Abstract
Two pilot-scale submerged-bed microbial biofilms were set up for the removal of Cr(III) and Pb(II) from groundwater, and the biological activities and structure of the bacterial communities developed in the presence of the heavy metals were analyzed. Artesian groundwater was polluted with Cr(III) or Pb(II) (15 mg/l) and amended with sucrose (250 mg/l) as carbon source. While Pb(II) was over 99% removed from groundwater during long-term operation (130 days), the efficiency of the removal of Cr(III) significantly decreased in time (95–73% after 60 days). Cr(III)-amended biofilms displayed significant lower sucrose consumption, ATP cell contents and alkaline phosphatase activity, compared to biofilms formed in the presence of Pb(II), while analysis of exopolymers demonstrated significant differences in their composition (content of carbohydrates and acetyl groups) in response to each heavy metal. According to transmission electron microscopy (TEM) and electron-dispersive X-ray analysis (EDX), Cr(III) bioaccumulated in the exopolymeric matrix without entering bacterial cells, while Pb(II) was detected both extra and intracellularly, associated to P and Si. Temperature-gradient gel electrophoresis (TGGE) profiling based on partial amplification of 16S rRNA genes was used to analyze the differences in the structure of the biofilm bacterial communities developed under exposure to each heavy metal. Prevalent populations in the biofilms were further identified by reamplification and sequencing of isolated TGGE bands. 75% of the sequences in the Pb(II) biofilter were evolutively close to the Rhodobacterales, while in the Cr(III) biofilter 43% of the sequences were found affiliated to the Rhizobiales and Sphingomonadales, and 57% to Betaproteobacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution of water poses serious environmental and public health concerns. Chromium (Cr) and lead (Pb) are included on the US Environmental Agency (EPA) list of priority pollutants (http://epa.gov/waterscience/methods/pollutants.htm), and in the European Union their discharge in water is regulated by the 76/464/EEC and 80/68/EEC Directives. Chromium compounds are classified by their valence states, being Cr(III) and Cr(VI) the most widely distributed in nature. Due to its widespread industrial use in tanning, electroplating and metallurgy, chromium raised as an important pollutant of soil and water (Zayed and Terry 2003). Trivalent and hexavalent forms of Cr exhibit fairly diverse chemical properties and both have been extensively studied for their toxicity (Cervantes et al. 2001; Eastmon et al. 2008; Zayed and Terry 2003). Cr(III) is widely accepted to be an essential trace element related to glucose and lipid metabolism (Eastmon et al. 2008; Fraga 2005), and historically has been considered much less toxic than Cr(VI). Nevertheless, at high concentrations Cr(III) can exhibit mutagenic and genotoxic effects (Eastmon et al. 2008), and recent studies indicate that its actual toxicity to aquatic organisms may be generally underestimated due to its intrinsic solubility properties in natural environments (Vignati et al. 2010). In this sense, Cr(VI) remediation practices often include its biological reduction to Cr(III) (Bencheik-Latmani et al. 2007; Ludwig et al. 2007; Priester et al. 2006).
Pb is a heavy metal with no known essential functions for living organisms and in humans is long-time known as a neurotoxicant (White et al. 2007). Lead toxicity has been thoroughly researched in comparison to that of other heavy metals, due to its extensive worldwide use in anthropogenic activities over centuries (manufacturing of glass, pigments, winery, fuel additives, electronics) and hence the enormous exposure of the environment to its compounds (White et al. 2007). While elementary lead does not dissolve in water under ordinary conditions, it may however occur dissolved as Pb(II). Toxicity of Pb for humans is complex and involves not only dysfunction of the central and peripheral nervous system, but also other effects (Alessio et al. 2007; White et al. 2007).
