Abstract
Mercury is a global pollutant that is released into our environment by natural and anthropogenic processes resulting in extensive studies of mercury cycling in aquatic ecosystems, and the issuance of human-health-based fish-consumption advisories. We examined total mercury concentrations in Walleye Sander vitreus from Upper and Lower Red Lakes, located in north central Minnesota, between 2019 and 2020. Sampled Walleye (n = 265) ranged from 158 to 610 mm in total length from an age range of young-of-the year to 16 years. Mercury concentrations within the Walleye ranged from 0.030 mg/kg to 0.564 mg/kg (x̄ = 0.179 ± 0.105 mg/kg; x̄ = mean ± sd, all fish-mercury concentrations expressed on wet-weight basis). The best supported model for predicting mercury concentrations in Red Lake Walleye included the independent variables: length, age, sex, and lake basin. This model indicated that there was a significant difference in mercury concentrations between Upper and Lower Red Lake (x̄ = 0.215 ± 0.117 and 0.144 ± 0.077 mg/kg, respectively), and also suggests that individuals who rely on fish for subsistence should target Walleye that are ≤ 400 mm from Lower Red Lake. Observed differences in mercury concentrations could be linked to wetland area influences, fish growth rates, and physicochemical parameters between the two basins. Given that our results illustrated a significant difference in fish-mercury concentrations between basins, future pollutant monitoring efforts should treat Upper and Lower Red Lake as separate lakes and not assume that data from one basin can apply to the other.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a global pollutant that is released into our environment by natural and anthropogenic processes which has resulted in extensive mercury contamination and studies on Hg cycling in aquatic ecosystems which has led to the issuance of human-health-based fish-consumption advisories (Brigham et al. 2009, 2003; Brumbaugh et al. 2001). Mercury is commonly found in three forms (elemental Hg0, inorganic Hg+2, and methylmercury MeHg), each displaying different physical and chemical properties, economic uses, and human-health risks (Park and Zheng 2012; US EPA 1997). Since 1990 through the Clean Air Act, the United States has been regulating Hg compound uses and emissions (Rustagi and Singh 2010; US EPA 2015); however, Hg can still be found in the most remote locations due to its ability to remain in the atmosphere for an extended period of time before depositing back to Earth’s surface through wet and dry deposition. Through co-metabolic processes in microbes, Hg+2 ions undergo a methylation process that converts the inorganic Hg+2 into bioavailable MeHg (Gilmour et al. 1992; Myrbo et al. 2017; Shao et al. 2012). Due to MeHg’s ability to biomagnify in food chains, MeHg poses the greatest health concerns for wildlife and humans (Fitzgerald and Clarkson 1991). Human-health risks of chronic and/or high-dose acute exposures to MeHg can result in neurological implications; especially for individuals in their early developmental stages of life (Bernhoft 2012; Minai 2016; Myers and Davidson 1998). Because of these implications and extensive epidemiology studies on Hg, the United States Environmental Protection Agency (EPA) established a reference dose (RfD) of 0.1 µg of Hg per kg of body weight per day (µg/kg/day) that should be considered for consumption advisories for sensitive populations (ATSDR 1999; Goldman et al. 2001; US EPA 1997). Sensitive populations consist of women of child-bearing age, breast-feeding women, and children under 15 years of age (US EPA 1997). In a nationwide study by Xue et al. (2015), MeHg exposure of tribal populations from fish were 3–10 times higher than the United States general population.
The origin of mercury inputs to our waters are well documented (Brigham et al. 2003; Krabbenhoft et al. 1998; Lamborg et al. 2002; Scudder et al. 2009). A review of past and present research has indicated that there are known environmental conditions that promote the production of MeHg which include wetland acreage, dissolved organic carbon (DOC), pH, dissolved oxygen (DO), acid neutralizing capacity (ANC or alkalinity), acid-volatile sulfides (AVS) in wetland sediment, and land use and land cover (LULC) (Gilmour et al. 1992; Rypel 2010; Scudder et al. 2009). Many of these variables are associated with wetland properties which, in general, exhibit high DOC and AVS sediments and low pH, DO, and ANC levels that are resultant of anaerobic conditions necessary for the reduction of sulfate. Sulfur, in general, enters aquatic ecosystems by rock erosion, atmospheric deposition, or human influences and can be readily oxidized to sulfate (SO4−2) which is essential for plant growth (Clayden et al. 2017). Although essential for plant growth, excess loading of SO4−2 to aquatic ecosystems has been shown to harm some sensitive plant species (e.g., wild rice), and exacerbate MeHg production from sulfate-reducing bacteria (Myrbo et al. 2017). Sulfate-reducing bacteria are typically found in low pH (<6) environments in the transitional zone of aquatic ecosystems, and available metal cations, such as Hg+2, become incorporated through co-metabolic processes resulting in the inadvertent production of organically available MeHg. The abundance of wetlands located around the Red Lake Indian Reservation in northcentral Minnesota would indicate that there is a potential for higher MeHg concentrations in fish than other locations in northern Minnesota. More specifically, Upper Red Lake is influenced by a large expanse of wetlands to the north. Most of these wetlands are connected to the Tamarac River that discharges into the northeastern portion of Upper Red Lake (Fig. 1) leading to the possibility of an influx of MeHg during high-flow conditions.
The ubiquity of Hg in our environment has led to scientists performing decades of regional, national, and global scale studies on Hg (Chalmers et al. 2011; Engstrom et al. 2007; Krabbenhoft et al. 1998). These studies prompted regulatory agencies to establish fish-mercury monitoring programs to inform health-based fish-consumption advisories. The Red Lakes, consisting of the connected Upper and Lower Red Lakes (referred to as basins throughout the remaining text), is the largest freshwater lake ecosystem contained entirely within Minnesota. The Walleye fishery within the Red Lakes is both a culturally and economically important subsistence resource for the Red Lake Nation, whose lands surround much of the Red Lakes. Additionally, the eastern half of Upper Red Lake is an important sport fishery and recreational resource for the state of Minnesota. Although some fish-mercury monitoring has been done in the Red Lakes, existing data are too limited, hindering the assessment of Hg concentrations with respect to: fish size (length), time, or space. In particular, existing data are inadequate to determine if the Red Lakes can be treated as a single ecosystem, or if fish-mercury concentrations differ between the Upper and Lower basins.
