Abstract
Worldwide, environmental tragedies involving mining dam ruptures have become more frequent. As occurred a few years ago in Brazil (on 5 November 2015, in Minas Gerais state) the Fundão Dam rupture released 60 million m3 of tailings into terrestrial and aquatic ecosystems. Since then, little information on the ecotoxicity of these tailings has been disclosed. In the laboratory, the acute, chronic and bioaccumulation effects of increased Fundão tailing concentrations on oribatid mites (Scheloribates praeincisus) were assessed. Additionally, the bioaccumulation of 11 trace metals (Al, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb and Zn) and the total density of oribatid mites found in the areas contaminated by the Fundão tailings were determined. The percentages of mite survival and reproductive inhibition were higher than 60% and 80%, respectively, in all contaminated areas with the highest concentration (100% mine tailings). Field studies showed an expressive reduction in the total density of oribatids per m−2 (up to 54 times) in the contaminated areas compared with the reference area. Metal accumulations in the field were 5.4 and 3.2 higher (for Ni and Hg, respectively) and up to two times higher (for most metals) than those in the laboratory for 42 days. The mite responses to the Fundão tailings found in this study suggest long-term interference in their biological development. In this sense, we can conclude that the introduction of mine tailings onto soils tended to compromise the functionality of the mites in the ecosystem, which causes imbalances to cascade other organisms of the trophic web.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental impacts of mine tailings on terrestrial ecosystems are still poorly elucidated (Figueiredo et al. 2019). These tailings are often enriched in metals and affect the surrounding areas (Agnieszka et al. 2014). They might have a negative overall impact on the environment, causing changes in landscapes and ecosystems, habitat destruction, plant or animal biodiversity losses, land degradation, and soil and water pollution (Gabarrón et al. 2018). The world’s greatest environmental tragedies have been associated with mining dam ruptures, such as in Aznalcóllar (Spain), Borsa (Romania) and Arizona (USA), in which adverse effects have been apparent for decades (Macklin et al. 2003; Cabrera et al. 2008; USDA 2017; Cánovas et al. 2019). Ecological and environmental impact assessments of mine tailing deposition on terrestrial ecosystems should not be restricted only to chemical evidence, such as by excessive amounts of one or more metals on soils in comparison with limits established at the national level in compliance with directives. The tailing dispositions in the environments must also be monitored due to toxicity effects to humans, animals and plants, and the potential implications for their development during life should be evaluated (Farmer 2012).
The Fundão dam rupture (in 2015) has been one of the greatest environmental tragedies recorded in recent decades in Brazil (CPRM and ANA 2015a, b; Fernandes et al. 2016). This dam, owned by Samarco Corporation, spilled over 60 million m3 of iron ore tailings that spread down the Rio Doce River, killing 20 people and affecting biodiversity across hundreds of kilometres of river, riparian lands and Atlantic coast (IBAMA 2017). Due to the Fundão dam rupture, the regional loss of environmental services was assumed to be ~ US$521 million per year, in addition to its countless socioeconomic consequences (Garcia et al. 2017). Fundão tailings contain high contents of Fe (above 50%), SiO2 (above 10%) and Al (above 10%) and different concentrations of trace metals such as As, Cr, Cd, Hg and Pb (Gomes et al. 2017; Pires et al. 2003). Their metal composition persists over long periods of time in the environment and may accumulate and pass from one species to the next through the food chain (Fernandes et al. 2016; do Carmo et al. 2017). Furthermore, their physical–chemical properties may make them very hazardous, e.g. by exhibiting more synergistic tendencies than antagonistic tendencies in terrestrial ecosystems, which raises future environmental concerns (Fernandes and Ribeiro 2017; Chibuike and Obiora 2014).
Currently, the toxicity of mine tailings is understudied, and information about their effects on terrestrial fauna is scarce, mainly on microarthropods (Wahl and Maboeta 2012). Edaphic mesofauna organisms such as mites and springtails represent approximately 95% of the microarthropod abundance in soils (Orgiazzi et al. 2016). Oribatid mites are the world’s most abundant soil arthropods and inhabit almost all types of land use. In the organic horizons of most soils, they can be found in densities of several hundred thousand individuals per square metre (Zaitsev and Van Straalen 2001). Most species are generalists that feed on decomposing plant debris and fungi, constituting important links in the food web as decomposer organisms (Schneider et al. 2004). They also accelerate the release of elements within ecosystems either directly or indirectly by stimulating the action of soil microbes. Moreover, they significantly contribute to the formation and maintenance of soil structure (Luo et al. 2015). Thus, these organisms provide an early indication of ecosystem health and play an important role in functional ecology and associated ecosystem services (Owojori et al. 2019).
Several studies have reported that oribatid mites have a higher ability to accumulate trace metals than do other soil arthropods (Caruso et al. 2009; Skubala and Zaleski 2012). However, the levels of sensitivity and resistance are quite variable among different species of mites and dependent on a variety of factors, including microhabitat choice, mode of feeding, uptake and elimination kinetics, and biochemical strategies as well as particular morpho-physiological traits of species (Van Straalen et al. 2001; Eeva and Penttinen 2009). With respect to the sensitivity of mite species in ecotoxicological assessments, few studies have been carried out with S. praeincisus, most of which were related to the biological aspects or effects of genetically modified plants (Subías 2004; Oliveira et al. 2007). To date, no study of this species has been performed in polluted soils (by mine tailing and/or trace metals). Toxic effects on their behaviour, metabolism and biological development should be further understood, since it is a predominant species in tropical soils (Ferreira et al. 2012; Ermilov and Anichkin 2014). The importance of toxicity testing with indigenous species that typically inhabit soils where contamination occurs or may occur has gained recognition in risk assessment for terrestrial fauna (Kuperman et al. 2009). Different sensitivity levels of mite species to pollutants may be an excellent factor to determine the maximum permissible values of soil pollutants to ensure their quality while ensuring they remain biologically functional.
Despite the worldwide need for studies focused on environmental tragedies involving mine tailings and dams, even four years after the Fundão dam rupture, the impacts of mine tailings on the Rio Doce basin are not properly understood. In this case, the use of an ecotoxicological approach is highly necessary to understand the effects that mine mud has on organisms living and feeding there. In addition, it complements existing information to help protect the structure and functioning of terrestrial ecosystems.
The aim of this study was to assess the toxicity of Fundão tailings to soil oribatid mites. The total density of oribatid mites and the bioaccumulation of trace metals were investigated in situ, and the acute, chronic and accumulation effects were evaluated in ex situ conditions for the pantropical mite Scheloribates praeincisus.