Remediation technologies applied to the removal of heavy metals from contaminated water are classically based on physicochemical processes of separation or treatment (Eccles 1999; Mulligan et al. 2001). Cr(III) and Pb(II) are most often removed by chemical precipitation (Esmaeili et al. 2005; Matlock et al. 2002), however, this approach is extremely expensive and may not completely reduce metal concentrations to the required levels (Wu et al. 2008). Biological methods based on the use of microbial biofilms have upraised as eco-friendly and economic alternatives to the conventional physicochemical processes (Eccles 1999; Harrison et al. 2007; Malik 2004; Mulligan et al. 2001). Microorganisms have developed metal-specific tolerance mechanisms which include enzymatic transformations (oxidation, reduction, methylation or alkylation), precipitation and bioaccumulation (Malik 2004). The nature of the bioaccumulation depends both on each particular metal and microorganism, and includes either passive binding of metals to cell walls and external surfaces (biosorption), or metabolically-mediated active uptake (Ahluwalia and Goyal 2007; Malik 2004). The use of growing cells adds several advantages to the performance of heavy metal removal. Systems using dead microbial biomass lack the intracellular bioaccumulation capacity, and biosorption processes on dead cells are particularly sensitive to changes in physical conditions such as pH or ionic strength (Malik 2004). Despite the development of metal-resistance mechanisms, continued exposure to the toxic ions affects the structure of the microbial communities, often resulting in decreased activity and species diversity (Malik 2004). Therefore, the success of microbial-based remediation technologies benefits from the knowledge and understanding of the shifts in the physiology and structure of the microbial communities developed in response to metal stress, and the identification of the prevalent populations under such conditions (Harrison et al. 2007; Vílchez et al. 2007).
Different types of biofilm reactors have been used for biological treatment of water and wastewater, from which submerged fixed-bed technology seems to be particularly appropriate for application to freshwater treatment (Vílchez et al. 2007). In this study, we evaluated the performance of pilot-scale submerged biofilters for the removal of Cr(III) and Pb(II) amended to groundwater at a concentration of 15 mg/l, and analyzed the biological activities and composition of exopolymeric substances in the biofilm matrix. We also examined the structure of the bacterial communities developed in the presence of each heavy metal, by a cultivation-independent approach based on TGGE fingerprinting.
Materials and methods
Description of the submerged biofilter system
The pilot-scale submerged biofilters used for the experiments were analogous to that described in previous work (Vílchez et al. 2007). A simplified drawing is depicted in Fig. 1. Briefly, they consisted of a methacrylate cylindrical column (0.5 m high, 0.15 m inner diameter), packed with clayey schists (0.8 mm average pore diameter) as carrier material for the formation of the fixed-bed biofilm. The total weight of carrier material used in the biofilter was 15 kg, and was kept constant throughout the study. The characteristics of the carrier material are described in detail elsewhere (Gómez et al. 2002). Absence of heavy metals in the carrier material was previously checked by electron-dispersive X-ray microanalysis (EDX) as described below. The biofilters had five sampling ports (numbered p-0 to p-4), evenly distributed at every 10 cm of its complete height.
The systems were fed with artesian groundwater, collected weekly from a well in Churriana (Granada, Spain), and disposed in a 200 l tank, from which it entered the biofilter column. The average characteristics of the groundwater used in the study were analyzed in accordance with the standard methods for the examination of water and wastewater (APHA 2001), and were as follow: phosphates: 0.40 ± 0.05 mg/l; NO3 −: 121.3 ± 0.8 mg/l; NO2 −: not detected; NH4 +: 2.2 ± 0.5 mg/l; SO4 2−: 178.90 ± 0.07 mg/l; Cl−: 50.70 ± 0.08 mg/l; Na+: 250.0 ± 0.6 mg/l; K+: 7.50 ± 0.05 mg/l: heavy metals (Cu, Cd, Hg, Cr, Pb): <0.0001 mg/l; COD: 180 ± 10 mg/l; BOD5: 145.2 ± 0.8 mg/l; total mesophilic microorganisms grown at 20°C (48 h): 104 ± 25 CFU/ml; Enteroccoci and E. coli: not detected. Chromium and lead were added as Cr(NO3)3 or Pb(NO3)2 to the influent water, to get a final concentration of 15 mg heavy metal ion per liter of groundwater. The biofilters were fed a steady flow with a constant metal load and no recirculation.
Inoculation
Due to the low microbial population present in the water to be treated (see Description of the submerged biofilter system), a biofilm was previously produced by inoculation of the biofilters with an activated sludge (Vílchez et al. 2007). The sludge was collected from an urban wastewater treatment plant (EDAR Los Vados, Granada, Spain), amended with sucrose (5 g/l), and recirculated in the system at a flow rate of 100 ml/min for 24 h. Then the groundwater to be treated was pumped into the biofilter together with sucrose (250 mg/l) as C source, and spiked with the heavy metals Cr(III) or Pb(II), as above described. The amount of sucrose added to the influent water was optimized in preliminary tests to 250 mg/1, on the basis of using a concentration that guaranteed the feeding of the microorganisms throughout the whole filter depth, minimizing the concentration of sucrose present in the effluent water.