Currently, the Red Lake Indian Reservation has a Hg dataset that dates to 2002. This 20-year-old dataset is what the Tribe uses to inform its members about Hg levels in fish caught from Tribal waters of Upper and Lower Red Lake. In reference to Xue et al. (2015) nationwide mercury study in tribal populations, Red Lake Tribal members who rely on fish for subsistence may be exposed to elevated mercury levels, prompting the need for this research. Additionally, the Minnesota Department of Natural Resources (MN DNR), in conjunction with the Minnesota Pollution Control Agency (MPCA) and Minnesota Department of Health (MDH), have been monitoring the State waters since 1987 with the last assessment made in 2012. However, this dataset is too small to accurately determine Hg concentration in fish populations due to small sample sizes ranging from 3 to 24 fish per sampling event. With inconsistent and generally small sample sizes per event, determining statistical differences in fish populations is challenging. Collecting consistent data is paramount for sound science and the results of this study will provide Tribal and State agencies with models needed to estimate Hg concentrations in Walleye Sander vitreus. The objectives of this research are to (1) develop models for Walleye to determine Hg concentrations by length, age, sex, and lake basin; (2) statistically determine temporal variability; and (3) determine how Hg concentrations from the Red Lakes compare to similar large-lake systems within Minnesota. Focusing our efforts on Walleye will give us an accurate representation of how much Hg is in the ecosystem and the potential MeHg concentrations being consumed by humans from the Red Lakes. Achieving these objectives should provide Tribal and State agencies the necessary tools for long-term monitoring of Hg along with the appropriate sample size to produce statistically significant results as well as up-to-date data for updating the fish-based consumption advisory for the Red Lakes.
Methods
Study area
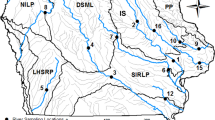
Located in north central Minnesota, USA, Upper and Lower Red Lakes are primarily within the Red Lake Indian Reservation. The Red Lake Indian Reservation is one of two “closed Reservations” in the United States, meaning that there is no outside law enforcement and the Red Lake Tribal Council governs over its members including their natural resources. The Reservation’s total land holdings are in excess of 3300 km2 consisting of approximately 2190 km2 of mixed forests and wetlands; 975 km2 of lakes (Upper and Lower Red Lake encompassing 963 km2 within the Reservation boundary); and over 597 km of rivers and streams. Situated within the Red Lakes Watershed which has an area of 5000 km2 (Fig. 1), Upper and Lower Red Lake form a contiguous water body consisting of two large basins (483.1 and 665.6 km2, respectively) that are naturally connected by a 1.4 km-wide strait. The watersheds’ land use and land cover consists primarily of open water/wetlands (mostly peatlands) and forested land, 79 and 13% respectively. Agricultural practices within the Reservation are minimal; approximately 6% of the Red Lake watershed land is utilized for cropland and grazing which occurs outside the Reservation boundary (in red, Fig. 1) in the eastern-most HUC 8 watershed.
There are 12 major streams, along with numerous ephemeral streams or ditches, that flow into Upper and Lower Red Lake. The Red Lakes form the headwaters of the Red Lake River which is controlled by a dam that is owned by the United States Army Corp of Engineers located at the outlet of Lower Red Lake. The Red Lake River is the only outlet of the two lakes and flows west for approximately 310 km before discharging into the Red River of the North in East Grand Forks, Minnesota. Upper and Lower Red Lake are classified as eutrophic lakes that rarely stratify; driven by prevailing west winds. The maximum depths for Upper and Lower Red Lake are 5.5 and 9.1 meters, respectively. The two lakes exceed Minnesota’s Clean Water standards for phosphorus which is set at 30 µg/L, however they are not listed as impaired waters. Both the State and Tribe recognized the need for site specific standards after a 2016 winter sediment-coring experiment performed by the St. Croix Watershed Research Station indicated that the phosphorus levels are consistent with historic diatom inferred phosphorus levels for this system (Burge 2021). In the mid-1990s, the Tribe voluntarily stopped Walleye harvest efforts after the Red Lakes experienced a crash in the Walleye population; by 1997 Walleye harvesting was prohibited. However, through cooperative management and recovery plans by fry-stocking, the fishery recovered by 2006. Today, the Red Lakes, especially Upper Red Lake, is considered one of the most productive Walleye fisheries within the United States. The major prey-base for Walleye include yellow perch (Perca flavescens), spottail shiner (Notropis hudsonius), river shiner (Notropis girardi), and emerald shiner (Notropis atherinoides). Other targeted game species that inhabit the Red Lakes include northern pike (Esox lucius), black crappie (Pomoxis nigromaculatus), and yellow perch.
The Red Lake Reservation is home to approximately 7500 Tribal members. Individuals’ primary livelihood include subsistence natural resource harvesting. Natural resources, historically, represented the most important source of employment to the Band members, with commercial fishing and timber harvesting representing the two most important industries. These two industries impact every member on the Reservation, therefore, preserving and restoring the Reservation’s rich aquatic ecosystem and abundance of other natural resources is critical to Band members’ health, welfare, traditional ways of life, economic viability, and is a high priority for the Band.
Sample size determination
Previous Walleye samples (n = 62) collected from Upper Red Lake by the MN DNR from 1987 to 2012 were used to generate linear regression models in R software (R Core Team 2021). These models were used to determine a mean maximum effect of varying samples sizes (n = 3–200). The mean maximum effect was determined from the maximum confidence interval width from 1000 simulations at each sample size. Plotting the confidence interval width at each sample size gave us an inflection point at 60 Walleye samples. Based off these calculations to efficiently and accurately measure Hg concentrations in the Red Lakes, a minimum sample size of 60 Walleye from Upper Red Lake and 60 Walleye from Lower Red Lake were needed to perform our statistical analysis and produce Hg models for the basins (Figure in Supplemental Information Section).
Walleye sampling
Walleye samples were collected through a collaborative effort working in conjunction with the MN DNR and Red Lake Department of Natural Resources (RL DNR) fisheries crews during the fall 2019 and 2020 experimental gill netting population assessments. These nets consist of five different mesh sizes (19, 25, 32, 38, and 51 mm) measuring 15.2 m each for a total span of 76.2 m. The RL DNR set four nets (paired net sets) per location each day from 3 September to 27 September 2019 and 1 September to 25 September 2020 (Fig. 1); two nets oriented in deep water and two nets oriented in shallow water for a total of 48 net sets (12 locations). The MN DNR used a grid pattern for their net sets for a total of 20 sets or 20 locations during the same sampling period as the RL DNR (Fig. 1). These net sets were fished for approximately 24 h before assessment at the DNR headquarters. A total of 265 Walleye samples, 131 Walleye (66 male and 65 female) from Upper Red Lake and 134 Walleye (62 male and 72 female) from Lower Red Lake were collected for Hg analysis. When possible, a minimum of three male and three female Walleye from 50 mm size classes were collected.
