Abstract
The work presents the historical evolution, objectives, goals, concepts, chemical and radiometric methods, results and conclusions for salt waters and natural peloids used in pelotherapy. This study assesses chemical composition, natural radioactivity concentrations and the radiological hazard in peloid and salt water samples, from ten places in the Techirghiol Lake from Romania. Pelotherapy is a very important procedure, and thus, the materials used for this purpose must be well characterized to guaranty safety use. Concentrations of elements such as Sr, Ba, Mn, Fe, Sb, Zn, Cu, Pb, Ti, Ni, Cr, As have been measured using ICP-OES analytical technique. The natural radionuclides such as 238U, 226Ra, 232Th and 40K have been determined by gamma-ray spectrometry. The average activity concentrations were of 0.48 ± 0.10 Bq/kg for 238U, 0.60 ± 0.10 Bq/kg for 226Ra, 0.30 ± 0.08 Bq/kg for 232Th and 17.5 ± 1.3 Bq/kg for 40K for salt water samples. Also, the mean activity concentrations for peloids were: 5.70 ± 1.00 Bq/kg for 238U, 6.85 ± 1.60 Bq/kg for 232Th, 15.3 ± 3.7 Bq/kg for 226Ra and 95.8 ± 5.5 Bq/kg for 40K. The results from this study contribute to the identification of possible contaminants in the salt water and peloid, and their association with the potential ecological and human health risk. In this context, of using salt water and peloid in a relatively long treatment period, several radiological indices have been calculated, to determine if the radionuclide’s content can be also harmful to human health. The assessment indicates that humans are not exposed to concentrations of metal contaminants higher than the international recommended values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peloids as defined by Gomes et al. (2013) are used for therapeutic treatments, rheumatic pathologies, arthritis, muscle and bone traumas, and for dermatological pathologies or aesthetic purposes (Baschini et al. 2010). Thus, the external application of peloids is called pelotherapy, which is a worldwide used method to treat numerous diseases (Kikouama and Balde 2010). The most known Romanian peloid is Techirghiol’s peloid. Due to its therapeutic effects, locals and people from all over the world have benefited from its healing effects (Ciobotaru et al. 2009; Marin et al. 2012).
The peloid of the Techirghiol Lake belongs to a group of organogenic underwater sediments, being characterized as a sapropelic coastal mud. This type of peloid is the product of extremely complex and long-lasting biochemical and chemical processes that mineral and organic substances from the lake suffer. The medical and scientific value Techirghiol Lake’s water and peloid have been the subject of numerous studies in the most diverse research areas. From a historical perspective, Alphons Saligny (1893) was the first researcher who studied the complex problems raised by the lake and the therapeutic use of the peloid with solid scientific proof, regarding its therapeutic actions and the methodology of treatments. In addition to this, professor Paul Bujor was the author of a complete study on the peloid followed by others (Munteanu and Dumitrascu 2011).
Subsequently, the peloid has been the subject of numerous research and clinical trials conducted by the physicians of the Spa, Balneary and Recovery Sanatorium Techirghiol, where many diseases are treated: degenerative rheumatic diseases or inflammatory type, post-traumatic sequelae, central and peripheral nervous system disorders, gynaecological disorders, dermatological disorders, peripheral vascular diseases, chronic back pain, fibromyalgia, ankylosing, spondylitis, rheumatoid arthritis, osteoarthritis, etc. (Ciobotaru et al. 2009; Marin et al. 2012).
One of the most intensive treatments is peloid packs—consisting of the application of 1–2-cm-thick layers of heated peloid to 38–46 °C on a limited region or on the entire body surface for 20–40 min, up to 10–14 days. This procedure can expose the patient to the ionizing radiation present in the peloid, mostly originating from radionuclides such as 238U and 232Th and from 40K. These primordial radionuclides and their progenies are of special interest because they are α-, β- and γ-ray emitters. The public exposure to the ionizing radiation is considered undesirable at all levels. From this point of view, the knowledge and understanding of natural radioactivity and induced doses of radiation along with the physico-chemical composition of the peloid and salt water are important and draw considerable attention from both physicists and physicians.
During the last decade, several studies on the chemical and radiometric characterization of peloids have been carried out in different regions of the world. For example, Carretero et al. (2014) compared the properties of three Spanish clays from the point of view of their mineralogy, geochemistry and physico-chemical properties to find out which is the most suitable for thermotherapy and pelotherapy. Quintela et al. (2012) investigated the clay sediments in order to achieve quality criteria establishment and certification of clayey products intended to be used, especially for peloids which have therapeutic actions. Diaz-Rizo et al. (2013) have studied radioactivity levels and radiation hazard of healing mud from the San Diego River, Cuba, showing the necessity of finding the geochemical abundance of toxic elements in peloids, including radioactive elements. Muñoz et al. (2015) provided a physico-chemical characterization and elemental specification of the complex system that composes the San Diego de los Baños peloid from Thermal Center, Cuba. Knorst-Fouran et al. (2012) have described the properties of the Dax peloid and its evolution when mixed with thermal and mineral water. Da Silva et al. (2015) looked into Peruíbe Black Mud characterization in natura and maturated forms by determining some of its physical properties, mineralogical and chemical composition, radioactivity content, as well as the assessment of radiation doses arising from the mud application over the human skin during the treatment. In Italy, Cantaluppi et al. (2014) have described radionuclides concentration in water and mud of Euganean Thermal District, and Veniale et al. (2007) analysed thermal muds. Karakaya et al. (2015) studied the radioactivity concentrations and dose assessments of therapeutic peloids from some Turkish spas. In Slovenia, Glavaš et al. (2017) presented the mineralogical, geochemical and thermophysical characterization of healing saline mud for use in pelotherapy and established quality criteria necessary for safe use. Shaltout et al. (2017) studied quantitative elemental analysis and natural radioactivity levels of mud and salt collected from the Dead Sea, Jordan. All these concerns for the determination of chemical and radioactive elements in peloids show their importance, from the point of view of the risks associated with human health and estimating doses for patients during treatment.