Materials and methods
Study areas and sampling collection
The Fundão dam located in Mariana city (Minas Gerais state, Brazil) was situated in a very steep area (20% to 40% slope; most portions were 850–1090 m altitude) (de Souza et al. 2005). This geomorphological characteristic favoured the formation of intense tailing waves in areas with lower slopes (850–38 m altitude) after the dam ruptured (CPRM and ANA 2015a, b).
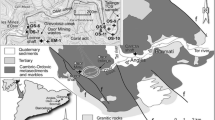
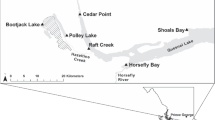
The study comprises 18 areas of the Rio Doce basin that were directly affected by the Fundão tailings spill along the 679-km stretch, between the Fundão dam and the Atlantic Ocean (Fig. 1). The geographical coordinates of the study areas and their distances from the ruptured areas of Fundão dam are available in Supplementary Material (Table S1). In addition, a reference area (RA) of native forest in Meloso (Conceição do Mato Dentro, Minas Gerais) that was unaffected by the Fundão tailings was included in the study (Table S1). In all study areas, soil samples were collected under riparian vegetation (when present). This vegetation type received a higher volume of Fundão tailings (Sánchez et al. 2018). In each area, four samples from ten subsamples (0–10 cm of depth) were collected with the aid of a flat hand shovel from a representative grid (20 × 20 m). The first sampling occurred between 7 and 17 days after the dam rupture in November 2015, and the second sampling occurred in October 2018. The soil physical and chemical properties were analysed according to analytical procedures recommended by EMBRAPA (2011). The soil physicochemical characterizations of the 19 study areas are shown in Table 1.
Trace metal determinations were carried out by inductively coupled plasma–mass spectrometry (ICP-MS) for Al, As, Cd, Cu, Cr, Fe, Pb, Mn, Ni and Zn according to USEPA (1994, 2001) and by atomic absorption spectrometry using cold vapour atomization for Hg according to USEPA (1986). Quality control was certified by São Joaquim 2709 reference material provided by the National Institute of Standards and Technology-NIST (U.S. Department of Commerce), and the results indicate a satisfactory recovery ranging from 85 to 97%. Trace metal concentrations are given in Table 2.
Ecotoxicological assessments—ex situ
Test species
The soil mite Scheloribates praeincisus (Berlese 1910) (Acari: Oribatida, Scheloribatidae) was used in the ecotoxicological assessments of mine tailings. This pantropical species is commonly abundant and representative in tropical forest soils (Subías 2004; Oliveira et al. 2005, 2017; Ferreira et al. 2012) and in agroecosystems (Oliveira et al. 2001; Simões et al. 2008). The species was obtained from the soil and litter of the Atlantic Forest of south-eastern Brazil and extracted using the Tullgren method. Specimens were cultured in the laboratory in Petri dishes with a layer of moist plaster of Paris mixed with activated charcoal 8:1, weight/weight (ISO 1999). Mites were kept in the laboratory at 20 °C ± 2 °C with a photoperiod of 12:12 h light:dark for up to 4 months prior to the experiments. Cultures were kept moistened with deionized water, and a small amount of biological dry yeast (Saccharomyces cerevisiae) was provided as a food source once per week.
Ecotoxicity tests
All toxicity tests were composed of five treatments of different tailing concentrations (dilution mixture), which consisted of the mixture of a natural soil (from RA) with the test soil (from the 18 areas contaminated by mine tailings). For each test soil, there were combinations of dilution mixtures, which were equivalent to 0% (only RA), 25%, 50%, 75% and 100% of the mine tailing concentrations. All treatments had five replicates. For effective comparisons between uncontaminated soils, tropical artificial soil (TAS) was adopted as a control treatment. The TAS was prepared following OECD guidelines (2016) and adapted according to Garcia (2004); specifically, the TAS was composed of 75% fine sand (washed and dried), 20% kaolin clay and 5% coir dust (dried at 60 °C), the latter representing the organic fraction, replacing the Sphagnum peat used in artificial soil for temperate regions. When necessary, the TAS pH value was adjusted to 6.0 ± 0.5 with calcium carbonate (CaCO3).
Acute and chronic tests were performed for 14 days and 35 days, respectively, and they were based on OECD guidelines (2016). The bioaccumulation tests were carried out for 42 days. All assessments were performed in cylindrical glass containers (10 cm diameter and 8 cm height) filled with 10 g dry mass of each test soil (from 19 areas). All containers were covered with parafilm and placed into incubators. The containers were aerated twice per week, and in this period, the moisture content was adjusted by monitoring the weight.
For toxicity expositions, adults of S. praeincisus (approx. 5 weeks old) from synchronized cultures (25 specimens in bioaccumulation tests and 10 specimens in acute and chronic tests) were used, which were transferred into each replicate test container with the aid of a stereomicroscope. In the acute and bioaccumulation tests, only adult males were exposed to a range of tailing concentrations mixed into the soil. In contrast, in the chronic exposure experiments, only females were introduced. All exposures were kept in the laboratory at 20 °C ± 2 °C with a photoperiod of 12:12 h light:dark. For each test container, a small amount of 2 mg of biological dry yeast (S. cerevisiae) was offered to the mites at the beginning of the acute and chronic tests and every 14 days in the bioaccumulation tests.
At the end of the tests, the number of surviving mites and the number of juveniles in each test container were counted 48 h after extraction with a modified Berlese–Tullgren apparatus at ± 45 °C (Aquino and Correia 2006).
Metal accumulation assessments on mites were carried out following the same analytical procedure described above for soil samples from the 18 contaminated areas (at 100% mine tailing concentration, i.e. undiluted test soils). A group of 50 surviving specimens was used in the analysis of metals, and the samples were pooled together and weighed three times to establish an accurate weight. For each treatment evaluated, the mites were dried and digested with concentrated nitric acid (7:1 by volume, Suprapur grade, Merck) and diluted with distilled and deionized H2O. The bioaccumulation factor (BAF) was calculated as the Ca/Cs ratio, where Ca is the metal concentration in surviving mites and Cs is the total metal concentration in the test soil.
Ecotoxicological assessments—in situ
For this study, the same RA and 10 other contaminated areas (FD1, FD2, Ges, BL, SSS, SJG, CN, V, GV and Tum) were assessed (previously described in Sect. 2.1). These 10 areas received higher Fundão tailing volumes (CPRM and ANA 2015b; Sánchez et al. 2018) and the highest chemical presence of heavy metals (Table 2). In the 11 sampling areas, five random topsoil samples (0–20 cm depth) were collected using a 5-cm-diameter corer. Sampling was carried out in October 2018 from a representative area (20 m × 20 m) of each site. Mites were separated from the soil using the Tulgren method.
