Abstract
The present investigation was conducted in order to evaluate the occurrence and distribution patterns of some potentially harmful trace elements in the borehole water of the Greater Giyani area, Limpopo, South Africa, and their possible implications on human health. Twenty-nine borehole water samples were collected in the dry season (July/August 2012) and another 27 samples from the same localities in the wet season (March 2013) from the study area. The samples were analysed for trace elements arsenic (As), cadmium (Cd), chromium (Cr), selenium (Se), and lead (Pb) using the inductively coupled plasma mass spectrometry technique. The average concentrations of As, Cd, Cr, Se, and Pb were 11.3, 0.3, 33.1, 7.1, and 6.0 µg/L in the dry season and 11.0, 0.3, 28.3, 4.2, and 6.6 µg/L in the wet season, respectively. There was evidence of seasonal fluctuations in concentrations of all analysed elements except for As, though Cd and Pb displayed low concentrations (<0.2 and <6.0 µg/L, respectively) in almost all sampled boreholes. Se and Cr concentrations slightly exceed the South African National Standard permissible limits for safe drinking water in few boreholes. A total of four boreholes exceeded the water quality guideline for As with two of these boreholes containing five times more As than the prescribed limit. The spatial distribution patterns of elevated As closely correlate with the underlying geology. The findings of this investigation have important implications for human health of the communities drinking from the affected boreholes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Borehole water is a primary source of drinking water supply in many parts of rural South Africa (DWAF 2004). In the Limpopo Province, according to DWAF (2000), groundwater accounts for about two-thirds of the total water supply. Since borehole water comes from underground, it is commonly considered pure and clean, but this is often not the case (Fawell and Nieuwenhuijsen 2003). All natural water contains some contaminants that may be deleterious to human and animal health either occurring at relatively low concentrations or in excessive amounts (Mehra and Juneja 2003; Afridi et al. 2006).
Globally, several studies provide evidence of the natural occurrence of high concentrations of toxic elements such as arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), and selenium (Se) in groundwater and associated health effects (Langard 1990; Smith et al. 2000; Duker et al. 2005; Ferreccio and Sancha 2006; Kapaj et al. 2006; Celik et al. 2008; Bernard 2008; McCarty et al. 2011; Chen 2012). The major health effects that can manifest after drinking water with anomalous As concentrations include cancer of the lungs, skin, liver, and bladder as well as non-carcinogenic health effects such as skin tumours, hyperkeratosis and hyperpigmentation, respiratory, and cardiovascular illness (Kapaj et al. 2006; Putila and Guo 2011; IARC 2012). Pb toxic effects are primarily on the haematological, renal, cardiovascular, gastrointestinal, endocrine, immune, central, and peripheral nervous system (Hutton 1987; WHO 2010). Young children are particularly vulnerable to the toxic effects of lead; at high levels of acute exposure, Pb attacks the brain and central nervous system and may cause coma, convulsions, and even death, while low-level long-term exposure to Pb is associated with neurobehavioral problems and cognitive difficulties such as reduced intelligence quotient, problems in paying attention, and increased antisocial behaviour (Finkelstein et al. 1998; WHO 2010). Umemura and Wako (2006) report that chronic Cd intoxication is characterised by renal dysfunction and osteopenic osteomalacia. The following studies by Langard (1990), Huvinen et al. (2002), Motzer and Engineer (2004) and Linos et al. (2011) suggest that exposure to Cr may cause respiratory diseases, cancer, ulceration, and perforation of the nasal membranes.
In South Africa, there are only a few studies that have examined the distribution of potential harmful trace elements (PHTEs) in the groundwater. For example, several boreholes in Beaufort West were found to contain potentially harmful levels of As, which is believed to be associated with uranium deposits (WRC 2009). Similarly, Meyer and Casey (2004) indicated that in the northern region of South Africa, arsenic (As), bromine (Br), cadmium (Cd), lead (Pb), mercury (Hg), molybdenum (Mo), and selenium (Se) are found in the drinking water of indigenous goats, occurring as localised anomalies at concentration levels exceeding local and international drinking water guideline values. Sami and Druzynski (2003) have identified geological units, e.g. Giyani Group, Giyani Greenstone Belt, Schiel Alkaline Complex, and Banderlierskop Complex that may be potential geological sources for anomalous As and Se based on their association with sulphidic gold mineralisation. Though these studies shed some light on the issue and clearly show that our groundwater resources might locally contain PHTEs that may be detrimental to human health, there is still a dire need for detailed studies on occurrences and distribution of these elements in South African groundwater resources, specifically those utilised for rural domestic purposes.
The main aim of the present study was to assess concentration levels and distribution patterns of some selected PHTEs such as, As, Cd, Cr, Pb, and Se in borehole water of rural communities in Greater Giyani local Municipality, Limpopo, South Africa, and the potential health implications associated with these elements. These trace elements were chosen because they are known to cause the most deleterious effects on humans. According to the International Agency for Research on Cancer (IARC) (2016), As, Cd, and Cr(VI) compounds are classified as group 1 human carcinogen. This ranking implies that there is enough evidence to suggest that exposure to these elements may cause cancer in humans. These trace elements were chosen because there is not enough information available on them in the study area in particular and South Africa in general.
The study area
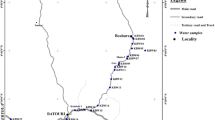
The study area is located in the Greater Giyani local Municipality (Fig. 1), which falls within the Mopani District in Limpopo Province, South Africa. The Municipality covers an area of approximately 25,344 13 km2 including part of the Kruger National Park (Mopani District Municipality 2008). The climate of the area varies from subtropical to semi-arid, and the temperature ranges from moderately warm to hot in the mountainous region to hot and extremely hot in the plains region (DWAF 2006). High rainfall occurs mainly between November and February (South African Weather Service 2011). Though the region receives high rainfall in summer, it often experiences severe drought conditions that result in serious water shortage (Mopani District Municipality 2008).