Thereby, the main objective of the study is to determine the activity concentrations of natural radionuclides and on this basis to estimate the doses received by the patients. The assessment of the gamma radiation dose from natural sources is of major importance as being one large contributor to the external dose of the population (Diaz-Rizo et al. 2013). In addition to this, the importance of determining the concentrations of some metals represents also a goal of this study, due to the fact that physico-chemical parameters such as salinity, pH, conductivity and temperature may influence these concentrations up to a toxic level. Some of these metals are becoming toxic when forming complexes with organic compounds, modifying the therapeutic properties, involving the transfer of chemical elements from the peloid to the human body, across the skin (Baron et al. 1990; Tateo et al. 2009).
Materials and methods
The study area
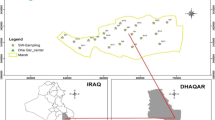
Situated on the Black Sea shore, between Techirghiol, Eforie Nord and Eforie Sud, Lake Techirghiol is the largest salt lake in Romania with a length of 7500 m (Fig. 1). It is located South–South–West from Constanta City and 150 m away from the Black Sea, in a climate specific to the Caspian–Pontic sea steppe. The lake has an irregular shape, consisting of a central basin and three branches. The Techirghiol Lake is fundamentally different from other lakes through its physico-geographic aspects, although the genesis is similar (river sea coast). From the point of view of origin, Lake Techirghiol is a former Black Sea lagoon, today separated from it. The complete disconnection of the sea is with the relatively recent date. The lake is 1.5 m below the Black Sea level, and it has a varying depth from 0.5 m to 5 m, sometimes reaching 9–10 m. The fauna consists of several species of arthropods, worms and protozoa. The flora consists of several algae species, and down to the bottom of the lake colourless sulphur bacteria predominate. Glossy and black peloid of Techirghiol Lake has a characteristic smell. It possesses physico-chemical properties that make it extremely useful in the treatment of various diseases along with salt water.
A general overview of the chemical composition of the lake shows, in the central part, homogeneous mineralization and salinity both horizontally and vertically of approximately 90 g/L. The general characteristics of water remain constant, being a chlorine–bromine–sodium sulphate and magnesium water. Chlorine is found in the form of sodium chloride and magnesium chloride. There is an appreciable amount of bromine, its presence being another indication of the marine origin of the lake (Munteanu 2012; Gâstescu et al. 2016). The absence of iodine seems to be related to metabolic actions of the zoo- and phytoplankton.
Sampling area and analytical techniques
Salt water samples preparation
Ten water samples were collected from Techirghiol Lake, mainly from the central part of the lake, where specific peloid exploitation areas exist. Most part of the lake is under the administration of the sanatorium, which also has the right to exploit the peloid. To observe the physical parameters of salt water samples, the pH was measured with a pH meter Consort P 901 on a 10% solution according to the SR EN ISO 10523:2012. The conductivity was measured with an Electrochemical Analyzer Consort K 912, according to SR EN 27888:1997, and the density according to SR ISO 758:1995. To determine the presence of metals in salt water samples, the Optima 2100 DV ICP-OES System (PerkinElmer) with dual-view optical (axial and radial) system was used. The calibration curves were obtained by dilution from the stock solutions of 1000 mg/L ICP Multi-Element Standard Certipur (Merck) and 100 mg/L Quality Control Standard 21 (PerkinElmer) (Sarojam and PerkinElmer 2010), according to the working procedures. These analyses focused on several elements, such as concentrations of strontium, barium, manganese, antimony, iron. Other metals were also considered such as copper, lead, titan, nickel, chromium, arsenic. However, these couldn’t be quantitatively determined in the salt water samples; the values obtained were below the quantification limit of the methods for determining the metals (Capra et al. 2016; Tanase et al. 2007). The optimized parameters for the operation of ICP-OES equipment are presented in Capra et al. (2016). Sample analysis was performed in duplicate, keeping the same conditions as for generating the calibration curve. The correlation coefficients of the calibration curves are between 0.9970 and 0.9999, fulfilling the acceptance criterion of r ≥ 0.997 (Tanase et al. 2007).
The major anions and cations, in salt water samples, have been determined using an ion chromatograph 850 Professional IC AnCat-MCS from Metrohm equipped with a Metrosep C4-150/4.0 column, ideal for the analysis of alkaline and earth alkaline metals in aqueous media and Metrosep C4-250/4.0 column for anions, being able to determine fluoride, hypophosphite, chlorite, bromate, chloride, nitrite, bromide, chlorate, nitrate, phosphate, sulphate and iodide anions (EPA 2007; Meng et al. 2008; Calisevici et al. 2009).
Peloid samples preparation
The peloid samples have been collected from the same points as the water samples, from depths between 6 and 9 m. These were dried at room temperature for 5 days, after which they were dried at 40 °C for 8 h, finely grounded, and then, 10 g was weighed to determine humidity at 105 °C. Subsequently, for the determination of metals by ICP-OES System (PerkinElmer), about 0.1 g of dried peloid at 105 °C was mineralized into the microwave digester (Multiwave 3000, Anton Parr GmbH, Austria) with a mixture of HNO3 (65%): HCl (37%): HF (50%) = 6: 2: 2 mL. As expected, more metals were found in peloid samples than in the salt water samples.
