Abstract
Chlorpyrifos is among the most widely sold organophosphates in the agriculture sector worldwide. Static bioassays were performed in the laboratory to compare the acute toxicity between the technical grade (94% a.i.) and commercial formulation (20% EC) of chlorpyrifos to four freshwater organisms: the crustacean zooplankton Cyclops viridis, the oligochaete worm Branchiura sowerbyi, the gastropod Pila globosa, and tadpole larvae of Duttaphrynus melanostictus. The recovery of actual chlorpyrifos concentrations in water after 2 h of exposure to the nominal concentrations ranged from 82.98% to 88.56%. The commercial formulation (F) of chlorpyrifos was found to be 1.94 to 2.76 times more toxic than the technical grade (T). Based on 96 h LC50 values of T and F chlorpyrifos, C. viridis was found to be most sensitive (0.56 and 0.25 μg/L) and P. globosa as most tolerant (1482 and 536 μg/L) to chlorpyrifos. Changes in LC50 values of both T and F chlorpyrifos were noted in respect of exposure hours for the three aquatic invertebrates and the tadpole larvae of the toad. In conclusion, the acute toxicity of chlorpyrifos to some non-target freshwater organisms differs between technical grade and commercial formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorpyrifos is a broad-spectrum chlorinated organophosphate insecticide that is commonly used to control a wide variety of pests (Deb and Das 2013). Several studies have found that it is highly hazardous to non-target organisms (Giddings et al. 2014; Huang et al. 2020; Rutkoski et al. 2020; Pal et al. 2021; Majumder 2023). The WHO (2019) classified chlorpyrifos among the moderately hazardous (Class II) technical grade active ingredients in pesticides. It works inside the body of an organism by blocking acetylcholinesterase, ultimately leading to an accumulation of acetylcholine and thereby leading to hyperexcitation and neurodisorders (Greer et al. 2019; Pal et al. 2021). Chlorpyrifos residues were found in surface water (Hashmia et al. 2020; Ganaie et al. 2023) and in fish tissues (Malhat and Nasr 2011). Because of the widespread presence of chlorpyrifos in the aquatic environment, as well as its ability to cause negative effects, it becomes an excellent choice for toxicological research.
The majority of the pesticide-related toxicity study was conducted using only the active ingredient of the test pesticide. But, commercially formulated products of pesticides, mixtures of active ingredients and other substances or ingredients labeled as ‘inert ingredients’, ‘adjuvants’ or ‘co-formulants’, are applied in agricultural fields to get better efficacy for pest control. ‘Inert ingredients’ or ‘adjuvants’ or ‘co-formulants’ may be surfactants, solvents, emulsifiers, propellant, antifoaming agents, carriers, diluents, stabilizer, dyes, preservatives, penetration agents, odour masking agent, etc. The composition of adjuvant is determined based on the physical and chemical properties of the active ingredient and the kind of formulation being produced (Mesnage and Antoniou 2018). Adjuvants or inert substances promote the absorption and stability of active ingredients, which is important for pesticide persistence and efficacy (Tu et al. 2023). The term “inert” does not imply non-toxic (U.S. EPA 2018). It is essential to comprehend how the ingredients in a commercial pesticide formulation interact with one another. They could work synergistically (Nagy et al. 2020). In many cases, such other ingredients make formulated commercial products more toxic to the organisms than the active ingredient of the pesticide itself (Puglis and Boone 2011; Majumder and Kaviraj 2019). Contribution of adjuvant to the toxicity of commercial pesticide formulation was reported by Demetrio et al. (2014). Exposure to the ubiquitous adjuvant or inert ingredient, α-(p-nonylphenyl)-ω- hydroxypoly(oxyethylene), has been linked to endocrine disruption, birth abnormalities, and toxicity in aquatic environments (Cox and Zeiss 2022). ‘Inert ingredient’ may pose threats to pollinators (Mullin et al. 2015). Therefore, research into pesticide risk assessment should include consideration of the possible dangers to non-target organisms from inert components (Tu et al. 2023). However, due to trade secrets, many manufacturers do not disclose the exact composition of the other ingredients in designed products (Cox and Surgan 2006). In consequence, the toxicity study on non-target organisms using adjuvants or inert ingredients is hampered (Cox and Surgan 2006). Therefore, determining the toxicity of both the active ingredient and the commercial formulation of the test pesticide may become more judicious in order to obtain an accurate picture of the ecotoxicological impact of insecticides on non-target organisms (Pereira et al. 2009; De Silva et al. 2010). Chlorpyrifos as an active ingredient in combination with organic adjuvants showed better performances in pest control during a field trial (Hoesain et al. 2023). Several studies documented that chlorpyrifos in its commercial form is more toxic than the active ingredient alone (Demetrio