Sample collection
Sample collection followed procedures similar to the US EPA (2000) and US Geological Survey (Scudder et al. 2008). Briefly, work surfaces were covered with a new plastic sheet or bag for each Walleye sample taken. All field personnel participating in processing samples wore Nitrile gloves. All total lengths (±1 mm), weights (±1.0 g), sexual identification, and aging structures (fin ray, scales, and otoliths) were taken before collecting a ~ 12.9 cm2 tissue sample. Skin-off tissue samples were taken on the left side anterior to the dorsal fin using a clean stainless-steel fillet knife. Tissue samples were rinsed with deionized water, weighed (wet weight) to the nearest 0.01 g, and placed in a clean sterile Whirl-Pak® plastic bag with the respective serial numbers from the netting assessment. Tissue samples were transferred to wet ice in an insulated cooler before being transferred to a laboratory freezer (−20 °C) to be stored until lyophilization and homogenization.
Sample preparation
Tissue samples were lyophilized with a Harvest Right® stainless-steel freeze dryer; approximately 28 h of run time from frozen to a freeze-dried sample. Ten percent of the samples were lyophilized a second time to determine equipment efficiency. Each sample was homogenized using porcelain mortar and pestles, weighed (± 0.0001 g) for wet vs. dry weight conversions, and placed in 40 mL borosilicate scintillation vials for dry storage.
Sample analysis/QAQC
Samples were analyzed using a Milestone TriCell Dual Beam Direct Mercury Analyzer (DMA-80evo) following EPA 7473 (US EPA 2007). Briefly, samples were introduced into the DMA-80evo quartz decomposition tube by a nickel sample boat. The decomposition tube is heated by two programmable furnaces to dry and thermally decompose the sample to release mercury vapors in an oxygenated environment. The mercury vapors are transported over the amalgamator that traps the mercury. Once all the mercury vapors are trapped, the amalgamator is rapidly heated to release the vapors in order to pass through the three absorbance cuvettes and spectrometer. Detection is then sent to the desktop controller. The instrument’s detection limit (0.0003 ng Hg) was 100 times lower than EPA 7473 requirement. A new calibration curve (created using stock 1000 mg/L Hg to gravimetrically dilute to 1.0, 0.1, 0.01 mg/L Hg) was created to span the width of all three cuvette cells at the beginning of the project. Two calibration standard samples were analyzed daily before the start of the sample run at 0.5 ng and 50 ng of Hg. Calibration standards were made daily or weekly depending on the change in percent absorbance; new check standards were made when percent absorbance exceeded 10% from the calibration curve. Certified reference material, DORM-4 (dried fish protein homogenate), was purchased from National Research Council Canada and used to verify EPA method 7473. National Research Council Canada certified DORM-4 at 0.412 ± 0.036 mg/kg (mean ± sd); DORM-4 analysis with the DMA-80evo illustrated x̄ = 0.392 ± 0.011 mg/kg (n = 30). Matrix spikes and matrix spike duplicates (MS/MSD) at an average spike of 30.834 ng Hg of the original sample were analyzed to test the DMA-80evo percent recovery of Hg (105.7–120.5%, x̄ = 111.7% from 26 samples). The method detection limit was 0.745 ng Hg and was estimated by analyzing 15 replicates of known Hg additions (50 ng Hg) to sterile sample boats and multiplying the standard deviation among replicates by 2.624, the single-tailed t value for a 99% confidence interval. A sample run consisted of 2 calibration check samples, 20 Walleye tissue samples, 3 equipment blanks, 2 DORM-4 samples, and 1 MS/MSD paired sample. All sample concentrations were converted back to wet weight. Sample boats were brushed clean of ash and ran back through the DMA-80evo for sterilization after each sample run and stored in a new zip-sealed bag.
Data analysis
All statistical analyses were performed in R (R version 4.1.1; R Core Team 2021) and significance was evaluated at α = 0.05. First, an exhaustive model selection procedure was used to evaluate a suite of variables for predicting mercury concentrations in Walleye from Upper and Lower Red Lake. These linear models were used to determine the significance of Hg concentration as a response to length, weight, age, sex, and basin. The best fit model for predicting Hg concentrations was based on the lowest Akaike information criterion (AIC). By theoretical definition, as a model becomes more complex, the AIC score will penalize those models (Rossi et al. 2020). Diagnostic plots were used to check for heteroscedasticity and verify the best fit model. Observed versus predicted plots with 95% confidence intervals were used to illustrate the data.
To compare mean length by age of Walleye between basins, we modeled the relationship between fish total length and fish age using the von Bertalanffy growth rate model (Ogle 2016) in R using packages FSA, FSAdata, plotrix, and dplyr (Lemon 2006; Ogle et al. 2021; Ogle, 2019; Wickham et al. 2021). A t-test was used to determine differences in mean length per age by basin.
Analysis of variance (Type III ANOVA; car package (Fox and Weisberg 2019)) was used to evaluate the interactive effect of length (continuous variable) and years sampled to determine temporal differences in Hg concentrations within the Red Lakes. We evaluated the interactive effect of length (continuous variable) and Minnesota’s top ten largest bodies of water (discrete variable) on fish Hg concentrations using two-way ANOVA (Type III ANOVA; car package (Fox and Weisberg 2019)). Mercury data in piscivorous fish from other lakes in Minnesota was supplied by Monson (2009). The significant interaction between length and body of water was evaluated with post-hoc comparisons using ANCOVA (emmeans package (Lenth 2021)) to estimate the mean Hg concentrations with a confidence level of 0.95. Finally, a Tukey pairwise comparison of estimated marginal mean Hg concentrations was used to determine significant differences between lakes.
Results
A total of 265 Walleye ranging from 158 to 610 mm were collected from Upper and Lower Red Lake (Table 1; Fig. 2) during the 2019 and 2020 study. Total mercury concentrations varied between 0.030 mg/kg to 0.564 mg/kg (x̄ = 0.179 ± 0.105 mg/kg; x̄ = mean ± sd, all fish-mercury concentrations expressed on wet-weight basis) with the highest Hg concentration found in a 10 year old, 517 mm female Walleye from Upper Red Lake. Total mercury concentration in Walleye were higher in Upper versus Lower Red Lake (Fig. 2; x̄ = 0.215 ± 0.117 and 0.144 ± 0.077 mg/kg, respectively). Mercury concentrations in Red Lake Walleye illustrated a positive linear relationship with length (Fig. 2) and age (Fig. 3). Age of Walleye ranged from young-of-the year (0) to 16 with a mean ± sd of 4.3 ± 3.3. Walleye at age-1, 2, and 6 through 10 from Lower Red Lake exhibited significantly faster growth rates than Walleye from Upper Red Lake (Fig. 3). Lastly, Hg concentrations in male and female Walleye from Upper and Lower Red Lake were similar (x̄ = 0.176 ± 0.103 and x̄ = 0.181 ± 0.107 mg/kg, respectively; Fig. 4).