All physical–chemical analyses were performed in a RENAR accredited laboratory (gravimetric, volumetric, electrochemical, ICP-OES, etc., samples of fertilizers, biocides, waste water and drinking water, etc.) from The National Research and Development Institute for Chemistry and Petrochemistry—ICECHIM.
Radiometric analyses: experimental set-up
For gross alpha and gross beta analysis, small quantities with masses between 3.4 g and 5.6 g of both salt water and peloid samples have been measured with alpha–beta global PROTEAN ORTEC MPC-2000-DP system, in UP ALPHA–BETA measurement geometry. The full system is described by Calin et al. (2013) and consists of a scintillation radiation detector ZnS dual detector phosphor. The system was calibrated regarding its efficiency using sets of standard radioactive sources of 241Am and of 90(Sr-Y) (Calin et al. 2013) and reference samples of KCl prepared in the laboratory in two sets, each containing 7 samples. Efficiency determination as a function of sample mass geometry was used. The samples were placed in metallic trays located at 3 mm below the probe detector, inside the lead castle system, directly facing the probe detector. These were measured in 10 intervals of 100 min, the total acquisition time being of 16.7 h. In addition, a measurement with an empty metallic tray for this specific geometry was performed to establish the background count rate (Calin et al. 2016; Radulescu et al. 2017; Pintilie et al. 2017). In order to calculate the gross α−β activity from samples, the following equation is used, Eq. 1 (Pintilie et al. 2017):
where A(α, β) is the gross α or β activity of the measured sample (Bq/kg), R is the rate of alpha or beta measurement for the sample (counts/s), R0 is the rate of α or β, respectively, measured for background (counts/s), m is the entire mass of the sample resulted after the evaporation of water from the sample (kg), M is the mass of the fresh sample (kg), ε is the efficiency of the detector and m1 is mass of sample transferred to a stainless steel tray for measurements (kg).
For the assessment of activity concentrations of 40K, 238U, 232Th and 226Ra, a low background coaxial p-type HPGe detector was used, with a relative efficiency of 35% and energy resolution of 1.85 keV at 1332.5 keV for 60Co. The detector is used for certified activities such as radiological monitoring of the environment. The geometry used for gamma spectrometry measurements was a cylindrical plastic container of 7.5 cm diameter and 4 cm height. The samples are kept at room temperature for a month to reach secular equilibrium. The calibration of the detector for energy, peak shape and efficiency was carried out by using certified volume sources for 60Co, 134Cs, 137Cs, 152Eu and 241Am supplied by the institute’s Radiation Metrology Laboratory (Radulescu and Calin 2014). The spectra were acquired for the live time between 86400 s and 300000 s, in the energy range 40–2650 keV for 16384 channels. The measurements and calibration procedures were conducted in conformity with the procedures of the accredited SR EN ISO/CEI 17025: 2005 and according to designation certificate LI1653/2018 (in compliance with the criteria for the evaluation of the testing laboratories).
Results and discussion
Chemical analysis results
The water pH was 10 at 20 °C, fairly constant for all 10 samples, as well as the conductivity, at 25 °C, where the average value was 139.2 ± 1.4 mS/cm. At the same time, the water density, for the selected points, did not vary statistically significant and the average value was 1.060 ± 0.004 kg/m3.
The concentrations of some of the elements Sr, Ba, Mn, Fe, Sb, Zn have been measured for the collected samples (Table 1). By analysing these concentrations determined in the salt water samples, it can be observed that in the case of strontium the recorded variation is not large, being between 80.8 mg/kg and 94.8 mg/kg, which is less than 15%. However, for the other metals the variations are higher than 50%, as in the case of manganese and antimony with variation between 0.16 mg/kg and 0.33 mg/kg, and 0.15 mg/kg and 0.32 mg/kg, respectively. Even greater variations are observed for barium and iron, that is 80%, from 0.15 mg/kg to 0.71 mg/kg and from 0.98 mg/kg to 4.91 mg/kg (Table 1). In terms of zinc concentrations, these were determined only in two salt water samples; thus, no variation could be highlighted. The values measured for the above elements are very low compared with international standards (Long et al. 1995), below which adverse biological effects are unlikely to occur. The determination of other metals, such as Co, Pb, Ti, Ni, Cr, As, was also desired, but their concentrations were below the quantification limit for this specific method.
Concentration values for several chemical parameters for naturally constituents present in salt waters such as ion concentrations: Na+, K+, Ca2+, Mg2+, F−, Cl−, Br−, \({\text{SO}}_{4}^{2 - } ,{\text{NO}}_{3}^{ - } ,\)\({\text{NO}}_{2}^{ - } ,\)\({\text{PO}}_{4}^{3 - } ,\)\({\text{NH}}_{4}^{ + }\) are shown in Table 2. As expected, this is highlighting the character of the water, where high concentrations are observed for Na+, Cl−, Mg2+ and \({\text{SO}}_{4}^{2 - }\), while for K+, Ca2+ and Br− concentrations are in the order of a few tens. Instead, concentrations of nitrates, nitrites and phosphates are rather low, since in some samples these were not determined, meaning that those values were lower than the determination limit. These samples were subject to assay the ammonium concentration. For the first three cations, namely Na+, K+ and Ca2+, the variations in their values are within 30%; for Mg2+, F−, Cl−, the variations are between 45 and 64%; for Br−, \({\text{SO}}_{4}^{2 - }\) these are lower, around 16%. Three anions, namely \({\text{NO}}_{3}^{ - } ,{\text{PO}}_{4}^{3 - }\) and \({\text{NH}}_{4}^{ + }\), have large variations of 98%, 83% and 77%, respectively. Ammonium anions are a waste product of the metabolism of animals, from fish and aquatic invertebrates, and its concentrations are normal values for the type of water.
