Abstract
Hexavalent chromium (Cr (VI)) is widely distributed in the marine environment of Hainan Province, China and poses a potential threat to its mangrove ecosystems. However, the mechanisms underlying Cr-induced stress and reproductive toxicity in clams remain largely unknown. In this study, the clams, Geloina erosa, were exposed to 4.34, 8.69, 17.38 and 34.76 mg/L Cr (VI) for 24, 48 and 72 h. The gonad-somatic index (GSI) was determined and histological alterations of the ovaries were quantified by light microscopy. The micronucleus test was performed which quantifies the genotoxic presence of small cytoplasmic bodies in eukaryotic cells. Enzymatic assays for catalase (CAT), glutathione reductase (GR), and malondialdehyde (MDA) activities were done. Quantitative real-time PCR (qRT-PCR) was used to quantify the expression of glutathione-S-transferase (GST), heat shock protein 70 (HSP70) and vitellogenin (Vtg) in ovaries of G. erosa. The results showed that the micronucleus frequency was significantly increased when clams were exposed to Cr (VI). Cr (VI) exposure induced the accumulation of MDA and affected CAT and GR enzyme activities. The high Cr (VI) concentration of 34.76 mg/L significantly increased the levels of GR activity, GST expression and HSP70 expression and inhibited Vtg expression and CAT activity. MDA content was significantly increased after 72 h at the high Cr (VI) exposure (34.76 mg/L). Therefore, Cr (VI) exposure may be toxic to the development of ovaries of G. erosa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, marine environmental pollution has become increasingly serious in China, especially for heavy metals. The concentrations of copper (Cu), cadmium (Cd) and chromium (Cr) found in edible shellfish in some marine locales in China exceed the known ecological thresholds of toxicity (Cheng et al. 2016). Huang et al. (2005) found that the concentrations of Cr and Pb in the surficial sediments at Dapeng Bay and Pearl River Estuary in Shenzhen exceeded the standard level (GB3097-1997: The standard level of Cr (VI) is Cr (VI) ≤ 0.005 mg/L). Mangroves are important sinks for toxic compounds because of their capacity for retaining various harmful substances. In mangrove areas, Cr contamination by human activities threatens the health of mangrove ecosystems (Shi et al. 2019). A previous study of sediments in Wenchang, Hainan Province, China, reported Cr levels as high as 109 mg/kg, a concentration that might affect local sediment dwelling biota (Vane et al. 2009). Another investigation of contamination at the same location in Wenchang reported a concentration of 54.83 mg/kg of Cr in the surface sediments of the mangrove (Ye et al. 2014).
Chromium can accumulate to elevated levels in benthic marine organisms (Rittschof and McClellan-Green 2005). Chromium exists in the environment as two valence states: hexavalent Cr (VI) and trivalent Cr (III). Cr (III) is generally recognized as nontoxic or may pose potential risk (Fan et al. 2015). In contrast, with its strong oxidative capacity, Cr (VI) can produce reactive oxygen species (ROS) and free radicals that can cause lipid peroxidation and induce oxidative stress which further damages organs (Lushchak 2011; Chen et al. 2015). Some previous studies have indicated that the toxic mechanism of Cr (VI) is genotoxic (Thompson et al. 2012; Yang et al. 2020). The direct heavy metal exposure of gametes showed the adverse effects on the reproduction of bivalves, including decreases of energy storage availability for reproduction (Smolders et al. 2004; Voets et al. 2006; Weng and Wang (2019)). Cr (VI) exposure can reduce recruitment to marine bivalve populations in mangrove areas due to reproductive toxicity.
Geloina erosa is a widely distributed clam species in intertidal and estuarine areas of tropical and subtropical mangroves. In China, G. erosa is mainly found in Hainan, Guangxi, and Guangdong provinces where it is the main economic shellfish species harvested by local residents because of its large size, rapid growth, and availability (Cai et al. 1995). Because of their ubiquity, sedentary life style, and bioaccumulation, bivalve mollusks are excellent bioindicator species (Kimbrough et al. 2008; Apeti et al. 2010; Venier et al. 2011). There have been several reports on the biological and ecological characteristics of G. erosa (Hiong et al. 2004; Cuong et al. 2005; Clemente and Ingole 2009, 2011), however few studies have focused on their response to heavy metals exposures. In 2011, Lai et al. (2011) studied the effects of Cd exposure on antioxidant enzymes, digestive enzymes and malondialdehyde (MDA) content of G. erosa. Mo et al. (2015) screened the internal reference gene (β-actin) of G. erosa by Quantitative Real-time PCR under polychlorinated biphenyls exposure. Finally, Xing et al. (2017) studied the response of oxidative stress biomarkers of G. erosa to short chain chlorinated paraffin exposures.