In the field, the mine tailing ecotoxicity on mites was assessed by mite total density and metal bioaccumulation. The total density of oribatid mites was expressed as the number of individuals per m2 in each area. The organisms extracted for heavy metal determinations were preserved in a mixture of water and glycerol with the addition of alcohol (approximately 5%) and kept in a refrigerator to avoid evaporation and micro-organism development. All oribatid mites found in the contaminated areas were analysed. (The species were not identified or separated by stage of development.) The bioaccumulation analysis of Al, As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb and Zn concentrations in mites, as well as the estimation of BAF values from study areas, was performed according to the ex situ study (previously described).
Statistical analysis
The effect concentrations (ECs) causing 10%, 50% and 90% of responses in acute and chronic tests in mites were estimated in their respective tests. Lethal concentration (LC) was estimated by regression techniques such as logistic regression functions and probit analysis using the PriProbit® programme version 1.5. EC values for reproduction were calculated using nonlinear regressions.
The lowest observed effect concentration (LOEC) was defined as the concentration that was significantly different from the control. The no observed effect concentration (NOEC) was defined as the highest test concentration that did not produce a statistically significant adverse effect on reproduction when compared to the control. The LOEC and NOEC values from the individual tests were obtained by performing analysis of variance (ANOVA) and then by comparing the mean number of juveniles from each test tailing concentration with the control using Dunnett’s test at the p < 0.05 level (software Minitab®17.1).
Results
Ecotoxicological assessments—ex situ
At the end of the toxicity tests (acute and chronic), the mean adult mortality per replicate in each test treatment was < 10% for the control soil and < 16% for the reference soil. These percentages fulfilled the validity criteria established by the OECD (2016).
After 14 days of acute exposure, the responses of S. praeincisus adults in the different mine tailing concentrations were similar among the 18 test soils (from contaminated areas). This result suggested a tendency for an increase in mortality in accordance with the increase in the tailing concentration. For all areas affected by the tailing spill, the survival of S. praeincisus adults was higher than 50% in all tested concentrations (Fig. 2a, b; Table 3). The LC50 values > 100% tailing concentrations indicated a low toxicity effect in the short term (Table 3). Although the mortality was slightly lower in 2018, there was no significant difference between treatments for the 2015 test soils. The highest values of mortality at 100% tailing concentration (undiluted test soils) were found in the GV, Per and Tum areas, with values of 46%, 44% and 42% for 2015 (Fig. 2a) and 44%, 40% and 38% for 2018, respectively (Fig. 2b). Additionally, higher mite mortalities were noted in the areas nearest to the ruptured areas of Fundão dam, such as the FD1 (40% and 38%), FD2 (38% and 36%), F (34% and 36%) and Pe (36% and 34%) areas (for the 2015 and 2018 sampling years, respectively) (Fig. 1a, b). The lowest lethal concentration causing a 10% effect (LC10) on mites was observed at 43%, 39.7% and 36.5% tailing concentrations in the areas of CN, GV and Tum, respectively (for the 2015 sampling year), and in the V, Per, GV and Tum areas with values of 44.1%, 43.9%, 39.3% and 36.2% tailing concentrations, respectively (for the 2018 sampling year) (Table 3). For most areas, the NOEC and LOEC values were from the 50% and 75% tailing concentrations, respectively (Table 3). The highest survival (> 75%) was noted in the SSS, SARD and Ges areas at 100% mine tailing concentration (Fig. 2).
Reproduction was a more sensitive endpoint than the acute test to assess Fundão tailing toxicity on mites. Moreover, the same tendency noted in the acute mite responses was evidenced in the chronic tests, for which the increase in reproductive inhibition followed the increase in Fundão tailing concentration in soil (Fig. 3a, b). In turn, the highest reproductive inhibitions of S. praeincisus were not associated with the areas closest to where the Fundão dam breaking occurred (Fig. 3a, b). At the end of the chronic test, the mean number of S. praeincisus juveniles was ≅ 210 and 226 specimens in the control and reference treatments, respectively (Fig. 3a, b), for the test soils sampled in 2015. In all test soils contaminated at ≥ 50% tailing concentrations, a number of juvenile mites ≤ 176 was observed (Fig. 3). In terms of the number of juvenile mites, there were no significant differences between the 2015 and 2018 sampling years (Fig. 3a, b). A decrease in reproduction was noted in test soils from the Tum, Gv, Per and CN areas at 100% tailing concentration, revealing a mean juvenile number of S. praeincisus < 10, thus differing statistically from other areas. The highest mean values of reproductive inhibition were found in the FD1, FD2 and F areas, with 54, 62 and 80 juvenile mites, respectively. This result is in contrast to that observed in the areas farthest from the areas where the Fundão dam rupture occurred, with juvenile counts of 90, 86 and 95 in the PO, SSS and SARD areas, respectively. Reproductive inhibitions ≥ 90% S. praeincisus were noted at ≤ 100% tailing concentrations, mainly in the GV and Tum areas (Table 4). The lowest EC10 values were noted in the areas of CN: 19.6% and 29.9%; Per: 22.2% and 28.2%; GV: 20.8% and 26%; and Tum: 21.5% and 26% tailing concentration (for the 2015 and 2018 sampling years, respectively) (Table 4). In all areas, the NOEC and LOEC values were from the 25% and 50% tailing concentrations, respectively (Table 4).
For the 18 test soils (undiluted) at 100% tailing concentration, the values of accumulated metals in S. praeincisus mites were as follows: Cd > As > Pb > Cu > Cr > Hg > Ni > Zn > Mn and Al > Fe, after 42 days (Fig. 4). The BAF values of S. praeincisus ranged from 0.56 to 0.9 for Al, 0.32 to 0.8 for Fe, 1.0 to 1.2 for Mn and 0.5 to 1.4 for Zn (Fig. 4a). For Cu, Cr and Ni, the BAF values ranged from 1.3 to 3.0, 1.0 to 2.6 and 0.8 to 1.7, respectively (Fig. 4b). For As, Cd, Hg and Pb, the BAF values ranged from 1.7 to 4.7, 2.9 to 7.5, 1.2 to 2.2 and 1.7 to 4.4, respectively (Fig. 4c). Although apparent higher metal accumulation in mites was noted in the test soils sampled in 2015, for most investigated areas, there were no significant differences between the 2015 and 2018 sampling years. In the reference area, the trace metal accumulations showed BAF values ≤ 1.3 in mites (Fig. 4). In general, the metal bioaccumulation in S. praeincisus mites was quite homogeneous for most contaminated areas, with the exception of the GV, Tum, CP, V, CN, FD1, FD2, F and Per areas, which tended to accumulate more metals (Fig. 4). The Mn, Al and Fe accumulations had a more uniform distribution along the sample gradient, showing low variability between the areas.