Geological and hydrological background
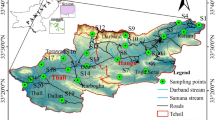
The study area is predominately underlain by Goudplaats Gneiss with the central area occupied by the Giyani Greenstone Belt, previously known as the Sutherland Greenstone Belt (Fig. 2). Shamariri Granite occurs to the north and to the south of the belt. Small outcrops of Schiel alkaline intrusion also occur at the western extremity of the study area. Goudplaats Gneiss is the oldest gneissic terrain in the area and generally forms flat ground and has overall poor exposure (Robb et al. 2006). Isotopic ages (207Pb/206Pb) for the granitoid gneisses obtained by single-grain zircon evaporation provide emplacement ages of between 2.8 and 3.3 Ma (Brandl and Kröner 1993; Kroner et al. 2000). The gneiss is generally characterised by alternating bands of leucocratic and melanocratic material of several centimetres in width and includes an assemblage of orthogneisses, tonalitic, trondhjemitic, and granodioritic (TTG) composition (e.g. Brandl 1986, 1987; Anhaeusser 1992; Baglow 2005; Robb et al. 2006).
The northern margin of the Giyani Greenstone Belt is characterised by a set of shear zones forming part of the Hout River shear system (Kreissig et al. 2001) that separates the Limpopo Complex from the Kaapvaal Craton, in which the Giyani Belt is situated. To the north of these shear zones, in the Southern Marginal Zone of the Limpopo Complex, the metamorphic grade of the Goudplaats Gneiss underwent granulite metamorphism at 2.73 Ga and subsequent patchy retrogression (Belyanin et al. 2014). A number of shear zone-hosted gold deposits are located mainly in the northern part of the Giyani Greenstone Belt, within the Hout River System (Gan and Van Reenen 1996). The gneisses in the area to the south of the Belt are also known as Klein Letaba or Baviaanskloof Gneiss. They are characterised by amphibolite facies metamorphism and form part of the Kaapvaal Craton (Robb et al. 2006). The north-east trending Giyani Greenstone Belt is a roughly sinusoidal-shaped supracrustal complex with a widened central area and a 30-km extension towards the west, which bifurcates into two arms, a southern Lwaji and north-western Khavagari arm (Brandl 1987; Kroner et al. 2000; Kramers et al. 2014). In the southern Lwaji arm, the base succession consists of ultramafic schists, which pass upward into a thick sequence of metasedimentary rocks including phyllite, quartz–sericite–chlorite schists, and quartzite (Kroner et al. 2000). The base succession of the northern Khavagari arm consists of ultramafic schists, probably metamorphosed komatiites, associated with cherts and banded iron formation (Kroner et al. 2000). In the central part, mafic rocks (metagabbros as well as volcanics) are associated with metapelites (Kramers et al. 2014). Generally, the belt is very poorly exposed, which prohibits lithostratigraphic subdivision. However, according to Brandl (1987), the succession can be divided into the following rocks types: ultramafic schists and amphibolite with subordinate metasedimentary rocks, iron formation, and acid metalava.
The hydrology of the study area is generally characterised by fracture-bound aquifers formed mainly within the rocks of the Goudplaats Gneiss, Giyani Greenstone Belt and to a smaller extent the Shamariri Granite and Schiel Alkaline Complex (Haupt and Sami 2006). The major rivers that transect the area are the Nsami, Klein Letaba, Shingwedzi, Middle Letaba, and Groot Letaba, which flow in an easterly direction towards the Indian Ocean. These Rivers form part of the secondary drainage region B8 and B9, which falls within the Letaba/Luvuvhu Water Management Area (WMA) (DWAF 2004). According to Du Toit and Lelyveld (2010), the groundwater quality of the aquifer hosted by the Goudplatas Gneiss, evaluated in terms of electrical conductivity (EC), using 611 analyses, shows that about 63 % of boreholes have EC values above the recommended limit of 70 mS/m for drinking water, whereas 5 % have values higher than the maximum allowable limit of 300 mS/m. In the Greenstone Belt aquifers, the groundwater is of good quality although some elevated nitrate and magnesium values were recorded. Their averages, however, are below the recommended upper limit for domestic use. The aquifer in the Schiel Complex is typically alkaline, and groundwater displays magnesium–sodium–calcium-bicarbonate character. No information is available for groundwater quality in the Shamariri Granite aquifer.
Methodology
As part of this investigation, geochemical groundwater datasets of Limpopo were obtained from Groundwater Resource Information Project (GRIP) Limpopo and Groundwater Project Management (GPM) consultants. GRIP is a project aimed at gathering and verifying groundwater data to improve management and development of rural groundwater resources (DWAF n.d.). Close inspection of the GRIP database indicated that trace elements in groundwater from the studied area were not analysed. Therefore, in order to achieve the objective of this investigation, a total of 45 villages were visited, and 88 % of these had a working community borehole and/or a school borehole used as primary source of drinking water supply. The remaining villages either had no borehole or depended on other sources of water. The groundwater is pumped into a storage reservoir and water flows from there to the communal taps. Among those villages with working borehole, only 6 % were insignificant to this study as surface water mixes with groundwater in the reservoirs, which hindered collection of representative water samples.
Twenty-nine water samples were collected from boreholes, including 15 community boreholes and 14 primary school boreholes in the Giyani area during the dry season (July/August 2012). Another 27 samples including 15 community boreholes and 12 school boreholes from the same localities were collected during the wet season (March 2013) for comparison. A global positioning system (GPS) was used for locating the boreholes, and a geographic information system (ArcMap 10.2.2) was used for mapping. The boreholes were selected based on the following criteria: each borehole had to represent a single village and serve at least ten individuals. Additionally, the borehole water had to be used primarily for drinking purposes. All boreholes selected satisfied these criteria. Field parameters pH, temperature (T), and oxidation–reduction potential (Eh) were measured in the field using a portable WTW Multi-parameter (3430) equipped with a SenTix pH electrode and conductivity TetraCon 95 probes.
