Abstract
This study presents the groundwater quality assessment in the north of Isfahan, Iran. In the study area, assessment and measurement of groundwater hydrochemical parameters such as pH, total dissolved solids (TDS), electrical conductivity (EC), sodium absorption ratio (SAR), total hardness, major cations (K+, Na+, Ca2+ and Mg2+) and major anions (Cl−, \({\text{HCO}}_{ 3}^{ - } ,{\text{CO}}_{3}^{2 - }\) and \({\text{SO}}_{4}^{2 - }\)) concentrations were performed. Accordingly, the 66 water samples from different locations were collected during April and May 2015. Water samples collected in the field were analyzed in the laboratory for cations and anions using the standard methods. In this research, the analytical results of physiochemical parameters of groundwater were compared with the standard guideline values as recommended by the world health organization (WHO) for drinking and public health purposes. The pH values of groundwater samples varied from 7.05 to 8.95 with a mean of 7.78, indicating a neutral to slightly alkaline water. TDS values showed that 14% of the samples exceeds the desirable limit given by WHO. EC values varied from 213 to 4320 µS/cm, while 23% of the samples were more than the standard limit. Gibbs diagram had shown that 90% of the samples in the study area fall in the rock weathering zone, and this means that chemical weathering of rock-forming minerals is the main factor controlling the water chemistry in the study area. Irrigation suitability and risk assessment of groundwater are evaluated by measuring EC, %Na, SAR and RSC. According to the dominant cations and anions, five types of water were identified in the water samples: Ca-HCO3, Ca-SO4, Na-Cl, Na-HCO3 and Na-SO4. The results show that the majority of samples (30 samples, 45%) belongs to the mixed Na-SO4 water type. Correlation analysis and principal component analysis was used to identify the relationship between ions and physicochemical parameters. Results indicated that 18 stations of the study area had the best quality and can be used for irrigation and drinking purposes in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogeological and geochemical studies are fundamental for groundwater quality assessment and groundwater resource management. Groundwater has become the major source of water supply for domestic, industrial and agricultural sectors of many countries, especially in Iran. Additionally, knowledge of the geochemical evolution of groundwater properties in arid regions can lead to improving knowledge of hydrochemical systems in such areas and eventually contribute to sustainable development and effective management of water resources. Nowadays, the importance of groundwater studies is inadequate about the natural phenomena in Iran that the chemical compositions of groundwater or anthropogenic factors govern. Water shortages have become a serious problem in Iran, especially in the arid and semiarid regions of western Iran (Jalali 2006). Iran is located in a semiarid area with an average annual precipitation less than one-third of that of the world (Baghvand et al. 2010). In Iran, groundwater is the primary source of water for irrigation as well as human consumption and industrial use. The quality of groundwater is important because it determines the suitability of water for drinking, domestic, agricultural and industrial purposes (Tatawat and Chandel 2008). Groundwater chemistry depends on diverse factors such as general geology, the degree of chemical weathering of the various rock types, quality of recharge water and inputs from sources other than water–rock interaction. The hydrogeochemical study is a useful technique to identify processes that are responsible for groundwater chemistry (Jeevanandam et al. 2007). Many factors and their interactions affect on complex groundwater quality (Guler and Thyne 2004; Giridharan et al. 2008). Understanding the temporal variation of groundwater quality is a vital factor for implementing the optimal management of water resources. Chemical reactions such as weathering, dissolution, precipitation, ion exchange and various biological processes commonly take place below the surface. The physical, chemical and microbiological characteristics of fresh water can be modified due to contamination. Effluent discharges from water sources generally result in changes in the pH, temperature and metal concentrations that present a danger to the aquatic life (Jordao et al. 2007). Natural weathering processes of rock-forming minerals affect the water quality of aquifers (Subramani et al. 2010; Ako et al. 2012; Kamtchueng et al. 2014). It is inevitable to know the patterns of natural groundwater evolution before delineating possible anthropogenic influence on groundwater quality of agricultural land use, industrial emissions, or domestic waste and sewage (Ludwig et al. 2011). Groundwater quality factors, such as dissolved ion content, are mostly affected by either the natural geochemical characteristics including climate, lithology, mineral weathering, the nature of the geochemical reactions, solubility of salts, dissolution/precipitation reactions, ion exchange, wet and dry deposition of atmospheric salt and evapotranspiration or various anthropogenic activities such as agriculture, sewage disposal, mining and industrial wastes (Jiang and Yan 2010). To assess the quality of groundwater, it is necessary to know the physicochemical parameters of groundwater such as pH, total dissolved solids (TDS), electrical conductivity (EC), sodium absorption ratio (SAR), total hardness (TH), major cations (K+, Na+, Ca2+ and Mg2+) and major anions (Cl−, \({\text{HCO}}_{ 3}^{ - } ,{\text{CO}}_{3}^{2 - }\) and \({\text{SO}}_{4}^{2 - }\)) concentrations. The common method widely used to describe the constraints on groundwater evolution is the hydrogeochemical approach. Multivariate statistical techniques such as Spearman’s correlation matrix are effective tools for interpreting and representing data set concern for the quality of water. They are frequently employed as a proxy to characterize the quality of natural water in a given environment (Mano et al. 2013).