Mercury concentrations by length from Upper (URL; red) and Lower (LRL; black) Red Lake collected by the Red Lake DNR and MN DNR during the 2019 and 2020 study. Point sizes are weighted based on the age of individual Walleye; larger points are older individuals. Young-of-the year Walleye received a 0.5 age in order to illustrate the points; three depicted age points are for reference, young-of-the year through age-16 fish are represented in the figure. The 99% confidence interval indicates that the linear regression will fall inside the interval bounds. Green horizontal lines indicate 2–3 serving/week of 0.15 mg/kg Hg in raw fish fillet; blue horizontal lines indicate a consumption of 1–2 servings/week at levels of ≤0.23 mg/kg Hg in raw fish fillet; below the red polygon indicates a consumption of 1 serving/week at levels ≤0.46 mg/kg Hg in raw fish fillet; the area in red indicates a consumption of 1 serving/month at levels ≥0.46 mg/kg Hg in raw fish fillet. A serving size is based on human body weight; for reference, a 90 kg person could consume approximately 298 g of wet weight fish (224 g cooked)
Mercury concentrations as a response to sex from the 2019 and 2020 study on Upper and Lower Red Lake. Plot A is Hg concentration in male (black) and female (red) Walleye from Upper Red Lake and plot B is Lower Red Lake. Size of data points are weighted based on age of male and female Walleye; three depicted age points are for reference, young-of-the year through age-16 fish are represented in the figure. Green horizontal lines indicate 2–3 servings/week of 0.15 mg/kg Hg in raw fish fillet; blue horizontal lines indicate a consumption of 1–2 servings/week at levels of ≤ 0.23 mg/kg Hg in raw fish fillet; below the red polygon indicates a consumption of 1 serving/week at levels ≤ 0.46 mg/kg Hg in raw fish fillet; the area in red indicates a consumption of 1 serving/month at levels ≥ 0.46 mg/kg Hg in raw fish fillet. A serving size is based on human body weight; for reference, a 90 kg person could consume approximately 298 g of wet weight fish (224 g cooked)
An interactive model with the main effects of length, age, sex, and basin produced the best AIC score and explained 80% of the Hg concentration variability in Walleye (Table 2, Fig. 5A). A simplified interactive model, which could be used by the general public to estimate Hg concentrations in Walleye, contained the main effects of length and basin. This model explains 53% of the Hg variability found in Walleye from the Red Lakes (Table 2, Fig. 5B). The assumption would be made that the general public cannot accurately estimate fish age and/or sex a Walleye from the Red Lakes due to fast growth rates and an abundance of visceral fat.
Predictive models to estimate Hg in Walleye from Upper and Lower Red Lake. Plot A is an interactive function with four different variables (length, age, sex, and basin) to estimate Hg concentrations in Walleye from Upper and Lower Red Lake (AIC = −852.5). Plot B is also an interactive function with two variables (length and basin) to estimate Hg concentrations in Walleye from Upper and Lower Red Lake (AIC = −637.6). Plot A is the best fit model for the data with a linear regression line that will fall within the confidence interval, in green, 95% of the time: F-statistic: 71.39 on 15 and 249 DF, p value: < 0.01
Individual Walleye samples for Hg analysis have been collected from 1997 to 2020. Sampling intervals have been inconsistent ranging from 1 to 7 years and sample sizes ranging from 3 to 224 individuals. Trends in Hg concentrations over time were not noticeable, and after adjusting for length (394 mm to 460 mm) to fit years sampled, there were no significant differences temporally (Fig. 6).
Scatter plot of Walleye mercury concentrations (mg/kg) by year sampled in Upper and Lower Red Lake adjusted for length as the covariate. A two-way ANOVA (Type III ANOVA) was used to evaluate the interactive effect of length (continuous variable) and years sampled to determine temporal differences in Hg concentrations within the Red Lakes. The ANOVA suggests that there are no significant differences in Hg concentrations between years sampled
Spatial comparisons of Hg concentrations in Walleye from Minnesota’s ten largest lakes in relation to the Red Lakes showed significant differences among lakes (Table 3). After adjusting for length (325 to 610 mm) to fit all water bodies, Lower Red Lake’s Hg concentrations were significantly lower than Otter Tail and Rainy Lake (x̄ = 0.140 ± 0.080 mg/kg; x̄ = 0.460 ± 0.236 mg/kg; x̄ = 0.477 ± 0.203 mg/kg; P value < 0.05, respectively), while Upper Red Lake’s Hg concentrations were lower than Rainy Lake (x̄ = 0.260 ± 0.118 mg/kg; x̄ = 0.477 ± 0.203 mg/kg; P values < 0.05, respectively).
Discussion
The Red Lakes in north central Minnesota are considered one of the most important fisheries within the state. Both basins are important fisheries for recreation, subsistence, and commercial fishing for the Red Lake Band of Chippewa Indians. The eastern half of Upper Red Lake is also an important sport fishery and recreational resource for non-Tribal anglers in Minnesota. Even with its popularity, scientific advancements in regards to pollutant monitoring have been limited, in part, due to cooperative management efforts. The robust dataset collected for this study on the Red Lakes allowed us to determine important factors influencing Hg concentrations observed in the Walleye population. Previous Hg studies conducted on the Red Lakes’ Walleye focused primarily on a certain size range near 400 mm. These individuals are known to be the most targeted or harvestable fish by anglers which coincides with a protected slot limit between 432 mm to 660 mm; current regulations for non-Tribal members allows anglers to harvest one Walleye over 432 mm. However, with data gaps from both smaller and larger individuals and virtually zero individuals collected from Lower Red Lake, making accurate assumptions about Hg concentrations in Walleye is challenging; this study provided those missing components. All size classes were represented from both Upper and Lower Red Lake in our dataset from young-of-the year (<254 mm) to 16 year old (>600 mm) individuals. Collecting lengths, weights, age, sex, and location (basin) provided us the ability to perform model predictions about the Hg concentrations found in Walleye. Based on AIC scores, the best predictive model incorporates length, sex, age, and basin; these factors can interact with other variables within the model which explained 80% of the Hg variability we observed in the Red Lake Walleye. Previous studies have shown that as fish grow, in length and age, Hg concentrations generally increase (Depew et al. 2013; Eagles-Smith et al. 2008; Mathers and Johansen 1985). However, explaining Hg differences in male and female walleye is complex due to sexual dimorphism or growth dilution, energy and reproductive requirements. Studies on smallmouth bass (Murphy et al. 2007) and on Walleye (Henderson et al. 2003) both illustrated differences in Hg concentrations between sexes; however, this was only after sexual maturity where sexual dimorphism is most noticeable. In this study, sexual differences in mercury concentrations was an important predictor variable for the model and is likely linked to the differences in energy requirements and growth dilution (Madenjian et al. 2016).
