Abstract
The economic benefits of mining industry have often overshadowed the serious challenges posed to the environments through huge volume of tailings generated and disposed in tailings dumps. Some of these challenges include the surface and groundwater contamination, dust, and inability to utilize the land for developmental purposes. The abandoned copper mine tailings in Musina (Limpopo province, South Africa) was investigated for particle size distribution, mineralogy, physicochemical properties using arrays of granulometric, X-ray diffraction, and X-ray fluorescence analyses. A modified Community Bureau of Reference (BCR) sequential chemical extraction method followed by inductively coupled plasma mass spectrometry/atomic emission spectrometry (ICP-MS/AES) technique was employed to assess bioavailability of metals. Principal component analysis was performed on the sequential extraction data to reveal different loadings and mobilities of metals in samples collected at various depths. The pH ranged between 7.5 and 8.5 (average ≈ 8.0) indicating alkaline medium. Samples composed mostly of poorly grated sands (i.e. 50% fine sand) with an average permeability of about 387.6 m/s. Samples have SiO2/Al2O3 and Na2O/(Al2O3 + SiO2) ratios and low plastic index (i.e. PI ≈ 2.79) suggesting non-plastic and very low dry strength. Major minerals were comprised of quartz, epidote, and chlorite while the order of relative abundance of minerals in minor quantities is plagioclase > muscovite > hornblende > calcite > haematite. The largest percentage of elements such as As, Cd and Cr was strongly bound to less extractable fractions. Results showed high concentration and easily extractable Cu in the Musina Copper Mine tailings, which indicates bioavailability and poses environmental risk and potential health risk of human exposure. Principal component analysis revealed Fe-oxide/hydroxides, carbonate and clay components, and copper ore process are controlling the elements distribution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mine waste are unwanted, currently uneconomic, solid and liquid materials or gaseous by-products of mining, mineral processing, and metallurgical extraction found at or near mine sites (Hudson-Edwards et al. 2011). Types of mine waste include mine waters, tailings, sulphidic wastes and waste rock (Lottermoser 2010). Mine tailings are the unused materials formed after the enrichment process of the ore and are usually stored in impoundments (Yang et al. 2009). Mine waste production is an environmental challenge worldwide. For instance, BRGM (2001) reported that more than 4700 million tonnes of mine wastes are stored all over the European Union (EU). Furthermore, in China over 2688 collectively state-owned and individually owned metal mines account for 70% of the total solid waste. Each year, these mine wastes occupy an additional 2000 hectares (ha) of land. Global assessments of tailings production are equally high: In the 1960s, tens of thousands of tonnes of tailings were produced each day, but by 2000, that amount had risen to hundreds of thousands of tonnes per day (Jakubick and McKenna 2003).
These fallow materials contain high contents of toxic and carcinogenic elements such as As, Cd, Cr, Cu, Hg, Pb, Ni, and Zn that may pose serious hazards to ecosystems and human health (Nehdi and Tariq 2007; Hudson-Edwards et al. 2011; Hudson-Edwards and Dold 2015). Metals cannot be degraded and thus they remain persistent in the environment posing a serious health problem by existing in soil, surface, and drinking water (Yang et al. 2009). Total metal concentrations in soil mirror the geology and weathering, and sometimes anthropogenic inputs of metals from industrial activities (Kersten and Förstner 1989). Ogola and Sebola (2010) reported maximum levels of Cd, Cu, Fe, Pb, and Zn in mine waste and soil within and in the immediate vicinity of the Musina Copper Mine tailings dam. Similarly, Singo (2013) established medium to low contamination index for heavy metals in soils from closed Musina Copper Mine. In addition to the total concentration of metals in soil as reported by some authors (Ogola and Sebola 2010; Singo 2013), it may also be imperative to evaluate the physicochemical forms, of the metals in the tailing dump that may affect their environmental mobility and bioavailability to organisms.
Madrid et al. (2007) have indicated that a single wet chemical extraction scheme does not provide a precise estimate of the quantities of metals released from soil. To solve this problem, selective sequential extraction scheme can be used to appraise the relative mobility of metals in soils including the level that metals may be adsorbed to soil phases (Rao et al. 2008). For instance, various chemical reagents can be used to evaluate metal mobility, as well as the use of reagents to release metals on bound to exchangeable or organic matter phases in soil (Rauret et al. 2001; Keller and Hammer 2004; Borgese et al. 2013). The selective sequential extraction procedures combine a succession of reagents in a sequential extraction scheme, in which the residue from one extractionable phase is used as the material for the next stage through a predetermined number of stages, thus revealing potential valuable information (Borgese et al. 2013).
Geopolymerization process has been employed as a technology to stabilize and consolidate (i.e. strengthen) mine tailings. During geopolymerization process, the silicon- and aluminium-rich mine tailings were suitable raw materials (Rao and Liu 2015). Geopolymerization could result in the immobilization of toxic metals by geopolymeric binders through physical encapsulation and chemical bonding (van Jaarsveld et al. 1999). Wide-ranging research has been carried out on the production of bricks using mining overburden, waste rock, mine tailings, slags, granulated blast furnace slag (GGBS), mine water, water treatment sludge, and gaseous waste (Saeed and Zhang 2012; Zhang 2013; Bennet et al. 2013). The use of mine tailings as road paving materials and construction bricks has also been investigated (Zou and Li 1999; Mahmood and Mulligan 2007). Some authors utilized dry copper mine tailings, fly ash and activator solutions to synthesize geopolymer pastes (Zhang et al. 2011; Ahmari et al. 2012). Pacheco-Torgal et al. (2007, 2008) also produced tungsten mine tailings-based geopolymer as a cementitious material with high early stage strength.