Ecotoxicological assessments—in situ
Field studies indicated a mean total density of 1268 oribatid mites m−2 (≥ 250 μm) in the reference area. Comparatively, this area showed a higher mean density than that found in contaminated areas, for which the total number of individuals did not exceed 300 oribatid specimens per m2 at each sampling area (Table 5). Along the sample gradient, the GV, Tum and FD1 areas showed the lowest mean total density values (23, 25 and 26 oribatid mites m−2, respectively), followed by the FD2 and CN areas (52 and 53 oribatid mites m−2, respectively). On the other hand, the highest mean total density values were 298 and 255 oribatid mites m−2, which were found in the V and BL areas, respectively.
Field assessments indicated the following metal accumulation sequence in oribatid mites: Cd > As > Pb > Ni > Hg > Cr > Cu > Zn > Mn > Al > Fe (Fig. 5). For most metals, the BAF values were higher under in situ assessments than those found in ex situ assays, with the exception of the BAF values of Al, Fe and Mn. The BAF values of Al, Fe and Mn were quite similar among the contaminated areas, ranging from 1.0 to 1.1 (Al), 0.8 to 1.0 (Fe) and 1.0 to 1.6 (Mn); however, the BAF values were significantly higher than those in the reference area (0.6 (Al), 0.5 (Fe) and 0.76 (Mn)) (Fig. 5a). In general, for Cr, Cu and Ni, the lowest BAFs were observed in areas closest to where the Fundão dam broke areas, and the highest BAFs were found in areas that presented higher contents of these metals in the soils (Fig. 5b). For As, Cd and Pb, the BAF values followed a higher bioaccumulation in the GV > Tum > V > FD1 > FD2 > SJG and CN > Ges > BL > SSS > RA areas (Fig. 5c). Among these areas, low variability between the BAFs was noted for Hg, which had the highest bioaccumulation in the GV and Tum areas (Fig. 5). Despite the low Cd and Hg concentrations in soils in most areas contaminated by mine tailings, these metals were bioaccumulated in oribatid mites up to fourteen and seven times higher, respectively, in the field than in laboratory conditions (Fig. 5c).
Discussion
Apparently, the Fundão tailings did not have significant short-term effects, as noted in the acute responses of S. praeincisus. This condition in the presence of mine tailing for soil mites has not been scientifically reported. Due to the predominance of a great mixture of trace metals in the composition of this tailing type, the literature provides several reports of their long-term effects on organisms (which will be discussed subsequently). We emphasize that trace metals have silent and cumulative effects on oribatid mites. This result does not mean that tailing do not negatively influence their behaviour and biological development (as noted in the chronic responses). Because of these findings, we can assume that in areas contaminated by mine tailings the selection of species and/or taxonomic groups that are more adapted or resistant may occur, reflecting their abundance, diversity and mainly their functionality to the environment.
The highest acute and chronic effects of Fundão tailings on S. praencisus may be associated with physical–chemical characterization of the tested contaminated soils, given that the highest mortalities were observed, e.g. in the FD1 and FD2 areas of higher bulk density, and in areas that presented higher metal concentrations, such as the GV and Tum areas. Previous studies have attributed the highest metal concentrations in these areas to a combination effect caused by the Fundão tailings and the intensive mining activities that have occurred in these regions since the 1930s (Maxine 2013).
Similar abilities to accumulate metals have been noted in species of the same genus, e.g. the bioaccumulation factors of Cd, Cu, Pb and Zn in S. praeincisus showed BAFs of 7.5, 3.0, 4.5 and 1.4, respectively (in the present study), while Scheloribates laevigatus (Koch) had BAFs of 7.8, 1.41, 4.76 and 1.31, respectively, according to El-Sharabasy and Ibrahim (2010). However, opposite responses in oribatid species that are closely related taxonomically can also be expected, mainly in iron accumulation, as noted by Zaitsev and Van Straalen (2001).
This study evidenced similar trends of metal accumulation in mine tailings to those reported in other studies with oribatids, even under the influence of different abiotic and biotic factors (Zaitsev and Van Straalen 2001; El-Sharabasy and Ibrahim 2010). The oribatid ability to accumulate high metal concentrations without dying may be associated with different detoxification strategies, such as by permanently storing metals in insoluble granules within the digestive tract (Ludwig et al. 1992; Lebrun and Van Straalen 1995). Oribatid life cycles can be another attenuating aspect of metal bioaccumulation and toxicity. Classically, oribatids have six distinct morphological stages, each separated by a moult. The pre-larvae usually have an inactive and short-lived state. The larva, protonymph, deutonymph, tritonymph and adults are active and free living. However, each stage has an inactive period: the pre-ecdysial resting stage (Lebrun and Van Straalen 1995). This inactive time allows oribatid mites to accumulate high metal concentrations in their cuticle/tissue and, after that, to eliminate through moulting with no short-term interference, especially in relation to the survival of these organisms (Kratzmann et al. 1993). Detoxification strategies of trace metals allow the animal to survive, but they require much energy (Van Straalen et al. 1989).
The ability to accumulate metals was not associated with the feeding habits of ten oribatid species, mainly for Cu, Cd, Pb and Fe accumulations (Zaitsev and Van Straalen 2001). However, there was a strong relation to Zn accumulation in phytophage and mycophage mites (Zaitsev and Van Straalen 2001). Fungi tend to accumulate more Zn in fungal cell content than in cell walls, and it is an essential element for their development (Khan et al. 2000).
Among the metals investigated in this study, Cd accumulated the most in mites (BAF = 7.5 ex situ and BAF = 13.2 in situ), even though Cd was found at low concentrations in the evaluated soils. This relation between Cd concentrations in soils and mites was also noted by El-Sharabasy and Ibrahim (2010), but with expressive variability in the bioaccumulation factors (from 0.79 to 25.92) for the four oribatid species tested. According to the Agency for Toxic Substances and Disease Registry (ATSDR), Cd is the seventh most toxic element, being a non-essential element to organisms; it affects the enzymatic systems of cells and stimulates oxidative stress and nutritional deficiency in plants (Patrick 2003; Irfan et al. 2013). The effects and mechanism of Cd toxicity are not understood clearly, but its effects on cells are known in invertebrates (Patrick 2003). Its concentration may be increased 3000 fold when it binds to cysteine-rich proteins such as metallothionein. Relating this element in the food web, the chemical complexation of Cd may induce hepatotoxicity and nephrotoxicity. In addition, it has the ability to bind with cysteine, glutamate, histidine and aspartate ligands, leading to iron deficiency (Castagnetto et al. 2002; Jaishankar et al. 2014). In food assessments, green algae (Protococcus sp.) contaminated at ≥ 247 mg Cd kg−1 soil indicated significant reductions in fertility and growth of Archegozetes longisetosus (tropical parthenogenetic oribatid), and it increased their mortality and the developmental time of their offspring (Seniczak and Seniczak 2002).