Observing a significant difference in Hg concentrations between Upper and Lower Red Lake Walleye was an important finding from a management and recreational standpoint. Historically, samples for Hg analysis were collected primarily from the easternmost portion of Upper Red Lake. These samples were then used to infer Hg levels throughout the entire Red Lake ecosystem. Observing differences in Hg concentrations could be explained by a couple of factors, even though the two basins lack any barrier to fish movement where they are connected. The first factor being the limnology of the two basins. Both basins are shallow, windswept basins with Lower Red Lake being twice the depth of Upper at ~10 m. Lower Red Lake provides more fish habitat to promote faster growth rates while Upper Red Lake is primarily sand, small cobble, and soft sediment. Second, wetlands are a dominant land cover type within the Red Lake watershed which are suitable sites for certain bacteria species (e.g., sulfate reducing bacteria) to methylate Hg (Hall et al. 2008; Jeremiason et al. 2006). Upper Red Lake is situated down gradient of a large wetland expanse, primarily peatland, to the north and east and has one major tributary, the Tamarac River to the northeast. There are also numerous perennial and semi-perennial streams and ditches along the north shore of Upper Red Lake that may facilitate the transport of MeHg from sites of methylation at the peatland-upland interface (Mitchell et al. 2008a, 2008b; Wang et al. 2021). In contrast, Lower Red Lake has six major tributaries located along the south and east shores which are influenced more by upland forest and agriculture. Studies have shown that wetlands contribute to increased bioavailable Hg to aquatic systems (Hall et al. 2008; Louis et al. 1994; Rypel 2010), whereas upland forests accumulate and retain total Hg (Demers et al. 2013). Due to the abundance of potential wetlands influencing Upper Red Lake, there is evidence to suggest that wetlands provide a disproportionate Hg load that impacts Upper Red Lake Walleye. Even though direct Hg deposition to surface water is an important source for MeHg concentrations found in fish (Harris et al. 2007), direct Hg inputs to the Red Lakes does not explain the Hg differences between the basins. The third factor that alludes to the Hg difference we found in this study are the growth rates in Walleye between the two basins. Walleye from age-classes 1 through 10 exhibited faster growth rates on average in Lower Red Lake than Upper Red Lake. Before sexual maturity, young-of-the year through age-4 Walleye from Lower Red Lake, on average, were 12 mm longer than Upper Red Lake Walleye. After sexual maturity (age-5 through 10), Lower Red Lake Walleye exhibit an average growth rate of 53 mm of increased length per age than Upper Red Lake Walleye. Although growth rates were not statistically different in all age-classes (age-classes young-of-the year and 3–5), the observed average growth differences from age-classes 1, 2, and 6 through 10 could contribute to the Hg differences we observed between the two basins. Simoneau et al. (2005) concluded that slower growing fish from Québec experienced increased Hg levels compared to faster-growing fish. Because basin was an important predictor variable for estimating Hg concentrations in Walleye and the observed growth rate differences by age, this dataset suggests that the Walleye populations do not frequently mix between the two basins which contradicts Smith Jr et al. (1952) study that indicated that ~20% of tagged Walleye utilize both basins. Observing these Hg trends in Walleye between the two basins warrants standardize sampling throughout Upper and Lower Red Lake.
Since the early 1990’s, the US EPA has been regulating Hg uses and emissions. The Toxic Release Inventory Program has shown steady declines in Hg emissions from ~57,000 kg in 2007 to ~14,000 kg in 2019 (US EPA 2019). However, the temporal variability of Hg within the Red Lake Walleye has been inconsistent with data showing no significant trends from 1997–2020 which is linked to limited data. What our data suggests is the need for frequent sampling of a specific number of individuals from a certain size class to determine temporal changes. Secondly, the Red Lakes are situated within a large complex of wetlands to the north and east; previous studies have shown that wetlands can act as a massive storage system for Hg and also increase the methylation rates of elemental Hg (Gabriel et al. 2009; Mitchell et al. 2009; Rypel 2010; Snodgrass et al. 2000). Due to wetlands’ ability to store and release MeHg into surface water, observing a significant increase or decrease in Hg levels found in fish could take decades.
Monson et al. (2011) compared Hg levels across all of Minnesota and found evidence to suggest that Hg increases from south to north and west to east. However, based on this study, Lower Red Lake Walleye have one of the lowest mean Hg concentrations of other large lakes (>52 km2) in Minnesota. Also, two distinctly different lakes, Otter Tail Lake and Rainy Lake, exhibit some of the highest Hg levels found in Walleye. Otter Tail Lake is located in west central Minnesota and is part of a chain of lakes. The shorelines are well established by residential and commercial uses and the surrounding watershed land cover is primarily agriculture, forest, and water. Studies have indicated that agricultural and forested land covers can contribute to high total Hg levels due to the retention of Hg in foliage (Brumbaugh et al. 2001; Krabbenhoft et al. 1998). Rainy Lake, in contrast, is located in north eastern Minnesota and is primarily undeveloped. The lake is situated within a boreal forest that exhibits shallow soils, bedrock, and peat bogs. Due to minimal anthropogenic processes, elevated Hg levels in Rainy Lake likely occur from atmospheric deposition, leaching geologic formation, and suitable physical and chemical water quality parameters for Hg methylation. In contrast to Monson et al. (2011) above, Simoneau et al. (2005) and Strandberg et al. (2018) illustrated that spatially comparing lakes for Hg is difficult due to different food availability and growth rates, water chemistry, watershed influences, and anthropogenic processes which is also what we experienced in this study. When adjusting length as a covariate between other large lakes in Minnesota, Lower Red Lake Walleye exhibit significantly lower Hg concentrations than Otter Tail and Rainy Lake, whereas Upper Red Lake Walleye only exhibit significantly lower Hg concentrations than Rainy Lake.