et al. 2014; Majumder and Kaviraj 2019; Majumder 2024). Accordingly, comparisons were made in the present study between the acute toxicity of the technical grade and commercial formulation of chlorpyrifos to four common freshwater organisms from four different taxa. As food for fish, the freshwater crustacean zooplankton, Cyclops viridis, plays a crucial role in the food chain of freshwater ecosystems and is a representative member of pelagic communities. In addition, its ease of culture in the laboratory, short life cycle, and high reproductive rate make it an ideal candidate for ecotoxicological research (Sarkar and Saha 2016). Branchiura sowerbyi, an oligochate, has long been preferred as a bio-indicator organism to assess sediment associated contamination due to its availability in aquatic environments, high individual biomass, and role as fish food (Lobo and Espindola 2014). Pila globosa, a gastropod mollusk, is considered an important aquatic resource in local freshwater bodies and is suitable to monitor the aquatic environment. It is a representative member of benthic habitats, important in the detritus food chain with its unique body plan, efficient adaptation, and immunological system (Pal et al. 2021). In most freshwater bodies in India, toad tadpole larvae are the most abundant larval representatives during the rainy season. They can play an important role in the food chain, and their appearance in fields is associated with water bodies and coincides with pesticide application time (David and Kartheek 2015). So, tadpole larvae of Duttaphrynus melanostictus have been selected as one of the test organisms in the present study. In most freshwater aquatic ecosystems in India, these species often form a sustainable direct or indirect food chain (Majumder and Kaviraj 2015). As a result, acute toxicity data for both technical grade and commercial formulations of chlorpyrifos for these species will provide an indication of the eco-toxicological risk of chlorpyrifos in aquatic ecosystems vulnerable to pesticide runoff from agricultural fields.
Materials and methods
Test organisms and their acclimatisation
Altogether four test organisms were used in the present study. Among them, the crustacean zooplanktons (C. viridis), tadpoles of D. melanostictus, and gastropod mollusks (P. globosa) were collected during rainy season from a local, natural pond in Kalyani, Nadia district, West Bengal, India, that is far from agricultural lands and industrial installations and there is no other source of contamination. Initially, a culture of the crustacean zooplanktons, C. viridis, was maintained in an outdoor vat with cow dung at its bottom and filled with 400 L of water. Before the bioassays, the zooplankton specimens (mean length: 0.009 ± 0.03 cm) were brought from the culture vats and were acclimatised to laboratory conditions for 96 h. Cow dung water was given. Another test organism, D. melanostictus tadpoles (mean length: 1.23 ± 0.15 cm; mean weight: 0.035 ± 0.008 gm) were transported in plastic containers filled with habitat water taken from the same pond. They were allowed to acclimatise to laboratory conditions for 96 h prior to the start of experiment. Every day, the water was replaced. Algae were given to the tadpoles as feed. Any dead tadpoles were removed using forceps to ensure healthy water quality. The feeding of the tadpoles was discontinued 24 h before the experiment began. For P. globosa, medium sized, healthy, active specimens (mean weight: 20±2 gm) were used, regardless of sex. Following collection, they were rinsed in freshwater and given a 0.1% KMnO4 solution treatment to prevent pathogenic infection. They were allowed to acclimatise to laboratory conditions for 96 h. The crushed leaves of aquatic plants were provided as food. To reduce debris loads, the water was changed every 24 h. Their feeding was stopped 24 h before start of the test. The fourth test organism, mature B. sowerbyi (mean length: 2.93 ± 0.35 cm; mean weight: 0.003 ± 0.0004 gm) were purchased from a nearby aquarium shop in Kalyani, West Bengal, India. After that, they had 96 h to acclimatise. Cow dung water was added. Only healthy organisms (2.93 ± 0.35 cm) were chosen and brought to the experimental setup once they had acclimatised. After the acclimatisation phase, test organisms were stocked at ten individuals of crustacean zooplanktons, or five individuals of tadpole larvae, or ten individuals of worms in a 500 ml glass beaker and at five individuals of gastropod mollusk in a 15 L aquarium in continuously aerated deep tube well water for the bioassay. All treatments, including control, were made in three replicates.
Test chemicals
Two test chemicals were used. Technical grade chlorpyrifos (94% active ingredient of O,O-diethyl O-3,5,6-trichloro-2-pyridinyl- phosphorothioate) was obtained from Krishi Rasayan Group of Companies, Kolkata-700020 (India) and a commercial formulation of chlorpyrifos under the brand name Dursban® (chlorpyrifos 20% EC; EC: emulsifiable concentrate) was procured from the Dow Agro Sciences India Pvt. Ltd., Mumbai-400079 (India).