The average concentration values for major elements such as magnesium, calcium, potassium and iron (Table 3) are (27.6 ± 1.3) × 103 mg/kg, (94.7 ± 7.5) × 103 mg/kg, (12.1 ± 0.3) × 103 mg/kg and (19.9 ± 0.5) × 103 mg/kg, respectively. According to the presented data, there are positive correlations between the concentrations of magnesium/calcium and iron/potassium, since between the magnesium/potassium and iron/magnesium the correlations are negative (Fig. 2). Values of continental crust (CC) and upper continental crust (UCC) for the major element’s concentrations are presented for comparison (Table 3), but these are different and only the magnesium values are close to the CC concentrations, most likely due to variations in regional background levels. Quantitative analysis results of peloids for Dead Sea (Shaltout et al. 2017) show similar values of (32.5 ± 9.8) × 103 mg/kg for Mg, (180 ± 17) × 103 mg/kg for Ca, (22.2 ± 2.0) × 103 mg/kg for K and (41.8 ± 4.1) × 103 mg/kg for Fe.
Elements and metals concentrations measured in the Techirghiol Lake’s peloid samples (Table 4) were in average 401 ± 38 mg/kg for Sr, 230 ± 7 mg/kg for Ba, 334 ± 25 mg/kg for Mn, 56.6 ± 1.5 mg/kg for Zn, 21.5 ± 0.8 mg/kg for Cu, 29.5 ± 1.1 mg/kg for Cr, 2.7 ± 0.6 mg/kg for Pb, 4.5 ± 1.0 mg/kg for Ni and 2242 ± 82 mg/kg for Ti. Then, these concentrations were compared with other values reported by other authors for the peloid samples. A good agreement exists between values reported by Maor et al. (2006) for the Dead Sea black mineral mud and values reported in this paper for Sr, Ba, Mn, Zn, Cu and Pb. The values for Cr and Ni presented in Table 4 are lower than those in Dead Sea black mineral mud. Da Silva et al. (2015) found slightly higher values in Peruibe Black Mud than in Techirghiol peloid. The metals concentrations reported in soil and sediment samples from Bedimahi river by Zorer et al. (2009) are considerably higher than the values reported in this paper. Moreover, the threshold effect level (TEL) and probable effect level (PEL) were considered to quantify if adverse effects can occur for reported values. It can be easily observed that all values are well below TEL for which adverse biological effects rarely occur, except for copper. However, the average value of copper is below value considered by US EPA for marine sediment quality guidelines (SQGs), that is, 25 mg/kg for non-polluted sediments (EPA 2012).
A direct comparison of the measured elements and metals concentrations shows that the lowest are those specific to lead and nickel (Fig. 3). For these two metals, in some samples, the concentration values were lower than the limit of quantification for this specific determination method. The maximum determined value for lead was 4.9 mg/kg, which is lower than 12.1 mg/kg previously obtained by Radulescu et al. (2014). Also, in the case of nickel, the maximum value was 8.6 mg/kg which is two-fold smaller than 17.5 mg/kg reported by Radulescu (2014). Only in the case of manganese, their value of 92.5 mg/kg is five-fold smaller than the maximum value reported in this paper, 466.6 mg/kg. The elements and metals concentrations, in the peloids, do not have a strong correlation with each other, as shown in Fig. 3 and Table 5. Only in few cases, there are some relatively weak correlations, as can be observed from the Pearson correlation coefficients (Table 5).
The most obvious positive correlation is between manganese and strontium (Figs. 3, 4, Table 5) where the correlation coefficient is 0.75. Another positive correlation is seen between chromium and titanium (Fig. 4). Negative correlations are observed between chromium/strontium and zinc/strontium (Fig. 4). Other dependences are not observed for elements determined in peloid samples.
Differences in the results are due to the fact that trace metals in peloid may exist in different chemical forms or ways of binding. Trace metals may be recycled via chemical and biological processes, within the sedimentary compartment and back to the water media (Leschber et al. 1985; Tessier and Campbell 1987). Trace metals can be deposited and incorporated into peloid after entering the aquatic system, being an integral and dynamic part of the lake. The mobility and bioavailability of the metals varied significantly with the peloid properties: particle size, organic matter, carbonates, pH, redox potential and water dynamic.
Elements and metal concentrations and distributions are, in general, influenced by regional background levels (Grigoras et al. 2012), where geological factors are major controls for their distribution. These trace metals in the sediments have distinct geochemical behaviours, possibly originating from anthropogenic sources through inputs of water and air (Mardomingo et al. 2015; Kusin et al. 2018).