This study applies the hydrogeochemical and multivariate statistical techniques to identify processes that control surface water and groundwater chemistry in the area. The main objectives of this research are determining the geochemical characteristics of Isfahan groundwater (Ardestan and Natanz areas), evaluate groundwater quality and assess its suitability for drinking and irrigation.
Study area
Location, topography and climate
The area of study is located in the north of Isfahan province in Iran. This region is located in the central part of Iran and is part of the Dagh-e-Sorkh basin that lies between longitudes 52°00′00″–52°40′00″E and latitudes 33°20′00″–33°35′05″N, covering an estimated area of about 2400 km2, which includes the cities of Natanz and Ardestan (Fig. 1). The northern part of the study area is situated in the Sanandaj–Sirjan zone, while its southern part is located in the central Iran zone. The study area has a warm climate, and the air temperature is highest in July (35 °C) and lowest in January (1 °C) with an annual average of 16.2 °C. The average annual rainfall is 150 mm in the region.
Groundwater is an important water resource for drinking and agricultural uses in the study area. It is noteworthy that in recent years, low precipitation and overexploitation of groundwater resources have caused an extensive groundwater level decline in this region. From the morphological point of view, mountainous terrains of the study area are formed by igneous rocks. The southern parts of the area are covered by coarse-textured alluvial fan sediments, which play a significant role in the absorption of runoffs and transferring them to the aquifer. Other areas possess smooth morphological conditions. The altitude of the area varies from 950 to 2250 m above sea level, and the topography is the mixture of rugged mountains with flat plains.
Geology and hydrogeology of the study area
The detailed geological information of the study area will be investigated in this section. The Laramide orogeny (late Cretaceous) created a regional unconformity at the base of the Eocene deposits throughout a vast part of Iran. The study area is mainly composed of Eocene volcanic and pyroclastic rocks (Fig. 2). In the Oligocene–Miocene, marl, limestone, sandstone, shale and gypsum units known as Qom Formation became widespread in the area, becoming even more extensive in depressions. In the Late Miocene, deposition of sandstone, marl, conglomerate and evaporate rocks known as the upper red formation developed in a molasses-type condition of a sedimentary environment as a result of slow positive movement of the basin (Babaahmadi et al. 2010). The fractured and weathered nature of the bedrock together with the overlying pyroclastic and basaltic materials gives a high porosity and permeability that favor the recharge of the aquifers. The study area water resources include spring, qanats and wells. Qanats and wells are the primary sources of water supply in the study area. Most of the wells are hand-dug wells (open and closed wells). According to groundwater level, the depth to water table ranges from 2 to 112 m.
Geological map of the study area (Amidi and Zahedi 1988)
Figure 3 depicts the 1:250,000 hydrogeological map of the study area. According to this figure, the vast majority of the basin recharge zone is covered by non-carbonated formations, while most of the basin discharge zones has delimited in recent alluvial sediments. The basin aquifer is unconfined and medium height, of the plain of the eastern part, is 948 m, while in central and eastern parts is 1050 m.
Materials and methods
Sample collection and analytical procedures
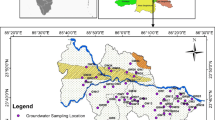
In order to achieve the research objectives, 66 water samples from different locations (35 qanats, 22 springs, 8 wells and 1 piezometer) were collected during April and May 2015 from representative sites in the study area. The selected qanats, springs, wells and piezometer are used for agricultural and domestic purposes. The minimum and maximum groundwater levels were 2–112 m during the sampling period. Location of sampling stations is shown in Fig. 4. In order to evaluate the quality of groundwater in the study area, groundwater samples were collected after 10 min of pumping, dry, clean and sterilized polyethylene bottles. The collected samples were labeled, sealed and transported to the laboratory and preserved in the refrigerator at a temperature of about 4 °C until analysis time. Water samples collected in the field were analyzed in the laboratory for major cations (K+, Na+, Ca2+ and Mg2+) and major anions (Cl−, \({\text{HCO}}_{ 3}^{ - } ,{\text{CO}}_{3}^{2 - }\) and \({\text{SO}}_{4}^{2 - }\)) using the standard methods within 48 h after sampling. Immediately after sampling, physicochemical parameters including temperature, pH, electrical conductivity (EC) and total dissolved solids (TDS) were measured in situ, using a multi-parameter portable meter (HATCH, Germany). Total hardness and calcium were determined titrimetrically, using standard EDTA. Magnesium was calculated on the basis of the difference in concentration between total hardness and Ca2+. Chloride (Cl−) was determined by the standard AgNO3 titration method (Mohr method). Carbonate (\({\text{CO}}_{3}^{2 - }\)) and bicarbonate (\({\text{HCO}}_{ 3}^{ - }\)) were determined by titration with HCl. Sodium (Na+) and potassium (K+) were measured by flame photometry and sulfate (\({\text{SO}}_{4}^{2 - }\)) by spectrophotometric turbidimetry (Rowell 1994). Total dissolved solids (TDS) were measured by evaporating a pre-filtered sample to dryness. The accuracy of the chemical analysis was verified by calculating ion-balance errors. The errors were generally around ±5%.