In this study (in the laboratory and the field), As and Pb were also highly accumulated in mites. In living organisms, As is a protoplastic poison since it primarily affects the sulphhydryl group of cells, causing malfunctioning of cell respiration, cell enzymes and mitosis (Jaishankar et al. 2014). The effects of As on mites are still poorly understood. With respect to Pb accumulations in mites, wide differences between species have been observed (BAF values for Pb ranged from 1.4 to 6.4), possibly diverging due their food strategies (Zaitsev and Van Straalen 2001; El-Sharabasy and Ibrahim 2010). Platynothrus peltifer seems more tolerant to Pb exposure in soils than other oribatid species (Luo et al. 2015).
Cu in oribatids has been described as a persistent accumulator element (Skubala and Kafel 2004). Zaitsev and Van Straalen (2001) showed little variation in Cu accumulation for among ten oribatid species. In a variety of soil invertebrates, such as snails, isopods and arachnids, the Cu concentrations may be used in haemocyanins to transport oxygen (El-Sharabasy and Ibrahim 2010). However, for oribatids, high Cu concentrations may indicate the presence of other substances capable of accumulating copper or may indicate this element has a crucial role in their metabolism, since the mites do not possess haemocyanin.
The lowest BAF values for Fe and Al found in mites (in this study) may be justified by the low absorption of these elements through the intestinal walls or by the fast excretion of oribatids (Janssen and Hogervorst 1993). Few metals can be removed through elimination functions, while most investigated metals tend to accumulate in the body and food chain, exhibiting chronic toxicity. According to Zaitsev and Van Straalen (2001), mites tend to accumulate Zn and Fe more intensively in non-contaminated areas because they need Zn and Fe for their metabolism.
The bioaccumulation in the field was five times higher than that in the laboratory for the 42-day experiments. This difference can be related to the longer exposure time to contaminants in situ conditions, as observed by Buch et al. (2017b). Although in the field assessments have not been possible to estimate how long the bioaccumulation of metals in soils has been occurring, the incontestable fact is that the presence of them in soils may lead to various adverse effects in the biological development of soil-living communities. Metal concentrations in soils lead to increased toxicity through the food web in terrestrial fauna due to biomagnification processes and to synergistic interactions caused by chemical changes (compounds or species). For example, Hg, through different environmental factors and interactions between soil organisms, can lead to methylation, potentiating its toxicity and availability in soils (Buch et al. 2016, 2017a, b, 2018).
Our field results showed a mean oribatid density of ≅ 1268 individuals m−2 in the reference area, while studies in Amazon forest soils have reported densities of 1800 oribatids m−2 (Moraes et al. 2011) and from 3157 to 7340 mites m−2 (Franklin et al. 2001). Many studies have shown that the composition of oribatid communities in soils polluted by heavy metals can be negatively influenced. Jamshidian et al. (2015) observed diversity reductions in areas polluted with high Zn and Cd concentrations, but no effect with any Cu concentrations. Moreover, these authors also evidenced no relationship between the metal levels on soils and the total density of oribatids, showing populations from 25,000 to 30,000 individuals m−2. On the other hand, El-Sharabasy and Ibrahim (2010) reported the smallest total density of oribatid mites (from 3024 to 659 individuals m−2) in soils impacted by high Cd, Cu, Pb and Zn concentrations. Some researchers have noted that the total oribatid density was often non-responsive, due to considerable inter-species differences in sensitivity. With an increasing metal load in the soil, sensitive species may be replaced by resistant species without changing the total number of mites (Skubala and Kafel 2004). In this study, the Fundão tailing toxicity to mites was associated with their characteristics, mainly with respect to metal composition. In the laboratory, the test soils that presented higher toxicity to S. praeincisus were those sampled in the areas with a higher indicated metal presence. Furthermore, the same influence was noted in field assessments (in accumulations and mite density fluctuations). Despite the complex interaction between abiotic and biotic factors in terrestrial environments contaminated by tailings, some studies have reported similar influences on the abundance and diversity of mites, e.g. in areas contaminated by mine tailings (Frouz et al. 2008) and by cyanide-laced tailings (high Pb, Zn and Cu concentrations on soils), where just one oribatid mite species was found (Oppiella uliginosa) (Feketeová et al. 2016). Caruso et al. (2009) reported that other spatially variable factors, such as feeding habits and macrofauna reductions, may also affect oribatid assemblages, which may be indirectly influenced by metals. It seems obvious to have a significantly higher mite abundance in reference areas (uncontaminated areas) than in areas polluted by metals, as evidenced in many studies (Seniczak et al. 1997; Frouz et al. 2008; Manu et al. 2017; McAdams et al. 2018); however, by variable abiotic factors (e.g. temperature, particular soil parameters), the opposite can also often be found (Skubala and Zaleski 2012; Franklin et al. 2013). Some studies have suggested that the total density of oribatid mites on soil will rarely be reduced to zero or to the existence of a single mite species (Khalil et al. 2009). In general, the mite behaviour to avoid soil layers that present metals is not expected due to slow dispersal capacity (Janssen et al. 1990; Minor 2011).
Conclusion
From the present study, it can be concluded that mine tailings spilled on riparian soils may affect the life development of oribatid mites, mainly due to chronic toxicity. The evidence of a higher metal bioaccumulation in oribatids in the field than that in the laboratory can indicate the cumulative influence over time (at least three years of elapsed time after the Fundão dam rupture). In the case of terrestrial ecosystem contamination by rupture of mine tailing dams, the particularity of the actions to be taken will undoubtedly be based on the tailings composition and, above all, the chemical composition and metal combined concentrations because each metal acts differently on different types of organisms, as discussed in this study.
Therefore, we stress the need for research on ecological risk assessments that provide ecotoxicological studies with other taxonomic orders of soil fauna. Inadequate management of mine tailings is an equal problem impacting sites worldwide.