Bioassay
Static bioassays were made according to the standard protocol (APHA 1995). The test medium for all tests was deep tube well (300 feet underground) water stored in an overhead tank: Temperature 27–32 °C, pH 7.4 ± 0.2; free CO2 3.42 ± 0.21 mg/L; dissolved oxygen 6.8 ± 0.3 mg/L; total alkalinity 85.75 ± 4.37 mg/L as CaCO3; total hardness 237.33 ± 6.01 mg/L as CaCO3. Bioassays for the crustaceans, worms, and tadpoles of the toad were conducted in 500 ml glass beakers, each containing 300 ml of water. Bioassays for the gastropod were carried out in 15-liter glass aquariums, each containing 2 liters of water. A stock solution of 100 mg/L of technical (T) or formulation (F) of chlorpyrifos was prepared by dissolving an appropriate amount of the pesticide in 10 ml of water or acetone. Necessary dilutions were made before actual treatment. The active ingredient of chlorpyrifos was mixed with acetone due to its low solubility before being added to the test medium. The actual chlorpyrifos concentrations used to determine LC50 values of the pesticide for different test species in the present study have been given in Table 1. Two separate bioassays were made for each test organism: one with the technical grade chlorpyrifos and the other with the formulation (emulsified concentrate). In each category, there was a control with an equal number of replicates. Negative controls with and without solvent were used. For the acetone control, 0.1 ml/L acetone was added to the test water because the maximum amount of acetone present in the highest concentration of this category of chlorpyrifos tested was less than 0.1 ml/L. No food was provided during the bioassay to avoid interference of excretory products of the test organisms with the test chemical.
Residue analysis of chlorpyrifos
After 2 h of treatment, 250 ml water samples were taken from each test container to quantify chlorpyrifos concentrations in water by Chromatographic methods. In a 500 mL conical flask, a 250 mL sample of water was added, along with 25 g of sodium chloride (NaCl). The solution was partitioned three times with 50 mL of a mixture of hexane and dichloromethane (80:20). The organic phase was collected over anhydrous sodium sulfate in a conical flask and evaporated in a rotary evaporator. The volume was made up to 10 mL with ethyl acetate. The extract was filtered with a syringe filter using 25 mm, 0.22 μ nylon filter paper and transferred into vials for chlorpyrifos determination in a gas chromatograph equipped with an ECD detector (Agilent 6890 N) with a wide-bore HP column (HP-5, 30 m, 0.32 mm ID, 0.25 µm film thickness) and a 7683 B Series auto injector. As a carrier, N2 gas was used. The concentration of chlorpyrifos was determined from the calibration curve prepared from standard chlorpyrifos concentrations, using ChemStation software. The instrument’s limit of detection (LOD) and limit of quantification (LOQ) were 5 μg/L and 150 μg/L, respectively.
Statistical analysis
Actual death of the test organisms was considered as mortality. The lethal concentrations of chlorpyrifos (LC50) at which 50% mortality of the test organisms occurred and its 95% confidence limits were estimated for 24, 48, 72, and 96 h from the mortality data using EPA-Probit analysis version 1.5 statistical software based on the probit analysis method of Finney (1971). LC50 data of the technical grade and formulation of chlorpyrifos were compared following the criteria described by Mayer and Ellersieck (1986), Schmuck et al. (1994), APHA (1995) and Demetrio et al. (2014).
Results
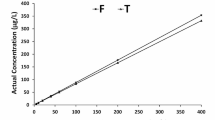
The actual recovery of chlorpyrifos in water after 2 h of exposure to both of the nominal concentrations of technical grade (T) as well as formulation (F) of chlorpyrifos is presented in Fig. 1. The recovery percentage for T chlorpyrifos was 82.98 ± 0.22%, whereas for F chlorpyrifos, it was 88.56 ± 1.90%, and there was a linear increase in 2 h chlorpyrifos concentration with the increase in nominal concentration exposure. Table 2 shows the 96 h lethal concentrations (μg/L) of technical grade (94% a.i) and commercial formulation (20% EC) of chlorpyrifos to crustacean zooplankton (C. viridis), worm (B. sowerbyi), gastropod mollusk (P. globosa), and tadpole larva of toad (D. melanostictus). The gastropod P. globosa was least sensitive, while the crustacean C. viridis was most sensitive to both the technical grade (T) and commercial formulation (F) of chlorpyrifos. The susceptibility of the test organisms to chlorpyrifos varied with the chemical form of the pesticide. The current study’s findings indicated that aquatic organisms are susceptible to technical grade (a.i.) as well as commercial formulations of chlorpyrifos in the following order: C. viridis > Tadpole larva of D. melanostictus > B. sowerbyi > P. globosa.