Borehole water sampling
The sampling procedures employed here are based on the recommendations outlined in Weaver et al. (2007). Prior to collection of a water sample, a new pair of disposable gloves was used to prevent cross-contamination between sample locations; and all sample bottles, lids, sampling buckets, and syringes were thoroughly washed with distilled water. Purging of the borehole is a most important and necessary practice during groundwater sampling. This technique removes stagnant water that can alter the chemistry of the water and result in unrepresentative samples (Weaver et al. 2007). All boreholes sampled in the study area were in use at the time of sampling as such, purging of borehole was not necessary. However, as a precautionary measure, all boreholes were purged for several minutes. A bucket was used to collect water from the boreholes. An aliquot of the sample was then withdrawn from the bucket using a syringe into a thoroughly rinsed 100-mL plastic bottle. To prevent suspended solids from being collected in the sample water, a 0.45-µm disposable membrane filter fitted onto the syringe was used to filter the sample water. The filtered samples were acidified to pH < 2 with nitric acid (HNO3) to preserve and reduce precipitation of trace elements. The acidified samples were kept cool at approximately 4 °C until dispatch (after less than a week) to the Council for Geoscience laboratory for analysis. The samples were then analysed for trace elements As, Cr, Cd, Se, and Pb using a PerkinElmer ELAN 6000 ICP-MS. In order to assess the quality of the borehole water, we compared PHTEs concentrations with the 2011 South African National Standard (SANS 241-1) for safe drinking water. The SANS maximum allowable concentrations for As, Cd, Cr, Se, and Pb in drinking water are 10, 3, 50, 10, and 10 µg/L, respectively (SANS 2011).
Quality assurance
Quality control and quality assurance are an integral part of water sampling activities as they ensure that a representative water sample is collected and the water results are accurate and reliable (Weaver et al. 2007). Quality control was ensured at every stage from sample collection to analyses. As indicated above, care was taken during sample collection to minimise potential contamination and appropriate preservation methods were followed to ensure representative samples. Sample water bottles were clearly labelled to prevent accidental mix-up of samples at the laboratory. Four blind field duplicate samples were collected (two in the dry season and another two in the wet season) to assess sampling precision and laboratory accuracy. Verification of the ICP-MS calibration was done by analysing two reference standard solutions, one a separately prepared solution of the Merck VI-standard (referred to as “QC STD”) and another a custom mixture of major and trace elements, purchased from a separate supplier, with a certificate of analysis tracing concentration of elements to NIST quantities, referred to as “QC MIX”. Total method blanks were analysed at regular intervals during the analytical batch to monitor possible contamination in the laboratory environment and consumables used. The limit of detection for the method applied was less than 4, 0.2, 1, 0.4, and 6 µg/L for As, Cd, Cr, Se, and Pb, respectively. Accuracy of the method was determined from the analysis of the two reference standard solutions “QC STD” and “QC MIX”, and the results expressed as recovery (%). The recovery results (Table 1) were calculated as the average measured value for the reference standard, divided by the certified or reference values, expressed as %. Recovery (in %) = measured value/reference value ×100 %. The certified values of the QC STD and QC MIX reference standards, as provided by certificate of analysis, were used as the reference values. Precision (or repeatability) was calculated from repeated measurements of the QC MIX-solution (n = 8 replicate measurements). Within-batch repeatability expressed as relative standard deviation, RSD (%) was found to be 1.13, 1.07, 1.44, 2.96, and 3.04 for As, Cr, Cd, Se, and Pb, respectively.
Borehole water results
The trace elements and physico-chemical properties of the water samples from boreholes in Giyani are shown in Tables 2 and 3. The mean trace element concentrations decrease in the order of Cr > As > Se > Pb > Cd and Cr > As > Pb > Se > Cd, in the dry and wet seasons, respectively. The borehole water of the Greater Giyani area is circum-neutral with pH ranging from 6.6 and 7.6 during the dry season and from 6.8 and 7.9 in the wet season. The highest pH value of 7.9 was recorded at borehole BH-08, whereas the lowest value of 6.6 was registered at borehole BH-02. The pH values for all the samples are within the SANS prescribed limits of 5.0–9.7 for safe drinking water (SANS 2011). The average temperatures recorded were 25 and 28 °C for the dry and wet seasons, respectively. The redox potential for the dry season lies between 154.4 and 252.4 mV, while in the wet season, it lies between 179.5 and 270.4 mV. The As concentrations during the sampling period varied widely; it ranged from less than 4.0 µg/L (laboratory detection limit) in 86 % of all samples tested to 112.3 µg/L with a mean concentration of 11.3 and 11.0 µg/L in the dry and wet seasons, respectively. In general, there is no discernible difference between As levels in the dry season as compared to the wet season, except for one borehole, BH-28, which presented As levels of 61.5 and 75.0 µg/L in the dry and wet seasons, respectively (Fig. 3). Of the 29 boreholes sampled, 14 % exceeded As levels of 10.0 µg/L, the SANS recommended limit for safe drinking water. Two of these boreholes, BH-07 and BH-28 gave As levels of more than five times higher than the SANS permissible limit for safe drinking water. The total Se content varied from 1.7 to 18.8 µg/L and from 0.4 to 12.0 µg/L in the dry season and wet seasons, respectively. Approximately, 24 % (7 boreholes) of the sampled boreholes exceeded the SANS maximum permitted limits for the amount of selenium level allowed in drinking water (Fig. 4). Cr concentrations in the borehole water of the study area are generally low, and only one borehole exceeded the SANS allowable limit for safe drinking water. Though the remaining samples fall below the SANS recommended upper limit for drinking water, a large proportion of borehole water samples gave Cr levels of more than 30.0 µg/L in both seasons. Similarly, Pb and Cd concentrations are generally low, mostly below the detection limits, and they fall far below the SANS for drinking water (Fig. 5). Only one sample (BH-08) in the wet season contains unusually high Pb (19.0 µg/L) concentrations; this sample also has a higher than average Cd concentrations. Seasonal variations are observed for all the trace elements under investigation except for As, though Cd and Pb levels were very low and are below the detection limits in most of the boreholes. The concentrations of Se, Pb, and Cr were significantly higher in most boreholes in the dry season. In the case of Cd, a few high peaks were observed in both dry and wet seasons. The spatial distribution patterns of As, Pb, Cr, Se, and Cd in borehole water in relation to the geology in Giyani area are shown in Figs. 6, 7, 8, 9 and 10.
