Abstract
Restrictions on second-generation anticoagulant rodenticides (SGARs) in the United States, which were partially implemented in 2011, prohibit the sale of SGAR products through general consumer outlets to minimize use by non-professional or non-agricultural applicators. This study analyzed liver tissue from four species of birds of prey admitted to a wildlife clinic in Massachusetts, USA, from 2012–2016 for residues of anticoagulant rodenticides (ARs). Ninety-four birds were analyzed; 16 were symptomatic for AR toxicosis, and 78 asymptomatic. Ninety-six percent of all birds tested were positive for SGARs: 100% of those diagnosed with AR toxicosis ante-mortem and/or post-mortem and 95% of subclinically exposed birds. Brodifacoum was found in 95% of all birds. Sixty-six percent of all birds contained residues of two or more SGARs. A significant increase in exposures to multiple SGARs occurred in later years in the study. Pesticide use reports (PURs) filed with the Massachusetts Department of Agricultural Resources were reviewed to determine the frequency of use of different ARs by pest management professionals (PMPs) across five years. This study finds that the three SGARs favored by PMPs—bromadiolone, difethialone, brodifacoum—were present in combination in the majority of birds, with increases in multiple exposures driven by increased detections of bromadiolone and difethialone. Continued monitoring of AR residues in nontarget species following full implementation of sales and packaging restrictions in the US is needed in order to elucidate the role of PMP use of SGARs in wildlife exposures and to evaluate the effectiveness of current mitigation measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anticoagulant rodenticides (ARs) interfere with blood clotting by inhibiting the enzyme vitamin K epoxide reductase, which functions to activate vitamin K. This inhibition results in the accumulation of an inactive form of vitamin K, which in turn is unable to activate the vitamin-K dependent clotting factors. The depletion of these activated clotting factors causes a coagulopathy and hemorrhage.
Exposure to and mortality from ARs in free-living birds of prey is well documented over many years in multiple countries (Hegdal and Colvin 1988; Stone et al. 2003; Berny and Gaillet 2008; Walker et al. 2008; Albert et al. 2010; Murray 2011; Langford et al. 2013; Stansley et al. 2014; Huang et al. 2016; Justice-Allen and Loyd 2017). Signs of toxicosis in free-living birds of prey have been described (Stone et al. 1999; Murray and Tseng 2008; Murray 2011). The US Environmental Protection Agency (EPA) has determined that second-generation ARs (SGARs), which are more potent and have longer half lives in comparison to first-generation ARs (FGARs), present a significant risk to wildlife through both primary and secondary exposures (Erickson and Urban 2004).
To address the risk of SGARs to nontarget wildlife, in 2008 the US EPA issued the risk mitigation decision for ten rodenticides (RMD) disallowing the sale of the SGARs brodifacoum, bromadiolone, difenacoum, and difethialone through general consumer retail outlets, effective June 2011 (US Environmental Protection Agency 2008). The RMD allows the sale of the FGARs diphacinone and chlorophacinone on the general consumer market, as FGARs are thought to pose less risk to nontarget species (Erickson and Urban 2004). The decision allows continued use of SGARs by pest management professionals (PMPs) and agricultural users. Other users may still potentially purchase SGARs outside of general retail outlets—though packaging requirements are designed to discourage such use (US Environmental Protection Agency 2008)—with the exception of residents of the state of California, which has designated SGARs as restricted pesticides (California Department of Pesticide Regulation 2013).
Full compliance with the RMD was not achieved in June 2011 due to one manufacturer, Reckitt Benckiser (RB), refusing to remove SGAR-containing products from the general consumer market. Reckitt Benckiser retained product registrations for the SGARs brodifacoum and difethialone through 2014 (US Environmental Protection Agency 2014). However, other AR manufacturers brought replacement products to the market in June 2011. Along with FGARs, the neurotoxic agent bromethalin is allowed for sale through general consumer retail outlets. Discontinuation of the distribution of SGARs by RB to general consumer outlets occurred in March 2015 under an agreement between the company and the US EPA (US Environmental Protection Agency 2014).
Along with less convenient availability of SGARs to general consumers and bait station requirements, the potential effectiveness of the US EPA regulations in protecting wildlife species further depends on the supposition that use of SGARs by PMPs will be a component of an integrated pest management (IPM) strategy. An IPM approach should decrease reliance on chemical rodenticides and therefore result in less risk of exposure to and toxicosis from SGARs in wildlife species (US Environmental Protection Agency 2008). However, a 2015 survey of PMPs in Massachusetts revealed that 97% of respondents use chemical rodenticides more than half of the time as part of an IPM approach to rodent control, raising the question of whether PMP use of SGARs may still be a source of significant exposure in non-target species (Memmott et al. 2017). Moreover, this survey found that respondents had a low level of knowledge regarding potency and half lives of ARs, and 50% of respondents indicated a neutral or low level of concern regarding the effects of ARs on wildlife, including birds of prey (Memmott et al. 2017).
The objective of this study is to evaluate four species of free-living birds of prey (red-tailed hawks [Buteo jamaicensis], barred owls [Strix varia], eastern screech-owls [Megascops asio], and great horned owls [Bubo virginianus]) admitted to a wildlife clinic in Massachusetts, USA, for AR residues both in birds diagnosed with AR toxicosis and in those that showed no clinical signs of coagulopathy. Additionally, a sampling of pesticide use reports (PURs) filed with the Massachusetts Department of Agriculture, Division of Crop and Pest Services, were reviewed to determine the frequencies of use of specific rodenticides by PMPs in the state in order to evaluate the extent to which residues of ARs favored by PMPs are present in these birds of prey.