Operation parameters of the biofilters
The influent water flow was kept at 2.3 l/h, regulated by a peristaltic Watson Marlow 505S pump. The columns were supplied with an airflow of 1 l/min (0.113 vvm), controlled by a Dynaval air rotameter. The flow of air was sterilized using a membrane filter of 0.2 mm (millipore), which was changed every 2 weeks. To avoid filter clogging and optimize the elimination of the heavy metals from water, cleaning cycles of the biofilters were carried out every 10 days by back-flushing from the column ends, as previously described (Vílchez et al. 2007).
Monitoring of sucrose concentration
Sucrose concentration in influent water, effluent water, and sampling ports p-1 to p-4, was measured by the method of Roe and Papadopoulos (1954), at the beginning and end of each cycle of operation of the biofilters (days 1 and 9), in order to measure the rate of consumption of the C source by the microorganisms in the biofilm at the different biofilter depths.
Monitoring of heavy metal concentration
Pb(II) and Cr(III) concentrations in water were daily monitored by atomic absorption using electrothermal atomization in a graphite furnace (Perkin-Elmer 4100-ZL).
Organic matter content (weight of volatile compounds)
Weight of organic matter in the biofilm was estimated by the loss on ignition (LOI) method, based on sequential heating of samples to calculate the weight of volatile compounds (Dean 1974). Wet pieces of carrier material with adhered biofilm were placed in empty pre-weighed porcelain crucibles. After oven-drying of the samples to constant weight (24 h at 120°C), organic matter was combusted at 550°C in a Selecta muffle furnace. LOI was calculated from the subtraction of both weights. All samples were cooled to room temperature in a desiccator before any weight measurements were made. Organic matter content of the biofilm is given as mg of volatile compounds/g dry weight of carrier material.
ATP extraction and determination of ATP content
15 g of carrier material with adhered biofilm were suspended in 5 ml of DMSO buffered at pH 7.8 (Tris HCl, 0.5 M). The suspension was sonicated for 15 min and then vortexed for 5 min. The decantate was collected, its volume measured, and then it was centrifuged at 1500 g for 10 min. Quantification of ATP in samples of 50 μl of the supernatant was achieved by the firefly bioluminescence assay as described by Karl (1980), using the Sigma ATP Bioluminescence Assay Kit, and a bioluminescence photometer (Turner designs). Results are given as ng ATP/mg organic matter.
Composition of cell-bound extracellular polymeric substances from the biofilm
Cell-bound exopolymeric substances were recovered from biofilms samples by the method previously described by Vílchez et al. (2007). Quantification of organic matter in the recovered exopolymers was determined by LOI as described above, and given as g/100 g dry weight. Content of total carbohydrates, proteins, uronic acids, and acetyl groups, was quantified by previously described methods (Blumenkrantz and Asboe-Hansen 1973; Bradfords 1976; Dubois et al. 1956; McComb and McCready 1957). In order to avoid interferences due to the presence of heavy metals in the samples, 0.1 M EDTA was added to both samples and standards when appropriated (Vílchez et al. 2007).
Scanning electron microscopy (SEM), transmission electron microscopy (TEM) and electron-dispersive X-ray microanalysis (EDX)
Pieces of carrier material with adhered biofilm were sampled from the column and fixed with 2.5% glutaraldehyde at pH 7.4 (sodium cacodylate buffer, 0.05 M) at 4°C (24 h for SEM, 4 h for TEM), washed, post-fixed in OsO4 and dehydrated in graded ethanol. Samples for SEM were critical-point dried using liquid CO2 in a Polaron CPD7501 apparatus, and carbon coated in an Hitachi evaporator. Samples for TEM were embedded in Epon resine and polymerized. Ultrathin sections were stained with uranyl acetate plus lead citrate (Cr(III) biofilm), or uranyl acetate alone (Pb(II) biofilm), before being observed.