Radiometric results
Activity concentrations
The gross alpha and gross beta activity together with isotopes concentrations of 40K, 238U, 232Th and 226Ra in Techirghiol Lake is calculated for the salt water samples (WS1–WS10) and in peloid samples (PS1–PS10) (Table 6). The activity concentrations for all types of measurements have values considerable higher in peloid than in salt water samples. The average activity concentrations for 40K, 238U, 232Th and 226Ra in peloid samples are 95.8 ± 5.5 Bq/kg, 5.7 ± 1.0 Bq/kg, 6.85 ± 1.60 Bq/kg and 15.3 ± 3.7 Bq/kg, respectively. The activity concentrations measured for all ten points are rather similar and cannot be concluded that there is any measurement point that shows abnormal values or is influenced by anthropogenic activities. Comparing the activities reported in peloids for 226Ra, the results are similar to data presented by Shaltout et al. (2017), where a value of 14 Bq/kg is measured for mud from the Dead Sea Jordan and also by Da Silva et al. (2015), wherein an average of 15 Bq/kg is obtained for Peruibe Black mud. Other authors reported values slightly higher (Diaz-Rizo et al. 2013; Jonas et al. 2018). All reported values for 40K are higher than those presented in Table 6, the same situation occurring for 232Th and 238U (Diaz-Rizo et al. 2013; Shaltout et al. 2017; Jonas et al. 2018). The concentration of each radionuclide is lower than the world average values (UNSCEAR 2000). As a novelty, gross alpha and gross beta activities were determined in these samples to provide basic information to consumers and competent authorities regarding the exposure risk due to accidentally intake of salt water. The values for gross alpha activities and gross beta activities were in the ranges of 0.13–0.22 Bq/kg and 0.99–4.52 Bq/kg, respectively. The composition of natural peloids depends on their origin, making comparison difficult with other samples of peloids from other parts of the world. The results can serve, in the future, as a comparison when evaluating contribution from radionuclides released to the environment as a result of any human practices in the studied area.
Radiological hazard indices
In the context of radiological risk assessment of using salt water and peloid in relatively long treatment periods, few radiological indices are calculated to observe if the radionuclides content can be harmful to human health (Table 7).
Radium equivalent activity (Raeq)
Due to the fact that radium can be strongly adsorbed in mineral oxides present in peloid or by organic material, the radium equivalent hazard index is first calculated. Few models have been developed in order to keep the radiation dose limit due to a certain material to 1.5 mGy (1 mSv). It has been concluded that an activity concentration of 370 Bq/kg (1 μCi/g) of 226Ra, uniformly distributed in material, will give an annual dose of 1.5 mGy. This index has been in practice for the last 40 years for the assessment of the radiological hazard in environmental materials (Tufail 2012). It is also used when comparing specific activities of samples containing different concentrations of 226Ra, 232Th and 40K. It is defined on the assumption that 10 Bq/kg of 226Ra, 7 Bq/kg of 232Th and 130 Bq/kg of 40K produce the same gamma radiation exposure dose rate which is calculated as in Eq. 2 (Shaltout et al. 2017; Beretka and Mathew 1985):
where CRa, CTh and CK are the activity concentrations for the three radionuclides in Bq/kg. It can be noted that all values of Raeq for peloid and salt water samples (Table 7) are lower than the recommended maximum value of 370 Bq/kg (ICRP 1991).
Absorbed gamma dose rate (D)
Another important index that takes into consideration the contribution of gamma radiations from naturally occurring radionuclides 226Ra, 232Th and 40K is the absorbed dose rate calculated in Eq. 3, based on guidelines provided by ICRP and UNSCEAR (ICRP 1991; UNSCEAR 1993, 2000).
Equation 3 uses conversion factors to compute absorbed gamma dose rate (D) of 0.462 nGyh−1/Bqkg−1 for 226Ra, 0.604 nGyh−1/Bqkg−1 for 232Th and 0.0417 nGyh−1/Bqkg−1 for 40K and the assumption that other radionuclides such as 137Cs or 235U decay series have a negligible contribution to the total dose. CRa, CTh and CK from Eq. 3 have the same meaning as in Eq. 2.
The experimental results for the absorbed dose rate in the air are ranging from 0.66 nGy/h to 1.19 nGy/h for salt water samples and from 9.38 nGy/h to 19.77 nGy/h for peloid samples.
Annual effective dose (AED)
To estimate the annual effective dose, the conversion coefficient (0.7 SvGy−1) from the absorbed dose in the air to the effective dose and the outdoor occupancy factor (20%) must be considered. The annual effective dose (AED) outdoors in units of μSv/y is calculated by the following formula, Eq. 4:
The values for the annual effective dose in the air are in average of 1.13 µSv/y for salt water samples and of 18.6 µSv/y for peloid samples. The maximum calculated value for peloid sample was 24.3 µSv/y for the sample 7 because of the high values activities concentrations for 40K and 232Th. The annual effective dose rate results are ranging from 0.80 to 1.45 µSv/y for salt water samples and from 11.50 μSv/y to 24.25 µSv/y for peloid samples. The annual equivalent world average for outdoor terrestrial gamma radiation is 70 µSv/y, higher than the presented values.
Gamma radiation hazard index (Iγr)
Radiation hazards due to the natural radionuclides of 226Ra, 232Th and 40K were assessed by another index called representative level index, Iγr (Beretka and Mathew 1985), calculated with Eq. 5:
where CRa, CTh and CK are the activity concentrations (Bq/kg). The average Iγr in salt water and peloid samples is 0.014 and 0.23, respectively.
External hazard index (Hex)
The external hazard index (Hex) for the samples under analysis is given by Eq. 6 (Beretka and Mathew 1985). The aim of this index is to keep the values less than unity in order to keep the health hazard risk as low as possible.
where CRa, CTh and CK (Bq/kg) are described above. This index has very low values for salt water samples with an average of 0.005, but for peloid samples the average value is 0.10.