Biomarkers are common tools used in environmental quality evaluation and risk assessment, since they can provide the early molecular and cellular responses to environmental disturbance (Zhang et al. 2010; Gueguen et al. 2017). In this study, G. erosa was used as the test organism for the assessment of the effect of Cr (VI) exposure on its reproduction. G. erosa clams were exposed to five different doses of Cr (VI) and the following assays were conducted to evaluate their responses. The micronucleus test was performed to evaluate the genotoxicity of Cr (VI) exposure. Oxidative stress biomarkers including catalase (CAT), glutathione reductase (GR), and glutathione-S-transferase (GST) activities were investigated in the ovaries of G. erosa. MDA content, heat shock protein 70 (HSP70) expression and vitellogenin (Vtg) expression in ovaries of G. erosa were evaluated. Histological changes were evaluated and the gonad-somatic index quantified under different doses of Cr (VI) exposure. The results will provide valuable data on the reproductive toxicity of Cr (VI) to bivalve mollusks in mangrove ecosystems and offer a reference for future studies in mangrove environmental monitoring.
Materials and methods
Treatments and exposure of clams
G. erosa clams were collected from Bamen Bay, Wenchang City, Hainan Province (110°47′38.84″E, 19°37′28.02″N). Mature females of similar sizes were selected for the experiments. The average fresh weight of individuals was 32.19 ± 6.37 g/individual (mean ± SD) and the average shell height was 32.15 ± 2.33 mm (mean ± SD). Clams were acclimated in plastic tanks (980 × 760 × 680 mm) containing artificial seawater (1 L/clam) for 5 days. The salinity of the seawater was 15 parts per thousand (ppt), the temperature was maintained at 26 ± 1 °C and the clams were fed with dried algal powder. Before the formal experiment, we carried out the pre-acute toxicology experiment and measured the 96-h LC50 of Cr (VI) in G. erosa (69.52 mg/L).
A stock solution of 1000 mg/L of pure K2Cr2O7 (XNK, China) was prepared and then diluted to five different doses. Based on the results of the preliminary acute toxicity test, groups of female G. erosa were separately exposed for 24 h, 48 h and 72 h to 4.34, 8.69, 17.38 and 34.76 mg/L of Cr (VI) (corresponding to 1/16, 1/8, 1/4 and 1/2 of the 96-h LC50).
To investigate the effect of Cr (VI) on the development of G. erosa ovaries, we designed the first experiment to analyze GSI and histopathological observation. In the first experiment, we designed three Cr (VI) concentration groups (0, 4.34, 34.76 mg/L) and 30 G. erosa individual were exposed in each Cr (VI) concentration group. Ten G. erosa (GSI measurement, n = 5; histopathological observation, n = 5) were sampled at 72 h for each Cr (VI) concentration group. Based on the results of the first experiment, we designed the second experiment for the detection of micronucleus, enzyme activity and gene expression. In the second experiment, we designed five Cr (VI) concentration groups (0, 4.34, 8.69, 17.38, 34.76 mg/l) with three biological replicates for each concentration group, and 18 G. erosa were exposed in each biological replicate. Samples were taken at 24 h, 48 h, and 72 h in each biological replicate for each Cr (VI) concentration.
GSI and histopathological observation
Ovarian tissue samples were collected. The GSI was expressed as wet gonad weight/wet soft tissue weight * 100%. Ovarian tissues were immediately placed in Bouin’s solution (saturated picric acid: 75 parts, 37–40% formaldehyde: 25 parts and glacial acetic acid: 5 parts) for histopathological observation. Samples for light microscopy were soaked in Bouin’s for 18 h, dehydrated with ethanol, embedded in paraffin, sliced into 4 µm thick slices, and stained with Ehrlich’s haematoxylin and eosin (H&E) and were observed with light microscopy (Olympus IX71).
Micronucleus test
Micronucleus tests were performed according to the methodology of Kumar et al. (2010) with slight modifications. A drop of blood was used to prepare smears which were fixed with methanol for 10 min. The smears were air-dried at room temperature and further stained with diluted Giemsa stain for 30 min and 1000 erythrocytes from each clam were counted from the experimental and control groups for each concentration and duration of exposure.
Extraction of oxidative stress biomarkers and measurement of relative enzyme activity
Assays for CAT, GR, and MDA were performed using kits provided by Nanjing Jiancheng Bioengineering Institute (Catalase assay kit, A007; glutathion reductases assay kit, A062; malondialdehyde assay kit, A003). Assays were conducted as instructed by the manufacturer. The ovarian tissues were homogenized (1:10, w/v) in ice-cold buffer and the pH was adjusted to 7.60 (50 mM phosphate buffer, 1 mM DDT, 1 mM EDTA and 150 mM KCl). Homogenates were centrifuged at 10,000 × g for 20 min (4 °C) to obtain the supernatants which were stored at −80 °C. CAT activity was measured at 405 nm and expressed as U (mg wet weight) −1. GR activity was measured at 340 nm and expressed as U (g wet weight) −1. The concentrations of MDA content were measured at 532 nm and expressed as nmol (mg wet weight) −1.