The main objectives of the present study were to (a) to estimate the concentrations of investigated metals and metalloids (i.e. Al, As, Fe, Ca, Cd, Co, Cu, Cr, K, Mg, Mn, Mo, Ni, Se, Ti and Zn) in the easily mobilized fractions using the BCR revised sequential extraction procedure; (b) to identify various geochemical processes controlling the elements distribution using principal component; and (c) to determine potential beneficial use of tailing dump materials as fillers in the construction industry’.
Local geology of Messina Copper Mine
Musina area is characterized by medium–high grade metamorphic rocks of which the Sand River Gneiss is the oldest (3.7 Ma) and forms the basement to a sequence of rock covers of the Beit Bridge Complex, which according to their dominant lithology are subdivided into the Mount Dowe, Malala Drift and Gumbu Group (Brandl 1981). The rocks have undergone several periods of deformation and metamorphism (tectonic events) between ~ 3.2 Ga and ~ 2.0 Ga. The Musina Layered Intrusion which is the oldest layered intrusion of earth is best preserved on the Farm Bokveld within the Reserve. The carbonate rocks were exposed in the eastern portion, while sapphirine-bearing rocks in the western portion. The Musina copper mineralization is mostly composed of a breccia filled with quartz and copper sulphide minerals. Blue quartz crystals from the upper levels of the mine are much valued by mineral collectors and fine clear quartz crystals occur in various places along the Dowe-Tokwe fault that crosses the Musina Nature Reserve.
Materials and methods
Field sampling and sample preparation
Over 30% of the world’s global mineral reserves are found in Africa continent with South Africa, Botswana and the Democratic Republic of Congo being the top three producers of minerals by value (Ekosse et al. 2004). In Limpopo Province (South Africa), there exists a small town known as Musina which is home to several successful mining companies, some of which are not working anymore. Amongst these is a closed down mine that belonged to the Messina (Transvaal) Development Company which was incorporated in London to exploit the copper in what was referred to as Messina back then (Beale 1985). The ore body was exploited as early as 1906 until the mine was closed in 1991 (Wilson 1998). Although 42 Mt of ore was mined, only 0.75 Mt of copper was produced (Wilson 1998). Five ore bodies were identified and mined in Musina; these include the Musina mine itself which was found at the Centre of the fault, Spence and Attornvilla located closely to each other on the northern limb and lastly Harper and Campbell which are in the northern space along the southward extension (Beale 1985).
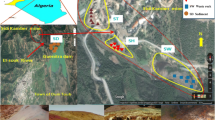
Copper mine tailings samples were collected from Copper Mine tailings facility in Musina. Five sampling points were selected according to the heterogeneity of the tailings considering colour, texture differences, and degree of concretion or contact with the underlying soil or tailings–soil interface. The tailings samples were collected at different depths to understand the mobility of metals down hole and possible dangers pose to ground water system. Samples were obtained by hand augering from surface and at 1-m intervals along depth at each designated point (Fig. 1). Samples were oven-dried at 100 °C and crushed with use of mortar and pestle. The samples were further milled in a tungsten–carbide milling pot to achieve particle sizes < 75 micron and kept in ziplock polythene bag for further laboratory analysis.
Optimum moisture content determination
The procedure for determination of optimum moisture content was adopted from ASTM D 2216 (1998). Representative tailing samples wet weight (total mass before heating or oven drying) was measured. The samples were oven-dried at 105 °C for 8 h, and the dry weight (total mass after heating or oven drying) was recorded. The difference between the two weights was taken as the optimum moisture content expressed in %. The procedure was duplicated.
Granulometric analysis, soil consistency and permeability tests
The grain size distributions were mechanically determined in Mining and Environmental Geology Laboratory, University of Venda, South Africa, using Retsch vibratory sieve shaker (Retsch AS 200 model) mounted with a set of sieves; 100 g of each sample was agitated for 20 min. British Standards were employed with a sieve set in the order of mesh sizes: 2.00, 1.18, 0.85, 0.60, 0.425, 0.30, 0.0025, 0.50, 0.10, 0.075 and 0.0063 mm. The fraction of each mesh size was weighed in grams for statistical analysis based on the procedure of Folk and Ward (1957). Soil consistency test was carried out according to AASHTO Designation T89 which has been modified for New York State Department of Transportation (Geotechnical Test Method-7 2015). Permeability test was conducted according to ASTM D 2434-68 (2006).
Pore water chemistry
Paste pH and EC were determined by weighing 20 grams of air-dried and homogenized washout samples and adding 20 mL of distilled water until a slurry was obtained. After mixing for 5 s, the slurry was left to stand for 10 min. The Thermo Scientific Orion Star ISE/pH/EC meter located in Ecology and Resources Management (ERM) Laboratory, University of Venda, South Africa, was calibrated with pH solutions of analytical grade purchased from Rochelle Chemicals & Lab Equipment CC, South Africa Ltd.
The pH/EC electrodes were then inserted in the slurry and after swirling slightly the pH and Eh were measured until a constant value was obtained. For generation of leachates, a 10 g tailings washout sample was suspended in a 100 mL MilliQ water in plastic bottles. Sample suspensions were agitated in a linear shaker (SSL2 model) for 24 h at a temperature of 22 ± 3 °C, at 0.90 g-force, until a stable reading of pH, electrical conductivity (EC), and total dissolved solid (TDS) was obtained.