Excess lifetime cancer risk (ELCR)
The excess lifetime risk of cancer (ELCR) was calculated using Eq. 7:
where AED is annual effective dose rate defined as in Eq. 4, DL is the lifetime (70 years) and RF is the risk factor (1/Sv) that is fatal cancer risk per Sievert. The 103 report of ICRP (2007) uses a value of 0.055 for the nominal risk coefficient adjusted to the detriment of cancer for stochastic effects after a small dose rate radiation exposure to the public (Gomes et al. 2018).
The highest values for excess lifetime risks of cancer are for samples 7 and 8 (Table 7). These samples are collected from the northern part of the lake, where increased activities concentrations of 226Ra are observed. This rise of values could be due to human activities in the coastal area. The lowest values were found in the southern part of the lake where there is less influence from human activities. Values presented in Table 7 are in the same order of magnitude or somewhat smaller than others presented in the literature Diaz-Rizo et al. (2013), Karakaya et al. (2015), El-Arabi (2005), Otansev et al. (2016), Shaltout et al. (2017) and El-Taher et al. (2018a, b).
All calculated values for the radiological indices (radium equivalent activity, absorbed gamma dose rate, annual effective dose, gamma radiation hazard index, external hazard index and excess lifetime cancer risk) are lower than world average or the maximum allowed level defined by UNSCEAR and ICRP.
Conclusions
In this paper, were analysed and investigated from both chemical and radiometric perspective ten samples of salt water and peloid originating from Techirghiol Lake, which represents the most known and intensively used area in Romania for therapeutic purposes. The chemical composition highlighted the chlorine–sodium, magnesium, sulphurous and brominated character of water, as these elements presented relatively high concentrations. The results of the chemical analyses of water and peloid samples show variations in the elements concentrations, but measured values are very low compared with international standards, for which adverse biological effects are unlikely to occur. These variations have rather week correlations between the elements, but also between the water and peloid from the same point of measurement. By analysing the results presented in Tables 1, 3 and 4, can assort that the increase in element’s concentrations does not highlight a clear influence from human activity, close to shore and settlements. The elements detected in peloids are within the limits, and their concentrations are below the maximum allowance level.
Pelotherapy is a very old therapeutic practice, whose efficacy can be documented by modern techniques and clinical observations. Experiments showed that the amount transferred across the skin depends on the element’s concentration in the water and peloid. However, the exchangeable fraction is negligible with respect to the high salinity of the water, but still, after 20 min of peloid application only few chemical elements cross the skin.
Another objective of the study was to determine the activity concentration of natural radionuclides and based on them to estimate the doses received by patients. The assessment of the gamma radiation dose from natural sources is of major importance, being one large contributor to the external dose of the population. The annual effective doses to the public were between 11.5 μSv/y and 24.3 µSv/y, representing two orders of magnitude lower than the reference level of 1 mSv per year. The radiological impact of natural radionuclides was estimated for the gamma-ray exposure of the full body. The measured specific activity concentrations for the 238U, 232Th decay series and 40K and radiation hazard parameters are lower than the maximum allowance level defined by UNSCEAR and ICRP. The salt water and peloid samples analysed from Techirghiol Lake do not pose a radiological threat to the population’s health.
References
Baron, J., Legret, M., & Astruc, M. (1990). Study of interactions between heavy metals and sewage sludges. Determination of stability constants and complexation capacities of complexes formed with Cu and Cd. Environmental Technology,11, 151–162.
Baschini, M. T., Pettinari, G. R., Vallés, J. M., Aguzzi, C., Cerezo, P., Galindo, A., et al. (2010). Suitability of natural sulphur-rich muds from Copahue (Argentina) for use as semisolid health care products. Applied Clay Science,49(3), 205–212.
Beretka, J., & Mathew, P. J. (1985). Natural radioactivity of Australian building materials, industrial wastes and byproduct. Health Physics,48, 87–95.
Calin, M. R., Druker, A. E., & Radulescu, I. (2013). The calculation of the detection efficiency in the calibration of gross alpha–beta systems. Journal of Radioanalytical and Nuclear Chemistry,295, 283–288.
Calin, M. R., Radulescu, I., Ion, A. C., & Sirbu, F. (2016). Radiochemical investigations on natural mineral waters from Bucovina region, Romania. Romanian Journal of Physics,61, 1051–1066.
Calisevici, M. N., Perju, D., Dumitrel, G. A., Glevitzky, M., & Moldovan, R. C. (2009). Determination of anions and cations content in Romanian drinking waters by HPIC method. Chemical Bulletin of Politehnica University,54, 26–30.
Cantaluppi, C., Fasson, A., Ceccotto, F., Cianchi, A., & Degetto, S. (2014). Radionuclides concentration in water and mud of euganean thermal district. International Journal of Environmental Research,8, 237–248.
Capra, L., Manolache, M., Ion, I., & Ion, A. C. (2016). Validation of a method for determination of antimony in drinking water by ICP-OES. UPB Scientific Bulletin, Series B: Chemistry and Materials Science,78, 103–112.
Carretero, M. I., Pozo, M., Legido, J. L., Fernández-González, M. V., Delgado, R., Gómez, I., et al. (2014). Assessment of three Spanish clays for their use in pelotherapy. Applied Clay Science,99, 131–143.
Ciobotaru, C., Minea, M., Surdu, O., & Surdu, T. V. (2009). Statistic evaluation of the clinical benefits of rehabilitation in patients with spine cord injury undergoing complex treatment with specific therapeutic factors from Techirghiol Health Resort. Advanced technologies for enhanced quality of life, 2009. AT-EQUAL’09. https://doi.org/10.1109/at-equal.2009.23.