Within Minnesota, the MPCA and MN DNR collect fish samples for Hg analysis every five years on average. The last known study conducted within Tribal waters was in 2002 resulting in the tribal community relying on data collected from State waters for fish consumption guidelines. The State waters account for approximately 25% of the entire Red Lake ecosystem and with inconsistency between sample sizes and time between collection events, there was a need to produce a robust Hg dataset that encompasses all of Upper and Lower Red Lake. This dataset provided the necessary information to create an updated Red Lake-specific Walleye consumption advisory and the coding required to produce an interactive web-based application for predicting Hg concentrations within an individual Walleye. Anticipated informational graphics will mirror those in Fig. 2. The current fish consumption guidelines that the state of Minnesota implements is an effective guideline to use for the Red Lakes. The Minnesota fish consumption guideline has minor differences from the EPA recommendations which are broken down into four categories for sensitive populations: no restrictions (≤0.05 mg/kg), one meal per week (0.05–0.2 mg/kg), one meal per month (0.2–1.0 mg/kg), and no consumption (≥1.0 mg/kg) (MDH 2004); whereas EPA’s guidelines are weekly fish servings: three 4 ounce servings per week (≤0.15 mg/kg), two 4 ounce servings per week (0.15–0.23 mg/kg), one 4 ounce serving per week (0.23–0.46 mg/kg), and zero servings per week (>0.46 mg/kg) (US EPA 2001). After standardizing the EPA consumption guidelines to mimic Minnesota’s guidelines, the advisories are similar with Minnesota’s guideline slightly more restrictive for sensitive populations. The Hg data collected from this study suggests that all Walleye sampled between 2019 and 2020 are within safe consumption levels, however moderation is still recommended to meet the consumption guidelines. For people who wish to consume Walleye frequently, they should limit their consumption to one meal per week (MN guidelines) or one to three servings per week (EPA guidelines), and they should target individuals that are ≤ 400 mm (Fig. 2).
In conclusion, collecting Walleye from a wide size distribution was beneficial for this project by providing insights on how mercury can accumulate within a species (e.g., growth, trophic status, energy acquisitions, etc.). The most important finding in this study suggests that management and pollutant monitoring (e.g., mercury) should be sampled throughout the entire Red Lake ecosystem. This study also allowed us to fill in the mercury-data gaps when making comparisons between Upper and Lower Red Lake Walleye. The development of models to estimate mercury levels by length, age, sex, and lake basin will help inform the general public about up-to-date Hg levels in Walleye.
References
Agency for Toxic Substance and Disease Registry (ATSDR (1999) Toxicological profile for mercury. US Department of Health and Human Services, Public Health Service, Atlanta, GA. Accessed 24 Mar 2019 https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=115&tid=24
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012:1–10. https://doi.org/10.1155/2012/460508. Accessed 21 Apr 2019
Brigham ME, Krabbenhoft DP, Hamilton PA (2003) Mercury in stream ecosystems-New studies initiated by the U.S. Geological Survey, 1–4. https://pubs.usgs.gov/fs/fs-016-03/. Accessed 14 Dec 2018
Brigham ME, Wentz DA, Aiken GR, Krabbenhoft DP (2009) Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ Sci Technol 43:1–6. https://doi.org/10.1021/es802694n. Accessed 18 Sep 2018
Brumbaugh WG, Krabbenhoft DP, Helsel DR, Wiener JG, Echols KR (2001) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients: Bioaccumulation in fish. Biological Science No. 2001–0009 USGS. http://pubs.er.usgs.gov/publication/bsr20010009. Accessed 9 Sep 2018
Burge DRL (2021) Managing the Red Lake Nation’s and Minnesota’s largest lake: Monitoring and paleolimnology support a site-specific standard for Upper and Lower Red Lakes (Red Lake Nation and Minnesota, USA). Modern Limnological and Paleolimnological Applications of Diatoms in Minnesota Lakes (Doctoral dissertation, University of Minnesota) 18–45
Chalmers AT, Argue DM, Gay DA, Brigham ME, Schmitt CJ, Lorenz DL (2011) Mercury trends in fish from rivers and lakes in the United States, 1969–2005. Environ Monit Assess 175:175–191 https://doi.org/10.1007/s10661-010-1504-6. Accessed 20 Oct 2018
Clayden MG, Lescord GL, Kidd KA, Wang X, Muir DCG, O’Driscoll NJ (2017) Using sulfur stable isotopes to assess mercury bioaccumulation and biomagnification in temperate lake food webs: Sulfur isotopes and mercury. Environ Toxicol Chem 36:661–670. https://doi.org/10.1002/etc.3615. Accessed 18 Sep 2018
Demers JD, Blum JD, Zak DR (2013) Mercury isotopes in a forested ecosystem: Implications for air-surface exchange dynamics and the global mercury cycle. Global Biogeochem Cycles 27:222–238. https://doi.org/10.1002/gbc.20021. Accessed 14 Nov 2018
Depew D, Burgess N, Anderson M, Baker R, Bhavsar S, Bodaly RA, Eckley C, Evans M, Gantner N, Graydon J, Jacobs K, LeBlanc J, St.Louis V, Campbell L (2013) An overview of Hg concentrations in freshwater fish species: a national fish mercury (Hg) dataset for Canada. Can J Fish Aquat Sci 70:1–16. https://doi.org/10.1139/cjfas-2012-0338. Accessed 13 Oct 2021
Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL (2008) Mercury trophic transfer in a eutrophic lake: the importance of habitat-specific foraging. Ecol Appl 18:A196–A212. https://doi.org/10.1890/06-1476.1. Accessed 25 Oct 2018
Engstrom DR, Balogh SJ, Swain EB (2007) History of mercury inputs to Minnesota lakes: influences of watershed disturbance and localized atmospheric deposition. Limnol Oceanogr 52:2467–2483. https://doi.org/10.2307/4502395. Accessed 14 Dec 2018
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: present and future concerns. Environ Health Perspect 96:159–166. https://doi.org/10.1289/ehp.9196159. Accessed 19 Mar 2019