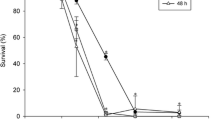
The results of the present study clearly indicate that the technical grade (94% a.i) chlorpyrifos was less toxic to all test organisms than its commercial formulation (20% EC). The quotient of LC50 values (LC50 T/LC50 F) for C. viridis, B. sowerbyi, and P. globosa in this study varies from 2.24 to 2.76, while it is 1.94 for tadpole larvae of the toad, D. melanostictus (Table 2). Regression parameters of the log concentration-probit mortality line for 96 h mortality data for all test organisms have been presented in Table 3 and the slopes have been presented in Fig. 2. The slopes were found more or less parallel between T and F. Figure 3 shows the changes in LC50 values of T and F chlorpyrifos with respect to exposure hour for the three aquatic invertebrates and the tadpole of the toad. The LC50 values decreased as the exposure period lengthened. The LC50 concentrations that were determined in the present study were based on actual lethality, not ecological lethality.
Changes in LC50 values of chlorpyrifos to different aquatic organisms (a: Cyclops viridis; b: Branchiura sowerbyi; c: Tadpole larva of Duttaphrynus melanostictus; d: Pila globosa) with respect to exposure period. Different lowercase letters above bars indicate differences in LC50 values at each time point as per the criteria of APHA (1995)
Within half an hour of being exposed to chlorpyrifos, all of the exposed test organisms became hyperactive. Neurotoxicants, like chlorpyrifos, can paralyse the exposed organisms and cause behavioural abnormalities as observed in this study. At first, C. viridis exhibited irregular, erratic movements, and later, they were seen at the bottom of the glass beaker with slow creeping movements. The tadpoles of the toad displayed increased surfacing, gradually erratic movements, and eventually balance loss. B. sowerbyi was observed to be coiled, wrinkled, and irritated during acute chlorpyrifos exposure, and fragmentation and degeneration of body parts occurred with increasing exposure period. To avoid chlorpyrifos exposure in the aquatic medium, P. globosa was found to seal their shell and release excess mucous.
Discussion
In this study, the crustacean zooplankton C. viridis was found to be the most susceptible to chlorpyrifos, with 96 h LC50 values of 0.25 and 0.56 μg/L respectively, for 20% EC and technical grade chlorpyrifos. The gastropod P. globosa, on the other hand, was the most tolerant (96 h LC50 being 536 and 1482 μg/L), followed by the oligochaete worm B. sowerbyi (96 h LC50 being 135 and 303 μg/L). The tadpole larvae of D. melanostictus were moderately susceptible to chlorpyrifos, with a 96 h LC50 ranging between 52 and 120 μg/L.
Crustaceans have close phylogenetic relationships with insects, which makes them more susceptible to insecticides (Roque et al. 2005). They have similar biological pathways to insects (Tamone and Harrison 2015), which makes them sensitive to neurotoxicants that affect acetylcholine, which is the mode of action of chlorpyrifos (Duarte-Restrepo et al. 2020; Ubaid Ur Rahman et al. 2021). Demetrio et al. (2014) found that the susceptibility of another crustacean zooplankton, Daphnia magna, to chlorpyrifos formulation (96 h LC50: 0.30 μg/L) is close to that found in the present study. Moore et al. (1998) reported the 48 h LC50 value of 0.6 μg/L for the a.i. of chlorpyrifos in Daphnia magna, which is similar to the LC50 value of chlorpyrifos in C. viridis (0.58 μg/L) found in the current study. There is little documentation comparing the sensitivity of B. sowerbyi and P. globosa to chlorpyrifos to that of the current study. When the susceptibilities of these two organisms to other toxicants were evaluated (Tripathi and Pandey 2014; Saha et al. 2016), it was revealed that organic substances released by B. sowerbyi might prevent toxicants from accumulating in their bodies. Likewise, P. globosa is structurally avoiding exposure to chlorpyrifos by shutting its shell and producing a protective layer of mucus, which is a defense mechanism.