Bulk chemical composition
The samples are milled in a tungsten–carbide milling pot to achieve particles sizes < 75 micron. The samples were dried at 100 °C and roasted to determine loss on ignition (LOI) values. 1 g of the samples was mixed with 6 grams of lithiumteraborate flux and fused at 1050 °C to make a stable fused glass bead. The Thermo Fisher ARL 9400 XP + Sequential XRF with OXSAS software was used for analyses. The technique reports the major elements concentration in % and trace elements in mg kg−1. The standards used for the calibration are traceable to Geological society of Japan JF-2-Feldspar, JG-2-granite Canadian certified reference materials, Geological Survey of Canada-660 (Diorite Gneiss SY-4(CCRMP/PCMR)), China National Reference Center, NCS certified reference material, NCS DC 70301-carbonate rock, and NCS DC 73304-rock. The instrument is calibrated every batch of 10 samples analysed. The X-ray fluorescence equipment located in Geology Department, University of Pretoria, South Africa, is checked every six months by the manufacturer (i.e. Bruker).
Mineralogical composition
Mineralogical composition of samples was determined by X-ray powder diffraction (XRD) analysis according to Akinyemi et al. (2012). The detailed procedure for the XRD analysis is as follows: after addition of 20% elemental silicon (Aldrich 99.9%) for quantitative determination of amorphous compounds, and micronizing in a McCrone micronizing mill, the sequential extraction residue samples were prepared for XRD analysis using a back-loading preparation method. They were analysed with a PANalytical X’Pert Pro powder diffractometer with X’Celerator detector and variable divergence—and fixed receiving slits with Fe-filtered Co Kα radiation. The phases were identified using X’Pert HighScore Plus software. The relative phase amounts (weights %) were estimated using the Rietveld method (Autoquan program). X-ray diffraction equipment situated in Geology Department, University of Pretoria, South Africa, is aligned twice a year by the supplier of the instrument (i.e. PANalytical Company). Lanthanum hexaboride (LaB6) was used for line profile and line position.
Geochemical fractionation
A three-step sequential extraction method proposed by the European Community Bureau Reference (BCR) (Tokalioglu et al. 2003) was adopted for this study. Five composited tailings samples from five depth profiles were taken for this study. All reagents used in this study were of analytical grade. They were purchased from Rochelle Chemicals & Lab Equipment CC, South Africa Ltd. The detailed procedure is as follows:
Fraction 1: water/acid soluble and exchangeable
Forty millilitres of 0.11 M acetic acid was added to 1 g of tailings material, shaken for 16 h. After shaking, the mixture was centrifuged at 5000 rpm for 10 min. The supernatant was filtered through a 0.45-µm-pore membrane filter. Two drops of concentrated nitric acid (HNO3) were added and then stored in the fridge at 4 °C until analysis for metal cations by inductively coupled plasma mass spectroscopy/atomic emission spectroscopy (ICP-MS/AES). The procedure was repeated several times to ensure there was enough residual material for the next extraction procedure. This fraction contains elements that are mobile and thus pose more danger to the environment (Quispe et al. 2012).
Fraction 2: reducible
0.5 M of hydroxylammonium chloride adjusted to pH 2 with 2 M nitric acid was used. Forty millilitres of the solution was added to 1 g of the tailings and shaken for 16 h. After shaking, the mixture was centrifuged at 5000 rpm for 10 min. The supernatant was filtered through a 0.45-µm-pore membrane filter and acidified with two drops of nitric acid. The samples were then stored in the fridge at 4 °C for metal cations analysis using inductively coupled plasma mass spectroscopy/atomic emission spectroscopy (ICP-MS/AES) located in Solutions Laboratory, Geology Department, University of Stellenbosch, South Africa. This fraction is composed of metal species bound to Fe and Mn oxyhydroxides that may be released if the environmental conditions change from oxic to anoxic.
Fraction 3: oxidizable fraction
Ten millilitres of 8.8 M hydrogen peroxide was added to one gram of the tailings, shaken manually for one hour, then at 85 °C for one hour. Further heating reduced the volume of the hydrogen peroxide to a few mLs on a hot plate. The same volume of hydrogen peroxide was added in small aliquots to the mixture, and the same procedure was repeated. Fifty millilitres of 1 M ammonium acetate was the added to the mixture and shaken for 16 h. The supernatant was filtered through a 0.45-µm-pore membrane filter. Two drops of concentrated nitric acid were added and then stored in the fridge at 4 °C until analysis for metal cations by inductively coupled plasma mass spectroscopy/atomic emission spectroscopy (ICP-MS/AES). This fraction extracts the metal species bound to organic matter and sulphides under oxidizing conditions. A summation of the first three fractions of the sequential extraction represents the total metal content associated with the mobile phase.
Fraction 4: residual fraction, aqua regia
0.5 g of residual material from fraction 3 was put in a volumetric flask and 36 ml of the aqua regia (HCl: HNO3: 3:1) added. The mixture was then put in a water bath for ≈90 min at a temperature of ≈110 °C. Five millilitres of HF was added into the mixture and allowed to digest under the temperature of 90 °C for 12 h. After boiling the mixture to near dryness, it was then left to cool, and then was diluted with 10 mL of 20% v/v HNO3. The mixture was then filtered and put in 100-mL polyethylene bottles which were then filled up to the mark with MilliQ water. To validate the results, a triple acid digestion (HCl: HNO3: HF) of the tailings was done in a procedure like fraction 4. This fraction consists of metal species strongly bound to amorphous and crystalline phases that can only be extracted by aqua regia digestion and are unlikely to be released except under extreme weathering conditions.