Discussion
Trace elements occurring at elevated concentrations in borehole water of the Giyani area
Out of five trace elements analysed, only As showed elevated concentrations in most boreholes as compared to other trace elements. Four boreholes had As concentrations greater than SANS permissible concentrations of 10.0 µg/L with two of these boreholes displaying As levels of approximately five times higher than the SANS recommended limit for safe drinking water. Elevated As concentrations were observed at pH values >7.1 and progressively increased with an increase in pH, although most water samples with high pH have low As contents (Fig. 11). Similar trends have been observed in groundwater of eastern New England (Ayotte et al. 2003) and several other areas in the USA (Welch et al. 2000). Given that Fe-oxides are a common constituent in the lateritic soils in the study area, the observed increase in As concentrations at higher pH suggests that desorption of iron oxides is favoured in this condition (Dzombak and Morel 1990; Welch et al. 2000). Desorption of As from iron oxides is pH dependent (Massecheleyn et al. 1991). At elevated pH, ferric oxides generate a negative surface charge which results in electrostatic repulsion of As, leading to a release of As in the water (Welch et al. 2000). Eh negatively correlates with As concentrations; thus, some high-As groundwaters are associated with low Eh, although most low Eh samples have low As contents (Fig. 12). This is in agreement with the high-As groundwater found associated with low Eh from Cu–W–As belt in SW Finland (Bodenan et al. 2004). Speciation of As was not determined; however, under this condition, arsenate is expected to be the dominant As species (Massecheleyn et al. 1991). Other As-enriched water with circum-neutral to alkaline pH conditions associated with sulphide minerals is found in the Mother Lode in Califonia (Welch et al. 2000).
Spatially, As appears to be strongly influenced by the underlying geology and the bedrock mineralisation. The boreholes with the highest As concentrations are found in the Giyani Greenstone Belt, mostly along its northern margins where gold mineralisation is also concentrated, while regional low As concentrations occur in the gneisses. As is a common constituent in more than 200 minerals, particularly, sulphide bearing mineral deposits associated with gold mineralisation (Edmunds and Smedley 1996; Duker et al. 2005). These observations are in agreement with that of Smedley et al. (2007) who found that high As in groundwater of Burkina Faso occurs close to sites of known gold mineralisation, while low-As groundwaters are reported away from the Au-mineralisation. Similarly, As-rich waters related to mineralised zones are reported in Thailand (As concentrations of up to 5000 µg/L), Brazil (As levels of up to 350 µg/L), and various areas in the USA (As levels of up to 48,000 µg/L) (Smedley and Kinniburgh 2002). Notably, low concentrations of As in the southern section of the Giyani Belt coincide with the lack of sulphide minerals and gold deposits.
Other trace elements in borehole water of the Giyani area
Se, Cr, Cd, and Pb levels were generally very low and mostly found below the laboratory detection limit, and those boreholes with relatively higher concentrations only slightly exceeded the SANS limit for drinking water. Globally, the levels of Se in groundwater are generally low, and most drinking water contains concentrations much lower than 10.0 μg/L, but these levels may increase in the presence of natural or anthropogenic source of Se (Bassil et al. 2014; WHO 2011). The results of our investigation show that only few boreholes (24 %) slightly exceeded the SANS limit for Se in drinking water and those boreholes with anomalous concentrations occur sporadically in the gneisses. These anomalies may be attributed to the presence of Se-bearing minerals such as selenopyrite (FeS2) in these rock types (Bailey et al. 2009). Other possible sources of Se include applications of Se and nitrate fertilisers to agricultural soil (Bailey et al. 2009; Hudak 2010). Nitrate has been shown to play an important role in mobilising Se in agricultural lands through oxidation reactions (Wright 1999). Se can be oxidised by dissolved nitrate in soil, releasing selenate (SeO4) in the groundwater (Bailey et al. 2009). For example, high-Se groundwaters are found to be associated with irrigated lands where nitrate is abundant (Bailey et al. 2009). Similarly, a survey of 79 wells in irrigated agricultural regions of South Texas indicated that about 4 % of sampled wells exceeded the 50 µg/L Se limit for drinking water (Hudak 2010). Furthermore, presence of high-sulphur coals may contribute mass loading of Se in the groundwater (Lenz and Lens 2009).