The LC50 value for tadpole larva of toad found in this study (24 h LC50: 163 μg/L) is close to the 24 h LC50 values obtained by Abbasi and Soni 1991 (177 μg/L) for Rana tigrina, but lower than the findings of Sparling and Fellers (2007) for Rana boylii (24 h LC50 values as 3005 μg/L) and Bernabό et al. (2011) for Rana dalmatina (96-h LC50 values as 5174 μg/L). On the other hand, Ramadani et al. (2022) reported 96 h LC50 value of commercial chlorpyrifos for Fejervarya limnocharis tadpoles as 2.86μg/L. However, susceptibility of tadpoles to pesticides varies with species and developmental stages (Berrill et al. 1998; Bridges and Semilitsch 2000) as well as testing protocols (Jones et al. 2009), thereby making comparisons of tadpole susceptibility to chlorpyrifos challenging.
The present study clearly showed that the toxicity of the commercial formulation of chlorpyrifos (20% EC) is higher than that of its technical grade (94% a.i.), regardless of species. Tilak and Veeraiah (2001), De Silva et al. (2010), Demetrio et al. (2014), and Majumder and Kaviraj (2019) found similar variations in toxicity between commercial formulation (F) and technical grade (T) chlorpyrifos in Labeo rohita, Perionyx excavatus, Daphnia magna, and Oreochromis niloticus, respectively. According to the Material Safety Data Sheet (MSDS), in the commercial formulation (chlorpyrifos 20% EC) under the brand name Dursban® used in this study, the ‘inert ingredient’ is the solvent naphtha (petroleum), heavy aromatic (Dow AgroSciences India Pvt. Ltd 2014). Solvent Naphtha (P) HA is used widely in pesticide formulations (Tu et al. 2023) and is listed as a neuro- and hepatotoxin (Haz-Map 2023). Therefore, the added inert ingredients work synergistically with the active ingredient chlorpyrifos, and may be responsible for the increased toxicity of the formulated product Dursban® in the present study. Adams et al. (2021) also documented that the presence of the solvent naphtha clearly influences the toxicity of the cycloxydim herbicide formulation.
The degree of the difference in toxicity between T and F chlorpyrifos, on the other hand, differed with species. The quotient of LC50 values (LC50 T/LC50 F) for C. viridis, B. sowerbyi, and P. globosa in this study varies from 2.24 to 2.76, while it is 1.94 for tadpole larvae of the toad, D. melanostictus. Mayer and Ellersieck (1986) proposed that a toxicant is more toxic when the quotient of two LC50 values (higher LC50 value/lower LC50 value) is greater than 1. Schmuck et al. (1994), on the other hand, assumed that natural variability causes the quotient to vary between 0.5 and 2.0, and indicated that when the quotient value approaches 2, a toxicant is more harmful. Based on the findings of this study, the formulation was more toxic than technical grade chlorpyrifos for all test organisms except the tadpole larva of D. melanostictus, according to the criteria of Mayer and Ellersieck (1986). The criteria of Schmuck et al. (1994) also satisfied the findings for all other test organisms. However, comparing sensitivity based on quotient is not universally accepted. Because the criteria proposed by Schmuck et al. (1994) and Mayer and Ellersieck (1986) only considered one single point (LC50) for the concentration–effect function, Demetrio et al. (2014) proposed accepting a criterion as valid only when the concentration effect lines were parallel. Table 3 shows the regression parameters of the log concentration-probit mortality line for 96 h mortality data for all test organisms. The slopes of different species in the present study, shown in Fig. 3, were not perfectly parallel and did not satisfy the criteria of Demetrio et al. (2014). Therefore, it could not be possible to unquestionably accept the difference in LC50 values between the technical grade and commercial formulation of chlorpyrifos based on quotient (LC50 T / LC50 F). Because the confidence limits of LC50 values do not overlap, the LC50 values for T chlorpyrifos and F chlorpyrifos are different at each time point according to the criterion of APHA (1995). Therefore, it is better to rely solely on LC50 value and compare toxicity based on LC50 values and their confidence limit, unless there is an overlap of the confidence limit between the two LC50 values.
Conclusions
In conclusion, organisms (zooplankton, worm, mollusk, and toad tadpole) exhibited varied sensitivities to chlorpyrifos exposure; results demonstrated all species were more susceptible to the formulated chlorpyrifos when compared to the technical grade chlorpyrifos. “Inert ingredients” are not necessarily inert. Their existence as adjuvants with the active ingredient in the formulated chlorpyrifos showed synergistic effects, which is the main reason for the enhanced toxicity of formulated chlorpyrifos in the present study. Crustaceans are the most sensitive of the test organisms since they are taxonomically and physiologically similar to insects. Because chlorpyrifos and other organophosphates were used to manage insect pest populations by inhibiting acetylcholinesterase activity and producing neurotoxicity, crustacean zooplanktons are equally susceptible to insecticides. Therefore, it is necessary to take into account the management of natural water bodies as well as the assessment of environmentally acceptable insecticide concentrations and their lethal values for aquatic organisms.