Results and discussion
Physiochemical characteristics of groundwater samples
The physicochemical parameters and statistical summary for all the 66 water samples are investigated. Table 1 illustrates the physicochemical parameters of groundwater samples which represent maximum, minimum, standard deviation and mean values.
In this research, the analytical results of physiochemical parameters of groundwater were compared with the standard guideline values as recommended by the world health organization (WHO 2004) for drinking and public health purposes. In the study area, temperature values at groundwater samples vary from 14.4 to 32.7 °C with a mean of 14.4 °C. pH values of groundwater samples vary from 7.05 to 8.95 with a mean of 7.78, indicating a neutral to slightly alkaline water. Nearly 11% (7 samples) of the samples display pH values above WHO standard (WHO 2004). Figure 5 shows the spatial distribution of pH values in the study area based on the inverse distance weighting interpolation method. Total dissolved solids (TDS) values range from 104 to 2250 mg/l, with a mean value of 568 mg/l. Groundwater with high TDS is not suitable for both drinking and irrigation purposes. The maximum allowable limit for the TDS in drinking water is 1000 mg/l according to WHO standard (WHO 2004). TDS in 14% (9 samples) of the samples exceeds the desirable limit given by WHO. Figure 6 shows the spatial distribution of TDS values in the study area based on the inverse distance weighting interpolation method. Water electrical conductivity (EC) values vary from 213 to 4320 µS/cm with an average of 1133 µS/cm. It is noteworthy that maximum allowable limit for EC in drinking water is 1500 µS/cm according to WHO standard (WHO 2004). As a result, the EC values of 23% (15 samples) of the samples are above the standard value. The degree of hardness (TH) in water is commonly based on the classification: (0–75) soft, (75–150) moderately hard, (150–300) hard, (>300) very hard (Todd and Mays 2005). TH values vary from 15.65 to 441 mg/l with an average of 123.15 mg/l. Accordingly, 20 samples had a soft degree, 30 samples had a moderate degree, 10 samples had a hard degree, and 6 samples had a very hard degree.
In this section, anions and cations behavior has investigated. In the study area, sodium (Na+) values vary from 12.07 to 1529 mg/l with an average of 364 mg/l. Sodium content in 61% (40 samples) of the analyzed samples exceeds the desirable limit of 200 mg/l (WHO 2004). In this region, the highest concentration of Na+ is probably the result of weathering of rock-forming minerals such as halite and sodium plagioclase along with anthropogenic sources including domestic and animal waste.
Chloride (Cl−) content varies from 9 to 1151.4 mg/l with an average of 169.7 mg/l. Nearly 18% (12 samples) of the analyzed sample display chloride content above the standard value (250 mg/l; WHO 2004). If halite dissolution is responsible for sodium, the Na+/Cl− ratio should be approximately equal to 1, whereas a ratio greater than 1 is typically interpreted as Na released from silicate weathering reactions (Meyback 1987). In the study area, the Na+/Cl− ratio of groundwater samples varies from 1.06 to 7.9 with an average of 2.90 (Fig. 7). In all samples, Na+/Cl− ratio is greater than 1 and indicated that an ion exchange process is common and might have come from silicate weathering. Figure 8 shows the concentrations of Na+ versus Cl−. Also, Fig. 9 shows the spatial distribution of Na+/Cl− ratio values in the study area based on the inverse distance weighting interpolation method.
Potassium (K+) varies from 0.21 to 28.64 mg/l with an average of 2.76 mg/l. In all samples, magnesium concentration is below the WHO allowable value. It is worth noting that during the water/rock interaction and rock weathering, Mg2+, \({\text{HCO}}_{ 3}^{ - }\), Ca2+, \({\text{SO}}_{4}^{2 - }\) and Cl− can be added to the groundwater. Calcium (Ca2+) values vary from 0.81 to 355 mg/l with an average of 99.70 mg/l. Calcium content in 9% (6 samples) of the analyzed samples exceeds WHO standard (WHO 2004). In this region, calcium can be derived from both weathering of carbonate minerals (calcite, CaCO3; dolomite Ca Mg (CO3)2; and aragonite, CaCO3), calcium-rich minerals like feldspars, pyroxenes, amphiboles and leakage of chemical fertilizers. Magnesium (Mg2+) values vary from 2.65 to 149.9 mg/l with an average of 23.44 mg/l. In all samples, magnesium concentration is below the WHO allowable value.
The chloro-alkaline index (CAI), CAI = [Cl–(Na + K)]/Cl is suggested by Schoeller (1977), which indicates the ion exchange between the groundwater and its host environment. If there is ion exchange of Na+ and K+ from water with Ca2+ and Mg2+ in the rock, the exchange is known as direct when the indices are positive. If the exchange is reversed, then the exchange is indirect and the indices are found to be negative. The negative CAI values in the study area show that Ca2+ and Mg2+ from water are exchanged with Na+ and K+ in rock favoring cation–anion exchange reactions (Fig. 10).