Methods
Birds included in study and diagnosis of anticoagulant rodenticide toxicosis
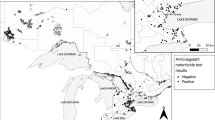
Birds included in this study are free-living red-tailed hawks (RTHAs), barred owls (BDOWs), eastern screech-owls (EASOs), and great horned owls (GHOWs) that were admitted to the Wildlife Clinic at Cummings School of Veterinary Medicine at Tufts University (CSVM) in North Grafton, Massachusetts, from late 2012 to early 2016. Locations of recoveries at the county level were examined to provide an indication of the predominant type of land use in the areas from which the birds were recovered.
Of the 16 birds diagnosed with AR toxicosis, ten were admitted to the clinic alive and were administered appropriate treatment (Murray and Tseng 2008) but died despite therapy; three others that were admitted alive were humanely euthanized due to grave prognoses and/or concurrent injuries; and three were recovered alive but were dead on arrival to the clinic. All birds diagnosed with AR toxicosis within the period of sampling were analyzed for residues of ARs. Subclinically exposed birds were sampled on an opportunistic basis, when birds from the included species died or were humanely euthanized, and were randomly chosen for analysis within each species group. These do not represent all mortalities among these species within the sampling period. These birds died soon after admission, were humanely euthanized due to the severity of the presenting injury or illness, or were dead on arrival.
Humane euthanasia was performed by intravenous injection of a veterinary euthanasia solution containing pentobarbital sodium and phenytoin sodium in cases in which the bird’s condition indicated a grave prognosis for survival or precluded release to the wild. No birds were euthanized for the purpose of this study. No institutional animal care and use protocol was required due to the study’s utilization of cadavers only for tissue sampling. Medical treatment was directed by wildlife veterinarians. Rehabilitation of birds of prey at Tufts Wildlife Clinic is conducted under appropriate and state and federal permits.
Gross post-mortem examinations were performed on all birds by a wildlife veterinarian board certified in avian medicine, and a cause of death was assigned when possible. Cause of death categories other than AR toxicosis include trauma, infectious disease, and unknown. A diagnosis of AR toxicosis was made based on post-mortem findings, in conjunction with ante-mortem signs, when possible. Diagnostic criteria have been described previously (Murray and Tseng 2008; Murray 2011). Briefly, ante-mortem diagnosis of AR toxicosis was based on factors including anemia and hypoproteinemia; delayed clotting as demonstrated by a modified whole blood clotting test; evidence of excessive hemorrhage (e.g., external bleeding, extensive intramuscular or subcutaneous hemorrhage) in the absence of concurrent traumatic injuries such as fractures, severe wounds, or ocular injury; depressed mentation; evidence of cardiovascular shock (pallor of mucous membranes, poor capillary refill time). Post-mortem criteria included evidence of extensive hemorrhage (subcutaneous, intramuscular, pulmonary, visceral, or intracoelomic hemorrhage, pallor of internal organs) without concurrent evidence of corresponding severe trauma. For AR analysis, liver tissue was collected and stored frozen at −17 °C.
Analysis for anticoagulant rodenticides
Analysis of liver tissue for ARs was performed at the California Animal Health and Food Safety Laboratory (Davis, CA). Screening included testing for brodifacoum, bromadiolone, chlorophacinone, difenacoum, difethialone, diphacinone, and warfarin.
Two methods were used for analysis of ARs during the course of this project. In the first method, tissue samples were homogenized in 5% ethanol in ethyl acetate. The extracts were exchanged into hexane, cleaned up using Florisil solid phase extraction, exchanged into methanol and then screened using electrospray HPLC-MS/MS on a Thermo LXQ linear ion trap interfaced to a Waters Acquity HPLC. Extracts containing detectible levels of any of the ARs were re-analyzed for quantitation using HPLC with diode array and fluorescence detection. Analysis of analyte standard solutions at varying levels were used to construct calibration curves for quantitation. In the second method, used from September 2015 onward, tissue samples were homogenized in 10% methanol in acetonitrile, cleaned up using dispersive solid phase extraction, and analyzed by electrospray HPLC-MS/MS on a Thermo Q-Exactive high resolution mass spectrometer interfaced to a Dionex Ultimate 3000 HPLC system. In order to provide calibration curves for accurate quantitation by electrospray LC-MS/MS, control tissue samples were fortified at varying levels and then extracted and analyzed as per this method. Quality control practices for both methods included analysis of extracts of negative control tissue and spiked control tissue samples.
Reporting limits for each AR detected in this study for the earlier and later methods in ppm (wet weight), respectively, are: brodifacoum 0.01, 0.02; bromadiolone 0.05, 0.02; chlorophacinone 0.25, 0.02; difethialone, 0.25, 0.02; diphacinone 0.25, 0.02; difenacoum 0.05, 0.02.
Review of pesticide use reports
The Massachusetts Department of Agricultural Resources (MDAR), Division of Crop and Pest Services, retains yearly PURs filed by all licensed applicators of pesticides for 6 years from the time of filing. The earliest and most current years available for review during the time this manuscript was prepared were 2008 and 2015. Information available from the PURs includes trade name of products employed, major site or crop treated (e.g., structural, turf, cranberry, etc.) and total amount of product used. The PURs do not include information on amount of active ingredient used. Only PURs that denoted structural use of rodenticides were included.
Because the PURs are not in electronic format, a complete review of all reports from each year was not undertaken. To estimate the total number of PMPs using chemical rodenticides within a year, a list of active pesticide license holders in 2015 obtained from MDAR via public records request was examined. License holders for which company names denoted tree, landscape, solely insect-related, or other services clearly unrelated to structural rodent control were excluded, resulting in approximately 1300 individual licenses remaining. However, this number could be an overestimate of the total sampling population due to inability to ascertain whether each of these license holders definitely offered rodent control services or was actively employed in that year.