The obtained data from 4 steps of the sequential extraction scheme was evaluated with principal component analysis (PCA) using Varimax rotation and Kaiser normalization. The PCA is used for data reduction and extraction of small number of principal components. In this study, principal component analysis was applied to the whole data set using the SPSS V17.0 for Windows. This was carried out to assess the element associations, and deductions were made with respect to processes that control the distribution of trace metals.
Accuracy and calibration of ICP-MS/AES techniques
Trace element analysis
Trace elements were analysed on an Agilent 7700 quadrupole ICP-MS. Samples are introduced via a 0.4 mL/min micromist nebulizer into a peltier-cooled spraychamber at a temperature of 2 °C, a carrier gas flow of 1.05 L/min, and using HMI mode to set very robust analysis conditions. The instrument was calibrated using NIST (National Institute of Standards and Technology, Gaithersburg MD, USA) traceable standards (i.e. IV-28 multi-element) purchased from Inorganic Ventures [INORGANIC VENTURES· 300 Technology Drive · Christiansburg, VA 24073] to quantify selected elements. A separate NIST-traceable standard (i.e. Merck VI) from Merck was analysed to verify the accuracy of the calibration before sample analysis, while a drift monitor standard was analysed every 12 samples. Internal standards were used to correct for instrument drift over the period of the analysis.
Major element analysis
Major elements were analysed on a Thermo ICap 6200 ICP-AES. The instrument was calibrated using NIST-traceable standards to quantify selected elements. A NIST-traceable quality control standard (ULME-2A) from De Bruyn Spectroscopic Solutions, Bryanston, South Africa, was analysed to verify the accuracy of the calibration before sample analysis, as well as throughout the analysis to monitor drift.
Quality assurance and quality control of analytical data
The limit of detection (LOD) calculated for X-ray fluorescence and induced plasma mass spectrometry (ICP-MS) measurements of investigated elements is three times the standard deviation for blank measurements. The duplicate of analysed samples was included to assess the intra-sample and intra-measurement variability of investigated metals. The higher values of relative standard deviation indicate the metal concentration is very close to the limits of detection (Table 1). Analysis of QA/QC data showed a satisfactory level of accuracy and precision (Table 2).
Results and discussion
Pore water chemistry
Table 3 depicts the obtained results from the Musina Copper Mine tailings paste. The pH ranged between 7.5 and 8.5 with an average value of 8.0 indicating alkaline medium. This could be ascribed to the presence of chlorite, plagioclase, muscovite, and calcite minerals in the tailings (Table 4). Electrical conductivities ranged from 87.0 to 1204 µS cm−1 with an average value of 666.9 µS/cm. Similarly, the total dissolved solids ranged between 52.0 mg L−1 and 733.0 mgL−1 with an average of 400.5 mg L−1 in the Musina Copper Mine tailings. Optimum moisture contents of the tailings ranged from 0.49 to 10.09% with an average value of 4.3%. The lowest per cent of optimum moisture content recorded in the sample code-named 1A is attributed to the evaporation from direct sunlight.
Particle size distribution
The particle distribution curve of Musina Copper Mine tailings is shown in Fig. 2. The tailings material is mostly poorly grated sands (i.e. 50% fine sand) with an average permeability of about 387.6 m/s. This property makes the tailings material not easily compactable thus not suitable for use as foundation filling material in engineering structures. The absence of enough clayey or cremating in the tailings material makes the dump easily susceptible to different form of erosion. The tailings material has a low plastic index (i.e. PI = 2.79) suggesting that the material is non-plastic, has very low dry strength, and will fall apart easily.
Bulk mineralogical and chemical composition
Table 4 shows the mineral phases and the respective quantities present in the Musina Copper Mine tailings as determined by XRD. The major mineral suites comprise of quartz, epidote, and chlorite while the order of relative abundance of other minor minerals are plagioclase > muscovite > hornblende > calcite > haematite. Table 5 shows the major oxides contents of Musina Copper Mine tailings as determined by XRF analysis. The results indicate that the major oxides in the mine tailings’ samples are SiO2, Al2O3, Fe2O3, and CaO. Silica content varies between 56.24 and 85.16 wt% with an average value of 64.88%. Though Al2O3 contents ranged from 11.36 to 14.80 wt% with an average value of 12.65 wt%, the SiO2/Al2O3 ratios vary between 3.80 and 5.66 with an average value of 5.16 (Table 6). The TiO2/Al2O3 ratios in the Musina Copper Mine tailings vary between 0.048 and 0.073 with an average value of 0.06. Although the Na2O/K2O ratios ranged from 0.45 to 2.17 with an average value of 1.30, the Fe2O3 + MgO vary between 8.30 and 14.55 with an average value of 11.00. The Na2O/(Al2O3 + SiO2) ratio ranged between 0.01 and 0.02 with an average value of 0.01. The SiO2/Al2O3 and Na2O/(Al2O3 + SiO2) ratios indicate the Musina Copper Mine tailings would therefore require blending with Al2O3-rich feedstock for them to develop maximum strength in the manufacture of bricks.