Sulfate (\({\text{SO}}_{4}^{2 - }\)) content ranges from 20 to 1240 mg/l whereby 36% (24 samples) of samples exceeds WHO standard (WHO 2004). In this region, the major source of sulfate in groundwater is weathering of sulfur-bearing minerals such as gypsum, anhydrite and sulfide minerals along with industrial and mining waste. The concentration of chloride and sulfate increases from the recharge zone to the discharge zone. Bicarbonate content ranges from 23.4 to 456.2 mg/l with an average of 171.4 mg/l. Nearly 29% (19 samples) of the analyzed samples display the bicarbonate content above the standard value (240 mg/l; WHO 2004). In this region, the primary source of \({\text{HCO}}_{ 3}^{ - }\) is the dissolution of carbonate minerals, and secondary sources can result from a dissolution of CO2 by anoxic biodegradation of organic matter from anthropogenic sources such as industrial and domestic sewage and buried waste in landfills. The mean concentration of cations (mg/l) in groundwater indicates that Na+ > Ca2+ > Mg2+ > K+ of the study area. Similarly, the mean concentration of anions (mg/l) displays the following decreasing trend: \({\text{SO}}_{4}^{2 - } > {\text{HCO}}_{ 3}^{ - } > {\text{Cl}}^{ - }\) (Fig. 11). Figures 12 and 13 show the spatial distribution of \({\text{SO}}_{4}^{2 - }\) and \({\text{HCO}}_{ 3}^{ - }\) values in the study area based on the inverse distance weighting interpolation method.
Processes controlling groundwater chemistry
One of the processes which influence on the water chemistry and groundwater quality is an interaction between groundwater and aquifer minerals. The concentration of dissolved ions is controlled by factors of the geochemical reactions, weathering, lithology, and solubility of interaction rocks in groundwater. The weathering of minerals is of initial importance in controlling the groundwater chemistry. Hence, Gibbs diagram was employed to assess hydrochemical controlling processes such as precipitation, rock weathering and evaporation on water chemistry in the study area. Accordingly, Gibbs diagram describes the variation in the ratio of Na/(Na + Ca) and Cl/(Cl + HCO3) with TDS (Gibbs 1970). Figure 14 shows that 90% of the samples in the study area fall in the rock weathering zone, and this means that chemical weathering of rock-forming minerals is the main factor controlling the water chemistry in the study area. It would be due to weathering of minerals such as carbonates and silicates. About 10% of the samples in the study area fall in the evaporation zone. It would be due to climate conditions. Since the study area is located in the arid region, thus groundwater evaporation is a common phenomenon. Evaporation increases salinity through increasing Na+ and Cl− content and subsequently TDS.
Mechanisms controlling groundwater chemistry of the study area (after Gibbs 1970)
Irrigation water quality parameters
In the study area, human activities are extensively done (especially agriculture). Therefore, irrigation mainly depends on groundwater properties. Knowledge of the quality of irrigation water is critical to understand what management is necessary for long-term agricultural productivity (Srinivasamoorthy et al. 2014). Irrigation suitability and risk assessment of groundwater are evaluated by measuring EC, %Na, SAR and RSC. The results of the computed indices are given in Table 2.
Electrical conductivity (EC)
The EC (salinity hazard) and Na concentration (sodicity or alkalinity hazard) are usually considered important in classifying irrigation water. High EC in groundwater leads to the formation of saline soil, whereas high Na content causes alkaline soil (Nagarajan et al. 2010). In the study area, EC values and classification are presented in Table 2. Figure 15 shows the spatial distribution of EC values in the study area based on the inverse distance weighting interpolation method.
Sodium percentage (%Na)
Sodium percentage (%Na) is a common index used for assessing the suitability of irrigation water. Sodium content in irrigation water involves exchange reactions with Ca2+ and Mg2+ of clay particles of the soil, which in turn leads to the reduction of soil permeability, internal drainage and air circulation. %Na is calculated using the following formula (Wilcox 1955):
Higher %Na in irrigation water is known to reduce the permeability of the soil. It is important for deciding the suitability of water for irrigation. %Na in water of the study area varies from 18.12 to 96.05 with an average of 63.43. The obtained results of the percent sodium calculations are presented in Table 2. As shown, the majority of the groundwater samples (33% of the samples) is “doubtful” for irrigation. A perusal of Wilcox’s (1955) diagram shows that out of 66 samples, 16 samples are “excellent” to “good”, 5 samples belong to “good to permissible”, 28 samples fall in the “permissible” to “doubtful” category, 13 samples are “doubtful” to “unsuitable”, and 4 samples belong to “unsuitable” category (Fig. 16). Figure 17 shows the spatial distribution of sodium percentage values in the study area based on the inverse distance weighting interpolation method.
Sodium adsorption ratio (SAR)
The sodium adsorption ratio (SAR) is an important parameter for assessing the suitability of groundwater for irrigation. The SAR, which indicates the effect of relative cation concentrations on Na+ accumulation in the soil, is used for evaluating the sodicity hazard of water. Soil permeability decreases due to increase in Na+ in relation to Ca2+ and Mg2+ contents and inhibits water intake by crops. The SAR is computed using the below formula (Hem 1991):
The SAR levels, expressed in meq/l, range from 0.47 to 34.90 (mean = 9.02) in the study area. The SAR data are plotted on the US Salinity Diagram (USSL 1954) (Fig. 18). Figure 19 shows the spatial distribution of SAR values in the study area based on the inverse distance weighting interpolation method.