In terms of Cr concentrations, only one borehole had high concentrations exceeding the SANS recommended limit of 10.0 µg/L for safe drinking water. The spatial distribution map of Cr concentrations does not reveal any apparent spatial or geology-related pattern. The localised anomaly in borehole BH-09 may be attributed to the presence of the Cr-bearing mineral chromite (Oze et al. 2007). Cd was detected in only a few samples, but most of the analyses were found to be below the laboratory detection limit of 0.2 µg/L. The maximum acceptable limit for drinking water was not exceeded in any borehole. Pb was found below the detection limit of 6.0 µg/L in almost all boreholes except in borehole BH-08 in the wet season, where the value is 19.0 µg/L. This is to be expected as the Pb content in the aqueous environment is generally very low (UNEP 2010). For instance, in groundwater of Yenagoa, Bayelsa State in Nigeria, Pb concentrations were detected below the detection limit except in three boreholes with Pb values ranging from 0.008 to 0.04 mg/L (Agbalagba et al. 2011). The source for the unusual high Pb concentrations in one borehole (BH-08) in our study area is unclear; however, we believe that it might not be geogenic. According to Katz et al. (1999), borehole casing material can affect the amount of Pb in the borehole water. In their study, they found that median concentrations of Pb in waters from black iron and steel-cased boreholes were significantly higher than those from PVC-cased boreholes (Katz et al. 1999). Similarly, a laboratory experiments designed to compare leaching of metal pollutants from PVC and stainless steel well casings by exposing them to groundwater for four periods ranging from 1 to 40 days showed that stainless steel released the greatest amount of Pb into groundwater (Hewit 1989). It is therefore highly likely that borehole casing materials are the source of the unusual Pb levels in borehole BH-08. This is further supported by the fact that most of the boreholes in the study area have been reported to have stainless steel casing. Spatially, no apparent variation with rock types was observed for Pb and Cd concentrations.
Seasonal variability is observed for all trace elements under investigation except for As, although Cd and Pb concentrations were very low and below the detection limit in most of the boreholes. The concentrations of Se and Cr were highest in the dry season and lowest in the wet season. The elevated concentrations of Se and Cr in the dry season are more likely the result of evapotranspiration effects due to the hot climate, high pH, and redox reactions which facilitate desorption of oxyanions in the groundwater (Thundiyil et al. 2007; Ayotte et al. 2015). In contrast, As appeared to be little affected by seasonal variability. This observation is however not uncommon; in the USA, a study in Western Nevada assessed temporal variability of As in 356 wells between 1982 and 2000, and the results showed no significant fluctuation of As concentrations over the study period (Thundiyil et al. 2007). Similar results were also reported by Steinmaus et al. (2005) from the same region.
Possible implications for human health
The findings of the current investigation carry important implications for the health of the communities of the study area. In general, the proportion of Pb, Cd, Cr, and Se in most boreholes in the study area only exceeded the guideline limit by less than order of magnitude above the SANS acceptable maximum limit for drinking water, likely with minimal health risks. However, four boreholes in our study area contained As concentrations above the SANS limit for safe drinking water; two of these exceeded the limit by more than five times the acceptable limit. These boreholes need further risk assessment by the health community in association with geochemists because continuing drinking water from these boreholes with elevated concentrations of As may result in As-related health issues including skin conditions such as pigmentation and keratosis (Mazumder 2008). In addition, chronic As toxicity may cause cancer of the bladder, kidney, lung, neurological effects and, even death (Smith et al. 1992, 2000). This is evident in numerous studies where a link was established between exposure to elevated As in drinking water and cancer (Hopenhayn-Rich et al. 1998; Celik et al. 2008; Sohel et al. 2009; Nath et al. 2013). Nath et al. (2013) assessed concentrations of As in drinking water and blood samples of residents on the banks of the river Ganges in Patna, India. This investigation revealed that there is a strong correlation between elevated As concentrations in groundwater and high incidence of breast cancer, liver cancer, and skin cancer. Further evidence of a link between cancer and As exposure was found in a mortality study in Cordoba, Argentina, during the period 1986–1991 (Hopenhayn-Rich et al. 1998). A significant increasing trend of standardised mortality ratio (SMR) for lung cancer (0.9, 1.5 and 1.8 for men, 1.2, 1.3 and 2.2 for women; p < 0.001 for both) and kidney cancer (0.9, 1.3 and 1.6 for men, 1.0, 1.4, and 1.8 for women, p < 0.001 for both) with arsenic exposure areas (ranging from low to high) was observed (Hopenhayn-Rich et al. 1998). In a lung cancer case-controlled study in Chile, a synergistic relationship between smoking and ingested arsenic in lung cancer was found. Odds ratio of 8.0 in non-smokers and 32.0 in smokers were reported for arsenic exposure above 200 µg/L (Ferreccio et al. 2000). Another study from the USA involving As concentrations of stream sediments and soil with estimates of smoking prevalence has found As-rich sediments were significantly associated with an increased risk of lung cancer, and smoking showed significant positive correlation with arsenic levels (p < 0.0001) (Putila and Guo 2011).
Conclusions
The present investigation shows that:
-
Cd, Cr, Pb, and Se concentrations are very low in most boreholes and fall below the SANS recommended limit for drinking water. Therefore, there is no immediate health risk associated with these trace elements.
-
Seasonal variability was observed in almost all trace elements under investigation except for As, which appeared to be little affected by these variations.
-
Serious borehole water quality problems exist in the Greater Giyani area with respect to As. Markedly increased As levels are found in several boreholes, and its distribution pattern reflects a very strong control by the underlying bedrock geology and Au mineralisations. Consequently, we believe that to continue drinking water from these boreholes may result in long-term deleterious health problems in this area. Furthermore, we recommend that further research by joint group consisting of environmental scientists (or geochemists) and health scientists, particularly, epidemiologists or toxicologists are highly warranted to assess the risk of exposure to PHTEs in drinking water in the study area.
References
Afridi, H. I., Kazi, T. G., Kazi, G. H., Jamali, M. K., & Shar, G. Q. (2006). Essential trace and toxic elements distribution in the scalp hair of Pakistani Myocardial Infarction patients and controls. Biological Trace Elements Research, 113, 19–33.
Agbalagba, O. E., Agbalagba, O. H., Ononugbo, C. P., & Alo, A. A. (2011). Investigation into the physic-chemical properties and hydrochemical processes of groundwater from commercial boreholes in Yenagoa, Bayelsa State, Nigeria. African Journal of Environmental Science and Technology, 5(7), 473–481.
Anhaeusser, C. R. (1992). Structures in granitoid gneisses and associated migmatites close to the granulite boundary of the Limpopo Belt, South Africa. Precambrian Research, 55, 81–92.