References
Abbasi SA, Soni R (1991) Evaluation of water quality criteria for four common pesticides on the basis of computer-aided studies. Indian J Environ Health 33:22–24
Adams E, Gerstle V, Schmitt T, Brühl CA (2021) Co-formulants and adjuvants affect the acute aquatic and terrestrial toxicity of a cycloxydim herbicide formulation to European common frogs (Rana temporaria). Sci Total Environ 789:147865. https://doi.org/10.1016/j.scitotenv.2021.147865
APHA (1995) Standard Methods for the Examination of Water and Wastewater, 19th ed. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington, D.C., USA, p 1193
Bernabό I, Sperone E, Tripepi S, Brunelli E (2011) Toxicity of chlorpyrifos to larval Rana dalmatina: acute and chronic effects on survival, development, growth and gill apparatus. Arch Environ Contam Toxicol 61(4):704–718. https://doi.org/10.1007/s00244-011-9655-1
Berrill M, Coulson D, McGillivray L, Pauli B (1998) Toxicity of endosulfan to aquatic stage of anuran amphibians. Environ Toxicol Chem 9:1738–1744. https://doi.org/10.1002/etc.5620170914
Bridges CM, Semilitsch RD (2000) Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns amphibian decline. Conserv Biol 14:490–1499. https://doi.org/10.1046/j.1523-1739.2000.99343.x
Cox C, Zeiss M (2022) Health, pesticide adjuvants, and inert ingredients: California case study illustrates need for data access. Environ Health Perspect 130(8):85001. https://doi.org/10.1289/ehp10634
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Perspect 114(12):1803–1806. https://doi.org/10.1289/2Fehp.9374
David M, Kartheek RM (2015) Malathion acute toxicity in tadpoles of Duttaphrynus melanostictus, morphological and behavioural study. J Basic Appl Zool 72:1–7. https://doi.org/10.1016/j.jobaz.2015.01.004
De Silva PMCS, Pathiratne A, Cornelis AM, Gestel V (2010) Toxicity of chlorpyrifos, carbofuran, mancozeb and their formulations to the tropical earthworm Perionyx excavatus. Appl Soil Ecol 44:56–60
Deb N, Das S (2013) Chlorpyrifos toxicity in fish: a review. Curr World Environ 8(1):77–84. https://doi.org/10.12944/CWE.8.1.17
Demetrio PM, Bonetto C, Ronco AE (2014) The effect of cypermethrin, chlorpyrifos and glyphosate active ingredients and formulations on Daphnia magna (Straus). Bull Environ Contam Toxicol 93:268–273. https://doi.org/10.1007/s00128-014-1336-0
Dow AgroSciences India Pvt. Ltd (2014) Material Safety Data Sheet: Dursban (TM) 20EC insecticide. Dow Agro Sciences India Pvt. Ltd., Mumbai-400079 (India), https://www.corteva.in/content/dam/dpagco/corteva/as/in/en/products/files/DF.Dursban.MSDS.pdf.pdf
Duarte-Restrepo E, Jaramillo-Colorado BE, Duarte-Jaramillo L (2020) Effects of chlorpyrifos on the crustacean Litopenaeus vannamei. PLoS One 15(4):e0231310. https://doi.org/10.1371/2Fjournal.pone.0231310
Finney DJ (1971) Probit Analysis. Cambridge University Press, London, UK, p 333
Ganaie MI, Jan I, Mayer AN, Dar AA, Mayer IA, Ahmed P, Sofi JA (2023) Health risk assessment of pesticide residues in drinking water of upper Jhelum region in Kashmir Valley-India by GC-MS/MS. Int J Anal Chem 2023:6802782. https://doi.org/10.1155/2023/6802782
Giddings JM, Williams WM, Solomon KR, Giesy JP (2014) Risks to aquatic organisms from use of chlorpyrifos in the United States. In: Giesy J, Solomon K (eds) Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States. Reviews of Environmental Contamination and Toxicology, vol 231. Springer, Cham, https://doi.org/10.1007/978-3-319-03865-0_5
Greer JB, Magnuson JT, Hester K, Giroux M, Pope C, Anderson T, Liu J, Dang V, Denslow ND, Schlenk D (2019) Effects of chlorpyrifos on cholinesterase and serine lipase activities and lipid metabolism in brains of rainbow trout (Oncorhynchus mykiss). Toxicol Sci 172(1):146–154. https://doi.org/10.1093/2Ftoxsci/2Fkfz167
Haz-Map (2023) Haz-Map-Information on hazardous chemicals and occupational diseases. https://haz-map.com (Accessed on May 15, 2024)