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd ed. SAGE Publications, Inc, Thousand Oaks, CA. Accessed 31 Aug 2021. https://us.sagepub.com/en-us/nam/an-r-companion-to-applied-regression/book246125
Gabriel MC, Kolka R, Wickman T, Nater E, Woodruff L (2009) Evaluating the spatial variation of total mercury in young-of-year yellow perch (Perca flavescens), surface water and upland soil for watershed–lake systems within the southern Boreal Shield. Sci Total Environ 407:4117–4126. https://doi.org/10.1016/j.scitotenv.2009.03.019. Accessed 13 Nov 2020
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in freshwater sediments. Environ Sci Technol 26:2281–2287. https://doi.org/10.1021/es00035a029. Accessed 25 Oct 2018
Goldman LR, Shannon MW, Committee on Environmental Health (2001) Technical Report: Mercury in the environment: implications for pediatricians. Pediatrics 108:197–205. https://doi.org/10.1542/peds.108.1.197. Accessed 21 Mar 2019
Hall BD, Aiken GR, Krabbenhoft DP, Marvin-DiPasquale M, Swarzenski CM (2008) Wetlands as principal zones of methylmercury production in southern Louisiana and the Gulf of Mexico region. Environ Pollut 154:124–134. https://doi.org/10.1016/j.envpol.2007.12.017. Accessed 4 Nov 2021
Harris HC, Rudd JWM, Amyot M, Babiarz CL, Beaty KG, Blanchfield PJ, Bodaly RA, Branfireun BA, Gilmour CC, Graydon JA, Heyes A, Hintelmann H, Hurley JP, Kelly CA, Krabbenhoft DP, Lindberg SE, Mason RP, Paterson MJ, Podemski CL, Robinson A, Sandilands KA, Southworth GR, St. Louis VL, Tate MT (2007) Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. PNAS 104:16586–16591. https://doi.org/10.1073/pnas.0704186104. Accessed 29 Jan 2022
Henderson B, Collins N, Morgan G, Vaillancourt A (2003) Sexual size dimorphism of walleye (Stizostedion vitreum vitreum). Can J Fish Aquat Sci 60:1345–1352. https://doi.org/10.1139/f03-115. Accessed 13 Oct 2021
Jeremiason JD, Engstrom DR, Swain EB, Nater EA, Johnson BM, Almendinger JE, Monson BA, Kolka RK (2006) Sulfate addition increases methylmercury production in an experimental wetland. Environ Sci Technol 40:3800–3806. https://doi.org/10.1021/es0524144. Accessed 13 Nov 2020
Krabbenhoft DP, Wiener JG, Brumbaugh WG, Olson ML, DeWild JF, Sabin TJ (1998) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients. U.S. Geological Survey Toxic Substance Hydrology Program-Proceedings of the technical meeting, Charleston, S.C., March 8–12, 1999. USGS Water-Resources Investigations Report 99-4018B, Vol 2, 147–160. https://pdfs.semanticscholar.org/8780/28e45ab5027ed861928eb0d2addca43fb387.pdf. Accessed 14 Dec 2018
Lamborg CH, Fitzgerald WF, Damman AWH, Benoit JM, Balcom PH, Engstrom DR (2002) Modern and historic atmospheric mercury fluxes in both hemispheres: global and regional mercury cycling implications. Glob Biogeochem Cycles 16:51-1–51–11. https://doi.org/10.1029/2001GB001847. Accessed 20 Oct 2018
Lemon J (2006) Plotrix: a package in the red light district of R. R-News 6(4):8–12
Lenth RV (2021) emmeans: estimated marginal means, aka least-squared means. R package version 1.6.2-1. https://cran.r-project.org/web/packages/emmeans/emmeans.pdf. Accessed 31 Aug 2021
Louis S, Rudd J, Kelly C, Beaty K, Bloom N, Flett R (1994) Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Can J Fish Aquat Sci 51:1–12. https://doi.org/10.1139/f94-106. Accessed 4 Nov 2021
Madenjian CP, Rediske RR, Krabbenhoft DP, Stapanian MA, Chernyak SM, O’Keefe JP (2016) Sex differences in contaminant concentrations of fish: a synthesis. Biol Sex Differ 7:1–16. https://doi.org/10.1186/s13293-016-0090-x. Accessed 4 Nov 2021
Mathers R, Johansen P (1985) The effects of feeding ecology on mercury accumulation in walleye (Stizostedion vitreum) and pike (Esox lucius) in Lake Simcoe. Can J Zool 63:2006–2012. https://doi.org/10.1139/z85-295. Accessed 13 Oct 2021
Minai M (2016) Methylmercury and human embryonic development. Embryo Project Encyclopedia 1–9. http://embryo.asu.edu/handle/10776/11335. Accessed 21 Mar 2019
Minnesota Department of Health (MDH) (2004) Eat fish often? A Minnesota guide to eating fish. http://www.health.state.mn.us/divs/eh/fish/index.html. Accessed 13 Oct 2021
Mitchell CPJ, Branfireun BA, Kolka RK (2008a) Assessing sulfate and carbon controls on net methylmercury production in peatlands: An in situ mesocosm approach. App Geochem 23:503–518. https://doi.org/10.1016/j.apgeochem.2007.12.020. Accessed 29 Jan 2022
Mitchell CPJ, Branfireun BA, Kolka RK (2008b) Spatial characteristics of net methylmercury production hot spots in peatlands. Environ Sci Technol 42:1010–1016. https://doi.org/10.1021/es0704986. Accessed 29 Jan 2022
Mitchell CPJ, Branfireun BA, Kolka RK (2009) Methylmercury dynamics at the upland-peatland interface: Topographic and hydrogeochemical controls: methylmercury at the upland-peatland interface. Water Resour. Res 45:1–12. https://doi.org/10.1029/2008WR006832. Accessed 13 Nov 2020
Monson BA (2009) Trend reversal of mercury concentrations in piscivorous fish from Minnesota Lakes: 1982-2006. Environ Sci Technol 43:1750–1755. https://doi.org/10.1021/es8027378. Accessed 3 Aug 2021
Monson BA, Staples DF, Bhavsar SP, Holsen TM, Schrank CS, Moses SK, McGoldrick DJ, Backus SM, Williams KA (2011) Spatiotemporal trends of mercury in walleye and largemouth bass from the Laurentian Great Lakes Region. Ecotoxicol 20:1555–1567. https://doi.org/10.1007/s10646-011-0715-0. Accessed 26 Jul 2021
Murphy GW, Newcomb TJ, Orth DJ (2007) Sexual and seasonal variations of mercury in smallmouth bass. J Freshw Ecol 22:135–143. https://doi.org/10.1080/02705060.2007.9664153. Accessed 13 Oct 2021
Myers GJ, Davidson PW (1998) Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect 106:841–847. https://doi.org/10.1289/ehp.98106841. Accessed 21 Mar 2019
Myrbo A, Swain EB, Johnson NW, Engstrom DR, Pastor J, Dewey B, Monson P, Brenner J, Shore MD, Peters EB (2017) Increase in nutrients, mercury, and methylmercury as a consequence of elevated sulfate reduction to sulfide in experimental wetland mesocosms. J of Geophys Res Biogeosci 122:2769–2785. https://doi.org/10.1002/2017JG003788. Accessed 19 Sep 2018
Ogle DH (2019) FSAdata: Fisheries stock analysis, datasets. R package version 0.3.8.