Da Silva, P. S. C., Torrecilha, J. K., de Macedo Gouvea, P. V., Máduar, M. F., de Oliveira, S. M. B., & Scapin, M. A. (2015). Chemical and radiological characterization of Peruíbe Black Mud. Applied Clay Science,118, 221–230.
Diaz-Rizo, O., Gelen-Rudnikas, A., Arado-Lopez, J. O., D’Alessandro, Rodriguez K., Gonzalez-Hernandez, P., Fagundo-Castillo, J. R., et al. (2013). Radioactivity levels and radiation hazard of healing mud from San Diego River, Cuba. Journal of Radioanalytical and Nuclear Chemistry,295, 1293–1297.
El-Arabi, A. M. (2005). Natural radioactivity in sand used in thermal therapy at the Red Sea Coast. Journal of Environmental Radioactivity,81, 11–19.
El-Taher, A., Alshahri, F., & Elsaman, R. (2018a). Environmental impacts of heavy metals, rare earth elements and natural radionuclides in marine sediment from Ras Tanura, Saudi Arabia along the Arabian Gulf. Applied Radiation and Isotopes,132, 95–104.
El-Taher, A., Zakaly, H. M. H., & Elsaman, R. (2018b). Environmental implications and spatial distribution of natural radionuclides and heavy metals in sediments from four harbors in the Egyptian Red Sea coast. Applied Radiation and Isotopes,131, 13–22.
Environmental Protection Agency (EPA). (2012). Summary of maximum allowable concentrations of chemical constituents in uncontaminated soil used as fill material at regulated fill operations. www.epa.state.il.us/land/ccdd/new-max-allowable-concentrations.
Environmental Protection Agency (EPA). (2007). Method 9056A determination of inorganic anions by ion chromatography. https://www.epa.gov/hw-sw846/sw-846-test-method-9056a-determination-inorganic-anions-ion-chromatography. Accessed 30 May 2019.
Gâştescu, P., Breţcan, P., & Teodorescu, D. C. (2016). The lakes of the Romanian Black Sea coast. Man-induced changes, water regime, present state. Romanian Journal of Geography,60, 27–42.
Glavaš, N., Mourelle, M. L., Gómez, C. P., Legido, J. L., Šmuc, N. R., Dolenec, M., et al. (2017). The mineralogical, geochemical, and thermophysical characterization of healing saline mud for use in pelotherapy. Applied Clay Science,135, 119–128.
Gomes, C., Carretero, M. I., Pozo, M., Maraver, F., Cantista, P., Armijo, F., et al. (2013). Peloids and pelotherapy: Historical evolution, classification and glossary. Applied Clay Science,75–76, 28–38.
Gomes, T., Song, Y., Brede, D. A., Xie, L., Gutzkow, K. B., Salbu, B., et al. (2018). Gamma radiation induces dose-dependent oxidative stress and transcriptional alterations in the freshwater crustacean Daphnia magna. Science of the Total Environment,628–629, 206–216.
Grigoras, G., Cuculeanu, V., Ene, G., Mocioaca, G., & Deneanu, A. (2012). Air pollution dispersion modeling in a polluted industrial area of complex terrain from Romania. Romanian Reports in Physics,64, 173–186.
ICRP-60. (1991). Recommendations of the international commission on radiological protection. Oxford: Pergamon Press. ISSN 0146-6453.
ICRP-103. (2007). Recommendations of the international commission on radiological protection (Vol. 37). Elsevier. ISSN 0146-6453.
Jonas, J., Somlai, J., Csordas, A., Toth-Bodrogi, E., & Kovacs, T. (2018). Radiological survey of the covered and uncovered drilling mud depository. Journal of Environmental Radioactivity,188, 30–37.
Karakaya, M. C., Dogru, M., Karakaya, N., Vural, H. C., Kuluozturk, F., & Bal, S. S. (2015). Radioactivity concentrations and dose assessments of therapeutic peloids from some Turkish spas. Clay Minerals,50, 221–232.
Kikouama, O. J. R., & Balde, L. (2010). From edible clay to a clay-containing formulation for optimization of oral delivery of some trace elements: A review. International Journal of Food Sciences and Nutrition,61, 803–822.
Knorst-Fouran, A., Casás, L. M., Legido, J. L., Coussine, C., Bessières, D., Plantier, F., et al. (2012). Influence of dilution on the thermophysical properties of Dax peloid (TERDAX®). Thermochimica Acta,539, 34–38.
Kusin, F. M., Azani, N. N. M., Hasan, S. N. M. S., & Sulong, N. A. (2018). Distribution of heavy metals and metalloid in surface sediments of heavily mined area for bauxite ore in Pengerang, Malaysia and associated risk assessment. CATENA,165, 454–464.
Leschber, R., Davis, R. D., & L’Hermite, P. (1985). Chemical methods for assessing bio-available metals in sludges and soils. London: Elsevier Applied Science Publishers.
MacDonald, D. D., Carr, R. S., Calder, F. D., Long, E. R., & Ingersoll, C. G. (1996). Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology,5, 253–278.
Maor, Z., Henis, Y., Alon, Y., Orlov, E., Sorensen, K. B., & Oren, A. (2006). Antimicrobial properties of Dead Sea black mineral mud. International Journal of Dermatology,45, 504–511.
Mardomingo, I. J., Jiménez-Hernández, M. E., Moreno, L., de la Losa, A., de la Cruz, M. T., & Casermeiro, M. A. (2015). Application of high doses of organic amendments in a Mediterranean agricultural soil: An approach for assessing the risk of groundwater contamination. CATENA,131, 74–83.