Hashmia TA, Qureshi R, Tipre D, Menon S (2020) Investigation of pesticide residues in water, sediments and fish samples from Tapi River, India as a case study and its forensic significance. Environ Forensics 21(1):1–10. https://doi.org/10.1080/15275922.2019.1693441
Hoesain M, Prastowo S, Wagiyana, Pradana AP, Alfarisy FK, Adiwena M (2023) The utilization of organic adjuvants to increase the effectiveness of chlorpyrifos insecticides against Spodoptera litura. Plant Sci Today 10(2):178–186. https://doi.org/10.14719/pst.2064
Huang X, Cui H, Duan W (2020) Ecotoxicity of chlorpyrifos to aquatic organisms: A review. Ecotoxicol Environ Saf 200:110731. https://doi.org/10.1016/j.ecoenv.2020.110731
Jones DK, Hammond JI, Relyea RA (2009) Very highly toxic effects of endosulfan across nine species of tadpole: lag effects and family level selectivity. Environ Toxicol Chem 28:1939–1945. https://doi.org/10.1897/09-033.1
Lobo H, Espindola ELG (2014) Branchiura sowerbyi Beddard, 1892 (Oligochaeta: Naididae) as a test species in ecotoxicology bioassays: a review. Zoosymposia 9:059–069. https://doi.org/10.11646/zoosymposia.9.1.11
Malhat F, Nasr I (2011) Organophosphorus pesticides residues in fish samples from the River Nile tributaries in Egypt. Bull Environ Contam Toxicol 87(6):689–92. https://doi.org/10.1007/s00128-011-0419-4
Majumder R, Kaviraj A (2015) Variation in acute toxicity between technical grade and commercial formulation of cypermethrin to some non-target freshwater organisms. Int J Curr Res 7(06):16755–16759
Majumder R, Kaviraj A (2019) Acute and sublethal effects of organophosphate insecticide chlorpyrifos on freshwater fish Oreochromis niloticus. Drug Chem Toxicol 42(5):487–495
Majumder R (2023) Effects of chlorpyrifos on histopathological biomarkers of the freshwater teleost Oreochromis niloticus. Fish Aquat Life 31:207–214. https://doi.org/10.2478/aopf-2023-0020
Majumder R (2024) Comparative acute toxicity studies of chlorpyrifos technical grade with its emulsifiable concentrate (20% EC) on Labeo rohita, a freshwater major carp, and Mystus vittatus, a freshwater catfish. Bull Environ Contam Toxicol 113:27. https://doi.org/10.1007/s00128-024-03936-4
Mayer Jr FL, Ellersieck MR (1986) Manual of acute toxicity: Interpretation and data base for 410 Chemicals and 66 species of freshwater animals. US Department of the Interior, Fish and Wildlife Service, Washington, D.C., USA, p 581
Mesnage R, Antoniou MN (2018) Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front Public Health 5:361. https://doi.org/10.3389/2Ffpubh.2017.00361
Moore MT, Huggett DB, Gillespie WB, Rodgers JH, Cooper CM (1998) Comparative toxicity of chlordane, chlorpyrifos, and aldicarb to four aquatic testing organisms. Arch Environ Contam Toxicol 34:152–157. https://doi.org/10.1007/s002449900299
Mullin CA, Chen J, Fine JD, Frazier MT, Frazier JL (2015) The formulation makes the honey bee poison. Pestic Biochem Physiol 120:27–35. https://doi.org/10.1016/j.pestbp.2014.12.026
Nagy K, Duca RC, Lovas S, Creta M, Scheepers PTJ, Godderis L, Ádám B (2020) Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ Res 181:108926. https://doi.org/10.1016/j.envres.2019.108926
Pal S, Maity S, Balachandran S, Chaudhury S (2021) In-vitro effects of chlorpyrifos and monocrotophos on the activity of acetylcholinesterase (AChE) in different tissues of apple snail Pila globosa (Swainson, 1822). Nat Environ Pollut Technol 20(3):1263–1268. https://doi.org/10.46488/NEPT.2021.v20i03.037
Pereira JL, Antunes SC, Castro BB, Marques CR, Goncalves AMM, Goncalves F, Pereira R (2009) Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicol 18:455–463. https://doi.org/10.1007/s10646-009-0300-y
Puglis HJ, Boone MD (2011) Effects of technical-grade active ingredient vs. commercial formulation of seven pesticides in the presence or absence of UV radiation on survival of green frog tadpoles. Arch Environ Contam Toxicol 60:145–155. https://doi.org/10.1007/s00244-010-9528-z