The weathering indices used to evaluate the decomposition of unstable minerals in the tailings materials are chemical index of alteration (CIA; Nesbitt and Young 1982), chemical index of weathering (CIW; Harnois 1988), and plagioclase index of alteration (PIA; Fedo et al. 1995). In the annotated formula in Table 5, CaO* is the amount of CaO incorporated in the silicate fraction of the studied sandstone samples. Correction for CaO from carbonate contribution was not done for the studied sandstone samples since there was no CO2 data. Consequently, to compute for CaO* from the silicate fraction, the assumption proposed by Bock et al. (1998) was adopted. In this regard, CaO values were accepted only if CaO ≤ Na2O; accordingly, when CaO > Na2O, it was assumed that the concentration of CaO equals that of Na2O (Bock et al. 1998). The CIA values ranged from 56.96 to 60.77% with an average value of 59.59%.
Chemical index of weathering values varies between 58.02 and 62.98% with an average value of 61.46%. The CIA and CIW values indicate weak weathering primary materials. The plagioclase index of alteration values ranged between 57.22 and 61.73% with an average value of 60.22% similarly suggests low degree of weathering. The mineralogical index of alteration (MIA) was also used to examine the degree of weathering of unstable minerals in the tailings dump. These ranges of MIA values indicate incipient (0–20%), weak (20–40%), moderate (40–60%), and intense to extreme (60–100%) weathering. The value of 100% means complete weathering of a primary material into its equivalent weathered product (Voicu and Bardoux 2002). The MIA values for the studied tailing samples vary between 13.91 and 22.15% with an average value of 19.18% (Table 6) which indicates incipient weathering of primary minerals into the tailings dump. This suggests poorly grated sand implying potential application as aggregates in conventional brick manufacture.
The concentration of metals and metalloids such as As and Pb in the tailings samples is shown in Table 7. The Cu contents varied between 301 mg kg−1 and 1472 mg kg−1 with an average value of 804.7 mg kg−1. The obtained average value of Cu in the tailings samples is above the crustal abundance (i.e. 25 mg kg−1). The Ni concentrations ranged between 0 mg kg−1 and 46 mg kg−1 with an average value of 16 mg kg−1. The contents of Zn varied from 24 mg kg−1 to 60 mg kg−1 in the studied tailings samples with an average value of 38 mg kg−1. Conversely, the average value of Ni and Zn is below the crustal abundance (i.e. Ni ≈ 56 mg kg−1 and Zn ≈ 65 mg kg−1). The concentrations of heavy metals such as Pb and As are shown in Table 5. The concentrations of As varied between 63 mg kg−1 and 79 mg kg−1 with an average value of 71 mg kg−1. Similarly, the contents of Pb ranged between 88 mg kg−1 and 106 mg kg−1 with average value of 95.3 mg kg−1. The As and Pb values in tailings samples are far above their crustal abundance (i.e. As ≈ 1.8 mg kg−1, Pb ≈ 14.8 mg kg−1).
Sequential extraction result
Elements distribution in geochemical fractions
Exchangeable fraction (F1)
Multivariate statistical evaluation of the data extracted from readily extractable fraction (F1) based on dendogram cluster analysis using major and trace elements and pH as variables classified the data into two groups (Fig. 3).
Group I comprises of Cd, Ca, Ni, Mn and pH indicating mobility and ease of extraction depend on pH. Metal hydroxide minerals have a low solubility under high pH conditions in water due to hydroxyl ion activity being inversely related to pH. As pH decreases, solubility increases and more metals are liberated solution (Salomons 1995).
Group II consists of Ti, Cr, Zn, Mo, Fe, Co, Al, K, Mg, Cu, Se and As which suggest these metals and metalloids formed precipitates with oxides.
Calcium shows highest percentage in the exchangeable fraction with an average of 34.56%. Cadmium shows high extractable percentage in the exchangeable fraction of the studied sample with exception of sample code-named S5 with an average value of 35.67%.
In specific, the average concentration of metals in easily extractable fractions of analysed samples was, respectively: Cu (60.62%) > Ca (34.56%) > Mn (17.14%) > K (12.98%) > Mg (1.61%) > Al (0.91%) > Fe (0.73%) > Cd (33.57%) > Zn (8.15%) > As (7.63%) > Sc (5.52%) > Ni (4.36%) > Mo (4.43%) > Co (3.26%) > Cr (3.22%) > Ti (0.12%). With respect to other metal, Cu is mostly present in the easily extractable fraction (i.e. bioavailable) (Cao et al. 2000) and poses potential risk of exposure. Metals such as Pb, Sr, Rb, Ba, Sb, and B are below detection limits. Rath et al. (2008) reported that soils that can release in readily extractable fraction more than 50% of their total metal content could be considered to pose greater environmental exposure risk.