Samples were observed and analyzed with a DSM 950 Zeiss SEM coupled to an Oxford ISIS 300 EDX system, or a TEM/STEM Phillips CM20 coupled to an EDAX EDX system. Images (1,024 × 800 pixels) were taken with a SIS MegaView III camera. Both SE (secondary electrons) and BSE (back-scattered electrons) detectors were used. The BSE signal is strongly dependent on the mean atomic number of the target, hence it records not surface morphologies but chemical compositions, as structures composed of heavier elements appear brighter than those of lighter ones (Friedmann et al. 2001). SEM–SE and SEM–BSE images of the same field allow for the study of the distribution of the heavy metals in samples.
Biofilm recovery from the carrier material
Biofilm samples were recovered from the carrier material by the following method: 10–100 g of carrier material with biofilm adhered were taken from each sampling point (Fig. 1) and placed in flasks with 50 ml of sterile saline (0.9% NaCl). In order to detach the biofilm from the carrier and disperse the cells, the suspensions were sonicated in a bath sonicator (Ultrasonic bath, Selecta) at room temperature for 2 min at 40 kHz (0.05 W/ml) and then placed in an orbital shaker at 155 rpm for 1 h. The process was repeated twice. The suspensions of biofilm material were then used for the determination of cultivable cell numbers, quantification of alkaline phosphatase activity, and DNA extraction.
Determination of cultivable mesophilic microorganisms
Recovered biofilm suspensions were serially diluted in sterile isotonic saline and plated on TSA medium (Triptic Soy Agar, Difco). Plates were incubated at 30°C for 48 h.
Alkaline phosphatase activity
Total alkaline phosphatase activity was measured in biofilm suspensions by the method previously described by Vílchez et al. (2007). Results were given as mg p-nitrophenol released/mg organic matter per h.
DNA extraction from biofilms
Total DNA was periodically extracted from biofilm material following a method previously described (Vílchez et al. 2007). Several DNA extractions of each sample were carried out separately, and the total DNA was pooled together before PCR amplification.
PCR amplification of partial 16S rRNA genes
The 16S-rRNA gene was used as target for the study of bacterial diversity. Fragments of the gene of a size adequate for TGGE separation (ca. 200 bp) were amplified by PCR, using a nested approach (Vílchez et al. 2007). V3-hypervariable region of the 16S-rRNA gene was selected for TGGE fingerprinting because of its high inter-species variability and the reliability of previously described universal eubacterial primers for DGGE/TGGE studies (Yu and Morrison 2004). Primers fD1 and rD1 (Weisburg et al. 1991) were used to amplify a 1.5 kb fragment of the 16S-rRNA gene, then the universal primers GC-p1 and p2 described by Muyzer et al. (1993) were used in a nested PCR to amplify the V3 region. All primers were purified by HPLC and purchased from Sigma.
One microliter (2–5 ng) of DNA was used as template for PCR, and subsequently 1 μl of the first PCR product was used as template for the nested PCR. Ampli Taq Gold polymerase (Applied Biosystems) was used through all experiments. Conditions for the PCR reactions were kept as previously described for both amplifications (Vílchez et al. 2007). Final PCR products were cleaned and/or concentrated (when needed) using Microcon YM cartridges. Final concentration of DNA samples was 60–100 ng/μl. Two to five microliters were loaded in each well for TGGE.
TGGE fingerprinting and analysis
TGGE runs were done on a TGGE Maxi system (Whatman-Biometra). Denaturing gels (6% PAGE with 20% deionized formamide, 2% glycerol and 8 M urea) were made and run with 2× TAE buffer. The temperature-gradient applied for efficient separation of bands was optimized at 43–63°C (Vílchez et al. 2007). The gels were run at 125 V for 18 h. Gel bands were visualized by silver staining using the Gel Code Silver Staining kit (Pierce), following the manufacturer’s indications, except for the omission of the stabilization step, as it increased background staining on gels. Stained gels were photographed with a canon digital camera. A six-species marker was included to aid normalization of the gel images (Vílchez et al. 2007).
Band patterns generated by TGGE were normalized, compared and clustered using the Gel Compar II image analysis software, version 5.101. (Applied Maths). Bands were automatically detected and matched, and further corrections were applied manually. For cluster analysis, TGGE profiles were compared using a band assignment-independent method based on the Pearson product-moment correlation coefficient. This analysis uses the whole densitometric curve, taking into consideration band intensity. Dendrograms relating band pattern similarities were automatically calculated with UPGMA algorithms (Unweighted pair group method with arithmetic mean). Significance of UPGMA clustering was estimated by calculating the cophenetic correlation coefficients (Sokal and Rohlf 1962).