Quantitative real-time PCR analysis of biomarkers
The quantitative real-time polymerase chain reaction (qRT-PCR) was used to quantify gene expression of GST, HSP70 and Vtg in ovaries of G. erosa. Total RNA was extracted from the samples using RNAiso Plus (Takara Corp, Dalian, China), according to the manufacturer’s instructions. The concentrations and purities of the isolated RNA were assessed using a NanoDrop 2000 (Thermo Fisher Scientific, USA). RNA was then reverse-transcribed to cDNA using PrimeScript™ RT reagent kits (Takara, Japan) following the manufacturer’s instructions. All primers were designed using Primer Premier 5.0 software and are presented in Table 1. In this study, β-actin was used as an internal reference gene. An ABI 7500 HT Real-time Detection System (Applied Biosystems, USA) was used in qRT-PCR along with SperReal Premix (SYBR Green) (TIANGEN, China). The whole reaction system consisted of 5 μL SYBR Premix Ex TaqTM, 0.4 μL (10 μmol/L) of the forward and reverse primers, 1 μL cDNA template diluted by 10 times, and 3.2 μL of RNase free H2O. qRT-PCR reaction conditions were: an initial denaturation at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 60 °C for 30 s, 72 °C for 30 s. The melting curve analysis was performed. After the qRT-PCR was completed, data were analyzed with ABI 7900HT SDS software 2.3 (Applied Biosystems, USA). Data normalization with the 2−△△CT method was conducted with the internal reference β-actin gene (Livak and Schmittgen 2001). A value of 100 for the normalized control group means 100%, this has been set to 1.0 (Fig. 5).
Data analysis
The results were presented as arithmetical mean ± standard deviation (SD). Data were analyzed with one-way analysis of variance (ANOVA) and least significant difference (LSD) using SPSS software (version 17.0).
Results
G. erosa reproductive development affected by Cr (VI) exposure
The condition of G. erosa gonads after 72 h of Cr (VI) exposure was quantitatively evaluated as GSI, and the results are shown in (Fig. 1). Compared with the control group, the GSI of G. erosa initially declined at 4.34 mg/L of Cr (VI) exposure and showed a significant decrease at 34.76 mg/L of Cr (VI) exposure.
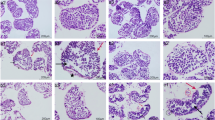
Figure 2 shows the negative histological effect of Cr (VI) exposure on ovaries of G. erosa as evidenced by the higher incidence of hemocyte aggregates with the higher Cr (VI) concentrations (Fig. 2B, C). Evidence of histopathological atretic oocytes are indicated by the black arrows (Fig. 2B–D).
Light micrographs of G. erosa ovary sections stained with hematoxylin/eosin. Atretic oocytes marked with black arrows. A Ovary section of an individual exposed to 0 mg l−1 Cr (VI) for 72 h. B Ovary section of an individual exposed to 4.34 mg l−1 Cr (VI) for 72 h. C Ovary section of an individual exposed to 34.76 mg l−1 Cr (VI) for 72 h. D Atretic oocytes. O oocyte, OF ovarian follicle, H hemocyte
Micronucleus frequency
The mean value of cell micronucleus permillage of G. erosa were significantly increased after 24 h Cr (VI) exposures at all doses. After 48 h exposures, the mean value of cell micronucleus permillage was 3.72 ± 0.03 when treated with 34.76 mg/L Cr (VI) and showed a significant effect compared to the control. After 72 h exposures, the mean value of cell micronucleus permillage was 3.75 ± 0.02 when treated with 4.34 mg/L and 5.6 ± 0.20 when treated with 34.76 mg/L. After different exposure times, the greatest effect was observed when clams were treated with 34.76 mg/L. The results are shown in (Fig. 3).
Results of micronucleus test in ovarian tissues of G. erosa exposed to four different doses of Cr (VI) for (A) 24 h, (B) 48 h, and (C) 72 h. Results are shown as means ± S.D. Different letters a, b, c, d signify effect of treatment at the same time interval with ‘a’ being significantly higher than ‘b’, ‘c’ as well as ‘d’ and ‘b’ being significantly higher than ‘c’ as well as ‘d’ and ‘c’ being significantly higher than ‘d’. (p < 0.05)
Biochemical biomarkers affected by Cr (VI) exposure
CAT activity in ovaries of G. erosa was significantly depressed after 24 h Cr (VI) exposure at all doses. However, after 48 h exposures, the activity of CAT in the 4.34 mg/L Cr (VI) group showed no effect compared to the control group. The lowest CAT activity was recorded as 33.84 ± 6.60 U (mg wet weight) −1 for the ovaries exposed to 34.76 mg L−1 of Cr (VI) for 24 h (Fig. 4A). The activities of GR were up-regulated responding to the different Cr (VI) exposure (Fig. 4B). The highest value of GR activity was recorded as 18.49 ± 2.22 U (mg wet weight) −1 at 34.76 mg/L Cr (VI) at 48 h. The MDA content remained unaltered after 24 h of Cr (VI) exposures compared to the control groups, but MDA content showed an upward trend with longer exposures and increasing concentrations and reached a maximum value of 534.21 ± 42.74 nmol (mg wet weight) −1 at 34.76 mg/L Cr (VI) at 48 h.