Marin, V., Surdu, O., Profir, D., & Demirgian, S. (2012). Peloidotherapy in osteoarthritis-modulation of oxidative stress. In Q. Chen (Ed.), Osteoarthritis—Diagnosis, treatment and surgery (pp. 143–156). Rijeka: In Tech. ISBN 978-953-51-0168-0.
Meng, H. B., Wang, T. R., Guo, B. Y., Hashi, Y., Guo, C. X., & Lin, J. M. (2008). Simultaneous determination of inorganic anions and cations in explosive residues by ion chromatography. Talanta,76, 241–245.
Muñoz, M. S., Rodríguez, C. M., Rudnikas, A. G., Rizo, O. D., Martínez-Santos, M., Ruiz-Romera, E., et al. (2015). Physicochemical characterization, elemental speciation and hydrogeochemical modeling of river and peloid sediments used for therapeutic uses. Applied Clay Science,104, 36–47.
Munteanu, C. (2012). Therapeutic peloid (in Romanian “Namolul therapeutic”) Ed. Balneara, Bucharest. ISBN 978-606-93159-1-0.
Munteanu, C., & Dumitrascu, M. (2011). Therapeutic muds. Balneo-Research Journal,2, 12–16.
Otansev, P. S., Taskin, H., Bassari, A., & Varinlioğlu, A. (2016). Distribution and environmental impacts of heavy metals and radioactivity in sediment and seawater samples of the Marmara Sea. Chemosphere,154, 266–275.
Pintilie, V., Ene, A., Georgescu, L. P., & Moraru, D. I. (2017). Gross alpha, gross beta and 40K activities and daily effective dose due to natural radionuclides from food supplements. Romanian Journal of Physics,62(703), 1–9.
Quintela, A., Terroso, D., Da Silva, E. F., & Rocha, F. (2012). Certification and quality criteria of peloids used for therapeutic purposes. Clay Minerals,47, 441–451.
Radulescu, I., & Calin, M. R. (2014). Reliability and performances of a high-purity gamma spectrometry system used for environmental measurements. Journal of Radioanalytical and Nuclear Chemistry,301, 141–146.
Radulescu, I., Calin, M. R., Ion, I., Ion, A. C., Capra, L., & Simion, C. A. (2017). Gross alpha, gross beta and gamma activities in bottled natural mineral water from Romania. Romanian Reports in Physics,69(710), 1–10.
Radulescu, C., Dulama, I. D., Stihi, C., Ionita, I., Chilian, A., Necula, C., et al. (2014). Determination of heavy metals in water and therapeutic mud by atomic absorption spectrometry. Romanian Journal of Physics,59, 1057–1066.
Rudnick, R. L., & Gao, S. (2003). Composition of the continental crust. In R. L. Rudnick (Ed.), The crust (pp. 1–64). Oxford: Elsevier-Pergamon.
Sarojam, P. (2010). ICP-optical emission spectroscopy, application note. Shelton, CT: PerkinElmer, Inc.
Shaltout, A. A., Ahmed, S. I., Abayazeed, S. D., El-Taher, A., & Abd-Elkader, O. H. (2017). Quantitative elemental analysis and natural radioactivity levels of mud and salt collected from the Dead Sea, Jordan. Microchemical Journal,133, 352–357.
Tanase, I. G., Pana, A., Radu, G. L., & Buleandra, M. (2007). Validation of analytical methods—Theoretical principles and studies case. Bucharest: Printech Publishing. (in Romanian).
Tateo, F., Ravaglioli, A., Andreoli, C., Bonina, F., Coiro, V., Degetto, S., et al. (2009). The in vitro percutaneous migration of chemical elements from a thermal mud for healing use. Applied Clay Science,44, 83–94.
Taylor, S. R. (1964). Abundance of chemical elements in the continental crust: A new table. Geochimica et Cosmochimica Acta,28, 1273–1285.
Tessier, A., & Campbell, P. G. C. (1987). Partitioning of trace metals in sediments: Relationships with bioavailability. Hydrobiologia,149, 43–52.
Tufail, M. (2012). Radium equivalent activity in the light of UNSCEAR report. Environmental Monitoring and Assessment,184, 5663–5667.
UNSCEAR. (1993). United nations scientific committee on the effects of atomic radiation. Sources and effects of ionizing radiation. Report to General Assembly, with Scientific Annexes, United Nations, New York.
UNSCEAR. (2000). United nations scientific committee on the effects of atomic radiation. Sources, effects and risks of ionizing radiation. Report to the General Assembly with annex B, United Nations, New York.
Veniale, F., Bettero, A., Jobstraibizer, P. G., & Setti, M. (2007). Thermal muds: Perspectives of innovations. Applied Clay Science,36, 141–147.
Zorer, O. S., Ceylan, H., & Dogru, M. (2009). Determination of heavy metals and comparison to gross radioactivity concentration in soil and sediment samples of the Bendamihi river basin (Van, Turkey). Water, Air, and Soil Pollution,196, 75–87.
Acknowledgements
This research was performed in the frame of ERA-NET SIINN, funded by the European Commission within the 7th Framework Program and supported by the Romanian Executive Agency for Higher Education and RDI Funding—UEFISCDI—and by the PNCDI III Program, Project No. PN 18 09 02 02/2018 (Romanian Ministry for Education and Research).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calin, M.R., Radulescu, I., Ion, A.C. et al. Investigations on chemical composition and natural radioactivity levels from salt water and peloid used in pelotherapy from the Techirghiol Lake, Romania. Environ Geochem Health 42, 513–529 (2020). https://doi.org/10.1007/s10653-019-00382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00382-8