For each of the years 2008, 2009, 2013, 2014, and 2015, random selections of 100 PURs filed by PMPs employing rodenticides for structural use were reviewed to evaluate the frequencies of use of specific rodenticides by PMPs in each year. The confidence interval estimating a total of 1300 potential license holders per year is less than +/− 0.1. The years 2008 and 2009 were chosen to correspond to years included in prior AR study in birds of prey (Murray 2011); the years 2010–2012 were not evaluated. The paper PURs were filed by the MDAR in no specific order other than by year. Within each year, PURs were selected from the files at random and sorted until 100 reports denoting structural use of chemical rodenticides were obtained.
Statistical analysis
Statistical analysis was performed in IBM SPSS Statistics, version 22. The change in laboratory methods of AR analysis applied to 20 out of the total of 94 samples in this study (eight samples from 2015 and all 12 samples from 2016). This change resulted in lower reporting limits and slightly increased sensitivity in these 20 samples for certain ARs (chlorophacinone, bromadiolone, difethialone, diphacinone, difenacoum). Due to this change in laboratory methods in late 2015, a Fisher’s exact test was used to compare the presence of one or multiple ARs in samples analyzed via the earlier and later methods for 2015 and 2015–2016 (n = 23, n = 20, respectively). No significant difference was found (p = 0.67). As a sensitivity analysis, samples analyzed via the earlier method in the year 2014 were combined with those from 2015 (n = 45) and compared against all samples analyzed via the later method using a Fisher’s exact test. This result also was not significant (p = 0.32). These results indicate that the change in laboratory methods likely did not lead to increased detections of certain ARs in late 2015 and 2016. Therefore, all samples were included in further analysis.
Descriptive statistics were used to evaluate the percentages of birds exposed to all ARs, to multiple ARs, and to specific ARs according to species, cause of death, and year of recovery. Descriptive statistics were also used to evaluate use patterns of specific ARs by PMPs in different years in the study.
A Fisher’s exact test was used to evaluate the presence of one or more than one AR in birds diagnosed with AR toxicosis compared to subclinically exposed birds. A Fisher’s exact test expanded for a 2 × 4 contingency table was used to evaluate whether species differences in exposure to multiple ARs were present. For temporal analysis of presence of multiple ARs, the years 2014–2016 were first examined using a Fisher’s exact test expanded for a 2 × 3 contingency table. The data were then collapsed into two time periods, 2012–2013 and 2014–2016, and compared using a Chi-square test.
A Kruskal–Wallis test was used to compare median brodifacoum liver concentrations among years included in this study and among species, followed by a Dunn procedure for pairwise comparisons where appropriate. As concentrations for bromadiolone, difethialone, and difenacoum were trace amounts below the limits of quantitation for the majority of detections for each, no statistical analyses were performed on concentration levels for these SGARs.
A Kruskal–Wallis test was used to compare cause of death to liver brodifacoum concentrations for each species independently, as species differences in sensitivity to ARs may exist (Petterino and Paolo 2001; Watanabe et al. 2015).
Results
Numbers of birds tested and geographic area of study
A total of 94 birds were tested for ARs: RTHAs n = 37, BDOWs n = 24, GHOWs n = 17, EASOs n = 16. Sixteen birds (14 RTHAs, 2 GHOWs) diagnosed with AR toxicosis and 78 birds without signs of AR toxicosis were analyzed. Numbers of birds tested in each year of the sampling period were as follows: 2012 n = 6; 2013 n = 21; 2014 n = 24; 2015 n = 31; 2016 n = 12. Because of the small number of birds tested in 2012 and as these samples were collected late in the year, samples from 2012 and 2013 were combined for statistical analysis focused on differences among years.
The majority of tested birds (89%) came from five counties: Worcester in central Massachusetts, where CSVM is located (37%), and four counties to the east: Middlesex (32%), Norfolk (10%), Suffolk (6%), and Essex (4%). Sixty-nine percent of tested birds were recovered from two counties, Middlesex and Worcester, which are the two largest counties in Massachusetts by population. The other most represented counties are the state’s next three most populous. These five counties are predominantly suburban to urban and include two major cities: Worcester in Worcester county and Boston in Suffolk county, with Middlesex, Norfolk, and Essex counties holding the western, southern, and northern suburbs of Boston, respectively.
Anticoagulant rodenticides
Of all birds tested, 90 (96%) were positive for ARs (97% of RTHAs, 88% of BDOWs, 100% of GHOWs, 100% of EASOs, Table 1). All birds suspected of suffering from AR toxicosis were positive. Of the 78 asymptomatic birds, 74 (95%) were positive. All positive birds had residues of SGARs. Brodifacoum was found in all positive birds except one (99%), a GHOW which was positive for bromadiolone only. Two birds had residues of FGARs: an EASO with residues of both diphacinone and brodifacoum and a GHOW with residues of chlorophacinone along with brodifacoum, bromadiolone, and difethialone.
The majority of birds across all years (66%) had residues of more than one AR (78% of RTHAs, 42% of BDOWs, 71% of GHOWs, 69% of EASOs). While BDOWs showed the lowest exposure to multiple ARs, differences among species were not significant (p = 0.087). No significant difference in the number of ARs present was found between symptomatic and asymptomatic birds (p = 0.37). Due to this lack of difference in number of ARs between these two groups, symptomatic and asymptomatic birds were combined for analysis of multiple AR exposures per year.
The percentages of birds positive for multiple ARs per year are as follows: 33% in 2012–13; 67% in 2014; 87% in 2015; 83% in 2016 (Fig. 1). Across the years 2014–2016, no significant increase in the presence of multiple ARs occurred (p = 0.48). For the time period 2014–2016, 79% of birds combined were positive for multiple ARs, a statistically significant increase from 2012–13 (p = 0.00).