Residual sodium carbonate (RSC)
An excess amount of \({\text{HCO}}_{ 3}^{ - }\) and \({\text{CO}}_{3}^{2 - }\) over the sum of Ca2+ and Mg2+ also influences the suitability of groundwater for irrigation as these ions cause damage to soil texture by dissolving organic matter in the soil (Kumar et al. 2007). In fact, RSC represents the sum of carbonate and bicarbonate over the sum of calcium and magnesium in water. Adsorption ratio of sodium increases with an increase in RSC value in the soil (Eaton 1950). RSC (expressed in meq/l) is employed to classify usability of water for irrigation and is given by the following relation (Raghunath 2003):
According to the US Salinity Laboratory (1954), an RSC value >2.5 is considered unsuitable for irrigation, a value between 1.25 and 2.5 is of doubtful quality, and a value less than 1.25 is safe for irrigation. RSC values vary from −18.2 to 4.7 (mean = −1.9) in the study area. The obtained results of the calculations and classification are presented in Table 2. Figure 20 shows the spatial distribution of RSC values in the study area based on the inverse distance weighting interpolation method.
Results indicate that the water samples collected in the study area (e.g., samples 27, 28, 33, 34, 35, 36, 39, 40, 45, 56, 58, 59, 60, 61, 62, 63, 64 and 66) are in the best quality and can be used for irrigation and drinking purposes in the future (Fig. 21).
Hydrogeochemical facies and classification (water types)
The term hydrochemical facies is used to describe the bodies of groundwater in an aquifer that different their chemical composition (Aghazadeh and Moghaddam 2011). The chemical composition of groundwater is dependent on the geology, especially lithology, and solution kinetics, flow patterns of the aquifer and anthropogenic activities which take place within the aquifer system. To find the dominant hydrochemical type and characterization of water in the study area, major cations and anions in meq/l were plotted on a trilinear Piper diagram (Piper 1944). According to the dominant cations and anions, five types of water could be identified in the water samples: Ca-HCO3, Ca-SO4, Na-Cl, Na-HCO3 and Na-SO4. The results show that the majority of samples (30 samples, 45%) belong to the mixed Na-SO4 water type. Ca-HCO3 water type (5 samples, 7%) indicates mineral dissolution (probably secondary carbonate and silicate minerals) with sufficient recharge from fresh water (Ako et al. 2012; Srinivasamoorthy et al. 2014). In Na-Cl water type (11 samples, 17%), based on the evolution of water types from the recharge zone to discharge zone (bicarbonate → sulfate → chloride), this evolution can be observed in water samples in the study area (the discharge zone). In addition to, water–evaporitic minerals interaction can also be other reasons for the presence of chloride type in this area (Todd and Mays 2005; Merkel and Planer-Friedrich 2008). Other types include Ca-SO4 water type (8 samples, 12%) and Na-HCO3 water type (12 samples, 18%). Figure 22 shows the distribution of water types in the groundwater of the study area. Also, Fig. 23 shows the spatial distribution of general water types in the study area.
In the study area, an abundance of alkali element (Na+) is probably the result of the dissolution of gypsum, halite, anhydride and alkali feldspar minerals in the aquifer matrix. Alkaline earth content (Ca2+) reflects the dissolution of calcite, dolomite and other Ca-bearing minerals.
Statistical analysis
In this research, multivariate statistical analyses apply to find out determine the relationship between groundwater parameters. Correlation analysis and principal component analysis (PCA) are performed using the commercial statistical software package SPSS version 17.0 for Windows. Principal component analysis (PCA) was implemented to reduce the number of variables and to detect the relationship between variables. This method allows us to display most of the original variability in a smaller number of dimensions and has been widely used in geochemical and hydrochemical studies (Razo et al. 2004). The hydrochemical variables measured consisting of temperature, TDS, EC, pH, Na+, K+, Ca2+, Mg2+, Cl−, \({\text{SO}}_{4}^{2 - }\) and \({\text{HCO}}_{ 3}^{ - }\) are used in this statistical study.
Correlation analysis
Correlation analysis has been widely applied in environmental studies. In this research, because the distribution of ions in groundwater samples is nonparametric, therefore the Spearman’s correlation analysis is used in this study to measure the correlation between physicochemical parameters. A Spearman’s correlation matrix was used to assess parameters associations. They provide an effective way to reveal the relationship between multiple variables and thus are helpful for understanding the influencing factors as well as the sources of chemical components. The correlation coefficient matrix for the physicochemical parameters is presented in Table 3. It is noteworthy that if the correlation coefficient (r) is greater than 0.7, two parameters are considered to be strongly correlated, whereas if the r value is between 0.5 and 0.7, it indicates a moderate correlation at a significance level p < 0.05 (Guey-Shin et al. 2011).
