properties: The case of Samian Earth. Journal of Archaeological Science, 57, 257–267.
Pourabolghasem, H., Ghorbanpour, M., & Shayegh, R. (2016). Antibacterial activity of copper-doped montmorillonite nanocomposites prepared by alkaline ion exchange method. Journal of Physical Science, 27(2), 1–12.
Rautureau, M., Gomes, C. S. F., Liewig, N., & Katouzian-Safadi, M. (2010). Argiles et Santé: Propriétés et Thérapies. Lavoisier: Édition Médicales Internationale.
Rautureau, M., Gomes, C. S. F., Liewig, N., & Katouzian-Safadi, M. (2017). Clays and health: Properties and therapeutic uses. Cham: Springer.
Roques, C. F. (2015). Mud therapy: Data for clinical evidence. Balnea, 10, 57–62.
Rossainz-Castro, L. G., De la Rosa-Gomez, I., Olguín, M. T., & Alcantara-Diaz, D. (2016). Comparison between silver-and copper-modified zeolite-rich tuffs as microbicidal agents for Escherichia coli and Candida albicans. Journal of Environmental Management, 183, 763–770. https://doi.org/10.1016/j.jenvman.2016.09.034.
Sandri, G., Bonferoni, M. C., Ferrari, F., Rossi, S., Aguzzi, C., Mori, M., et al. (2014). Montmorillonite–chitosan–silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydrate Polymers, 102, 970–977.
Sawai, J. (2003). Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO, CaO) by conductimetric assay. Journal of Microbiological Methods, 54, 177–182.
Sizaire, V., Nackers, F., Comte, E., & Portaels, F. (2006). Mycobacterium ulcerans infection: Control, diagnosis, and treatment. The Lancet Infectious Diseases, 6(5), 288–296.
Svensson, S. L., Behroozian, S., Xu, W., Surette, M. G., Li, L., & Davies, J. (2017). Kisameet Glacial Clay: An unexpected source of bacterial diversity, 8(3), e00590–17.
Syafawani, N., Nizam, N. A., & Chun, C. (2016). Antimicrobial activity of copper-kaolinite and surfactant modified copper-kaolinite against Gram-positive and Gram-negative bacteria. Jurnal Teknologi (Sciences nd Engineering), 76(3–2), 127–132.
Tong, G., Yulong, M., Peng, G., & Zirong, X. (2005). Antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis. Veterinary Microbiology, 105, 113–122.
Top, A., & Ulku, S. (2004). Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity. Applied Clay Science, 27, 13–19. https://doi.org/10.1016/j.clay.2003.12.002.
Tuba, T. (2018). Antibacterial clay compositions for use as a topical ointment U.S. patent application no. 15/216,940. Washington, DC: U.S. Patent and Trademark Office.
Unuabonah, E. I., Adewuji, A., Kolawole, M. O., Omovojie, M. O., Olatunde, O. C., Fayemi, S. O., et al. (2017a). Disinfection of wáter with new chitosan-modified hybrid clay composite absorbent. Heliyon, 3, e00379.
Unuabonah, E. I., Kolawole, M. O., Agunbiade, F. O., Omorogie, M. O., Koko, D. T., Ugwuja, C. G., et al. (2017b). Novel metal-doped bacteriostatic hybrid clay composites for point-of-use disinfection of water. Journal of Environmental Chemical Engineering, 5, 2128–2141.
Unuabonah, E. I., & Taubert, A. (2014). Clay-polymer nanocomposites (CPNs): Adsorbents of the future for water treatment. Applied Clay Science, 99, 83–92.
Veniale, F., Bettero, A., Jobstraibizer, P., & Setti, M. (2007). Thermal muds: Perspectives of innovation. Applied Clay Science, 36, 141–147.
Viseras, C., Carazo, E., Borrego-Sanchez, A., Garcia-Villen, F., Sánchez-Espejo, R., Cerezo, P., et al. (2019). Clay minerals in skin drug delivery. Clay and Clay Minerals. https://doi.org/10.1007/s42860-018-0003-7.
