Abstract
Residue levels, distribution patterns and ecological risk assessment of OCPs in water and sediment samples collected from two upstream feeding tributaries of the River Chenab, Pakistan were monitored. ΣOCPs levels in water and sediment ranged between 8 and 76 ng L−1 and 17 and 224 ng g−1, respectively. The mean concentration of ΣHCH (hexachlorocyclohexane) was 3.3 ± 3.2 ng L−1 and 8.4 ± 9 ng g−1 for water and sediment samples, respectively, while ΣDDT (dichlorodiphenyltrichloroethane) exhibited the average concentration of 9.07 ± 6.15 ng L−1 and 40.3 ± 26.2 ng g−1 for water and sediment samples, respectively. The concentration of DDT and HCHs in both water and sediment samples were about 80 % of total OCPs and DDTs were the predominant organochlorines in the investigated matrix. DDTs and HCHs in sediment samples posed higher ecotoxicological risk and results were significant when compared with the quality guidelines. Results of the present study should be taken seriously by higher authorities as there is a serious threat to ecological integrities by OCPs exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorines pesticides (OCPs) are imperative groups of organic pollutants which have been of great concern internationally as of their high production, usage and ubiquitous and persistent nature. OCPs are toxic environmental contaminants and their compounds like DDT, HCH, endosulfan and heptachlor are still used extensively in agricultural and industrial sectors of developing countries. Due to the high bio-accumulative potential and toxicity in living organisms OCPs may be serious threat to ecological integrities and human. Such hazardous compounds enter into the freshwater ecosystem via different sources like, domestic and metropolitan effluents, industrial wastewater, agricultural runoff, atmospheric deposition and some other means (Syed et al. 2013a; Zhou et al. 2005).
Long range transport of OCPs could be another possible route of contamination. OCPs become concentrated in different environmental matrix via biogeochemical processes that further deposit on the bottom of sediments. These compounds have a strong affinity with organic matters due to low water solubility and leach to bottom of tributaries in sediments, which act like a sink (Yang et al. 2005). Consequently, the levels and distribution in water and sediments can provide a valued record of pollution in the aquatic ecosystems (Syed and Malik 2011).
In Pakistan, pesticides were introduced in 1954 and thousand stones of agro-chemical pesticides were imported from USA and Europe during 1960–1970 (Ahad et al. 2010). Pesticides (DDT and HCH) manufacturing units were established in 1973 at Lahore district, Punjab province, Pakistan (Syed et al. 2013b). Pesticides imported from other countries and locally manufactured were distributed throughout the country and stored at different strategic locations for further distributions under Plant Protection Provincial Department. After the ban of OCPs usage throughout the globe, manufacturing units were closed and pesticide stores were eradicated but still, Pakistan holds a largest stockpile of out dated pesticides (Eqani et al. 2012). In Pakistan few published reports on OCPs level and distributions are available (Jan et al. 2008; Ahad et al. 2010; Eqani et al. 2011, 2012; Syed and Malik 2011; Syed et al. 2013a, b; Alamdar et al. 2014). Recently, few reports showed the high level of OCP from obsolete pesticide dumping sites (Ahad et al. 2010; Syed et al. 2013b; Alamdar et al. 2014).
Ahad et al. (2010) reported the eighteen obsolete pesticide dumping sites in populated cities of Pakistan. Two obsolete pesticide dumping sites are located in the catchment area (Simbrial and Wazirabad) of the study area, Gujranwala division, Punjab Province, Pakistan. Two tributaries (Nullah Aik and Nullah Palkhu) of the River Chenab pass from Sialkot, Simbrial and Wazirabad, which receive a huge efflux of industrial and urban wastewater along with chemical runoff from pesticide dumping stores. The stretch of about 203 km along these tributaries is purely agricultural belt where wheat and rice is cultivated and traded to the other parts of the country. These food crops are irrigated with the wastewater of Nullah Aik and Nullah Palkhu throughout agricultural belt along these Nullahs. To the best of our knowledge there is no reported study to assess the levels, distribution and risks of OCPs from water and sediments of these Nullahs (stream locally called [as] Nullah). On the basis of these observations the current study was aimed to assess the residue level, distribution pattern of OCPs and risk assessment to ecological integrities by OCPs in water and sediments from two tributaries of river Chenab, Pakistan.