A comparison of the effects of four different doses of Cr (VI) exposure on (A) CAT activity, (B) GR activity, and (C) malondialdehyde content compared to negative controls in G. erosa ovaries over three different times (24, 48, and 72 h). Data are presented as mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001
After Cr (VI) exposure, the mRNA expressions of GST and HSP70 were increased in G. erosa while the transcriptional expression of Vtg was suppressed (Fig. 5). GST expression was significantly increased and reached the maximum value after 48 h at 34.76 mg/L Cr (VI) exposure, which was almost 3 times higher than the control. Expression of HSP70 was induced in response to Cr (VI) exposures, and it expression reached the highest level at 34.76 mg/L Cr (VI) after 72 h, which was almost 3 times higher than the control. Expression of Vtg increased in the control group over time, while Vtg expression exhibited a negative association with increasing concentrations of Cr (VI) exposure. Vtg expression exhibited a minimum value after 72 h at an Cr (VI) level of 34.76 mg/L, that level was almost 4 times lower than that of the control.
Discussion
In this study, Geloina erosa clams were exposed to four different doses of Cr (VI) for three different times (24, 48, and 72 h) and a variety of biomarkers responses from the molecular through the histopathological were assessed. For example, with increasing Cr (VI) concentrations, the frequency of micronuclei increased, which may affect the growth and development of G. erosa ovum. The micronucleus test is used to evaluate the genotoxic potential of pollutants under field and laboratory conditions (Canedo et al. 2021). In the micronucleus test, micronucleated cell frequency is taken as evidence of genotoxic and cytotoxic damage. In this study, cell micronucleus permillage were considered and the maximum effect was observed when clams were treated with 34.76 mg/L of Cr (VI). Similarly, a study performed by Osman et al. (2011) revealed the occurrence of nuclear lesions along with micronuclei in Nile tilapia Oreochromis niloticus niloticus and African catfish Clarias gariepinus in river water with high heavy metal concentrations (Pb, Cr, Mn, Cd, Hg, Fe, Zn, and Cu).
The higher concentration of Cr (VI) and the lower GSI of G. erosa were consistent with the histopathological observations. The lower GSI indicated that Cr (VI) exposure could reduce recruitment to G. erosa populations in mangrove areas. Cr (VI) can cause inhibition of sperm production, changes in ultrastructure of gonads, leakage of cell junctions, damage of germ cells and reduction in gamete motility (Marouani et al. 2012). From previous studies, Cr (VI) could alter the expression of germ cell-related genes (Schulz et al. 2009) and reproductive function (Chen et al. 2016). Our histopathology results suggested that Cr (VI) negatively impacted the hemocyte aggregations and by elevating the frequency of atretic oocytes in ovaries of G. erosa. Many other anthropogenic pollutants and ocean acidification induced this phenomenon in bivalves such as Mytilus galloprovincialis and Adamussium colbecki (González-Fernández et al. 2016; Dell’Acqua et al. 2019). Abnormal hemocyte aggregation is an important biomarker in the affected tissues that is induced in response to external stress of metal contamination (Dorange and Le Pennec 1989). It can also be induced by parasite infections, nutritional deficits and organic pollutants (Donaghy et al. 2009; Ortiz-Zarragoitia and Cajaraville 2010; Cuevas et al. 2015). The direct heavy metal exposure of gametes showed the potential adverse effects on the reproduction of bivalves (Smolders et al. 2004; Voets et al. 2006; Weng and Wang 2019).
In this study, the change of CAT activity in response to low concentrations of Cr (VI) exposure suggested that this change was reversible, which was similar to a previous study of mercury-exposed goldfish (Carassius auratus) (Kong et al. 2012). In Mytilus galloprovincialis mussels exposed to sublethal concentrations of Cr (VI), it has been demonstrated that Cr (VI) inhibits CAT activity, which is consistent with our results (Ciacci et al. 2012; Franzellitti et al. 2012). Cr (VI) as a variable valence metal can easily enter cells through cell membranes, producing reactive oxygen species (ROS) and free radicals causing oxidative stress effects in organisms (Lushchak 2011; Chen et al. 2015). CAT is an important antioxidant enzyme that can scavenge oxygen free radicals and protect organisms. After Cr (VI) is absorbed by organisms, the production of free radicals will be significantly increased. As the main antioxidant enzyme in the first line of defense of the antioxidant system, CAT is one of the earliest antioxidant enzymes to participate in detoxification reactions. SOD decomposes superoxide anion radical (O2-) into H2O2 and O2, and CAT decomposes H2O2 into H2O and O2, thus reducing the concentration of H2O2 in organisms. CAT activity was also decreased in the bivalve Chlamys farreri exposed to lead and mercury (Zhang et al. 2010).