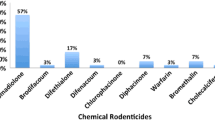
The percentages of positive birds with liver residues of each SGAR per year are shown in Fig. 2. The percentages of positive birds with residues of two, three, or four SGARs, respectively, for all years combined are as follows: 39, 27, 3%. The most frequent combinations of SGARs among positive birds were as follows: brodifacoum, bromadiolone, and difethialone (23%); brodifacoum and difethialone (19%); brodifacoum and bromadiolone (18%).
Range and median brodifacoum concentrations, respectively, in liver tissue (ppm) were as follows: all species combined <0.01–0.90, 0.11; RTHA <0.01–0.56, 0.12; BDOW <0.01–0.39, 0.041; GHOW <0.01–0.90, 0.17; EASO <0.01–0.83, 0.051.
GHOWs had significantly greater brodifacoum concentrations than BDOWs (p = 0.007). No significant differences were found among other species comparisons. No significant increase in brodifacoum concentrations across the years included in this study was found (p = 0.19).
Concentrations of other SGARs detected were trace amounts below the reporting limit for 69% of positive bromadiolone cases (<0.05 ppm, n = 18; <0.02 ppm, n = 11), 81% of positive difethialone cases (<0.25 ppm, n = 22; <0.02 ppm, n = 12), and all difenacoum cases. Range and median concentrations, respectively, among the quantitated cases for bromadiolone and difethialone for all species combined in liver tissue (ppm) are as follows: bromadiolone, 0.058–0.38, 0.13 (n = 13); difethialone, 0.05–0.67, 0.18 (n = 8).
Cause of death and signs of toxicosis
Of the 16 birds diagnosed with AR toxicosis, 13 (81%) had residues of >1 AR. Among RTHAs, which comprised 14 of the 16 toxicosis cases, 87% had residues of >1 AR (Table 2). Only SGARs were present in symptomatic birds, and these were present in the following combinations: brodifacoum and difethialone n = 6; brodifacoum, bromadiolone, and difethialone n = 4; brodifacoum and bromadiolone n = 2; brodifacoum, bromadiolone, difenacoum n = 1. The remaining three birds had residues of brodifacoum only. The concentration range of brodifacoum in liver tissue of symptomatic RTHAs was large: 0.078–0.56 ppm. Of the 12 RTHA toxicosis mortalities that had residues of >1 SGAR, quantitated bromadiolone concentrations ranged from 0.15 to 0.31 ppm (n = 3), and quantitated difethialone concentrations ranged from 0.05 to 0.67 ppm (n = 4).
Cause of death categories among birds that were not diagnosed with AR toxicosis include trauma (78%), infectious/systemic disease (3%), and unknown (19%). No significant differences were found in liver brodifacoum concentrations among all cause of death categories, including AR toxicosis, for each species (for RTHAs, p = 0.13).
Signs of toxicosis observed were similar to those reported in Murray (2011). In 13/13 birds presented alive, dull mentation, pale mucous membranes, and delayed capillary refill time were present. Anemia was documented in 9/9 birds for which a packed cell volume (PCV) was obtained, with PCVs ranging from 7 to 26%. Delayed clotting was seen in 7/7 birds for which a modified whole blood clotting time was performed (Murray and Tseng 2008). Other signs observed ante-mortem included: excessive bleeding from a laceration (n = 6), subcutaneous hemorrhage (n = 6), blood in the oral cavity (n = 2), bleeding from the nares (n = 1), bleeding from the vent (n = 2), hyphema (n = 1), severe periorbital swelling (n = 1). Signs observed on gross post-mortem examination included: pallor of internal organs (n = 9), coelomic hemorrhage (n = 9), pulmonary hemorrhage (n = 7), petechiation or bruising of the sternum (n = 6), hemorrhage into the central nervous system (n = 4), hemorrhage of the female reproductive tract with the presence of an egg (n = 2), hemorrhage of the gastrointestinal tract (n = 2), hemorrhage in air sacs (n = 1), blood pooling in the cloaca (n = 1); hemorrhage into the pectoral muscles (n = 1); hemorrhage into the pericardial cavity (n = 1).
Pesticide use reports
The percentages given here represent the number of reports of each toxicant per 100 PURs that indicated structural use of chemical rodenticides. The majority of reporting PMPs employed more than one type of AR in each year. The non-anticoagulant rodenticides bromethalin and zinc phosphide are included to present a more complete picture of chemical rodenticide use among PMPs (Table 3). In all years evaluated, bromadiolone was the most frequently reported AR. Reports of use of difethialone increased notably from 2008 to 2015, from 14 to 70%, respectively. The year 2015 was also the only year for which reports of use of all three of the most frequently employed SGARs, bromadiolone, brodifacoum, and difethialone, were over 50% for each SGAR.
Discussion
The data presented here demonstrate widespread AR exposure among four species of birds of prey in predominantly suburban and urban areas of Massachusetts, USA, with 96% of tested birds positive for AR residues in liver tissue. Each of the species included in this study showed extensive exposure to SGARs. All 16 birds diagnosed with AR toxicosis based on ante-mortem and/or post-mortem signs were positive for SGARs, and 95% of 78 asymptomatic birds were positive for ARs, mainly SGARs. The percentage of exposed birds in this study represents an increase over prior research in these same four species in the same geographic area in Massachusetts, which found that 86% of 161 birds were positive for SGARs (Murray 2011).