Ogle DH (2016) Introductory fisheries Analyses with R, 1st Ed., Chapman and Hall/CRC. 337. https://doi.org/10.1201/9781315371986 Accessed 15 Mar 2019
Ogle DH, Doll JC, Wheeler P, Dinno A (2021) FSA: Fisheries stock analysis. R package version 0.9.1. https://github.com/droglenc/FSA. Accessed 13 Oct 2021
Park J-D, Zheng W (2012) Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health 45:344–352. https://doi.org/10.3961/jpmph.2012.45.6.344. Accessed 18 Mar 2019
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 18 Mar 2021
Rossi R, Murari A, Gaudio P, Gelfusa M (2020) Upgrading model selection criteria with goodness of fit tests for practical applications. Entropy 22:447. https://doi.org/10.3390/e22040447. Accessed 13 Oct 2021
Rustagi N, Singh R (2010) Mercury and health care. Indian J Occup Environ Med 14:45–48. https://doi.org/10.4103/0019-5278.72240. Accessed 18 Mar 2019
Rypel AL (2010) Mercury concentrations in lentic fish populations related to ecosystem and watershed characteristics. Ambio 39:14–19. https://doi.org/10.1007/s13280-009-0001-z. Accessed 20 Oct 2018
Scudder BC, Chasar LC, DeWeese RL, Brigham ME, Wentz DA, Brumbaugh WG (2008) Procedures for collecting and processing aquatic invertebrates and fish for analysis of mercury as part of the National Water-Quality Assessment Program. USGS Open-File Report No. 2008–1208, 1–46. https://pubs.usgs.gov/of/2008/1208/pdf/ofr20081208.pdf. Accessed 25 Mar 2019
Scudder BC, Chasar LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, Krabbenhoft DP (2009) Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. USGS, Scientific Investigation Report 2009-5109, 1-74. https://pubs.usgs.gov/sir/2009/5109/pdf/sir20095109.pdf. Accessed 9 Sep 2018
Shao D, Kang Y, Wu S, Wong MH (2012) Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments. Sci Total Environ 424:331–336. https://doi.org/10.1016/j.scitotenv.2011.09.042. Accessed 18 Mar 2019
Simoneau M, Lucotte M, Garceau S, Laliberté D (2005) Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res 98:73–82. https://doi.org/10.1016/j.envres.2004.08.002. Accessed 20 Oct 2018
Smith Jr LL, Krefting LW, Butler RL (1952) Movements of marked Walleyes, Stizostedion vitreum vitreum (Mitchill), in the fishery of the Red Lakes, Minnesota. Trans Am Fish Soc 81:179–196
Snodgrass JW, Jagoe CH, Bryan Jr AL, Brant HA, Burger J (2000) Effects of trophic status and wetland morphology, hydroperiod, and water chemistry on mercury concentrations in fish. Can J Fish Aquat Sci 57:171–180. https://doi.org/10.1139/cjfas-57-1-171. Accessed 29 Mar 2019
Strandberg U, Bhavsar S, Parmar TP, Arts M (2018) Spatial and length-dependent variation of the risks and benefits of consuming Walleye (Sander vitreus). Environ Int 112:251–260. https://doi.org/10.1016/j.envint.2017.12.029
United States Environmental Protection Agency (US EPA) (2019) 2019 Toxics Release Inventory National Analysis. US EPA. https://www.epa.gov/trinationalanalysis/introduction-2019-tri-national-analysis. Accessed 13 Oct 2021
United States Environmental Protection Agency (US EPA) (2015) Clean Air Act Text. US EPA. https://www.epa.gov/clean-air-act-overview/clean-air-act-text. Accessed 18 Mar 19
United States Environmental Protection Agency (US EPA) (2007) Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry. Method 7472, US EPA. https://www.epa.gov/sites/production/files/2015-07/documents/epa-7473.pdf. Accessed 25 Mar 2019
United States Environmental Protection Agency (US EPA (2001) Water Quality Criterion for the Protection of Human Health: Methylmercury. Office of Science and Technology, EPA-823-R-01-001, Washington, DC. Accessed 13 Oct 2021. http://www.epa.gov/waterscience/criteria/methylmercury/
United States Environmental Protection Agency (US EPA) (2000) Quality assurance project plan for analytical control and assessment activities in the national study of chemical residues in lake fish tissue. Report No. EPA-823-R-02-006.
United States Environmental Protection Agency (US EPA) (1997) Mercury study report to congress (Executive Summary No. EPA-452/R-97-003). US EPA. https://www3.epa.gov/airtoxics/112nmerc/volume1.pdf. Accessed 21 Mar 2019
Wang B, Zhong S, Bishop K, Nilsson MB, Hu H, Eklof K, Bravo AG, Akerblom S, Bjorn E, Skyllberg U (2021) Biogeochemical influences on net methylmercury formation proxies along a peatland chronosequence. Geochimica et Cosmochimica Acta 308:188–203. https://doi.org/10.1016/j.gca.2021.06.010. Accessed 29 Jan 2021
Wickham H, François R, Henry L, Müller K (2021) dplyr: a grammar of data manipulation. R package version 1.0.7. https://CRAN.R-project.org/package=dplyr
Xue J, Zartarian V, Mintz B, Weber M, Bailey K, Geller A (2015) Modeling tribal exposures to methyl mercury from fish consumption. Sci Total Environ 533:102–109. https://doi.org/10.1016/j.scitotenv.2015.06.070. Accessed 3 Aug 2021
Acknowledgements
A special thank you to Pat Brown, Herb Mountain, and Kevin Spears at the RL DNR and Tony Kennedy and his staff at the MN DNR, Bemidji Area Fisheries Office for allowing me to collect portions of Walleye samples for this study. Special thank you to Emily Rapp, intern and temporary RL DNR employee, Ola Cobenais and Jennifer Malinski (RL DNR) for assisting in the data collection and recording efforts. Bemidji State University for providing me the opportunity to obtain my Masters in Science degree. Mark Brigham (USGS) for finding equipment for my research and providing valuable feedback throughout this study. Dr. Jeff Jeremiason, Gustavus Adolfus College in St. Peter, MN, for training of what I could expect to see throughout my sample analysis. There were no additional funds received throughout the development of this manuscript.
Author information
Authors and Affiliations
Contributions
The research study and manuscript was prepared by the primary author, TO, as part of his Master’s research project. The co-authors supplied suggestions and edits throughout the research and formation of the manuscript before the first submission. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Red Lake Band of Chippewa Indians and Bemidji State University before any data was collected. Fish samples were obtain through experimental gill net assessments for fish population size. All fish within this study were euthanized in accordance to Red Lake Fisheries SOPs, however, this study did not require an ethics approval by any participating party.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Orgon, T.J., Hafs, A.W., Isaacson, C.W. et al. Spatial and temporal variability of mercury in Upper and Lower Red Lake Walleye. Ecotoxicology 32, 811–823 (2023). https://doi.org/10.1007/s10646-023-02689-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02689-w