The activities of GR were upregulated in response to the different Cr (VI) exposures in G. erosa. GR is a critical molecule in resisting oxidative stress and maintaining cell integrity that has been used as a biomarker of marine contamination in bivalves (Cheung et al. 2001; Manduzio et al. 2004). Similar to our results, PAHs (Polycyclic aromatic hydrocarbons) contamination increased the levels of GR activity and inhibited CAT activity in Mytilus galloprovincialis during the reproductive stage (González-Fernández et al. 2016).
The MDA content of G. erosa remained unaltered after 24 h of Cr (VI) exposures compared to the control groups, but MDA content increased with longer exposures and higher concentrations and reached a maximum value at 34.76 mg/L Cr (VI) at 48 h. Commonly, changes in antioxidant enzymes activities are associated with significant changes in MDA. MDA is a lipid peroxidation product and has a strong destructive effect on cell membranes. MDA content can reflect the production of free radicals in organisms. Bebianno et al. (2005) suggested that high content of MDA was the result of the loss of antioxidant defense in organisms. After Cr (VI) exposure, the MDA content in tissues of goldfish Carassius auratus was increased with damaged the function of its liver and kidneys (Velma and Tchounwou 2013). A significant and delayed accumulation of MDA content was reported in zebrafish Danio rerio after Cr (VI) metal exposure (Yin et al. 2018) and the MDA content was observed significantly increased in the lead-exposed group (Zhang et al. 2010), which were similar to our results.
The significant increases of GST with dose that we observed indicated that they were involved in protecting against the deleterious effects of Cr (VI) exposure in G. erosa. GST has the effects of detoxification and inhibition of lipid peroxidation. GST mainly exists in cell fluid and is the second line of defense of the antioxidant system (Gueguen et al. 2017). GST not only participates in free radical scavenging, but also scavenges lipid peroxides. In addition, it can prevent covalent binding between exogenous chemicals and biological macromolecules and plays an antidotal role (Li 2013). It was reported that elevated levels of GST were induced by Cr (VI) to combat toxicity in ovaries of Japanese medaka (Oryzias latipes) (Chen et al. 2016).
HSP70 is a cytosolic protein that is induced in response to several external stressors such as heat stress, metal contamination, and bacterial infection in bivalve mollusks (Gupta et al. 2010; Taylor et al. 2013). In this study, HSP70 was up-regulated after Cr (VI) exposure, which indicated that Cr (VI) can induce the immune and apoptosis activities of G. erosa as an external stress. This result was consistent with our previous transcriptome analysis (Wang et al. 2020). Commonly, HSP70 expression is up-regulated with low concentrations of metal exposures in bivalves (Taylor et al. 2013; de Boissel et al. 2017). For example, Cr (VI) exposure induced small but significant increases in the transcriptional level of HSP70 at 1 and 10 μg L−1 Cr (VI) in the gills of female Mytilus galloprovincialis (Ciacci et al. 2012).
After Cr (VI) exposure, the mRNA expression of Vtg was decreased in G. erosa. The reduced expression of Vtg indicated that Cr (VI) exposure affected the expression of hormone-regulated gene and caused obvious reproductive toxicity in female G. erosa. Vtg is the vitellin precursor in almost all oviparous animals. Vtg levels is used as an indicator of estrogenic exposure and heavy metals exposures in bivalves (Falfushynska et al. 2013).
Conclusion
Cr (VI) exposure induced histological changes of hemocyte aggregations and elevations of atretic oocytes in ovaries of G. erosa, accompanied by antitoxic and antioxidant molecular responses. The significant changes in antioxidant enzyme activities and significant induction of HSP70 mRNA by Cr (VI) exposure indicated that antioxidant enzymes and HSP70 were involved in protecting against the deleterious effects of Cr (VI). The reduction in GSI, increase in the number of micronucleus cells, accumulation of MDA content and inhibition of Vtg levels in G. erosa ovaries suggested that lipid peroxidation was induced by Cr (VI) exposure and caused ovarian tissue damage. The reproductive toxicity of Cr (VI) might affect gametes directly and may reduce recruitment to G. erosa populations. Our results provided new information to guide future investigations to assess the toxic effects of hexavalent chromium on marine invertebrates and suggested the possible use of G. erosa as an informative indicator species of contamination of mangrove ecosystems.
Data availability
All data generated or analyzed during this study are included in this article.