DNA sequencing of TGGE-isolated bands, phylogenetic and molecular evolutionary analyses
Portions of individual bands on silver stained TGGE gels were picked up with sterile pipette tips, placed in 10 μl of filtered (0.22 μm) and autoclaved water, and directly used for reamplification with the appropriate primers. PCR products were purified by gel running and extraction with the Quiaex-II kit (Quiagen). DNA recovered was directly used for automated sequencing in an ABI PRISM 3100 Avant genetic analyzer.
DNA sequences were analyzed using the biocomputing tools provided on-line by the European Bioinformatics Institute (http://www.ebi.ac.uk). The BLASTn program (Altschul et al. 1997) was used for preliminary sequence similarity analysis. The ClustalX version 2.0.3 software (Jeanmougin et al. 1998) was used for the aligning of sequences. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4 (Kumar et al. 2001). A p-distance based evolutionary tree was inferred using the Neighbor-Joining algorithm (Saitou and Nei 1987). The bootstrap test was conducted to infer the reliability of branch order (Felsenstein 1985), with a round of 1,000 reassemblings. Bootstrap values below 50% are not shown in the tree.
Statistics
The effects of the presence of heavy metals and the depth of sampling in the biofilter columns, on the parameters studied, were evaluated by either one-way or multifactor analysis of variance (ANOVA), using the software package STATGRAPHICS 5.0 (STSC Inc., Rockville, MD, USA) to identify significant differences between measurements. A significance level of 95% (P < 0.05) was selected.
Results
Efficiency of heavy metal removal by the biofilters
Continuous operation of the biofilter system with the heavy metal-amended groundwater was optimal in cycles of 10 days for both Cr(III) and Pb(II), after which a cleaning by back-flushing was required. The system was operated for 6 complete cycles of 10 days (2 months) in the case of Cr(III), and for 13 complete cycles of 10 days (over 4 months) in the case of Pb(II).
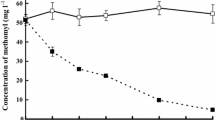
Cr(III) was efficiently removed from groundwater by the fixed-bed biofilm, showing a maximal removal rate of 95%, which was achieved during the first 3 cycles. However, further operation resulted in the biofilter system becoming progressively saturated with the heavy metal, significantly reducing the efficiency of Cr(III) removal from the water to 73% after 60 days of continuous operation (Fig. 2a).
a Pb(II) retention in the biofilm biomass (mg Pb(II)/g dry biofilm) during a whole cycle of operation of the biofilter (10 days). b Efficiency of Cr(III) removal from ground water by the biofilter, after six complete cycles of operation (60 days). Bars in both graphics indicate least significant differences (LSD) between means (ANOVA analysis, P < 0.05)
Pb(II) was over 99% removed form water by the biofilter during the whole duration of the experiment, as the concentration of soluble Pb(II) detected in water samples retrieved along all the sampling ports was always below 50 μg/l. Thus, the ability of the biofilm to remove the heavy metal was completely recovered after excess biomass and retained Pb(II) were detached by back-flushing at the end of each cycle of operation, for the full duration of the experiment. The concentration of Pb(II) retained by the biofilm was also analyzed during a whole cycle (Fig. 2b). Pb(II) accumulation by the biomass increased along the 10 days of the cycle and was significantly higher in biofilm samples retrieved from sampling ports 1 and 2, corresponding to the upper half of the biofilter bed. The biomass displayed a maximal removal capacity of 700 mg/g dry biofilm (measured at sampling port 1, after 10 days of operation).
Physiology and biological activities of the biofilms, and analysis of exopolymer composition
The parameters used to monitor the physiology and biological activities of the biofilms exposed to either Cr(III) or Pb(II) are summarized in Table 1A. Organic matter content of the biofilm, ATP cell content, mesophilic viable counts, and alkaline phosphatase activity were measured in the second half of the cycle of operation (days 5–7), while exopolymer extraction was carried out on the last day of the cycle of operation (day 9). All the parameters were measured at least during three complete cycles and showed no significant temporal variation, thus, the average values are reflected in Table 1A.