Brodifacoum was the most frequently detected SGAR and was found in all positive birds except one (99%). This finding is consistent with previous research which also found that 99% of positive birds had residues of brodifacoum (Murray 2011). In the United States, two additional published studies demonstrated widespread exposure to ARs, particularly brodifacoum, among certain raptor populations prior 2011, when the US EPA stipulated the removal of SGARs from the general consumer market (Stone et al. 2003; Stansley et al. 2014). In both reports, the SGAR brodifacoum was the most frequently identified compound. Stone et al. (2003) found brodifacoum in 84% of 130 positive birds sampled from 1998 to 2001 in the state of New York. Stansley et al. (2014) found brodifacoum in 93% of 103 positive birds sampled from 2008 to 2010 in the state of New Jersey.
While information on sales of general consumer rodenticide products is not readily available, brodifacoum has been noted to have been the most commonly purchased rodenticide from general retail outlets in the state of California pre-2011 (Krueger et al. 2015). Brodifacoum was still attainable on the general consumer retail market through much of the time period and in the geographic area of this study, allowing the likelihood that nonprofessional use contributed to this high percentage of exposures, particularly given that brodifacoum does not appear to be the most preferred SGAR among PMPs in Massachusetts according to PUR data presented here as well as to survey data (Memmott et al. 2017). If sales in California are representative of other states, brodifacoum is the SGAR most likely to be affected by EPA restrictions on points of sale and packaging in terms of potential decline in nontarget species exposures.
The review of PURs filed by PMPs with the state of Massachusetts reveal that PMPs have consistently favored bromadiolone in the years included in and prior to this study, with consistent but less frequent reports of brodifacoum use. Reports of difethialone use among PMPs, however, steadily increased over the years examined from 14% in 2008 to 70% in 2015. Reports of bromadiolone and brodifacoum use in 2015 were 85 and 60%, respectively, which are higher than other years included (Table 3). These data indicate that the majority of PMPs in Massachusetts used all three of these SGARs in 2015 with greater frequency than in earlier years. Given the limitations inherent in the information available on the PURs, it was not possible to accurately determine relative amounts of each SGAR used (total product or active ingredient) among years. However, the exposure and PUR data presented here show that Massachusetts PMPs employing chemical rodenticides for structural use favor the three SGARs (bromadiolone, difethialone, brodifacoum) found in varying combinations in 65% of tested birds across all years of this study. Of birds tested in 2015, coinciding with the year of highest reported use of each, 81% of birds were exposed to combinations of these three SGARs.
Difenacoum is the newest SGAR on the US PMP market, first registered in 2007 (US Environmental Protection Agency 2008). Reports of use by PMPs in Massachusetts were first detected in 2009 and were infrequent or zero in all subsequent years in the sampling of PURs examined indicating it is currently used by PMPs in Massachusetts far less frequently than other SGARs. However difenacoum was found in 7% of birds in combination with other SGARs, with the majority of detections occurring in 2015 and 2016 (Fig. 2).
A statistically significant increase in multiple SGAR exposures in birds of prey was found for the time period 2014–2016 compared to 2012–2013. This increase was driven by exposures to bromadiolone and difethialone combined with continued high exposure to brodifacoum (Fig. 2). The high percentage of birds overall with residues of more than one SGAR is in marked contrast to the three prior studies in the US. Previously in Massachusetts, less than 2% of positive birds were found to contain residues of more than one SGAR (brodifacoum and difethialone, Murray 2011). In New York, 12% of positive birds were noted to contain brodifacoum and bromadiolone with no other SGARs detected (Stone et al. 2003). In New Jersey, 19% of positive birds had residues of both brodifacoum and bromadiolone, with the SGAR difenacoum being found in one bird, and no other SGARs detected (Stansley et al. 2014).
The reason for the timing of the observed increase in multiple SGAR exposures in this study after 2012–2013 is not entirely clear. However, industry-sponsored US market studies from 2014 to 2016 identify a trend toward increased demand for rodent control services among structural pest control companies in each of these years (Pest Control Technology 2014, 2015, 2016a). This demand is suggested by industry sources to be influenced by increased rodent populations secondary to milder winters resulting in less seasonality to reproductive rates as well as by expansions in urbanized areas (Pest Control Technology 2016a). Additionally, 57% of 528 PMPs surveyed in 2014 and 62% of 285 surveyed in 2015 indicated that they believed the restriction on sale of SGARs through the general consumer market resulted in growth opportunities for their companies’ rodent control services (Pest Control Technology 2014, 2015). Given the data available in this study, it is not possible to correlate the increase in exposures to multiple ARs in birds of prey to use of increased quantities of ARs by PMPs. However, the SGAR exposures reported here in conjunction with the PUR data support the need for further study of PMP chemical rodenticide use practices (Memmott et al. 2017) and the extent to which these practices pose a risk to wildlife species, particularly if various market influences continue to spur growth in demand for professional structural rodent control services.
In previous research in Massachusetts, no exposures to FGARs were detected (Murray 2011). In this present study, chlorophacinone and diphacinone were detected in one GHOW and in one EASO, respectively, with both detections in 2015, in which year reports of use of these FGARs were 22 and 10%, respectively, among PMPs in Massachusetts. These FGARs are allowable for sale through general consumer retail outlets under EPA regulations. While thought to pose less risk of secondary toxicosis than SGARs, the FGARs diphacinone and chlorophacinone have been detected in the livers of raptors (Stone et al. 1999, 2003; Albert et al. 2010). Diphacinone has been demonstrated experimentally to cause secondary toxicosis in golden eagles (Aquila chrysaetos, Savarie et al. 1979) as well as in great horned owls and northern saw-whet owls (Aegolius acadicus, Mendenhall and Pank 1980). Moreover, laboratory studies in American kestrels (Falco sparverius) and EASOs determined that these birds of prey are significantly more sensitive to the toxic effects of diphacinone and chlorophacinone than other avian species previously used to determine lethal doses of FGARs in birds and that the risk to birds of prey from FGAR use may be underestimated (Rattner et al. 2011, 2012, 2014a, 2015; Vyas and Rattner 2012). The question of whether general consumer use of FGARs will result in exposures and mortalities in wildlife is an area requiring continued study.