This study reinforces the urgency of efforts in decision-making by responsible players in the Fundão dam tragedy and by supervisory state agencies to try to minimize, mitigate or reclaim environmental damage from tailing spills that compromise soil quality and human, animal and environmental health.
References
Agnieszka, B., Tomasz, C., & Jerzy, W. (2014). Chemical properties and toxicity of soils contaminated by mining activity. Ecotoxicology, 23(7), 1234–1244.
Aquino, A. M., & Correia, M. E. F. (2006). Amostragem da mesofauna edáfica utilizando funis de Berlese-Tülgren modificado. Circular técnica 17. ISSN 1519-7328. Embrapa-Seropédica, Rio de Janeiro, 1–4.
Berlese, A. (1910). Lista di nuove specie e nuovi generi di Acari. Redia, 6, 242–271.
Buch, A. C., Brown, G. G., Correia, M. E. F., Lourençato, L. F., & Silva-Filho, E. V. (2017a). Ecotoxicology of mercury in tropical forest soils: Impact on earthworms. Science of the Total Environment, 589(1), 222–231.
Buch, A. C., Niemeyer, J. C., Correia, M. E. F., & Silva-Filho, E. V. (2016). Ecotoxicity of mercury to Folsomia candida and Proisotoma minuta (Collembola: Isotomidae) in tropical soils: Baseline for ecological risk assessment. Ecotoxicology and Environment Safety, 127, 22–29.
Buch, A. C., Schmelz, R. M., Niva, C. C., Correia, M. E. F., & Silva-Filho, E. V. (2017b). Mercury critical concentrations to Enchytraeus crypticus (Annelida: Oligochaeta) under normal and extreme conditions of moisture in tropical soils—Reproduction and survival. Environmental Research, 155, 365–372.
Buch, A. C., Sisinno, C. L. S., Correia, M. E. F., & Silva-Filho, E. V. (2018). Food preference and ecotoxicological tests with millipedes in litter contaminated with mercury. Science of the Total Environment, 633, 1173–1182.
Cabrera, F., Ariza, J., Madejón, P., Madejón, E., & Murillo, J. M. (2008). Mercury and other trace elements in soils affected by the mine tailing spill in Aznalcóllar (SW Spain). Science of the Total Environment, 390, 311–322.
Cánovas, C. R., Caro-Moreno, D., Jiménez-Cantizano, F. A., Macías, F., & Pérez-López, R. (2019). Assessing the quality of potentially reclaimed mine soils: Environmental implications for the construction of a nearby water reservoir. Chemosphere, 216, 19–30.
Caruso, T., Migliorini, M., Bucci, C., & Bargagli, R. (2009). Spatial patterns and autocorrelation in the response of microarthropods to soil pollutants: The example of oribatid mites in an abandoned mining and smelting area. Environmental Pollution, 157, 2939–2948.
Castagnetto, J. M., Hennessy, S. W., Roberts, V. A., Getzof, E. D., Tainer, J. A., & Pique, M. E. (2002). MDB: The metalloprotein database and browser at the Scripps Research Institute. Nucleic Acids Research, 30(1), 379–382.
Chibuike, G. U., & Obiora, S. C. (2014). Heavy metal polluted soils: Effect on plants and bioremediation methods. Applied and Environmental Soil Science. https://doi.org/10.1155/2014/752708.
CPRM and ANA - Companhia de Pesquisa de Recursos Minerais-Serviço Geológico do Brasil and Agência Nacional de Águas. (2015a). Monitoramento especial da bacia do rio Doce. Relatório 1-Acompanhamento da onda de cheia. CPRM, Belo Horizonte- MG, Dezembro 2015. Retrieved February, 2016 from www.cprm.org.br.
CPRM and ANA - Companhia de Pesquisa de Recursos Minerais-Serviço Geológico do Brasil and Agência Nacional de Águas. (2015b). Monitoramento especial da bacia do rio Doce. Relatório 2-Geoquímica. CPRM, Belo Horizonte-MG, Dezembro 2015. Retrieved February, 2016 from www.cprm.org.br.
de Souza, L. A., Sobreira, F. G., & Prado Filho, J. F. (2005). Cartografia e diagnóstico geoambiental aplicados ao ordenamento territorial do município de Mariana-MG. Revista Brasileira de Cartografia, 57, 190–203.
do Carmo, F. F., Kamino, L. H. Y., Tobias, R., Jr., de Campos, I. C., do Carmo, F. F., Silvino, G., et al. (2017). Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspectives in Ecology and Conservation, 15, 145–151.
Eeva, T., & Penttinen, R. (2009). Leg deformities of oribatid mites as an indicator of environmental pollution. Science of the Total Environment, 407, 4771–4776.
El-Sharabasy, H. M., & Ibrahim, A. (2010). Communities of oribatid mites and heavy metal accumulation in oribatid species in agricultural soils in Egypt impacted by waste water. Plant Protection Science, 46(4), 159–170.
EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. (2011). Manual de métodos de análise de solo (p. 212). Segunda edição. Rio de Janeiro: Centro Nacional de Pesquisa de Solos. Centro Nacional de Pesquisa de Solo (Rio de Janeiro, RJ).
Ermilov, S. G., & Anichkin, A. E. (2014). A new species of Scheloribates (Scheloribates) from Vietnam, with notes on taxonomic status of some taxa in Scheloribatidae (Acari, Oribatida). International Journal of Acarology, 40(1), 109–116.
Farmer, A. M. (2012). Manual of European environmental policy (p. 1043). London.: Routledge.
Feketeová, Z., Sládkovicová, V. H., Mangová, B., Pogányová, A., Simkovic, I., & Krumpál, M. (2016). Biological properties of extremely acidic cyanide-laced mining Waste. Ecotoxicology, 25, 202–212.
Fernandes, G. W., Goulart, F. F., Ranieri, B. D., Coelho, M. S., Dales, K., Boesche, N., et al. (2016). Deep into the mud: Ecological and socio-economic impacts of the dam breach in Mariana, Brazil. Natureza e Conservação, 14, 35–45.
Fernandes, G. W., & Ribeiro, S. P. (2017). Deadly conflicts: Mining, people, and conservation. Perspectives in Ecology and Conservation, 15, 141–144.
Ferreira, R. N. C., Franklin, E., de Souza, J. L. P., & de Moraes, J. (2012). Soil oribatid mite (Acari: Oribatida) diversity and composition in semi-deciduous forest fragments in eastern Amazonia and comparison with the surrounding savanna matrix. Journal of Natural History, 46(33–34), 2131–2144.