Ayotte, J. D., Belaval, M., Alson, S. A., Burow, K. R., Flanagan, S. M., Hinkle, S. R. H., et al. (2015). Factors affecting temporal variability of arsenic in groundwater used from drinking water supply in the United States. Science of Total Environment,. doi:10.1016/j.scitotenv.2014.02.057.
Ayotte, D., Montgomery, D. L., Flanagan, S. M., & Robison, K. W. (2003). Arsenic in groundwater in eastern New England: Occurrence, control, and human health implications. Environmental Science and Technology, 15(10), 83–20157.
Baglow, N. (2005). A geological description of sheets 2329CD Pietersburg and 2329DC Mankweng. Explanation of 1:50,000 scale Sheet 2329CD Pietersburg and 2329DC Mankweng.
Bailey, R. T., Cody, B. C., & Gates, T. K. (2009). Mobilization and reactive transport of selenium in a stream-aquifer system: From field monitoring towards remediation modelling. Hydrology days. Department of Civil and Environmental Engineering. Colorado State University. http://hydrologydays.colostate.edu/Papers_2009/Bailey_paper.pdf. Accessed January 8, 2013.
Bassil, J., Naveau, A., Bodin, J., Fontaine, C., Di Tullo, P., Razarc, M., et al. (2014). The nature of selenium species in the hydrological experimental site of Poitiers. Procedia Earth and Planetary Science, 10, 159–163.
Belyanin, G. A., Kramers, J. D., Vorster, C., & Knoper, M. W. (2014). The timing of successive fluid events in the Southern Marginal Zone of the Limpopo Complex, South Africa: Constraints from 40Ar–39Ar geochronology. Precambrian Research, 254, 169–193.
Bernard, A. (2008). Cadmium and its adverse effects on human health. The Indian Journal of Medical Research, 128(4), 64–557.
Bodenan, F., Baranger, P., Piantone, P., Lassin, A., Azaroual, M., Gaucher, E., et al. (2004). Arsenic behaviour in gold-ore mill tailings, Massif Central, France: Hydrogeochemical study and investigation of in situ redox signatures. Applied Geochemistry, 19, 1785–1800.
Brandl, G. (1986). The geology of the Pietersburg area. Explanation of 1: 250,000 scale Sheet 2328 Pietersburg. Geological Survey South Africa (pp. 4–6 & 13–15).
Brandl, G. (1987). The geology of the Tzaneen area. Explanation of 1:250,000 scale Sheet of 2330 Tzaneen. Geological Survey South Africa (pp. 3–5, 15–19, 25–25, 30–31).
Brandl, G., & Kröner, A. (1993). Preliminary results of single zircon studies from various Archaean rocks of north-eastern Transvaal. Abstract. 16th Coll. African Geology, Mbabane, Swaziland (Vol. 1, pp. 54–56).
Celik, I. L., Gallicchio, L., Boyd, K., Lam, T. K., Matanoski, G., Tao, X., et al. (2008). Arsenic in drinking water and lung cancer: A systematic review. Environmental Research,. doi:10.1016/j.envres.
Chen, J. (2012). An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asian Pacific Journal of Clinical Nutrition, 21(3), 6–320.
Department of Water Affairs (DWAF). (n.d.). Limpopo groundwater resource information project (GRIP). http://www.dwaf.gov.za/Groundwater/GroundwaterOffices/Limpopo/GRIP_Limpopo.pdf. Accessed November 2, 2012.
Department of Water Affairs and forestry (DWAF). (2000). Policy and strategy for Groundwater quality management in South Africa. Pretoria, Republic of South Africa. https://www.westerncape.gov.za/text/2003/groundwaterpol.pdf. Accessed November 15, 2010.
Department of Water Affairs and forestry (DWAF). (2004). South Africa’s water situation and strategies to balance supply and demand. National Water Resource Strategy, http://www.dwaf.gov.za/nwrs/LinkClick.aspx?fileticket=15NoZWfpDjY%3D&tabid=63&mid=412. Accessed November 15, 2010.
Department of Water Affairs and forestry (DWAF). (2006). Letaba catchment reserve determination study-groundwater report final. Republic of South Africa. https://www.dwa.gov.za/rdm/documents/Briefing%20Document.pdf. Accessed May 19, 2014.
Duker, A. A., Carranza., E .J. M. & Hale, M. (2005). Arsenic geochemistry and health. Environmental International, 31, 631–641. http://www.dwaf.gov.za/Groundwater/GroundwaterOffices/Limpopo/GRIP_Limpopo.pdf. Accessed November 2, 2012.
Du Toit, W. H., & Van Lelyveld, M. (2010). An explanation of the 1: 500 000 general hydrological Map. Phalaborwa 2330. Unpublished report.
Dzombak, M. J., & Morel, F. M. M. (1990). Surface complexation modelling-hydrous ferric oxide. New York: Wiley.
Edmunds, W. M., & Smedley, P. L. (1996). Groundwater geochemistry and health: An overview. In J. D. Fuge & G. J. H. McCall (Eds.), Environmental geochemistry and health (pp. 91–105). London: Geological Society Special Publication.
Fawell, J., & Nieuwenhuijsen, M. J. (2003). Contaminants in drinking water. British Medical Bulletin, 68, 199–208.
Ferreccio, C., Gonzalez, C., Milosavjlevic, V., Marshall, G., & Smith, A. H. (2000). Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology, 11(6), 9–673.
Ferreccio, C., & Sancha, A. M. (2006). Arsenic exposure and its impacts on health in Chile. International Centre for Diarrhoeal Disease Research, 24(2), 164–175.
Finkelstein, Y., Markowitz, M. E., & Rosen, J. F. (1998). Low-level lead induced neurotoxicity in children: An update on central nervous system effects. Brain Research Review, 27, 168–179.