The highest correlation occurs between EC and TDS (r = 0.99) because all of the dissolved components cause increased ionic concentration as well as increased EC concentration. EC and TDS are highly related to Na+ (r = 0.85 and 0.84, respectively), Cl− (r = 0.95 and 0.95, respectively), \({\text{SO}}_{4}^{2 - }\) (r = 0.91 and 0.90, respectively), but moderately related to Mg2+ (r = 0.59 and 0.58, respectively) and the weak positive correlation with Ca2+ (r = 0.31 and 0.30, respectively) and K+ (r = 0.45 and 0.44, respectively). The results indicated that these ions involve various physical and chemical reactions, for example, oxidation/reduction reactions and ion exchange in groundwater aquifers, which suggest that the same factor strongly affects them (Rao 2002, 2003). The weak positive correlation (r = 0.281) of sulfate (\({\text{SO}}_{4}^{2 - }\)) with bicarbonate (\({\text{HCO}}_{ 3}^{ - }\)) suggests that weathering of rock-forming minerals is not the main source that accounts for the \({\text{SO}}_{4}^{2 - }\) concentration in solution. The high correlation between sodium (Na+) and chloride (Cl−) (r = 0.84) is generally used to identify the mechanism of salinity in arid and semiarid regions (Ganyaglo et al. 2010). The observed high concentration of these ions measured in the majority of the samples indicates the predominance of chemical weathering and leaching of chloride salts (mostly halite). The correlation between calcium (Ca2+) and bicarbonate (\({\text{HCO}}_{ 3}^{ - }\)) in the study area is poor (r = 0.43) and represents that in addition to calcite, dissolution of other mineral phases is also important. Calcium (Ca2+) and sulfate (\({\text{SO}}_{4}^{2 - }\) ) ions have a positive correlation (r = 0.323) and represent the dissolution of sulfate minerals in groundwater, especially gypsum and anhydrite. The positive correlation between sulfate (\({\text{SO}}_{4}^{2 - }\)) and magnesium (Mg2+) (r = 0.63) and sulfate (\({\text{SO}}_{4}^{2 - }\)) with sodium (Na+) (r = 0.72) suggests that part of these ions are derived from the weathering of magnesium and sodium sulfate minerals.
Principal component analysis (PCA)
Principal component analysis (PCA) is a widely used multivariate statistical technique, which has been frequently applied in hydrogeochemical studies (Adams et al. 2001). PCA is a versatile tool for the data reduction. This method is applied for the reduction of dimensionality of data sets into uncorrelated principal components (PCs) based on the correlation matrix of variables (Jolliffe 2002). Two matrices of scores and loadings are delivered by PCA. The former explains the relationship between components and samples, while the latter manifests the relationship between components and variables. In fact, the PCA is a linear combination of variables. For factor loadings, a high loading was defined as greater than 0.75, and a moderate loading was defined as 0.40–0.75. Loadings of less than 0.40 were considered insignificant (Evans et al. 1996). PCA and varimax rotation in order to determine the optimum number of factors have been performed using SPSS software (version 17). Two significant components were retained based on the eigenvalues of greater than one (Kaiser 1958). Figure 24 depicts the scree plot of components, according to which significant component was extracted. Table 4 demonstrates the total variances explained by the extracted components. The results show that the two factors for more than 75% of the variance, which is quite good and can be relied upon to identify the main sources of variation in the hydrochemistry. The first component (PC1) explains 54.8% of the total variance (Table 4) and indicates that EC, TDS, Cl−, Ca2+ and Na+ have high loading while temperature, \({\text{SO}}_{4}^{2 - } ,{\text{HCO}}_{ 3}^{ - }\) and Mg2+ have moderate loading (Table 5). It represents halite and evaporates from the underlying geologic formations (sulfate and chloride water types). EC and TDS have loadings of 0.87 and 0.87 and control the overall mineralization. Calcium has a loading of 0.766, and the high loading may be attributed to its abundance in the earth’s crust or as the by-product of the weathering of feldspars, amphibole and pyroxenes. Sodium and magnesium have loadings of 0.908 and 0.637, respectively, and sodium could be derived from the weathering of plagioclase feldspar and also cation exchange process while magnesium is derived from the weathering of mafic minerals. Chloride has a loading of 0.867, and it is derived from anthropogenic sources or the source of chloride in the study area could be from water trapped during magnetic activities. Sulfate in groundwater could be reduced to H2S during degradation of organics. The second component (PC2) explains 21.2% of the total variance and indicates that the pH has high loading, while \({\text{SO}}_{4}^{2 - }\) and K+ have moderate loadings.