References
Apeti DA, Lauenstein GG, Christensen JD, Kimbrough K, Johnson WE, Kennedy M, Grant KG (2010) A historical assessment of coastal contamination in Birch Harbor, Maine based on the analysis of mussels collected in the 1940s and the Mussel Watch Program. Mar Pollut Bull 60:732–742. https://doi.org/10.1016/j.marpolbul.2009.11.021
Bebianno MJ, Company R, Serafim A, Camus L, Cosson RP, Fiala-Médoni A (2005) Antioxidant systems and lipid peroxidation in Bathymodiolus azoricus from Mid-Atlantic Ridge hydrothermal vent fields. Aquat Toxicol 75:354–373. https://doi.org/10.1016/j.aquatox.2005.08.013
Cai Y, Huang X, Wu D (1995) Studies on the ecology of Polymesoda erosa (Solander). J Trop Oceanogr 14:94–98
Canedo A, de Jesus LWO, Bailão EFLC, Rocha TL (2021) Micronucleus test and nuclear abnormality assay in zebrafish (Danio rerio): Past, present, and future trends. Environ Pollut 290:118019. https://doi.org/10.1016/j.envpol.2021.118019
Chen HX, Wu X, Bi R, Li LX, Gao M, Li D, Xie LT (2015) Mechanisms of Cr (VI) toxicity to fish in aquatic environment: a review. Chin J Appl Ecol 26:3226–3234. https://doi.org/10.13287/j.1001-9332.20150921.031
Chen H, Cao J, Li L, Wu X, Bi R, Klerks PL, Xie L (2016) Maternal transfer and reproductive effects of Cr (VI) in Japanese medaka (Oryzias latipes) under acute and chronic exposures. Aquat Toxicol 171:59–68. https://doi.org/10.1016/j.aquatox.2015.12.011
Cheng J, Zhang X, Zhuo Q, Liu T, Tang Z (2016) Accumulation and health risks of heavy metals in edible marine shellfishes from China. Chin J Food Hyg 28:175–181. https://doi.org/10.13590/j.cjfh.2016.02.008
Cheung CC, Zheng GJ, Li AM, Richardson BJ, Lam PK (2001) Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat Toxicol 52:189–203. https://doi.org/10.1016/S0166-445X(00)00145-4
Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D’Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L (2012) Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquat Toxicol 120-121:109–118. https://doi.org/10.1016/j.aquatox.2012.04.015
Clemente S, Ingole B (2009) Gametogenic development and spawning of the mud clam, Polymesoda erosa (Solander, 1876) at Chorao Island, Goa. Mar Biol Res 5:109–121. https://doi.org/10.1080/17451000802317709
Clemente S, Ingole B (2011) Recruitment of mud clam Polymesoda erosa (Solander, 1876) in a mangrove habitat of Chorao Island, Goa. Braz J Oceanogr 59:153–162. https://doi.org/10.1590/s1679-87592011000200004
Cuevas N, Zorita I, Costa PM, Franco J, Larreta J (2015) Development of histopathological indices in the digestive gland and gonad of mussels: Integration with contamination levels and effects of confounding factors. Aquat Toxicol 162:152–164. https://doi.org/10.1016/j.aquatox.2015.03.011
Cuong DT, Bayen S, Wurl O, Subramanian K, Wong KKS, Sivasothi N, Obbard JP (2005) Heavy metal contamination in mangrove habitats of Singapore. Mar Pollut Bull 50:1732–1738. https://doi.org/10.1016/j.marpolbul.2005.09.008
de Boissel PGJ, Fournier M, Rodriguez-Lecompte JC, McKenna P, Kibenge F, Siah A (2017) Functional and molecular responses of the blue mussel Mytilus edulis’ hemocytes exposed to cadmium—an in vitro model and transcriptomic approach. Fish Shellfish Immunol 67:575–585. https://doi.org/10.1016/j.fsi.2017.06.001
Dell’Acqua O, Ferrando S, Chiantore M, Asnaghi V (2019) The impact of ocean acidification on the gonads of three key Antarctic benthic macroinvertebrates. Aquat Toxicol 210:19–29. https://doi.org/10.1016/j.aquatox.2019.02.012
Donaghy L, Lambert C, Choi KS, Soudant P (2009) Hemocytes of the carpet shell clam (Ruditapes decussatus) and the Manila clam (Ruditapes philippinarum): Current knowledge and future prospects. Aquaculture 297:10–24. https://doi.org/10.1016/j.aquaculture.2009.09.003
Dorange G, Le Pennec M (1989) Utrastructural study of oogenesis and oocytic degeneration in Pecten maximus from the Bay of St. Brieuc. Mar Biol 103:339–348. https://doi.org/10.1007/BF00397268
Falfushynska HI, Gnatyshyna LL, Stoliar OB (2013) Effect of in situ exposure history on the molecular responses of freshwater bivalve Anodonta anatina (Unionidae) to trace metals. Ecotoxicol Environ Saf 89:73–83. https://doi.org/10.1016/j.ecoenv.2012.11.024
Fan WT, Zhao XN, Cheng J, Liu YH, Liu JZ (2015) Oxidative stress and hepatocellular injury induced by oral administration of Cr3+ in chicken. J Biochem Mol Toxicol 29:280–287. https://doi.org/10.1002/jbt.21697