Red-tailed hawks represent the largest proportion of birds in the study and of birds diagnosed with AR toxicosis, consistent with prior study (Murray 2011). Red-tailed hawks are diurnal birds that readily utilize human-dominated landscapes (Morrison et al. 2016) and are the bird of prey admitted to Tufts Wildlife Clinic in highest numbers each year. Given this study’s reliance on admissions to a wildlife clinic for sampling, the species distribution among AR toxicosis cases cannot be interpreted to reflect relative risk among species, as various behavioral, environmental, and population factors will influence the likelihood of an individual bird being found and transported for veterinary care following injury or illness and will affect these data.
Much is still unknown about factors that contribute to the development of toxicosis among individual birds exposed to ARs (Rattner et al. 2014b; Watanabe et al. 2015; Huang et al. 2016). Consistent with prior study (Murray 2011) and other studies in free-living birds of prey (Stone et al. 1999, 2003; Justice-Allen and Loyd 2017), the range of brodifacoum in liver tissue in birds that showed signs of AR toxicosis was large (0.078–0.56 ppm in RTHAs). Also consistent with prior study, no association between cause of death and liver brodifacoum concentration was found for each species. Interpreting liver brodifacoum concentrations in relation to signs of toxicosis is further complicated by the high percentage of birds in this study exposed to multiple SGARs.
Among birds that died due to AR toxicosis, 81% were exposed to two or more SGARs. A study on AR exposure in barn owls (Tyto alba) in southwestern Canada found an increase in mortalities from AR toxicosis along with an increase in owls exposed to multiple SGARs between two time periods: 2006–2013 and 1992–2003 (Huang et al. 2016). No significant difference in the number of SGARs present in liver tissue in symptomatic vs. asymptomatic birds was found in the study reported here. However, the nature of this study, which does not allow knowledge of the amount and timing of ARs ingested, prevents a full understanding of how exposures to multiple ARs may interact in causing toxicosis. Specifically, it is impossible to know if the exposures to multiple ARs observed here resulted from ingestion of ARs as an acute dose combined within one prey animal or as sequential, chronic exposures via several contaminated prey items. Laboratory studies in rats have found that exposure to brodifacoum increases the level of sensitivity to subsequent exposure to warfarin. This effect is due to brodifacoum causing subclinical but prolonged partial inhibition of vitamin K epoxide reductase, the enzyme affected by ARs (Mosterd and Thijssen 1991). This research in rats suggests the possibility that subsequent exposure to another AR following sublethal exposure to an SGAR may be more likely to result in mortality. Further study is required to determine the effects of sequential exposures and exposures to multiple ARs in birds of prey (Rattner et al. 2012, 2014b).
The ante-mortem and post-mortem findings presented here contribute to a small body of literature describing the effects of ARs on free-living birds of prey exposed in their natural environments (Merson et al. 1984; Hegdal and Colvin 1988; Stone et al. 1999; Murray and Tseng 2008; Murray 2011; Huang et al. 2016; Justice-Allen and Loyd 2017). These reports combined identify subcutaneous hemorrhage, intramuscular hemorrhage, pallor of tissues and/or internal organs, and coelomic hemorrhage as being the most frequent post-mortem lesions observed in free-living birds of prey. Detailed ante-mortem signs in free-living birds of prey are less frequently described (Murray 2011; Murray and Tseng 2008). The findings presented here along with prior research (Murray 2011) support dull mentation, pallor of mucous membranes, anemia, excessive bleeding from a laceration, and subcutaneous and/or intramuscular hemorrhage as commonly observed ante-mortem signs of AR toxicosis in birds of prey suffering secondary toxicosis via natural prey items. These signs are consistent with those observed ante-mortem in birds of prey exposed to FGARs or SGARs by primary or secondary routes in experimental settings (Savarie et al. 1979; Radvanyi et al. 1988; Rattner et al. 2011, 2012, 2014a, 2015; Salim et al. 2014).
As the US EPA has concluded that exposure to SGARs presents a substantial risk to wildlife species warranting certain restrictions on sale and use (US Environmental Protection Agency 2008), the pervasive nature of SGAR exposure reported here is cause for concern, in particular, the increased detections of the SGARs bromadiolone and difethialone compared to earlier study (Murray 2011) and among later years in this study. These findings support the need for additional approaches toward risk mitigation. Strategies beyond restrictions on sale and packaging have been proposed for decreasing risks from SGARs, including examination of use practices, development of educational stewardship programs, and the implementation of an eco-fee, or polluter-pays, system to fund research and other efforts to ameliorate the effects of SGARs on wildlife (Elliott et al. 2016). Areas have been identified in which enhanced education regarding ecologically conscious use of SGARs may be beneficial for PMPs and the general public, which exerts consumer pressure on PMPs (Memmott et al. 2017). Exploration of chemical rodent control that may present less risk to nontarget species, while limited, has been undertaken and requires further study (Damin-Pernik et al. 2016; Pest Control Technology 2016b).