Reducible (F2) +oxidizable (F3) +residual (F4) fractions
Statistical evaluation of the data from less extractable fractions (F2 + F3 + F4) based on dendogram cluster analysis shows two groups (Fig. 4). The first group (group 1) revealed strong association of Mg, Ni, Cr, Mn, Co and Zn. The second group (Group 2) comprised of Ti, Al, Ca, Fe, K, Se, Cu, Cd, As, and Mo. The adsorption and desorption of these associated metals depend on pH and particulate binding surface. The highest percentage of cadmium is obtained in the reducible fraction of sample code-named S5 (≈ 36.36%). Cadmium has higher adsorption edges at higher pH’s compared to Fe and Cu and thus are more mobile and widely dispersed (Fadiran et al. 2014). Aluminium and iron show the highest percentage in the residual fraction with an average of 95.78 and 96.17%, respectively. Calcium shows highest percentage in the residual fraction with an average of 53.95%. Potassium and magnesium show highest percentage in the residual fraction with an average of 75.49 and 95.47%, respectively. Manganese reveals highest percentage in the residual fraction with average of 76.73%. Copper shows highest percentage in the residual fraction in samples code-named S1, S2 and S3, but highest percentage of copper was obtained in the reducible fraction of sample code-named S4 and S5. Molybdenum shows highest percentage in the reducible and residual fractions with average values of 56.96 and 33.95%, respectively. Chromium and arsenic show highest percentage in the residual fraction with average values of 86.44 and 68.23%, respectively. Selenium and zinc reveal highest percentage in the residual fraction with average values of 68.87 and 66.46%, respectively. Nickel and cobalt show highest percentage in the residual fraction with average values of 91.37 and 98.18%, respectively. Relatively high metal concentrations in the residual fraction suggest that considerable large fraction of metals in the tailings are strongly associated with chlorite. Therefore, the metals associated with residual, strong acid extractable fraction may be considered biologically unavailable (Borgese et al. 2013). Metals extracted from residual fraction have the strongest associations with crystalline structures of primary and secondary minerals, i.e. they are most difficult to extract (Rodgers et al. 2015).
The concentrations of Ti, Cr, Co, Ni, As, Se, Zn, Mo, and Cd in the less extractable fractions (i.e. F2 + F3 + F4) were normalized to the Al concentration in the less extractable fractions for all the analysed samples. The concentrations of investigated metal are correlated between the normality indicating the same geogenic origin (Borgese et al. 2013). The extremely high recoveries that were calculated as indicated in Tables 8 and 9 could have been due to inhomogeneity of the analysed samples.
Principal component analysis
Principal component analysis was performed on data of F1 and F2 + F3 + F4 fraction for all the analysed samples. The results are shown in Tables 10 and 11. The PCA performed on data from exchangeable fraction (F1) identified four principal components controlling their variability (Table 10). First component accounts for 30.03% of total data variations and consists of higher loading values of Fe, Co, Cr, and Al, but lower loading values of Zn, Mg, Mo, and K. This positive correlation of these elemental suites uncovered by factor analysis clearly suggests less variations in the origin of these metals (Table 10). This observation is consistent with the fact that the samples were collected at different depths of the tailings dump. The second component constitutes 26.13% to the total variance and comprises of higher loading values of Ni, Mn, Cd, Ca, K, and Mg. Third component contributes 21.84% to the total data variation in the exchangeable geochemical fraction and consists of higher loading values of Se, Cu and K indicating contribution from the primary copper ore. The fourth component contains 11.24% of the total variance and comprises of higher loadings of Ti which indicate relative abundance of TiO2 in the clay fraction of the tailings (Table 10).
Table 11 shows four principal components extracted from the F2 + F3 + F4 fractions of analysed samples. First component accounts for 31.31% of the data variability and consist of higher loadings of Cr, Mn, Mg, Ni, Zn, Co and Al. This indicates association with Fe and Mn oxide/hydroxides component of the tailing sample. Second component contributes 24.28% of the data variation and comprise of higher loading of Se, Fe, K, Ca, and Al indicating contribution from calcitic composition of the tailings sample. Third component accounts for 21.29% of the data variability and consists of higher loadings of Cu, Cd, and As suggesting derivation from copper ore. Fourth component contributes 11.71% to data variation and comprises of higher loadings of Mo and Ti which suggest derivation from clay composition of the tailing samples.
Adverse effects of arsenic and copper on human health
Reviews of literature to date indicate that inorganic arsenic is a conventional potent human carcinogen (IARC 2004). In addition, ingestion through drinking water had been implicated in several non-cancer diseases such as pigmentation changes and hyperkeratosis, peripheral vascular disease, respiratory, neurological and liver disorders, high blood pressure, diabetes mellitus, chronic disease, and all-cause infant and adult disease mortality (WHO/IPCS 2001; Rahman et al. 2006a, Rahman et al. 2006b; Yunus et al. 2011). Lasting exposure to copper can cause irritation of the nose, mouth and eyes and it causes headaches, stomach aches, dizziness, vomiting and diarrhoea. Furthermore, high uptakes of copper may cause liver and kidney damage and even death. Whether copper is carcinogenic has not been determined.
Conclusions
The huge volumes of tailings in the abandoned Musina Copper Mine are alkaline in nature. The tailing material is mostly poorly grated sands with medium permeability. The major element ratios such as SiO2/Al2O3 and Na2O/(Al2O3 + SiO2) and low plastic index implied that the material is non-plastic and has very low dry strength. The mineral assemblage consists of quartz, epidote, and chlorite, while the order of relative abundance of minerals in minor quantities is plagioclase > muscovite > hornblende > calcite > haematite. Considerable high concentrations of Mo, Se, Cr, As, and Cd were strongly bounded to less extractable fractions. Relative abundance of Cu and As in the readily extractable fraction indicates potential bioavailability for organisms, and thus generates potential environmental and health risk of exposure. Iron & Mn oxide/hydroxides, clay and carbonate components, and copper ore processes control the distribution of elements. Therefore, the inherent toxic metals in the tailings need to be immobilized through geopolymerization process prior to application in brick manufacture.