Franzellitti S, Viarengo A, Dinelli E, Fabbri E (2012) Molecular and cellular effects induced by hexavalent chromium in Mediterranean mussels. Aquat Toxicol 124-125:125–132. https://doi.org/10.1016/j.aquatox.2012.07.011
González-Fernández C, Albentosa M, Campillo JA, Viñas L, Franco A, Bellas J (2016) Effect of mussel reproductive status on biomarker responses to PAHs: Implications for large-scale monitoring programs. Aquat Toxicol 177:380–394. https://doi.org/10.1016/j.aquatox.2016.06.012
Gueguen Y, Denis S, Adrien S, Kevin M, Pierre G, Solène B, Marine N, Patrick B, Herehia H, Serge P, Gilles LM (2017) Response of the pearl oyster Pinctada margaritifera to cadmium and chromium: Identification of molecular biomarkers. Mar Pollut Bull 118:420–426. https://doi.org/10.1016/j.marpolbul.2017.03.012
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384. https://doi.org/10.1016/j.lfs.2009.12.015
Hiong KC, Peh WYX, Loong AM, Wong WP, Chew SF, Ip YK (2004) Exposure to air, but not seawater, increases the glutamine content and the glutamine synthetase activity in the marsh clam Polymesoda expansa. J Exp Biol 207:4605–4614. https://doi.org/10.1242/jeb.01334
Huang X, Zhang S, Huo Z (2005) Heavy metal distribution in seawater in Dapeng Bay of Shenzhen and Pearl River estuary. Transactions Oceanol Limnol 4:38–44. https://doi.org/10.13984/j.cnki.cn37-1141.2005.04.006
Kimbrough KL, Johnson WE, Lauenstein GG, Christensen JD, Apeti DA (2008) An assessment of two decades of contaminant monitoring in the Nation’s Coastal Zone. NOAA Technical Memorandum NOS NCCOS 74:1–105
Kong X, Wang S, Jiang H, Nie G, Li X (2012) Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat Toxicol 120–121:119–125. https://doi.org/10.1016/j.aquatox.2012.05.005
Kumar R, Nagpure NS, Kushwaha B, Srivastava SK, Lakra WS (2010) Investigation of the genotoxicity of malathion to freshwater teleost fish Channa punctatus (Bloch) using the micronucleus test and comet assay. Arch Environ Contam Toxicol 58:123–130. https://doi.org/10.1007/s00244-009-9354-3
Lai TH, He BY, Fan HQ, Zhou RQ, Yang Y (2011) Effects of cadmium stress on the activities of antioxidant enzymes, digestive enzymes and the membrane lipid peroxidation of the mangrove mud clam Geloina coaxans (Gmelin). Acta Ecol Sin 31:3044–3053
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Li Y (2013) The effects of three typical organic pollutants on Carassius auratus using antioxidant defense system. Dissertation, Nanjing University
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Manduzio H, Monsinjon T, Galap C, Leboulenger F, Rocher B (2004) Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: Major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquat Toxicol 70:83–93. https://doi.org/10.1016/j.aquatox.2004.07.003
Marouani N, Tebourbi O, Mahjoub S, Yacoubi MT, Sakly M, Benkhalifa M, Rhouma KB (2012) Effects of hexavalent chromium on reproductive functions of male adult rats. Reprod Biol 12:119–133. https://doi.org/10.1016/s1642-431x(12)60081-3
Mo Z, Che Z, Liao S, Yang M, Yan B (2015) Reference gene selection for qRT-PCR in mangrove mud clam (Polymesoda erosa) under PCBs stress. Guangxi Sci 22:350–356. https://doi.org/10.13656/j.cnki.gxkx.20150401.003
Ortiz-Zarragoitia M, Cajaraville MP (2010) Intersex and oocyte atresia in a mussel population from the Biosphere’s Reserve of Urdaibai (Bay of Biscay). Ecotoxicol Environ Saf 73:693–701. https://doi.org/10.1016/j.ecoenv.2010.04.002
Osman AGM, Abd El Reheem AM, Moustafa MA, Mahmoud UM, Abuel-Fadl KY, Kloas W (2011) In situ evaluation of the genotoxic potential of the river Nile: I. Micronucleus and nuclear lesion tests of erythrocytes of Oreochromis niloticus niloticus (Linnaeus, 1758) and Clarias gariepinus (Burchell, 1822). Toxicol Environ Chem 93:1002–1017. https://doi.org/10.1080/02772248.2011.564496
Rittschof D, McClellan-Green P (2005) Molluscs as multidisciplinary models in environment toxicology. Mar Pollut Bull 50:369–373. https://doi.org/10.1016/j.marpolbul.2005.02.008
Schulz RW, de França LR, Lareyre JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, Miura T (2009) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411. https://doi.org/10.1016/j.ygcen.2009.02.013
Shi C, Ding H, Zan Q, Li R (2019) Spatial variation and ecological risk assessment of heavy metals in mangrove sediments across China. Mar Pollut Bull 143:115–124. https://doi.org/10.1016/j.marpolbul.2019.04.043
Smolders R, Bervoets L, De Coen W, Blust R (2004) Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ Pollut 129:99–112. https://doi.org/10.1016/j.envpol.2003.09.027