Gan, S., & Van Reenen, D. D. (1996). Geology of gold deposits in the Southern Marginal Zone of the Limpopo belt and adjacent Sutherland greenstone belt, South Africa: Klein Letaba. South African Journal of Geology, 100, 73–83.
Haupt, C. J., & Sami, K. (2006). Letaba catchment reserve determination study-groundwater. Final report. Department of water affairs and forestry. South Africa. http://www.dwaf.gov.za/nwrs/LinkClick.aspx?fileticket=15NoZWfpDjY%3D&tabid=63&mid=412. Accessed November 15, 2010.
Hewit, A. D. (1989). Leaching of metal pollutants from four well casing used for groundwater monitoring U.S. Army Corps of Engineers Coid Region Research and Engineering. Laboratory Special Report, 89-32, 1.
Hopenhayn-Rich, C., Biggs, M., & Smith, A. H. (1998). Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba Argentina. International Epidemiological Association, 27, 561–569. http://asrg.berkeley.edu/index_files/Publications_PDF/98HopenhaynLungKidneyCancer.pdf. Accessed August 21, 2014.
Hudak, P. F. (2010). Nitrate, arsenic and selenium concentrations in the Pecos valley aquifer, West Texas, USA. International Journal of Environmental Research, 4(2), 229–236.
Hutton, M. (1987). Human health concerns of lead, mercury, cadmium and arsenic. In T. C. Hutchnson & K. M. Meema (Eds.), Lead, mercury, cadmium and arsenic in the environment (pp. 53–68). New York: Wiley.
Huvinen, M., Uitti, J., Oksa, P., Palmroos, P., & Laippala, P. (2002). Respiratory health effects of long-term exposure to different chromium species in stainless steel production. Occupational Medicine, 52(4), 204–212. http://occmed.oxfordjournals.org/content/52/4/203.full.pdf+html Accessed August 21, 2014.
International Agency for Research on Cancer (IARC). (2012). A review of human carcinogens: Arsenic, metals, fibres, and dusts. Lyon: World Health Organization Press. http://monographs.iarc.fr/ENG/Monographs/vol100C/. Accessed January 20, 2016.
International Agency for Research on Cancer (IARC). (2016). IARC monograph on the evaluation of carcinogenic risks to humans. World health Organization. http://monographs.iarc.fr/ENG/Classification/. Accessed February 22, 2016.
Kapaj, S., Peterson, H., Liber, K., & Bhattacharya, P. (2006). Human health effects from chronic arsenic poisoning—A review. Journal of Environmental Science and Health part A, 41, 2399–2428. http://monographs.iarc.fr/ENG/Classification/. Accessed February 22, 2016.
Katz, B. G., Berndt, M. P., Bullen, T. D. & Hansard, P. (1999). Factors controlling elevated lead concentrations in water samples from aquifer system in Florida. Water Resources Investigations Report 99-4020. United State Geological Survey, Florida. http://fl.water.usgs.gov/PDF_files/wri99_4020_katz.pdf. Accessed August 21, 2014.
Kramers, J. D., Henzen, M., & Steidle, L. (2014). Greenstone belts at the northernmost edge of the Kaapvaal Craton: Timing of tectonic events and a possible crustal fluid source. Precambrian Research, 253, 96–113.
Kreissig, K., Holzer, L., Frei, R., Villa, I. M., Kramers, J. D., Kröner, A., et al. (2001). Geochronology of the Hout River Shear Zone and the metamorphism in the Southern Marginal Zone of the Limpopo Belt, Southern Africa. Precambrian Research, 109, 145–173.
Kroner, A., Jaeckel, P., & Brandl, G. (2000). Single zircon ages for felsic to intermediate rocks from the Pietersburg and Giyani greenstone belts and bordering granitoid orthogneisses, northern Kaapvaal Craton, South Africa. Journal of African Earth Science, 30(4), 773–793.
Langard, S. (1990). One hundred years of chromium and cancer: A review of epidemiological evidence and selected case reports. American Journal of Industrial Medicine, 17(2), 189–215.
Lenz, M., & Lens, P. N. L. (2009). The essential toxin: The changing Perception of selenium in environmental science. The Science of the Total Environment,. doi:10.1016/j.scitotenv.2008.07.056.
Linos, A., Petralias, A., Christophi, C. A., Christoforidou, E., Kouroutou, P., Stoltidis, M., et al. (2011). Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—An ecological study. Environmental Health, 10, 50.
Massecheleyn, P. H., Delaune, R. D., & Patrick, W. H. (1991). Effect of redox potential and pH. On arsenic speciation and solubility in a contaminated soil. Environmental Science and Technology, 25, 1414–1419.
Mazumder, D. N. G. (2008). Chronic arsenic toxicity and health. Indian Journal of Medicine, 28, 436–447.
McCarty, K. M., Hanh, H. T., & Kim, K. W. (2011). Arsenic geochemistry and human health in South East Asia. Reviews Environmental Health, 26(1), 71–78.
Mehra, R., & Juneja, M. (2003). Adverse health effects in workers exposed to trace/toxic metals at work place. Indian Journal of Biochemistry & Biophysics, 40, 133–135.
Meyer, J. A., & Casey, N. H. (2004). Exposure assessment of potentially toxic trace elements in indigenous goats in the rural communal production systems of the northern region of South Africa. South African Journal of Animal Science, 34, 219–222.
Mopani District Municipality. (2008). Reviewed integrated development plan 2006/2011. Republic of South Africa. http://www.kruger2canyons.org/037%20-%20Mopani%20IDP%202008.pdf. Accessed April 6, 2014.
Motzer, W. & Engineers, T. (2004). Chemistry, geochemistry and geology of chromium and chromium compounds. In J. Guertin, J. A. Jacobs & C. P. Avakian (Eds.), Chromium (VI) handbook. Library of Congress Cataloguing-in Publication Data (pp. 23–761). United States of America.