References
Ahmari, S., Zhang, L., & Zhang, J. (2012). Effect of activator type/concentration and curing temperature on alkali-activated binder based on copper mine tailings. Journal of Materials Science, 47, 5933–5945.
Akinyemi, S. A., Akinlua, A., Gitari, W. M., Nyale, S. M., Akinyeye, R. O., & Petrik, L. F. (2012). An investigative study on the chemical, morphological and mineralogical alterations of dry disposed fly ash during sequential chemical extraction. Energy Science and Technology, 3(1), 28–37.
American Society for Testing and Materials (ASTM D 2216). (1998). Standard test methods for laboratory determination of water (moisture) content of soil and rock by mass.
American Society for Testing and Materials (ASTM D 2434-68). (2006). Standard test method for permeability of granular soils (constant head).
Beale, C. O. (1985). Copper in South Africa-Part 11. Journal of the South African Institute of Mining and Metallurgy, 85(4), 109–124.
Bennet, J. M., Sudhakar, M., & Natarajan, C. (2013). Development of coal ash—GGBS based geopolymer bricks. European International Journal of Science and Technology, 2(5), 133–139.
Bock, B., McLennan, S. M., & Hanson, G. N. (1998). Geochemistry and provenance of the Middle Ordovician Austin Glen Member (Normanskill Formation) and the Taconian Orogeny in New England. Journal of Sedimentology, 45, 635–655.
Borgese, L., Federici, S., Zacco, A., Gianoncelli, A., Rizzo, L., Smith, D. R., et al. (2013). Metal fractionation in soils and assessment of environmental contamination in the Vallecamonica, Italy. Environmental Science and Pollution Research International, 20(7), 5067–5075.
Brandl, G. (1981). The geology of the Messina area. Tech. Rep. Explanation sheet 2230, Geological Survey of South Africa, Messina, Italy.
Cao, X., Wang, X., & Zhao, G. (2000). Assessment of the bio-availability of rare earth elements in soils by chemical fractionation and multiple regression analysis. Chemo-sphere, 40, 23–28.
Ekosse, G., Van Den Heever, D. J., De Jager, L., & Totolo, O. (2004). Minerology of Tailings dump around Selebi Phikwe nickel-copper plant, Botswana. Journal of Applied Science in Environmental Management, 8(1), 37–44.
Fadiran, A. O., Tiruneh, A. T., & Mtshali, J. S. (2014). Assessment of mobility and bioavailability of heavy metals in sewage sludge from Swaziland through speciation analysis. American Journal of Environmental Protection, 3, 198–208.
Fedo, C. M., Nesbitt, H. W., & Young, G. M. (1995). Unraveling the effects of potassium metasomatism in sedimentary rock sand paleosols, with implications for paleoweathering conditions and provenance. Geology, 23, 921–924.
Folk, R. L., & Ward, W. O. (1957). Brazos River bar: A study in the significance of grain size parameters. Journal of Sedimentary Petrology, 27, 3–26.
Rao, F., & Liu, Q. I. (2015). Geopolymerization and its potential application in mine tailings consolidation: A review. Mineral Processing and Extractive Metallurgy Review, 36, 399–409. https://doi.org/10.1080/08827508.2015.1055625.
Geotechnical Test Method-7. (2015). Test method for liquid limit, plastic limit, and plasticity index. State of New York Department of Transportation Geotechnical Engineering Bureau.
Harnois, L. (1988). The CIW index: A new chemical index of weathering. Sedimentary Geology, 55, 319–322.
Hudson-Edwards, K. A., & Dold, B. (2015). Mine waste characterization, management and remediation. Minerals, 5, 82–85.
Hudson-Edwards, K. A., Jamieson, H. E., & Lottermoser, B. G. (2011). Mine wastes, Past, Present, Future. Elements, 7, 375–380.
International Association of Risk Compliance (IARC). (2004). Some drinking-water disinfectants and contaminants, including arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 84, 1–477.
Jakubick, A., & McKenna, G. (2003). Stabilisation of tailings deposits: international experience. Mining and the Environment III, Sudbury, Ontario, Canada, 25–28 May, 2003, pp 1–9.
Keller, C., & Hammer, D. (2004). Metal availability and soil toxicity after repeated croppings of Thlaspi caerulescens in metal contaminated soils. Environmental Pollution, 131, 243–254.
Kersten, M., & Forstner, U. (1989). Speciation of trace elements in sediments. In G. E. Batley (Ed.), Trace element speciation: Analytical methods and problems (pp. 245–318). Boca Raton: CRC Press.
Lottermoser, B. G. (2010). Mine wastes: Characterization, treatment, and environmental impacts (3rd ed., p. 400). Berlin Heidelberg: Springer.
Madrid, F., Reinoso, R., Florido, M. C., Díaz Barrientos, A., Ajmone-Marsan, F., Davidson, C. M., et al. (2007). Estimating the extractability of potentially toxic metals in urban soils: A comparison of several extracting solutions. Environmental Pollution, 147, 713–722.
Mahmood, A. A., & Mulligan, C. N. (2007). Investigation of the use of mine tailings for unpaved road base construction. In Proceedings of the annual international conference on soils, sediments, water and energy, 12, Article 11.
Nehdi, M., & Tariq, A. (2007). Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementations materials: A review. Journal of Environmental Engineering and Science, 6(4), 423–436.
Nesbitt, H. W., & Young, G. M. (1982). Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature, 299, 715–717.