Conclusions
In this research, the assessment of water quality and hydrogeochemistry studies has been investigated in the north of Isfahan. The hydrogeochemical study is a useful tool to identify processes that are responsible for groundwater chemistry. To assess the quality of groundwater, hydrochemical parameters of groundwater such as pH, total dissolved solids (TDS), electrical conductivity (EC), sodium absorption ratio (SAR), total hardness (TH), major cations (K+, Na+, Ca2+ and Mg2+) and major anions (Cl−, \({\text{HCO}}_{ 3}^{ - } ,{\text{CO}}_{3}^{2 - }\) and \({\text{SO}}_{4}^{2 - }\)) concentration were measured. In order to achieve the research objectives, 66 water samples from different locations (35 qanats, 22 springs, 8 wells and 1 piezometer) were collected during April and May 2015 from representative sites in the study area. In this research, the analytical results of physiochemical parameters of groundwater were compared with the standard guideline values as recommended by the world health organization (WHO) for drinking and public health purposes. In the study area, obtained results indicated that pH values of groundwater samples varied from 7.05 to 8.95 with a mean of 7.78, reagent a neutral to slightly alkaline water. Total dissolved solids (TDS) values were ranging from 104 to 2250 mg/l, with a mean value of 568 mg/l. Water electrical conductivity (EC) values varied from 213 to 4320 µS/cm with an average of 1133 µS/cm. TH values varied from 15.65 to 441 mg/l with an average of 123.15 mg/l. In all samples, Na+/Cl− ratio is greater than 1 and indicated that an ion exchange process was common and might come from silicate weathering. Gibbs diagram had shown that 90% of the samples in the study area fall in the rock weathering zone, and this means that chemical weathering of rock-forming minerals was the main factor controlling the water chemistry in the study area. Irrigation suitability and risk assessment of groundwater were evaluated by measuring EC, %Na, SAR and RSC. Most of the samples in the study area fall in the “good” and “doubtful” range based on SAR and %Na values, respectively. From Wilcox plot, it is observed that about 74% of the samples of the study area fall in the “excellent” to “good” and “good” to “permissible” categories, while remaining samples are “permissible” to “doubtful”, “doubtful” to “unsuitable” and “unsuitable” categories for irrigation purpose. According to the dominant cations and anions, five types of water could be identified in the water samples: Ca-HCO3, Ca-SO4, Na-Cl, Na-HCO3 and Na-SO4. The results show that the majority of samples (30 samples, 45%) belong to the mixed Na-SO4 water type. Based on statistical studies, the hydrochemistry of groundwater in the study area is influenced by many factors, including the water types, the degree of dissolution and similarity of hydrochemical processes. Correlation analysis and principal component analysis (PCA) were used to identify the relationship between ions and physicochemical parameters. Results indicate that the water samples collected in the study area (e.g., samples 27, 28, 33, 34, 35, 36, 39, 40, 45, 56, 58, 59, 60, 61, 62, 63, 64 and 66) are in the best quality and can be used for irrigation and drinking purposes in the future. The main reason for the better quality of samples is that these samples are located near the aquifer recharging zones.
References
Adams, S., Titus, R., Pietersen, K., & Harris, G. (2001). Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. Journal of Hydrology, 241(1–2), 91–103.
Aghazadeh, N., & Moghaddam, A. A. (2011). Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environmental Monitoring and Assessment, 176, 183–195.
Ako, A. A., Shimada, J., Hosono, T., Kagabu, M., Ayuk, A. R., Nkeng, G. E., et al. (2012). Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environmental Geochemistry and Health. doi:10.1007/s10653-012-9453-3.
Amidi, S. M., & Zahedi, M. (1988). Geology map of Kashan 1:250,000 sheets. Tehran: Geology Survey of Iran.
Babaahmadi, A., Safaei, H., Yassaghi, A., Vafa, H., Naeimi, A., Madanipour, S., et al. (2010). A study of Quaternary structures in the Qom region, West Central Iran. Journal of Geodynamics, 50, 355–367.
Baghvand, A., Nasrabadi, T., Bidhendi, N. G., Vosoogh, A., Karbassi, A., & Mehrdadi, N. (2010). Groundwater quality degradation of an aquifer in Iran central desert. Desalination, 260, 264–275.
Eaton, E. M. (1950). Significance of carbonate in irrigation water. Soil Science, 69, 12–133.
Evans, C. D., Davies, T. D., Wigington, P. J., Jr., Tranter, M., & Kretser, W. A. (1996). Use of factor analysis to investigate processes controlling the chemical composition of four streams in Adirondack Mountains, New York. Journal of Hydrology, 185, 297–316.
Ganyaglo, S. Y., Banoeng-Yakubo, B., Osae, S., Dampare, S. B., & Fianko, J. R. (2011). Water quality assessment of groundwater in some rock types in parts of the eastern region of Ghana. Environmental Earth Sciences, 62(5), 1055–1069.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Giridharan, L., Venugopal, T., & Jayaprakash, M. (2008). Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environmental Monitoring and Assessment, 143, 161–178.
Guey-Shin, S., Bai-You, C., Chi-Ting, C., Pei-Hsuan, Y., & TsunKuo, C. (2011). Applying factor analysis combined with Kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. International Journal of Environmental Research and Public Health, 8, 1084–1109.
Guler, C., & Thyne, G. D. (2004). Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian wells-Owens valley area, Southeastern California, USA. Journal of Hydrology, 285, 177–198.
Handa, B. K. (1969). Description and classification of media for hydro-geochemical investigations. Roorkee: Symposium on Ground Water Studies in Arid and Semiarid Regions.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water. Book 2254 (3rd ed.). Jodhpur: Scientific Publishers.