Nath, A., Vendan, P. S. E., Kumar, S., Kumar, A., & Singh, J. K. (2013). Toxicity due to arsenic in Gangetic Zone of Patna, India and its linkage to cancer. Journal of Environmental and Analytical Toxicology, 3, 6.
Oze, C., Bird, D. K., & Fendorf, S. (2007). Genesis of hexavalent chromium from natural sources in soil and groundwater. In PNAS. Proceedings of the National Academy of Sciences, 104 (16), 6544–6549. http://www.pnas.org/content/104/16/6544.full.pdf+html. Accessed July 13, 2013.
Putila, J. J., & Guo, N. L. (2011). Association of arsenic exposure with lung cancer incidence rates in the United States. http://www.plosone.org/article/fetchObject.action?uri=info:doi/10.1371/journal.pone.0025886&representation=PDF. Accessed May 13, 2015.
Robb, L. J., Brandl, G., Anhaeusser, C. R., & Poujol, M. (2006). Archaean granitoid intrusions. In M. R. Johnson, C. R. Anhaeusser & R. J. Thomas (Eds.), The geology of South Africa (pp. 0–73). Pretoria: Geological Society of South Africa, Council for Geoscience.
Sami, K., & Druzynski, A. L. (2003). Predicted spatial distribution of naturally occurring arsenic, selenium and uranium in groundwater in South Africa Reconnaissance survey. Water Research Commission. Report No 1236/1/03, Pretoria, South Africa. http://www.wrc.org.za/Knowledge%20Hub%20Documents/Research%20Reports/1236-1-03.pdf. Accessed January 21, 2014.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic material in natural waters. Applied Geochemistry, 17, 517–568.
Smedley, P. L., Knudsen, J., & Maiga, D. (2007). Arsenic in groundwater from mineralized Proterozoic basement rocks of Burkina Faso. Applied Geochemistry, 22, 1074–1092.
Smith, A. H., Hopenhayn-Rich, C., Bates, M. N., Geoden, H. M., Hertz-Picciotto, I., Duggan, H. M., et al. (1992). Cancer risks from arsenic in drinking water. Environmental Health Perspectives, 97, 259–267.
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking water by arsenic in Bangladesh: A public health emergency. Bulletin of the Word Heal Organization, 78(9). http://www.who.int/bulletin/archives/78(9)1093.pdf. Accessed February 16, 2016.
Sohel, N., Persson, L. A., Rahman, M., Streatfield, P. K., Yunus, M., & Vahter, M. (2009). Arsenic in drinking water and adult mortality: A population based-cohort study in rural Bangladesh. Epidemiology,. doi:10.1097/EDE.0b013e3181bb56ec.
South African National Standard (SANS). (2011). South African National Standard of Drinking water Part 1: Microbiological, physical, aesthetic and chemical determinands. Pretoria: SABS Standard Division.
Steinmaus, C. M., Yuan, Y., & Smith, A. H. (2005). The temporal stability of arsenic concentrations in wells in water in western Nevada. Abstract, Pubmed. http://www.ncbi.nlm.nih.gov/pubmed/16194666. Accessed February 16, 2016.
Thundiyil, J. G., Yuan, Y., Smith, A. H., & Steinmaus, C. (2007). Seasonal variations of arsenic in wells in Nevada. Environmental Research,. doi:10.1016/j.envres.2007.02.007.
Umemura, T., & Wako, Y. (2006). Pathogenesis of osteomalacia in itai–itai disease. Journal of Pathology, 19, 69–74.
United Nations Environmental Programme (UNEP). (2010). Final review of scientific information on cadmium. http://www.unep.org/hazardoussubstances/Portals/9/Lead_Cadmium/docs/Interim_reviews/UNEP_GC26_INF_11_Add_2_Final_UNEP_Cadmium_review_and_apppendix_Dec_2010.pdf. Accessed July 10, 2013.
Water Research Commission (WRC). (2009). Tracking trace constituents in groundwater. Technical brief 1–2. http://www.wrc.org.za/Knowledge%20Hub%20Documents/Briefs/TB%20trace%20constituents%20in%20groundwater.pdf. Accessed September 30, 2010.
Weaver, J. M. C., Cave, L. & Talma, A. S. (2007). Groundwater sampling: A comprehensive guide for sampling methods (2nd ed.). Water Research Commission, Report no TT 303/07. Republic of South Africa.
Welch, A. H., Westjohn, B. B., Helsel, D. R., & Wanty, R. B. (2000). Arsenic in groundwater of the United States: Occurrence and geochemistry. Groundwater, 38(4), 589–604.
World Health Organisation (WHO). (2010). Childhood lead poisoning. http://www.who.int/ceh/publications/leadguidance.pdf. Accessed May 16, 2016.
World Health Organisation (WHO). (2011). Selenium in drinking water. Background document for development of WHO guidelines for drinking water quality. World Health Organization. Geneva. Switzerland. http://www.who.int/water_sanitation_health/dwq/chemicals/selenium.pdf. Accessed July 10, 2013.
Wright, W. G. (1999). Oxidation and mobilization of selenium by nitrate in irrigation drainage. Journal of Environmental Quality, 28(4), 1182–1187. http://www.swhydrologic.com/reports/wright_jeq_se-no3.pdf. Accessed July 18, 2013.
Acknowledgments
The authors are grateful to the Council for Geoscience and the National Research Foundation (Incentive Funding for Rated Researchers to HM, Grant No. 91059) for providing financial support for this investigation and the anonymous reviewers and the Editor for their valuable comments and suggestions, which helped to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munyangane, P., Mouri, H. & Kramers, J. Assessment of some potential harmful trace elements (PHTEs) in the borehole water of Greater Giyani, Limpopo Province, South Africa: possible implications for human health. Environ Geochem Health 39, 1201–1219 (2017). https://doi.org/10.1007/s10653-016-9887-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9887-0