Materials and methods
Sampling strategy
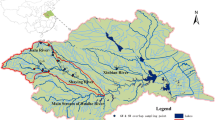
Water and sediment samples were collected during January–March 2013 from fourteen sampling sites located along Nullah Aik and Palkhu, tributaries of River Chenab, Punjab, Pakistan. Twelve sites (six on each Nullah) were located on Nullah Aik and Palkhu while two sites were located on River Chenab. Study area was divided into three zones; up-stream zone containing sites S8, S9, S10 and S11, mid-stream zone containing sites S6, S7, S12 and S13 and down-stream zone containing sites S1, S2, S3, S4, S5 and S14. Figure 1 describes the detail map of study area showing the locations of each site of allocated zone. This zonation was based on the origin of Nullahs and their gradual drain towards the River Chenab. Up-stream zone was purely rural and agricultural area where Nullahs receive rural sewage/wastewater and no major anthropological activity was seen. Mid-stream zone was the urban and industrial area of Sialkot district where Nullahs receive a huge efflux of municipal, industrial and urban wastewater. Down-stream zone is located in urban and peri-urban areas. Two obsolete pesticide dumping sites (Simbrial and Wazirabad) are also located in the catchment area of mid-stream and down-stream of Nullah Aik and Palkhu.
The criteria for site selection was based upon the anthropogenic activities across the study area, varying in habitat, presence or absence of solid and pesticides dumping sites.
Water sampling
Water samples (n = 28) were collected from 14 selected sites of Nullah Aik and Palkhu, during January–March 2013. Each sample was the composite of five sub-samples, collected from a depth of 2–3 m below the top surface of water in 5 L pre-cleaned (washed with acetone) sampling jars. After collection samples were placed in ice containing cooler and transferred immediately to the Environmental Biology and Ecotoxicology Laboratory, Quaid-I-Azam University, Islamabad, Pakistan. At laboratory water samples was filtered with glass wool to remove debris and other small particles and finally stored in −8 °C until further analysis.
Sediment sampling
Composite sediment samples (n = 28) were collected by combining 4–5 subsamples from the bottom of Nullahs. From each site, stretch of 500 m across both banks of Nullahs was marked for sample collection. Samples were stored in polythene bags, labeled and transported to the Environmental Biology and Ecotoxicology Laboratory, Quaid-I-Azam University, Islamabad, Pakistan. Samples were freeze dried, sieved with 2 mm sieve and transported to the State Key Laboratories of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, China where stored in freezer until further analysis.
Extraction of sediment samples
About 20 g of sediment was Soxhlet-extracted for 24 h with dichloromethane (DCM). A mixture of decachlorobiphenyl (PCB 209) and 2,4,5,6-tetrachloro-m-xylene (TCmX) was added as surrogate standard in each sample prior to extraction. For the removal of elemental sulfur, activated copper granules were added in the collection flasks. The extract was concentrated and the solvent phase was changed from DCM to hexane through rotary evaporator.
Extraction of water samples
Water samples were extracted through liquid–liquid extraction in separating funnel. 1 L of filtered water was mixed with the 25–35 mL of DCM and shacked vigorously for 2–4 min and allowed to settle down for 10 min to get two layers. Lower transparent layer of organic solvent containing pollutants was collected on anhydrous Na2SO4 and a mixture of PCB 209 and TCmX was added as surrogate standard in each sample. The extract was concentrated via rotary evaporator and the solvent phase was changed from DCM to hexane.
Clean-up procedure
Sediment samples were washed with H2SO4 prior to column clean up. For column clean-up silica alumina column was used. Silica gel, anhydrous Na2SO4 and alumina were soxhelt extracted with DCM for 48 h and finally backed at 450 °C for 6 h before using. The volume of samples fraction was reduced to 0.2 mL under the gentle nitrogen (high purified) stream after adding 30 μL of iso-octane as solvent keeper. PCB-54 was added as internal standard in each sample prior to GC–MS analysis.
Gas-chromatography-mass spectrometry analysis
OCPs including DDTs, HCHs, hexachlorobenzene (HCB), heptachlor, chlordane, mirex and endosulfan were detected in water and sediment samples via gas chromatography and electron ionization mass spectrometry (GC-EI-MS). CP-Sil 8 CB. 50 m, 0.25 mm, 0.25 µm column was used to detect OCPs by GC-EI-MS (Varian). Temperature of injector was 250 °C. Initially, temperature of oven was set at 150 °C for 3 min and then temperature raised up to 290 °C at the rate of 4 °C min−1 and held for 10 min. Isomers of OCPs were identified with three fragment ions with electron impact spectrometry in the selected ion monitoring (SIM) mode. MSD source temperature was 230 °C and quadruple temperature was 150 °C.