The content in organic matter of the biofilm was significantly lower in the Pb(II)-amended biofilter at all depths of the column, while cell ATP contents were significantly higher, compared to the Cr(III)-exposed biofilm. The use of sucrose was kept in a similar range in both biofilters when measured at sampling port 1, closest to the water influent entrance. However, sucrose consumption by the microorganisms in the Pb(II)-exposed biofilter gradually increased along the column depth until the sugar concentration was 96% reduced at sampling port 4, while the rate of uptake of the carbon source by the microorganisms in the Cr(III)-amended biofilter was significantly lower. Alkaline phosphatase activity was remarkably high in the Pb(II)-exposed biofilm, with average values often triplicating those measured in the presence of Cr(III).
There were significant differences in the composition of the biofilm exopolymers between the two biofilters, as shown in Table 1B. Content of carbohydrates and acetyl groups were 38% and 122% higher, respectively in the exopolymers from the Cr(III)-exposed biofilm, compared to the Pb(II) biofilter.
Biofilm structure and characterization of the sites of heavy metal accumulation
Microscopic studies and EDX analyses of the biofilms developed on the biofilter were always done on day 9 of the cycle of operation, before the back-flushing step. SEM images of the biofilms formed in the biofilter exposed to either Cr(III) or Pb(II) displayed an heterogeneous composition, showing microorganisms of different morphologies with surrounding exopolymers (Fig. 3). The comparison of images of a same SEM field in SE and BSE modes revealed that the electron-dense material located on the surface of the microorganisms, and not on the carrier. This was confirmed by the detection of Cr(III) and Pb(II) by EDX. Hence, microbes and their surrounding exopolymers acted as the physical barrier retaining the metal ions.
SEM images and EDX analysis of Cr(III) and Pb(II)-removing biofilms. Biofilm formed in the presence of Cr(III) (a) and Pb(II) (b) were photographed in SE mode (left) and BSE mode (right), showing the main areas of accumulation of the heavy metal ions. Scale bars: 20 μm. Due to the sample preparation method, Cu occurs in EDX of Cr(III)-exposed biofilm samples, and Au and Os occur in Pb(II)-exposed biofilm samples
In the case of the Cr(III)-exposed biofilm, TEM-EDX of individual cells confirmed that all the metal ions were bound to cell envelopes (walls/extracellular matrix material), while no Cr(III) was detected intracytoplasmically in any of the cells analyzed in the experiment (examples are shown in Fig. 4a). EDX analysis of electron-dense stained zones surrounding the cell membranes displayed the Cr peaks (Kα = 5.4 keV and Kβ = 5.93 keV). Si (Kα = 1.74 keV) was concomitantly detected with Cr(III).
TEM images and EDX analyses of microorganisms in the biofilm formed in the presence of heavy metals. Circles marked as EDX in pictures indicate points of positive detection of lead or chromium. a and b Cr(III) is accumulated on the cell envelopes of microorganisms, but is not detected in the cytoplasms. c Example of EDX analysis of a, showing Cr associated to the presence of Si. Pb and Cu occur in EDX due to sample preparation. d–f Pb(II) is detected by EDX both inside and outside the cells in the biofilm. g Example of EDX analysis of cytoplasmic structure shown in d, showing the presence of Pb associated to Si and P
In contrast, EDX analysis of the biofilm samples taken from the Pb(II)-exposed biofilter (Fig. 4b) demonstrated that the heavy metal occurs both extra and intracellularly (Pb Mα = 2.35 keV and Lα = 10.53 keV). Either extracellularly bound or located in the cytoplasm, Pb(II) was found associated to Si (Kα = 1.74 keV) and P (Kα = 2.01 keV).
Bacterial community structure of heavy metal-removing biofilms and identification of prevalent bacterial populations
In order to gain insight into the structure of the bacterial communities developed in the heavy metal-removing biofilter, DNA was extracted from samples of biofilms taken before and after the back-flushing step between cycles of operation (days 39 and 41 for the Cr(III) biofilter, days 49 and 51 for the Pb(II) biofilter). Additionally, the Pb(II)-exposed biofilter was sampled after 112 days of operation for comparison, as this experiment was conducted for a longer time than in the case of Cr(III).