Figueiredo, J., Vila, M. C., Góis, J., Biju, B. P., Futuro, A., Martins, D., et al. (2019). Bi-level depth assessment of an abandoned tailings dam aiming its reprocessing for recovery of valuable metals. Minerals Engineering, 133, 1–9.
Franklin, E., de Moraes, J., Landeiro, V. L., de Souza, J. L. P., Pequeno, P. A. C. L., Magnusson, W. E., et al. (2013). Geographic position of sample grid and removal of uncommonspecies affect multivariate analyses of diverse assemblages: The case of oribatid mites (Acari: Oribatida). Ecological Indicators, 34, 172–180.
Franklin, E. N., Morais, J. W., & dos Santos, E. M. R. (2001). Density and biomass of Acari and Collembola in primary forest, secondary regrowth and polycultures in central Amazonia. Andrias, 15, 141–153.
Frouz, J., Prach, K., Pizl, V., Hánel, L., Starý, J., Tajovsky, K., et al. (2008). Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. European Journal of Soil Biology, 44, 109–121.
Gabarrón, M., Faz, A., Martínez-Martínez, S., & Acosta, J. A. (2018). Change in metals and arsenic distribution in soil and their bioavailability beside old tailing ponds. Journal of Environmental Management, 212, 292–300.
Garcia, M. V. B. (2004). Effects of pesticides on soil fauna: Development of ecotoxicological test methods for tropical regions. Ecology and development series (Vol. 19). Bonn: University of Bonn.
Garcia, L. C., Ribeiro, D. B., Roque, F. O., Ochoa-Quintero, J. M., & Laurance, W. F. (2017). Brazil’s worst mining disaster: Corporations must be compelled to pay the actual environmental costs. Ecological Applications, 27(1), 5–9.
Gomes, L. E. O., Correa, L. B., Sá, F., Neto, R. R., & Bernardino, A. F. (2017). The impacts of the Samarco mine tailing spill on the Rio Doce estuary. Eastern Brazil Marine Pollution Bulletin, 120, 28–36.
IBAMA - Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. (2017). Operação Áugias - Fase Argos - Etapa III. Relatório geral de vistoria. Diretoria de Uso Sustentável da Biodiversidade e Florestas. 1–65.
Irfan, M., Hayat, S., Ahmad, A., & Alyemeni, M. N. (2013). Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi Journal of Biological Sciences, 20(1), 1–10.
ISO - International Organization for Standardization. (1999). ISO 11267:1999. Soil Quality - Inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. ISO, Geneve, Switzerland. www.iso.org.
Jaishankar, M., Mathew, B. B., Shah, M. S., & Gowda, K. R. S. (2014). Biosorption of few heavy metal ions using agricultural wastes. Journal of Environment Pollution and Human Health, 2(1), 1–6.
Jamshidian, M. K., Saboori, A., Akrami, M. A., & Van Straalen, N. M. (2015). Oribatid mite communities in contaminated soils nearby a lead and zinc smelting plant in Zanjan, Iran. Systematic and Applied Acarology, 20(3), 251–262.
Janssen, M. P. M., & Hogervorst, R. F. (1993). Metal accumulation in soil arthropods in relation to micronutrients. Environmental Pollution, 79, 181–189.
Janssen, M. P. M., Joose, E. N. G., & Van Straalen, N. M. (1990). Seasonal variation in concentration of cadmium in litter arthropods from a metal contaminated site. Pedobiologia, 34, 257–267.
Khalil, M., Janssens, T. K. S., Berg, M. P., & Van Straalen, N. M. (2009). Identification of metal-responsive oribatid mites in a comparative survey of polluted soils. Pedobiologia, 52(3), 207–221.
Khan, A. G., Kuek, C., Chaudhry, T. M., Khoo, C. S., & Hayes, W. J. (2000). Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere, 41, 197–207.
Kratzmann, M., Russell, D., Ludwig, M., Petersen, U., Wien, C., Storch, V., et al. (1993). Untersuchungen zur Bodenarthropodenfauna zweier Buchenwaldstandorte im Einflußbereich geogener Schwermetalle. Verhandlungen der Gesellschaft für Ökologie, 22, 413–418.
Kuperman, R. G., Checkai, R. T., Garcia, M. V. B., Rombke, J., Stephenson, G. L., & Sousa, J. P. (2009). State of the science and the way forward for the ecotoxicological assessment of contaminated land. Pesquisa Agropecuária Brasileira, 44(8), 811–824.
Lebrun, P., & Van Straalen, N. M. (1995). Oribatid mites: Prospects for their use in Ecotoxicology. Experimental and Applied Acarology, 19, 361–379.
Ludwig, M., Kratzmann, M., & Alberti, G. (1992). The influence of some heavy metals on Steganacarus magnus (Acari, Oribatida). Zeitschrift für angewandte Zoologie, 79, 455–467.
Luo, W., Verweij, R. A., & van Gestel, C. A. M. (2015). Toxicity of Pb contaminated soils to the oribatid mite Platynothrus peltifer. Ecotoxicology, 24(5), 985–990.
Macklin, M. G., Brewer, P. A., Balteanu, D., Coulthard, T. J., Driga, B., Howard, A. J., et al. (2003). The long term fate and environmental significance of contaminant metals released by the January and March 2000 mining tailings dam failures in Maramures County, upper Tisa Basin, Romania. Applied Geochemistry, 18, 241–257.
Manu, M., Băncilă, R. I., Iordache, V., Bodescu, F., & Onete, M. (2017). Impact assessment of heavy metal pollution on soil mite communities (Acari: Mesostigmata) from Zlatna Depression—Transylvania. Process Safety and Environmental Protection: Transactions of the Institution of Chemical Engineers Part B, 108, 121–134.
Maxine, M. (2013). Goodbye, Brazil: Émigrés from the land of soccer and samba (1st ed., p. 289). Madison: University of Wisconsin Press.
McAdams, B. N., Quideau, S. A., Swallow, M. J. B., & Lumley, L. M. (2018). Oribatid mite recovery along a chronosequence of afforested boreal sites following oil sands mining. Forest Ecology and Management, 422, 281–293.
Minor, M. (2011). Spatial patterns and local diversity in soil oribatid mites (Acari, Oribatida) in three pine plantation forests. European Journal Soil Biology, 47, 122–128.
Moraes, J., Franklin, E., de Morais, J. W., & de Souza, J. L. P. (2011). Species diversity of edaphic mites (Acari: Oribatida) and effects of topography, soil properties and litter gradients on their qualitative and quantitative composition in 64 km2 of forest in Amazonia. Experimental and Applied Acarology, 55, 39–63.