Quality control and quality assurance (QC/QA)
Quality control procedure was strictly followed for the all samples to ensure the quality of results. Calibration standards were used daily for instruments calibration. All the chemicals used during the experimentation were of analytical grade and purchased from Merck (Germany). Standards for PCBs were purchased from Dr. Ehrenstorpher GmbH, Germany. Field, procedural and solvent blanks were analyzed by the similar methodology, adopted for original samples. Glassware was double washed with distilled water and backed at 450 °C for more than 6 h. Agilent MSD Productivity Chemstationsoftware was used for data processing and acquirement. Detection limits were estimated as three times the standard deviation of the blank. Surrogate recoveries in all samples for TCmX ranged between 50 and 67 % and average recovery for PCB-PCB-209 was 76 %.
Statistical analysis
Statistica software (version 5.5) was used for basic descriptive statistics and one way ANOVA (P > 0.05). Arc-GIS software (version 9.3) was used for distribution pattern of OCPs from respective sites of the study area after sites identifications.
Results and discussion
OCP levels in sediments
Descriptive statistical values for studied OCPs in sediment samples are presented in Table 1. ΣOCP concentration was ranged between 16.6 and 224 ng g−1 with mean concentration of 95.5 ± 67 ng g−1. Among studied OCPs higher level of ΣDDT (sum of p,p′-DDT, o,p′-DDT, p,p′-DDD, o,p′-DDD, p,p′-DDE and o,p′-DDE) was found with 77 % of total OCP which showed a concentration of 40.3 ± 26.2 ng g−1, followed by the ΣHCH (8.4 ± 9.2 ng g−1), cis-chlordane (1.72 ± 2.65 ng g−1) and HCB (0.81 ± 1.26 ng g−1). Among DDTs and HCH compositional analysis showed that p,p′-DDT, o,p′-DDT, p,p′-DDD, p,p′-DDE and α-HCH were the dominant compounds which accounted 32, 17, 13, 10 and 7 % of contamination load, respectively. Among DDT isomers, order appeared as; p,p′-DDT>, o,p′-DDT>, p,p′-DDE>, p,p′-DDD>, o,p′-DDD>, o,p′-DDE. For HCH isomers, order appeared as; α-HCH> β-HCH> γ-HCH> δ-HCH. Results showed a significant difference (P > 0.05) for OCPs concentrations in up, mid and down streams (SI Table 1). The ΣOCPs level in sediment samples from up-stream, mid-stream and down-stream was ranged between 24–69.6, 70–186 and 40.3–207 ng g−1. The order for OCPs contamination among sampling zone appeared as mid-stream > down-stream > up-stream (Fig. 2).
Results of the present study were similar in OCP distribution pattern to the previously reported study by Alamdar et al. (2014) and Ahad et al. (2010). They reported the higher level of DDT from surrounding soil and sediments of different locations near the obsolete pesticides stores from Pakistan. Contamination level of ΣDDT and ΣHCH from the rural, urban/industrial and peri-urban areas of study area in the current study was found higher than those previously reported studies conducted on different areas of Pakistan (Bano and Siddique 1991; Ali and Jabbar 1991). The results of current study are in accordance or slightly lower than the previously reported data on sediments samples of down-stream of river Chenab (Eqani et al. 2011) and sediment samples collected from obsolete pesticides dumping site near Lahore city, Pakistan (Syed et al. 2013b). Compared with the OCPs contamination level of sediment samples from India and Vietnam reported by Minh et al. (2006), the present study showed relatively higher concentration of OCPs. When compared with other parts of the world the present study showed the higher or some-what similar pattern of DDT and HCH (Chen et al. 2006; Hu et al. 2009, 2010; Yang et al. 2013). Comparison of current study with previously reported data is given in Table 2.