Wang, X., Dong, H., Zeng, Q., Xia, Q., Zhang, L., & Zhou, Z. (2017). Reduced iron-containing clay minerals as antibacterial agents. Environmental Science and Technology, 51, 2401–2408.
Williams, L. B. (2017). Geomimicry: Harnessing the antibacterial action of clays. Clay Minerals, 52, 1–24.
Williams, L. B., & Haydel, S. E. (2010). Evaluation of the medicinal use of clay minerals as antibacterial agents. International Geology Review, 52, 745–770.
Williams, L. B., Haydel, S. E., & Ferrell, R. (2009). Bentonite, bandaids and borborygmi. Elements, 5, 99–104.
Williams, L. B., Haydel, S. E., Giese, R., & Eberl, D. D. (2008). Chemical and mineralogical characteristics of French Green Clays used for healing. Clays and Clay Minerals, 56, 437–452.
Williams, L. B., & Hillier, S. (2014). Kaolins and health: From first grade to first aid. Elements, 10, 207–211.
Williams, L. B., Holland, M., Eberl, D. D., Brunet, T., & De Courrsou, L. B. (2004). Killer clays! Natural antibacterial clay minerals. Mineralogical Society Bulletin, 139, 3–8.
Williams, L. B., Metge, D., Eberl, D. D., Harvey, R., Turner, A., Prapaipong, P., et al. (2011). What makes natural clay antibacterial? Environmental Science and Technology, 45, 3768–3773.
Wilson, M. J. (2003). Clay mineralogical and related characteristics of geophagic materials. Journal of Chemical Ecology, 29, 1525–1547.
Xu, J., Campbell, J. M., Zhang, N., Hickey, W., & Sahai, N. (2012). Did mineral surface chemistry and toxicity contribute to evolution of microbial extracellular polymeric substances? Astrobiology, 12(8), 785–798.
Ye, Y., Zhou, Y. H., Xia, M. S., Hu, C. H., & Ling, H. F. (2003). A new type of inorganic antibacterial material: Cu-bearing montmorillonite and discussion on its mechanism. Journal of Inorganic Materials, 18, 569–574.
Young, S. L. (2011). Craving earth. New York: Columbia University Press.
Yuan, P., Tan, D., & Bergaya, F. (2015). Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Applied Clay Science, 112–113, 75–93.
Yuen, J. W. M., Chung, T. W. K., & Loke, A. Y. (2015). Methicillin-resistant Staphylococcus aureus (MRSA) contamination in bedside surfaces of a hospital ward and the potential effectiveness of enhanced disinfection with an antimicrobial polymer surfactant. International Journal of Environmental Research and Public Health, 12, 3026–3041.
Zarate-Reyes, L., López-Pacheco, C., Nieto-Camacho, A., Apán, M. T. R., Palacios, E., López-Vidales, V., et al. (2017a). Naturally occurring layered-mineral magnesium as a bactericidal against Escherichia coli. Applied Clay Science, 149, 87–96.
Zarate-Reyes, L., López-Pacheco, C., Nieto-Camacho, A., Palacios, E., López-Vidales, V., Kaufhold, S., et al. (2017b). Antibacterial clay against gram-negative antibiotic resistant bacteria. Journal Hazardous Materials, 342, 625–632.
Zatta, P., Kiss, T., Suwalsky, M., & Berthon, G. (2002). Aluminium(III) as a promoter of cellular oxidation. Coordination Chemistry Reviews, 228(2), 271–284.
Acknowledgements
The authors would like to thank Marta Tacão and Isabel Henriques, staff members of CESAM and Department of Biology, University of Aveiro, 3800-193 Aveiro, Portugal, and of CESAM and Department of Life Sciences, Faculty of Science and Technology, University of Coimbra, Portugal, respectively, for their contribution with regard to the microbiological studies of two Portuguese clays looking at any eventual bacteriostatic and bactericidal properties.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, C.F., Gomes, J.H. & da Silva, E.F. Bacteriostatic and bactericidal clays: an overview. Environ Geochem Health 42, 3507–3527 (2020). https://doi.org/10.1007/s10653-020-00628-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00628-w