Jalali, M. (2006). Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamedan, Iran. Environmental Geology, 51, 433–446.
Jeevanandam, M., Kannan, R., Srinivasalu, S., & Rammohan, V. (2007). Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environmental Monitoring and Assessment, 132, 263–274.
Jiang, Y., & Yan, J. (2010). Effects of land use on hydrochemistry and contamination of Karst groundwater from Nandong underground river system, China. Water, Air, and Soil pollution, 210, 123–141.
Jolliffe, I. T. (2002). Principal component analysis (2nd ed., p. 487). New York: Springer.
Jordao, C. P., Ribeiro, P. R. S., Matos, A. T., Bastos, R. K. X., Fernandes, R. B. A., & Fontes, R. L. F. (2007). Environmental assessment of watercourses of the Turvo Limpo River basin at the Minas Gerais State, Brazil. Environmental Monitoring and Assessment, 127, 315–326.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23, 187–200.
Kamtchueng, B. T., Fantong, W. Y., Ueda, A., Tiodjio, E. R., Anazawa, K., Wirmvem, M. J., et al. (2014). Assessment of shallow groundwater in Lake Nyos catchment (Cameroon, Central-Africa): Implications for hydrogeochemical controls and uses. Environment and Earth Science, 72, 3663–3678.
Kumar, M., Kumari, K., Ramanathan, A., & Saxena, R. (2007). A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Journal of Environmental Geology, 53, 553–574.
Ludwig, F., Stober, I., & Bucher, K. (2011). Hydrochemical groundwater evolution in the bunter sandstone sequence of the Odenwald Mountain Range, Germany: A laboratory and field study. Aquatic Geochemistry, 17, 165–193.
Mano, J. K., Ghosh, S., & Padhy, P. K. (2013). Characterization and classification of hydrochemistry using multivariate graphical and hydrostatistical techniques. Research Journal Chemical Sciences, 3, 32–42.
Merkel, B., & Planer-Friedrich, B. (2008). Groundwater geochemistry—A practical guide to modeling of natural and contaminated aquatic systems. Berlin: Springer.
Meyback, M. (1987). Global chemical weathering of surficial rocks estimated from river dissolved loads. American Journal of Science, 287, 401–428.
Nagarajan, R., Rajmohan, N., Mahendran, U., & Senthamilkumar, S. (2010). Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environmental Monitoring and Assessment, 171, 289–308.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Eos, Transactions American Geophysical Union, 25(6), 914–928.
Raghunath, H. M. (2003). Groundwater. New Delhi: New Age International (P) Ltd.
Rao, N. S. (2002). Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environmental Geology, 41, 552–562.
Rao, S. V. L. (2003). Cluster analysis of groundwater quality data of Venkatagiri Taluq, Nellore district, Andhra Pradesh. Journal of the Geological Society of India, 62, 447–454.
Razo, I., Carrizales, L., Castro, J., Díaz-Barringa, F., & Monroy, M. (2004). Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollution, 152, 129–152.
Richards, L. A. (1954). Diagnosis and improvement of saline- alkali soils: Agriculture, Handbook 60 (vol. 160). Washington, DC: US Department of Agriculture.
Rowell, D. L. (1994). Soil science: Methods and applications. New York: Longman and Scientific Technical.
Schoeller, H. (1977). Qualitative evaluation of ground water resources (in methods and techniques of groundwater investigations and development), water resources series (vol. 33, pp. 44–52), UNESCO.
Srinivasamoorthy, K., Gopinath, M., Chidambaram, S., Vasanthavigar, M., & Sarma, V. S. (2014). Hydrochemical characterization and quality appraisal of groundwater from Pungar sub-basin, Tamilnadu, India. Journal of King Saud University-Science, 26, 37–52.
Subramani, T., Rajmohan, N., & Elango, L. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in hard rock region, Southern India. Environmental Monitoring and Assessment, 162, 123–137.
Tatawat, R. K., & Chandel, S. C. P. (2008). A hydrochemical profile for assessing the groundwater quality of Jaipur City. Environmental Monitoring and Assessment, 143, 337–343.
Todd, D. K., & Mays, L. W. (2005). Ground-water hydrology. New York: Wiley.
USSL (United States Salinity Laboratory). (1954). Diagnosis and improvement of saline and alkaline soils. Washington: US Department of Agriculture.
WHO. (2004). Guidelines for drinking water quality, vol. 1 recommendations (3rd ed.). Geneva: WHO.
Wilcox, L. V. (1955). Classification and use of irrigation water. USDA circular 969. US Department of Agriculture, Washington, DC, p. 19.
Acknowledgements
The authors would like to thank the Amirkabir University of Technology (Polytechnic Tehran) for supporting this research. The contribution of Adonis Fard Mousavi, Omid Mazaheri and Mohammad Parsa is appreciated. Funding was provided by Amirkabir University of Technology (Grant No. 95930).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, A., Hassani, H. Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan, Iran. Environ Geochem Health 40, 583–608 (2018). https://doi.org/10.1007/s10653-017-0003-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0003-x