OCP levels in water
Mean concentrations and basic descriptive statistical values of OCPs studied in water samples of two tributaries (Nullah Aik and Palkhu), River Chenab, Pakistan are presented in Table 1. ΣOCPs concentrations ranged between 7.68 and 76.4 ng L−1 with the mean concentration of 30.7 ± 21.2 ng L−1. This concentration is lower than the previously reported value of OCP from down-stream water samples of River Chenab, Pakistan (Eqani et al. 2011). The current study also reported the lower concentration of OCPs than the previously reported concentration for water (Table 2) of the Jinjiang River, Yangtze River,Huihe River, China, the Giomti River, India and the Kucuk Menderes River, Turkey (Yang et al. 2013; Feng et al. 2011; Singh et al. 2005; Turgut 2003; Tang et al. 2008). Among OCPs components, DDTs were the dominant with 49 %, followed by the HCB 21 %, ΣHCH (18 %), ΣEndosufan (08 %) and heptachlor. The compositional analysis between ΣDDT and ΣHCH showed that ΣDDT exhibited higher contamination load as compared to the ΣHCH and the results of one way ANOVA were also found significant for these isomers (P > 0.05). Among HCHs, α-HCH was the dominant isomer with mean concentration of 1.61 ng L−1 followed by the γ-HCH (0.85 ng L−1), β-HCH (0.77 ng L−1). Among DDTs, p,p′-DDE was the dominant with mean concentration of 2.93 ng L−1 followed by the p,p′-DDD (2.26 ng L−1), o,p′-DDT (2.01 ng L−1) and p,p′-DDT (1.29 ng L−1). Among sampling zones up, mid and down-stream zone exhibited 13.1–27.2, 22.5–46.6 and 14–53.2 ng L−1, respectively. A significant difference (P > 0.05) was observed among sampling zones and the order appeared as mid-stream > down-stream > up-stream.
Distribution pattern of OCPs
Figures 3 and 4 reflected the distribution pattern of OCPs in investigated matrix from each site of allocated zones of Nullah Aik and Palkhu, tributaries of River Chenab, Pakistan. The visual inspection of results (Figs. 3, 4) revealed that the OCP contamination load is higher at site 6, 12 and 13, located in mid-stream zone compared with the down and up-stream zone. Mid-stream zone is located in Sialkot city that it is an industrial area and one obsolete pesticide dumping site is located in Simbrial (Ahad et al. 2010) about 15–20 km far from these sites. Furthermore, site 2, 3, 4 and 14 also exhibited higher OCPs contamination load and these sites are also in vicinity of Wazirabad obsolete pesticide dumping sites (Ahad et al. 2010). Down-stream was located in the peri-urban and industrial area of study area. Up-stream zone was purely agricultural area where OCPs concentration was found relatively low compared to the mid and down-stream zones. Same results were concluded in the previously published reports on OCP level from pesticides dumping sites of Hyderabad and Lahore, Pakistan (Alamdar et al. 2014; Syed et al. 2013b).
The distribution pattern of OCPs level in water samples from each sampling site of study area is presented in Fig. 4. Site 6, 12 and 13 located in mid-stream zone exhibited higher OCPs contamination load. Site 1 showed the highest contamination load among all the sampling sites and it was located on the River Chenab. Draining of wastewater, dumping sites along the banks, agricultural run-off and foliar spray on crops may be the reason of high OCP contamination load in River water. The distribution pattern of OCPs in water samples was similar to the sediments distribution pattern in study area. Urban fraction was observed in both water and sediment samples and high contamination load was found in industrial and urban sampling sites. OCPs have a good fascination and affinity to organic matters due to the low water solubility and leaching to the sediments as they act like a sink for these compounds (Yang et al. 2005), this may be the reason of low contamination load in water.
Source identification of OCPs
DDT
Technical DDT mixture contain <85 % of p,p′-DDT and >15 % of o,p′-DDT (Zheng et al. 2009). After the banned of technical DDT in Pakistan, dicofol that contains 3–7 % DDTs was practiced widely. DDTs are degradable into DDD through reductive dechlorination in anaerobic environment and into DDE under aerobic environmental conditions. The ratio of p,p′-DDE/p,p′-DDD and (p,p′-DDE+ p,p′-DDD)/DDT can be useful to investigate the extent of DDT degradation along with the environment of degradation, and to identify the new input of DDT to environment (Eqani et al. 2011; Sarkar et al. 2008). The ratio of (p,p′-DDE+ p,p′-DDD)/DDT higher than 1 concluded that DDT were the main source of contamination while the ratio less than 1 suggested that the fresh input of DDT was the main source. p,p′-DDE/p,p′-DDD is used to check the technical DDT or dicofol contamination sources. The ratio of p,p′-DDE/p,p′-DDD higher than the 1.3 suggested that dicofol is the source while the ratio ranged between 0.2 and 0.3 indicated the use of technical DDT (Li et al. 2008; Qiu et al. 2005). The ratios of current study are presented in the SI Table 2.