Taylor DA, Thompson EL, Nair SV, Raftos DA (2013) Differential effects of metal contamination on the transcript expression of immune- and stress-response genes in the Sydney Rock oyster, Saccostrea glomerata. Environ Pollut 178:65–71. https://doi.org/10.1016/j.envpol.2013.02.027
Thompson CM, Fedorov Y, Brown DD, Suh M, Proctor DM, Kuriakose L, Haws LC, Harris MA (2012) Assessment of Cr (VI)-induced cytotoxicity and genotoxicity using high content analysis. PLoS ONE 7:e42720. https://doi.org/10.1371/journal.pone.0042720
Vane CH, Harrison I, Kim AW, Moss-Hayes V, Vickers BP, Hong K (2009) Organic and metal contamination in surface mangrove sediments of South China. Mar Pollut Bull 58:134–144. https://doi.org/10.1016/j.marpolbul.2008.09.024
Velma V, Tchounwou PB (2013) Oxidative stress and DNA damage induced by chromium in liver and kidney of goldfish, Carassius auratus. Biomark Insights 8:43–51. https://doi.org/10.4137/BMI.S11456
Venier P, Varotto L, Rosani U, Millino C, Celegato B, Bernante F, Lanfranchi G, Novoa B, Roch P, Figueras A, Pallavicini A (2011) Insights into the innate immunity of the Mediterranean mussel Mytilus galloprovincialis. BMC Genomics 12:69. https://doi.org/10.1186/1471-2164-12-69
Voets J, Talloen W, de Tender T, van Dongen S, Covaci A, Blust R, Bervoets L (2006) Microcontaminant accumulation, physiological condition and bilateral asymmetry in zebra mussels (Dreissena polymorpha) from clean and contaminated surface waters. Aquat Toxicol 79:213–225. https://doi.org/10.1016/j.aquatox.2006.06.001
Wang G, Zhang C, Huang B (2020) Transcriptome analysis and histopathological observations of Geloina erosa gills upon Cr (VI) exposure. Comp Biochem Physiol C Toxicol Pharmacol 231:108706. https://doi.org/10.1016/j.cbpc.2020.108706
Weng N, Wang WX (2019) Seasonal fluctuations of metal bioaccumulation and reproductive health of local oyster populations in a large contaminated estuary. Environ Pollut 250:175–185. https://doi.org/10.1016/j.envpol.2019.04.019
Xing Y, Nong Y, Lu Y, Yang M, Yan B (2017) Response characteristics of oxidative stress biomarkers of Polymesoda erosa to exposure of SCCPs. China Environ Sci 37:3962–3971
Yang Q, Han B, Xue J, Lv Y, Li S, Liu Y, Wu P, Wang X, Zhang Z (2020) Hexavalent chromium induces mitochondrial dynamics disorder in rat liver by inhibiting AMPK/PGC-1α signaling pathway. Environ Pollut 265:114855. https://doi.org/10.1016/j.envpol.2020.114855
Ye X, Wang P, Li X, Wang J, Zhao Z (2014) A study on enrichment of heavy metals in the surface sediments of mangrove wetland from northeast Hainan Island. Ecol Environ 30:318–321,328
Yin J, Wang AP, Li WF, Shi R, Jin HT, Wei JF (2018) Time-response characteristic and potential biomarker identification of heavy metal induced toxicity in zebrafish. Fish Shellfish Immunol 72:309–317. https://doi.org/10.1016/j.fsi.2017.10.047
Zhang Y, Song J, Yuan H, Xu Y, He Z, Duan L (2010) Biomarker responses in the bivalve (Chlamys farreri) to exposure of the environmentally relevant concentrations of lead, mercury, copper. Environ Toxicol Pharmacol 30:19–25. https://doi.org/10.1016/j.etap.2010.03.008
Funding
This study was supported by Science and Technology Basic Resources Investigation Program of China (Project Number: 2017FY100703), Outstanding young teachers program of the State Ministry of Education (Project Number: 002007007), Global Environmental Facility Study on degradation mechanism of mangrove wetland in Dongzhai harbor, Hainan province (Project Number: 41176084), Midwestern construction projects in Hainan University (ZXBJH-XK006).
Author information
Authors and Affiliations
Contributions
HM: Investigation, Formal analysis, Visualization, Revision. GW: Investigation, Writing—original draft, Formal analysis, Visualization. XF: Investigation, Revision. SC: Writing—original draft. JW: Investigation, Revision. BH: Conceptualization, Revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The animal experimental processes were approved by the Ethnic Committee of Hainan University and conducted in strict accordance to the standard of the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of the People’s Republic of China in 2006.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, H., Wang, G., Huang, B. et al. Effect of hexavalent chromium exposure on the reproductive status and biomarker responses of female Geloina erosa. Ecotoxicology 32, 736–745 (2023). https://doi.org/10.1007/s10646-023-02668-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02668-1