Conclusions
Many aspects of the effects of SGARs on wildlife and ecosystems remain poorly understood or minimally investigated, including exposure pathways under different use scenarios, effects of repeated exposures to the same or multiple ARs, and the persistence of SGARs in ecosystems (Vyas and Rattner 2012; Elliott et al. 2014; Rattner et al. 2014b; Hindmarch and Elliott 2015; Liu et al. 2015; Pitt et al. 2015). Specific limitations of this study include the inability to correlate the exact locations of PMP use of SGARs with specific locations of bird recoveries; the inability to quantify amounts of SGARs used by PMPs across years examined; the inability to determine the potential for SGARs intended for agricultural use or obtained through internet sales to contribute to exposures in birds; and the reliance on birds presented to a wildlife hospital for sampling, which do not fully represent the prevalence of exposure and mortality in the state’s populations of these species.
Despite these limitations, the widespread exposure among birds of prey to SGARs that are frequently used by PMPs, as shown by the PUR data as well as by a survey of PMPs in the state (Memmott et al. 2017), suggests the potential for PMP use of SGARs to be a significant route of exposure. Continued monitoring of at risk nontarget species and examination of SGAR sales and use practices are needed to elucidate the contribution of PMP use of SGARs to exposures and mortalities in wildlife and to evaluate the effectiveness of current mitigation approaches.
References
Albert CA, Wilson LK, Mineau P, Trudeau S, Elliott JE (2010) Anticoagulant rodenticides in three owl species from western Canada, 1988–2003. Arch Environ Contam Toxicol 58:451–459
Berny P, Gaillet J (2008) Acute poisoning of red kites (Milvus milvus) in France: data from the SAGIR network. J Wildl Dis 44:417–426
California Department of Pesticide Regulation (2013) DPR 13-002 Designating brodifacoum, bromadiolone, difenacoum, and difethialone (second generation anticoagulant rodenticide products) as restricted materials. http://www.cdpr.ca.gov/docs/legbills/rulepkgs/13-002/13-002.htm. Accessed 29 April 2017
Damin-Pernik M, Espana B, Besse S, Fourel I, Caruel H, Popowycz F, Benoit E, Lattard V (2016) Development of an ecofriendly anticoagulant rodenticide based on the stereochemistry of difenacoum. Drug Metab Dispos 44:1872–1880
Elliott JE, Hindmarch S, Albert CA, Emery J, Mineau P, Maisonneuve F (2014) Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ Monit Assess 186:895–906
Elliott JE, Rattner BA, Shore RF, Van Den Brink NW (2016) Paying the pipers: Mitigating the impact of anticoagulant rodenticides on predators and scavengers. BioScience 66:401–407
Erickson W, Urban D (2004) Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach. Office of prevention, pesticides, and toxic substances. United States Environmental Protection Agency, Washington, DC, http://www.fwspubs.org/doi/suppl/10.3996/052012-JFWM-042/suppl_file/10.3996_052012-jfwm-042.s4.pdf. Accessed 17 Feb 2017
Hegdal PL, Colvin BA (1988) Potential hazard to eastern screech-owls and other raptors of brodifacoum bait used for vole control in orchards. Environ Toxicol Chem 7:245–260
Hindmarch S, Elliott JE (2015) When owls go to town: the diet of urban barred owls. J Raptor Res 49:66–74
Huang AC, Elliott JE, Hindmarch S, Lee SL, Maisonneuve F, Bowes V, Cheng KM, Martin K (2016) Increased rodenticide exposure rate and risk of toxicosis in barn owls (Tyto alba) from southwestern Canada and linkage with demographic but not genetic factors. Ecotoxicology 25:1061–1071
Justice-Allen A, Loyd KA (2017) Mortality of western burrowing owls (Athene cunicularia hypugaea) associated with brodifacoum exposure. J Wildl Dis 53:165–169
Krueger L, Newton J, Semrow A, Levy L, Nguyen K, Morgan T, Sun S, Sims J, Koenig S, Shaw L, Cummings R (2015) An analysis of the largest publically funded rodent control program in California: orange county mosquito and vector control district’s rodent control program, 2004–2014. Proc Papers Mosq Vector Control Assoc Calif 83:52–56
Langford KH, Reid M, Thomas KV (2013) The occurrence of second generation anticoagulant rodenticides in non-target raptor species in Norway. Sci Total Environ 450–451:205–208
Liu J, Xiong K, Ye X, Zhang J, Yan Y, Ji L (2015) Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida. Chemosphere 135:250–256
Memmott K, Murray M, Rutberg A (2017) Use of anticoagulant rodenticides by pest management professionals in Massachusetts, USA. Ecotoxicology 26:90–96
Mendenhall VM, Pank LF (1980) Secondary poisoning of owls by anticoagulant rodenticides. Wildl Soc Bull 8:311–315
Merson MH, Byers RE, Kaukeinen DE (1984) Residues of the rodenticide brodifacoum in voles and raptors after orchard treatment. J Wildl Manage 48:212–216
Morrison JL, Gottlieb IGW, Pias KE (2016) Spatial distribution and the value of green spaces for urban red-tailed hawks. Urban Ecosyst 19:1373–1388
Mosterd JJ, Thijssen HHW (1991) The long-term effects of the rodenticide, brodifacoum, on blood coagulation and vitamin K metabolism in rats. Br J Pharmacol 104:531–535
Murray M (2011) Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey presented to a wildlife clinic in Massachusetts, 2006–2010. J Zoo Wildl Med 42:88–97
Murray M, Tseng F (2008) Diagnosis and treatment of secondary anticoagulant rodenticide toxicosis in a red-tailed hawk (Buteo jamaicensis). J Avian Med Surg 22:41–46
Pest Control Technology (2014) State of the rodent market. Pest control technology, PCT http://www.pctonline.com/FileUploads/file/State-Rodent-Market.pdf. Accessed 17 Feb 2017