In the current study the ratio of (p,p′-DDE+ p,p′-DDD)/DDT was greater than 1 for water samples which indicated that technical DDTs are the main source of contamination in study area while this ratio was less than 1 for sediment samples which suggested that fresh input of DDTs was introduced in the study area. p,p′-DDE/p,p′-DDD ratio for both water and sediments was higher than 1 which indicated the dicofol usage in study area. DDTs may have been used from many years in the study area for agricultural or acaricide purposes. Presence of two obsolete pesticide dumping sites located in the catchment areas of study area may be the possible source of dicofolto the rivulet ecosystem (Syed et al. 2013b).
HCH
Technical composition of HCH mixture is 60–70 % of α-HCH, 5–12 % of β-HCH, 10–15 % of γ-HCH, 6–10 % of δ-HCH and 3–4 % of ε-HCH. α-HCH and γ-HCH have low partition coefficient and high volatility among all HCH isomers while β-HCH has the lowest vapor pressure among other HCH isomers (Syed et al. 2013a). In the present study, lower magnitude of β-HCH as compared to the other HCHs in sediments (30 %) and water (23 %) showed the minimum transformation and limited historical use of HCH isomers in the studied environmental matrices. Generally, it is considered that α/γ-HCH ratio > 4 in environment is the indication of recent usage of HCH (Kalantzi et al. 2001), though the high γ-HCH percentages specify lindane contamination. α/γ-HCH ratios for water and sediment samples of the current study were found lower than 3 (SI Table 2), that indicate, new/fresh intake of technical HCH isomers may not be the major source of HCH isomers in the study area. However, α-HCH and γ-HCH in sediments and water accounted 61 and 75 % respectively, of total HCH isomers; this high proportion indicates the point source of lindane in the study area. It is important to note that a strong correlation between γ-HCH and α-HCH was observed in both sediments and water samples which reflect the degradation of γ-HCH to α-HCH (Walker et al. 1999). The reason for this degradation may be due to the fact that transformation of γ-HCH to α-HCH take place in day time during sunlight depends upon anaerobic conditions of soil and biological degradation (Buser and Muller 1995). Pakistan, being the part of subtropical zone, the summer temperature is very high which enhance the anaerobic conditions of soil that perhaps a reason of γ-HCH transformation into α-HCH. Results of the current study also agreed with the previously reported study from Pakistan (Syed et al. 2013a).
Risk assessment to ecological integrities
To assess the ecological risk of OCPs in sediments and water, the results were counter checked and compared with the Guidelines of Chinese Environmental Quality Standard, European Union and Canadian Council of Ministry of the Environment Guidelines(CCME 2002; IRIS et al. 2004) (Table 3). Potential risk of OCPs residues in sediments of Nullah Aik and Palkhu to ecological integrities was investigated by comparing the mean concentrations with the threshold effect level (TEL) and probable effect level (PEL) of Canadian Council of Ministry of the Environment Guidelines (CCME 2002). Results of the current study were also compared with the effect range of low (ERL) value (Long et al. 1998; Yang et al. 2013). Comparison between the concentrations of OCPs isomers and different guidelines is presented in Table 3. Contamination level of γ-HCH, o,p′-DDT, p,p′-DDT and ΣDDT was higher than the TEL and great number of sediment samples showed the higher contamination load as compared to the PEL values. Same was the case when results of sediments were compared with the ERL values. These results suggested that OCPs contamination level in sediments may cause adverse biological and ecotoxicologicalrisks. However, DDTs and HCH concentration were more toxic compared to the other OCP isomers, so, DDT and HCH might be of great concern.
The concentration of DDTs and HCHs in water samples was 3.21 ± 0.95 and 3.31 ± 3.18 ng L−1, respectively, which is less than the environment quality standard for surface water of Chinese guideline (HCH < 5000 ng L−1 and DDT < 1000). European Union standard values for DDT and HCH in water were 25 and 20 ng L−1, respectively, the results of current study are within the range of European Union (EU) guidelines (Yang et al. 2013). Highest HCH concentration was exhibited by the Site 1 i.e. 11.86 ng L−1 and this value was under the permissible limits of EU standards. Among fourteen sampling sites, all the water samples were under the Chinese as well as EU guidelines, directed that OCPs in water of Nullah Aik and Palkhu are free from ecological risks.