OECD - Organization for Economic Co-Operation and Development. (2016). Test no. 226: Predatory mite (Hypoaspis (Geolaelaps) aculeifer) reproduction test in soil. OECD Guidelines for testing of chemicals. Paris: OECD.
Oliveira, A. R., Argolo, P. S., de Moraes, G. J., Norton, R. A., & Schatz, H. (2017). A checklist of the oribatid mite species (Acari: Oribatida) of Brazil. Zootaxa, 4246, 1–89.
Oliveira, A. R., Castro, T. R., Capalbo, D. M. F., & Delalibera, I., Jr. (2007). Toxicological evaluation of genetically modified cotton (Bollgard®) and Dipel® WP on the non-target soil mite Scheloribates praeincisus (Acari: Oribatida). Experimental and Applied Acarology, 41, 191–201.
Oliveira, A. R., Norton, R. A., & Moraes, G. J. (2005). Edaphic and plant inhabiting oribatid mites (Acari: Oribatida) from Cerrado and Mata Atlântica ecosystems in the State of São Paulo, southeast Brazil. Zootaxa, 1049, 49–68.
Oliveira, A. R., Prieto, D., & Mores, G. J. (2001). Some oribatid mites (Acari, Oribatida) from the State of Sao Paulo, Brazil. Revista Brasileira de Zoologia, 18(1), 219–224.
Orgiazzi, A., Bardgett, R. D., Barrios, E., Behan-Pelletier, V., Briones, M. J. I., Chotte, J.-L., et al. (Eds.). (2016). Global soil biodiversity atlas (p. 176). Luxembourg: European Commission, Publications Office of the European Union.
Owojori, O. J., Ademosu, O. T., Jegede, O. O., Fajana, H. O., Kehinde, T. O., & Badejo, M. A. (2019). Tropical oribatid mites in soil toxicity testing: Optimization of test protocol and the effect of two model chemicals (cadmium and dimethoate) on Muliercula inexpectata. Chemosphere, 218, 948–954.
Patrick, L. (2003). Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Alternative Medicine Review, 8(2), 106–128.
Pires, J. M. M., Lena, J. C., Machado, C. C., & Pereira, R. S. (2003). Polluting potential of Samarco Mineração S.A. solid waste: A Germano dam case study. Revista Árvore, 27(3), 393–397.
Sánchez, L. E., Alonso, A. L., Barbosa, F., Brito, M. C. W., Laureano, F. V., May, P., et al. (2018). Impacts of the Fundão dam failure. A pathway to sustainable and resilient mitigation. Rio Doce Panel Thematic Report no. 1. Gland: IUCN.
Schneider, K., Renker, C., Scheu, S., & Maraun, M. (2004). Feeding biology of oribatid mites: A minireview. Phytophaga, 14, 247–256.
Seniczak, S., Dabrowski, J., & Dlugosz, J. (1997). Effect of copper smelting air pollution on the mites (Acari) associated with young Scots pine forests polluted by a copper smelting works at Giogow, Poland. 1. Arboreal mites. Water, Air, and Soil pollution, 94, 71–84.
Seniczak, A., & Seniczak, S. (2002). The effect of cadmium on Archegozetes longisetosus (Acari, Oribatida) in laboratory conditions. European Journal of Soil Biology, 38, 315–317.
Simões, R. A., Silva-Filho, M. C., Moura, D. S., & Delalibera, I., Jr. (2008). Effects of soybean proteinase inhibitors on development of the soil mite Scheloribates praeincisus (Acari: Oribatida). Experimental and Applied Acarology, 44, 239–248.
Skubala, P., & Kafel, A. (2004). Oribatid mite communities and metal bioaccumulation in oribatid species (Acari, Oribatida) along the heavy metal gradient in forest ecosystems. Environmental Pollution, 132, 51–60.
Skubala, P., & Zaleski, T. (2012). Heavy metal sensitivity and bioconcentration in oribatid mites (Acari, Oribatida) Gradient study in meadow ecosystems. Science of the Total Environment, 414, 364–372.
Subías, L. S. (2004). Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758–2002). Graellsia, 60, 303–305.
USDA - United States Department of Agriculture. (2017). Pinto valley mine environmental impact statement draft-final scoping and issues report. 219 pp. Accessed July, 2019 from http://www.pintovalleymineeis.us/documents/PVM_Scoping%20Report_Draft-Final_2017_0928.pdf.
USEPA - United States Environmental Protection Agency. (1986). Mercury in solid or semisolid waste (Manual Cold-Vapor Technique). Method 7471A. Springfield: United States of Environmental Protection Agency.
USEPA - United States Environmental Protection Agency. (1994). Determination of trace elements in waters and wastes by inductively coupled plasma – Mass Spectrometry. Method 2008. Cincinnati: United States of Environmental Protection Agency.
USEPA - United States Environmental Protection Agency. (2001). Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry. Method 2007. Cincinnati: United States of Environmental Protection Agency.
Van Straalen, N. M., Butovsky, R. O., Pokarzhevskii, A. D., Zaitsev, A. S., & Verhoef, S. C. (2001). Metal concentrations in soil and invertebrates in the vicinity of a metallurgical factory near Tula (Russia). Pedobiology, 45, 451–466.
Van Straalen, N. M., Schobben, J. H. M., & de Goede, R. G. M. (1989). Population consequences of cadmium toxicity in soil microarthropods. Ecotoxicology and Environmental Safety, 17(2), 190–204.
Wahl, J. J., & Maboeta, M. S. (2012). Soil mesofauna as bioindicators to assess environmental disturbance at a platinum mine in South Africa. Ecotoxicology and Environmental Safety, 86, 250–260.
Zaitsev, A. S., & Van Straalen, N. M. (2001). Species diversity and metal accumulation in oribatid mites (Acari, Oribatida) of forests affected by a metallurgical plant. Pedobiologia, 45, 467–479.
Acknowledgements
This study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) with a scholarship to A. Buch (202.453/2017) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The authors are grateful to Universidade Federal Fluminense (UFF), Empresa brasileira de Pesquisa Agropecuária (EMBRAPA), Empresa de Assistência Técnica e Extensão Rural (EMATER-MG) and Companhia de Pesquisa de Recursos Minerais (CPRM-MG). The authors also thank Bianca Prestes for help with laboratory and field work and Tales de Campo Piedade, who helped with digital satellite image processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buch, A.C., Sautter, K.D., Marques, E.D. et al. Ecotoxicological assessment after the world’s largest tailing dam collapse (Fundão dam, Mariana, Brazil): effects on oribatid mites. Environ Geochem Health 42, 3575–3595 (2020). https://doi.org/10.1007/s10653-020-00593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00593-4