Pest Control Technology (2015) State of the rodent market. Pest control technology, PCT. http://www.pctonline.com/article/exclusive-market-research-december-2015/. Accessed 17 Feb 2017
Pest Control Technology (2016a) State of the rodent market. Pest control technology, PCT. http://www.pctonline.com/fileuploads/file/2016_State_of_the_rodent_market.pdf. Accessed 17 Feb 2017
Pest Control Technology, PCT (2016b) Contrapest rodent control product earns EPA approval. http://www.pctonline.com/article/senestech-contrapest-rodent-product-epa-approval/. Accessed 17 Feb 2017
Petterino C, Paolo B (2001) Toxicology of various anticoagulant rodenticides in animals. Vet Hum Toxicol 43:353–360
Pitt WC, Berentsen AR, Shiels AB, Volker SF, Eisemann JD, Wegmann AS, Howald GR (2015) Non-target species mortality and the measurement of brodifacoum rodenticide residues after a rat (Rattus rattus) eradication on palmyra atoll, tropical pacific. Biol Conserv 185:36–46
Radvanyi A, Weaver P, Massari C, Bird D, Broughtont E (1988) Effects of chlorophacinone on captive kestrels. Bull Environ Contam Toxicol 41:441–448
Rattner BA, Horak KE, Warner SE, Day DD, Meteyer CU, Volker SF, Eisemann JD, Jonston JJ (2011) Acute toxicity, histopathology, and coagulopathy in American kestrels (Falco sparverius) following administration of the rodenticide diphacinone. Environ Toxicol Chem 30:1213–1222
Rattner BA, Horak KE, Lazarus RS, Eisenreich KM, Meteyer CU, Volker SF, Campton CM, Eisemann JD, Johnston JJ (2012) Assessment of toxicity and potential risk of the anticoagulant rodenticide diphacinone using Eastern screech-owls (Megascops asio). Ecotoxicology 21:832–846
Rattner BA, Horak KE, Lazarus RS, Goldade DA, Johnston JJ (2014a) Toxicokinetics and coagulopathy threshold of the rodenticide diphacinone in eastern screech‐owls (Megascops asio). Environ Toxicol Chem 33:74–81
Rattner BA, Horak KE, Lazarus RS, Schultz SL, Knowles S, Abbo BG, Volker SF (2015) Toxicity reference values for chlorophacinone and their application for assessing anticoagulant rodenticide risk to raptors. Ecotoxicology 24:720–734
Rattner BA, Lazarus RS, Elliott JE, Shore RF, van den Brink N (2014b) Adverse outcome pathway and risks of anticoagulant rodenticides to predatory wildlife. Environ Sci Technol 48:8433–8445
Salim H, Noor HM, Hamid, Omar NH, Kasim D, Abidin CMRZ (2014) Secondary poisoning of Captive barn owls, Tyto alba javanica, through feeding with rats poisoned with chlorophacinone and bromadiolone. J Oil Palm Res 26:62–72
Savarie PJ, Hayes DJ, McBride RT, Roberts JD (1979) Efficacy and safety of diphacinone as a predacide. In: Kenaga EE (ed) Avian and mammalian wildlife toxicology. STP 693. American Society for Testing and Materials, Philadelphia, PA, p 69–79
Stansley W, Cummings M, Vudathala D, Murphy LA (2014) Anticoagulant rodenticides in red-tailed hawks, Buteo jamaicensis, and great horned owls, Bubo virginianus, from New Jersey, USA, 2008–2010. Bull Environ Contam Toxicol 92:6–9
Stone WB, Okoniewski JC, Stedelin JR (1999) Poisoning of wildlife with anticoagulant rodenticides in New York. J Wildl Dis 35:187–193
Stone WB, Okoniewski JC, Stedelin JR (2003) Anticoagulant rodenticides and raptors: recent findings from New York, 1998–2001. Bull Environ Contam Toxicol 70:34–40
US Environmental Protection Agency, EPA (2008) Risk mitigation decision for ten rodenticides. USEPA office of prevention, pesticides and toxic substances. http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2006-0955-0764. Accessed 17 Feb 2017
US Environmental Protection Agency, EPA (2014) Product cancellation order for certain rodenticide registrations. Federal register: the daily journal of the United States Government. https://www.federalregister.gov/articles/2014/08/06/2014-18361/product-cancellation-order-for-certain-rodenticide-registrations. Accessed 17 Feb 2017
Vyas NB, Rattner BA (2012) Critique on the use of the standardized avian acute oral toxicity test for first generation anticoagulant rodenticides. Hum Ecol Risk Assess 18:1069–1077
Walker LA, Turk A, Long SM, Wienburg CL, Best J, Shore RF (2008) Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Sci Total Environ 392:93–98
Watanabe KP, Kawata M, Ikenaka Y, Nakayama SM, Ishii C, Darwish wS, Saengtienchai AS, Mizukawa H, Ishizuka M (2015) Cytochrome P450-mediated warfarin sensitivity in avian species: in vitro assays in several birds and in vivo assays in chickens. Environ Toxicol Chem 34:2328–2334
Acknowledgements
Funding for this research was provided by the Ruby Memorial Research Fund, administered by the Cummings School of Veterinary Medicine at Tufts University and supported by numerous generous donors, as well as the Blake-Nuttall Fund, Nuttall Ornithological Club. The author thanks Drs. Robert Poppenga and Adrienne Bautista for assistance with sample analysis, Michael Filigenzi for assistance with sample analysis and manuscript preparation, the Massachusetts Department of Agricultural Resources for assistance with access to public pesticide use records, and Jef C. Taylor for valuable input on this project.
Funding
This study was partially funded by the Nuttall Ornithological Club (grant number N/A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Murray, M. Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey in Massachusetts, USA, 2012–2016, in relation to use of rodenticides by pest management professionals. Ecotoxicology 26, 1041–1050 (2017). https://doi.org/10.1007/s10646-017-1832-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1832-1