Conclusion
Screening levels ecological risk assessment, spatial distribution pattern of OCPs along with the point source identifications have been investigated in the current report. Congener specific analysis reflected that DDTs were the dominant organic pollutants observed followed by the HCHs. Discharge of industrial wastewater into the tributaries of River Chenab, presence of two obsolete pesticides dumping sites and foliar spray of pesticides in agricultural land in the catchment areas of Nullah Aik and Palkhu are the possible reasons for high OCP levels. Technical DDTs, dicofol and lindane may be the possible sources of contamination of water and sediment in the study area. Urban/industrial fraction was observed by reporting the high contamination load from urban/industrial sampling sites of the study area. Regarding to the risks to ecological integrities, sediments were found more potent rather than water from tributaries of the River Chenab. It is recommended that government authorities should take serious steps for careful handling for proper removal/disposal of chemicals from obsolete pesticides stores.
References
Ahad K, Mohammad A, Khan H, Ahmad I, Hayat Y (2010) Monitoring results for organochlorine pesticides in soil and water from selected obsolete pesticide stores in Pakistan. Environ Monit Assess. doi:10.1007/s10661-009-0995-5
Alamdar A, Syed JH, Malik RN, Katsoyiannis A, Liu J, Li J, Zhang G, Jones KC (2014) Organochlorine pesticides in surface soils from obsolete pesticide dumping ground in Hyderabad City, Pakistan: contamination levels and their potential for air–soil exchange. Sci Total Environ. doi:10.1016/j.scitotenv.2013.09.053
Ali M, Jabbar A (1991) Government of Pakistan. Pakistan Council of Research in Water Resources (PCRWR), Islamabad
Bano A, Siddique SA (1991) Chlorinated hydrocarbons in the sediments from the coastal waters of Karachi Pakistan. Pak J Sci Ind Res 34:70–74
Buser HR, Muller MD (1995) Isomer and enantioselective degradation of hexachlorocyclohexane isomers in sewage-sludge under anaerobic conditions. Environ Sci Technol 29:664–672
CCME (Canadian Council of Ministers of the Environment) (2002) Canadian sediment quality guidelines for the protection of aquatic life. Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg
Chen SJ, Luo XJ, Mai BX, Sheng GY, Fu JM, Zeng EY (2006) Distribution and mass inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in sediments of the Pearl River Estuary and the Northern South China Sea. Environ Sci Technol 40:709–714
Eqani SAMAS, Malik RN, Mohammad A (2011) The level and distribution of selected organochlorines pesticides in sediments from River Chenab, Pakistan. Environ Geochem Health 33:33–47
Eqani SAMAS, Malik RN, Katsoyiannis A, Zhang G, Chakraborty P, Mohammad A et al (2012) Distribution and risk assessment of organochlorine contaminants in surface water from River Chenab, Pakistan. J Environ Monit 14:1645–1654
Feng JL, Zhai MX, Qun L, Sun JH, Guo JJ (2011) Residues of organochlorines pesticides (OCPs) in upper reach of the Huaihe River, East China. Ecotoxicol Environ Saf 74:2252–2259
Hu LM, Zhang G, Zheng BH, Qin YW, Lin T, Guo ZG (2009) Occurrence and distribution of organochlorine pesticides (OCPs) in surface sediments of the Bohai Sea, China. Chemosphere 77:663–672
Hu WY, Wang TY, Khim JS, Luo W, Jiao WT, Lu YL, Naile JE, Chen CL, Zhang X, Giesy JP (2010) HCH and DDT in sediments from marine and adjacent riverine areas of North Bohai Sea, China. Arch Environ Contam Toxicol 59:71–79
IRIS, DDT, DDE, DDD (2004) In: U. Integrated risk information system (Ed.), US Environmental Protection Agency, US EPA integrated risk information system. http://www.epa.gov/iris/. Accessed 07 July 2010
Jan MR, Shah J, Khwaja MA, Gul K (2008) DDT residue in soil and water in and around abandoned DDT manufacturing factory. Environ Monit Assess 155:31–38
Kalantzi OI, Alcock RE, Johnston PA, Santillo D, Stringer RL, Thomas GO et al (2001) The global distribution of PCBs and organochlorine pesticides in butter. Environ Sci Technol 35:1013–1018