Ogola, J. S., & Sebola, A. M. (2010). Investigation of heavy metals dispersion around the Messina copper mine tailings dam, South Africa. In Proceedings of the IASTED international conference, Vol. 888, pp. 15–17
Pacheco-Torgal, F., Castro-Gomes, J., & Jalali, S. (2007). Investigation about the effect of aggregates on strength and microstructure of geopolymeric mine waste mud binders. Cement and Concrete Research, 37, 933–941.
Pacheco-Torgal, F., Castro-Gomes, J., & Jalali, S. (2008). Properties of tungsten mine waste geopolymeric binder. Construction and Building Materials, 22, 1201–1211.
Quispe, D., Pérez-López, R., Silva, L. F. O., & Nieto, J. M. (2012). Changes in mobility of hazardous elements during coal combustion in Santa Catarina power plant (Brazil). Fuel, 94, 495–503. https://doi.org/10.1016/j.fuel.2011.09.034.
Rahman, M., Vahter, M., Sohel, N., Yunus, M., Wahed, M. A., Streatfield, P. K., et al. (2006a). Arsenic exposure and age and sex specific risk for skin lesions: A population-based case-referent study in Bangladesh. Environmental Health Perspectives, 114, 1847–1852.
Rahman, M., Vahter, M., Wahed, M. A., Sohel, N., Yunus, M., Streatfield, P. K., et al. (2006b). Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh. Journal of Epidemiology and Community Health, 60(3), 242–248.
Rao, R. M., Sahuquillo, A., & Lopez Sanchez, J. F. (2008). A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water, Air, and Soil Pollution, 189, 291–333.
Rath, P., Panda, U. C., Bhatta, D., & Sahu, K. C. (2008). Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—A case study: Brahmani and Nandira Rivers, India. Journal of Hazardous Materials, 163(2–3), 632–644. https://doi.org/10.1016/j.jhazmat.2008.07.048.
Rauret, G., López-Sánchez, J. F., Bacon, J., Gómez, A., Muntau, H., & Quevauviller, P. (2001). Report EUR 19774 EN. European Commission; Brussels: Certification of the Contents (Mass Fractions) of Cd, Cr, Cu, Ni, Pb and Zn in an Organic-Rich Soil Following Harmonised EDTA and Acetic Acid Extraction Procedures, BCR-700, p. 61
Saeed, A., & Zhang, L. (2012). Production of ecofriendly bricks from Cu mine tailings through geopolymerization. Construction and Building Materials, 29, 323–331.
Salomons, W. (1995). Environmental impact of metals derived from mining activities: Processes, predictions, prevention. Journal of Geochemical Exploration, 52, 5–23.
Singo, N. K. (2013). An assessment of heavy metal pollution near an old copper mine dump in Musina, South Africa. M.Sc. Thesis. The University of South Africa.
Tokalioglu, S., Kartal, S., & Irol, G. B. (2003). Application of a three-stage sequential extraction procedure for the determination of extractable metal contents in highway soils. Turkish Journal of Chemistry, 27, 333–346.
van Jaarsveld, J. G. S., van Deventer, J. S. J., & Schwartzman, A. (1999). The potential use of geopolymeric materials to immobilize toxic metals: part II. Material and leaching characteristics. Minerals Engineering, 12, 75–91.
Voicu, Gn, & Bardoux, M. (2002). Geochemical behaviour under tropical weathering of the Barama–Mazaruni greenstone belt at Omai gold mine, Guiana Shield. Applied Geochemistry, 17, 321–336.
Voicu, G., Bardoux, M., Harnois, L., & Grepeau, R. (1997). Lithological and geochemical environment of igneous and sedimentary rocks at Omai gold mine, Guyana, South America. Exploration and Mining Geology, 6, 153–170.
Wilson, M. G. C. (1998). Copper. In M. G. C. Wilson & C. R. Anhaeusser (Eds.), The mineral resources of South Africa (pp. 209–227). Pretoria: Council of Geosciences.
World Health Organisation (WHO)/International Programme Chemical Safety (IPCS). (2001). Environmental health criteria 224, arsenic and arsenic compounds (2nd ed.). Geneva: World Health Organization.
Yang, J. S., Lee, J. Y., Baek, K., Kwon, T. S., & Choi, J. (2009). Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. Journal of Hazardous Materials, 171(1–3), 443–451.
Yunus, M., Sohel, N., Hore, S. K., & Rahman, M. (2011). Arsenic exposure and adverse health effects: A review of recent findings from arsenic and health studies in Matlab, Bangladesh. The Kaohsiung Journal of Medical Sciences, 27(9), 371–376.
Zhang, L. (2013). Production of bricks from waste materials—A review. Construction and Building Materials, 47, 643–655.
Zhang, L., Ahmari, S., & Zhang, J. (2011). Synthesis and characterization of fly ash modified mine-based geopolymers. Construction and Building Materials, 25, 3773–3781.
Zou, D. H., & Li, L. P. (1999). Strengthening of solidified dilute tailing slurry. Journal of Geotechnical and Geoenvironmental Engineering, 125, 11–15.
Acknowledgements
The authors would want to acknowledge the support from ESKOM Foundation, NRF, and THRIP for funding this study through student bursaries and consumables.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gitari, M.W., Akinyemi, S.A., Ramugondo, L. et al. Geochemical fractionation of metals and metalloids in tailings and appraisal of environmental pollution in the abandoned Musina Copper Mine, South Africa. Environ Geochem Health 40, 2421–2439 (2018). https://doi.org/10.1007/s10653-018-0109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0109-9