Ramadani S, Marhendra APW, Wiadnya DGR, Kurniawan N (2022) Effect of acute toxicity of commercial organophosphate insecticide based on chlorpyrifos on Fejervarya limnocharis tadpoles (Anura: Dicroglossidae). J Trop Life Sci 12(2):231–240. https://doi.org/10.11594/jtls.12.02.09
Roque A, Abad S, Betancourt-Lozano M, de la Parra LM, Baird D, Guerra-Flores AL, Gomez-Gil B (2005) Evaluation of the susceptibility of the cultured shrimp Litopenaeus vannamei to vibriosis when orally exposed to the insecticide methyl parathion. Chemosphere 60(1):126–34
Rutkoski CF, Macagnan N, Folador A, Skovronski VJ, do Amaral AMB, Leitemperger J, Costa MD, Hartmann PA, Müller C, Loro VL, Hartmann MT (2020) Morphological and biochemical traits and mortality in Physalaemus gracilis (Anura: Leptodactylidae) tadpoles exposed to the insecticide chlorpyrifos. Chemosphere 250:126162. https://doi.org/10.1016/j.chemosphere.2020.126162
Saha NC, Giri SK, Chjatterjee N, Biswas SJ, Bej S (2016) Acute toxicity of dichlorvos to Branchiura sowerbyi (Beddard, 1982). Glob J Res Anal 5(5):138–139
Sarkar C, Saha NC (2016) A study on acute toxicity of an insecticide triazophos on zooplankton Cyclops viridis (Jurine, 1820) along with the changes in their behaviour. Glob J Res Anal 11(5):240–241
Schmuck R, Pfleeger W, Grau R, Hollihn U, Fischer R (1994) Comparison of short-term aquatic toxicity: formulation vs active ingredients of pesticides. Arch Environ Contam Toxicol 26:240–250. https://doi.org/10.1007/BF00224811
Sparling DW, Fellers G (2007) Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environ pollut 147:535–539. https://doi.org/10.1016/j.envpol.2006.10.036
Tamone SL, Harrison JF (2015) Linking insects with crustacea: physiology of the Pancrustacea: an introduction to the symposium. Integr Comp Biol 55(5):765–770. https://doi.org/10.1093/icb/icv093
Tilak KS, Veeraiah K (2001) Toxicity and effect of chlorpyrifos to the freshwater fish Labeo rohita (Hamilton). Pollut Res 20(3):443–445
Tripathi N, Pandey R (2014) Effect of monocrotophos on the mortality of apple snails. IOSR J Environ Sci, Toxicol Food Technol 8(7):14–18. https://doi.org/10.9790/2402-08721418
Tu LH, Grieneisen ML, Wang R, Watanabe H, Zhang M (2023) Assessment of agricultural pesticide inert ingredient transport following modeling approach: case study of two formulation agents in Sacramento River watershed. J Environ Manage 330:117123. https://doi.org/10.1016/j.jenvman.2022.117123
Ubaid Ur Rahman H, Asghar W, Nazir W, Sandhu MA, Ahmed A, Khalid N (2021) A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: evidence of mechanisms, exposures and mitigation strategies. Sci Total Environ 755(Pt 2):142649. https://doi.org/10.1016/j.scitotenv.2020.142649
US EPA (2018) Basic information about pesticide ingredients. https://www.epa.gov/ingredients-used-pesticide-products/basic-information-about-pesticide-ingredients (Accessed 12 May 2024)
WHO (2019) WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition. World Health Organization; 2020, Geneva, Licence: CC BY-NC-SA 3.0 IGO
Acknowledgements
The author extends his sincere gratitude to Professor Anilava Kaviraj, Department of Zoology, University of Kalyani, for giving him precious guidance during this study. Special thanks to the Head, Department of Zoology, University of Kalyani, and the Principal, Vivekananda Mahavidyalaya, Haripal for providing necessary laboratory facilities.
Author information
Authors and Affiliations
Contributions
RM conceptualised, designed, and performed this research work. He also wrote the manuscript, analysed data, prepared figures, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Ethical approval
Animal care was done in accordance with the University of Kalyani’s animal care protocols.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Majumder, R. Acute toxicity of chlorpyrifos to some non-target freshwater organisms: which one is more toxic—technical grade or commercial formulation?. Ecotoxicology (2024). https://doi.org/10.1007/s10646-024-02806-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s10646-024-02806-3