Li X, Wang W, Wang J, Cao X, Wang X, Liu J, Liu X, Xu X, Jiang X (2008) Contamination of soils with organochlorine pesticides in urban parks in Beijing, China. Chemosphere 70:1660–1668
Long ER, Field LJ, MacDonald DD (1998) Predicting toxicity in marine sediments with numeral sediment quality guidelines. Environ Toxicol Chem 17:714–727
Minh NH, Minh TB, Kajiwara N, Kunisue T, Subramanian A, Iwata H, Tana TS, Baburajendran R, Karuppiah S, Viet PH, Tuyen BC, Tanabe S (2006) Contamination by persistent organic pollutants in dumping sites of Asian developing countries: implication of emerging pollution sources. Arch Environ Contam Toxicol 50:474–481
Qiu XH, Zhu T, Yao B, Hu JX, Hu SW (2005) Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol 39:4385–4390
Sarkar SK, Bhattacharya BD, Bhattacharya A, Chatterjee M, Alam A, Satpathy KK et al (2008) Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ Int 34:1062–1071
Singh KP, Malik A, Mohan D, Takroo R (2005) Distribution of persistent organochlorines pesticide residues in Gomti River, India. Bull Environ Contam Toxicol 74:146–154
Syed JH, Malik RN (2011) Occurrence and source identification of organochlorine pesticide (OCPs) in the surrounding surface soils of the Ittehad Chemical Industries Kalashah Kaku, Pakistan. Environ Earth Sci 62:1311–1321
Syed JH, Malik RN, Liu D, Xu Y, Wang Y, Li J et al (2013a) Organochlorine pesticides in air and soil and estimated air–soil exchange in Punjab, Pakistan. Sci Tot Environ 444:491–497
Syed JH, Malik RN, Muhammad A (2013b) Organochlorine pesticides in surface soils and sediments from obsolete pesticides dumping site near Lahore city, Pakistan: contamination status and their distribution. Chem Ecol. doi:10.1080/02757540.2013.829051
Tang ZW, Yang ZF, Shen ZY, Niu JF, Cai YP (2008) Residues of organochlorines pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan, China. Environ Monit Assess 137:427–439
Turgut C (2003) The contamination with organochlorine pesticides and heavy metals in surface water in Kucuk Menderes River in Turkey, 2000–2002. Environ Int 29:29–32
Walker K, Vallero DA, Lewis RG (1999) Factors influencing the distribution of lindane and other hexachlorocyclo hexanes in the environment. Environ Sci Technol 33:4373–4378
Yang RQ, Lv AH, Shi JB, Jaing GB (2005) The level and distribution of organochlorines pesticides (OCPs) in sediments from the Haihe River, China. Chemosphere 61:347–354
Yang D, Qi S, Zhang J, Wue C, Xing X (2013) Organochlorine pesticides in soil, water and sediment along the Jinjiang River main stream to Quanzhou Bay, south east China. Ecotoxicol Environ Saf 89:59–65
Zheng XY, Liu XD, Liu WJ, Jiang GB, Yang RQ (2009) Concentrations and source identification of organochlorine pesticides (OCPs) in soils from Wolong Natural Reserve. Chin Sci Bull 54:743–751
Zhou Y, Liu H, Xi Z, Cheng H, Xu X (2005) Organochlorine pesticides (DDTs and HCHs) in soils from the outskirts of Beijing, China. Chemosphere 60:770–778
Acknowledgments
Authors would like to acknowledge the Higher Education Commission of Pakistan (HEC) for providing support under the IRSIP (International Research Support Initiative Program). We also express our thanks to Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, China. We are highly thankful to Prof. Dr. Xiandong Li (Polytechnic University, Hong Kong) for providing assistance for samples transportation to Guangzhou Institute of Geochemistry, Guangzhou, China. Authors can never forget to acknowledge Mr. Irfan Ali Ch. who guided to collect samples from far areas during sampling.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahmood, A., Malik, R.N., Li, J. et al. Levels, distribution pattern and ecological risk assessment of organochlorines pesticides (OCPs) in water and sediments from two tributaries of the Chenab River, Pakistan. Ecotoxicology 23, 1713–1721 (2014). https://doi.org/10.1007/s10646-014-1332-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1332-5