Pearson’s correlation coefficient-based cluster analysis of the TGGE profiles demonstrated that the composition of the bacterial community was remarkably stable in all the studied biofilms both when treating Cr(III) or Pb(II) polluted groundwater, regardless of the back-flushing operation or column depth. All fingerprints of samples coming from a same biofilter clustered together over 85% similarity (Fig. 5). In particular, there was little spatial variation of the structure of the bacterial communities within the biofilter column, since samples taken at four different depths in a same day mostly clustered together over 90% similarity.
TGGE community profiles of biofilms samples taken from Pb(II) and Cr(III)-exposed biofilters. Profiles are based on amplification and separation of the V3-hypervariable region of the 16S-RNAr gene. The time (days) and port of sampling in the biofilter (1–4) is indicated near each lane. a Dendrogram generated by UPGMA clustering (Pearson coefficient-based analysis) of band patterns generated from all samples taken from the Cr(III)-exposed biofilter. b Dendrogram generated by UPGMA clustering (Pearson coefficient-based analysis) of band patterns generated from all samples taken from the Pb(II)-exposed biofilter. The scale bars indicate the percentage of similarity. Numbers in nodes represent the cophenetic correlation coefficient values. Numbered white circles indicate bands that were reamplified and sequenced for the phylogenetic analysis shown in Fig. 6
Prevalent TGGE bands were isolated from fingerprints of biofilm samples of the biofilter exposed to each heavy metal, and a total of 22 TGGE bands were successfully reamplified and sequenced (14 from Cr(III)-exposed biofilms, 8 from Pb(II)-exposed biofilms). The phylogenetic analysis of the sequences showed that the identified bacteria were all related to Alpha and Betaproteobacteria classes (Fig. 6). In most cases, the closest relatives found in the EMBL database were uncultured environmental sequences. 75% of the sequences identified in the Pb(II) biofilter were evolutively close to the Rhodobacterales, while in the Cr(III) biofilter 43% of the sequences were found affiliated to the Rhizobiales and Sphingomonadales, and 57% to Betaproteobacteria. All the Betaproteobacterial sequences identified in both Cr(III) and Pb(II)-exposed communities were related to the Burkholderiales, particularly to members of the Comamonadaceae in the case of Cr(III). The band classes displaying the highest abundance (=band intensity) in all Cr(III)-exposed samples were related to the Variovorax/Acidovorax group (sequences C2, C3, C4). In the Pb(II) biofilter, the prevalent band class in samples taken after 49 or 51 days of operation (sequence P1) was shown phylogenetically related to sequences of unclassified Burkholderiales isolated from freshwater sources, while after 112 days of operation, the most abundant band class was that represented by sequence P9, evolutively close to the Alphaproteobacteria of the genus Rhodobacter.
Phylogenetic Neighbor-Joining trees of 16S rRNA gene partial sequences (V3 hypervariable region), showing the affiliations of the sequences amplified from excised TGGE bands (see Fig. 5). Accession numbers of bacteria retrieved from the EMBL database are indicated in the trees. A superscript T designates type strains. Only bootstrap values above 50% are shown
Discussion
Technically and economically viable application of living biofilms for the remediation of toxic metals in water require that the microorganisms maintain a constant removal capacity after multiple bioaccumulation–desorption cycles, and hence the optimization of biofilter design and setting up of the basic operation parameters (Malik 2004). The design of the laboratory-scale biofilter system used in the study was selected on the basis of its previous efficiency for the removal of Cu (II) from groundwater (Vílchez et al. 2007). Through the present study, the system was proven also efficient for the removal of Cr(III) and Pb(II). Pb(II) was mostly accumulated by the microbial biomass on the upper half of the biofilter fixed-bed, particularly in the first 10 cm. A similar behavior was previously reported for Cu (II) in an analogous biofilter system (Vílchez et al. 2007). An accumulation capacity of up to 700 mg Pb/g dry biofilm was recorded (Fig. 2). This rate is higher than those previously described for most processes designed for the removal of lead based on retention by bacterial, algae or fungal biomass (Ahluwalia and Goyal 2007). In contrast to the high performance of the Pb(II) biofilter, Cr(III) removal efficiency gradually decreased during long-term operation. The longer mobility of Cr(III) ions through the column suggests that an increase in the dimensions of the biofilter could contribute to improve the performance of removal of this heavy metal in long-term operation, an alternative which will be considered in future work.
In this study, several approaches were combined to characterize the physiology of the microbial biofilms in response to the heavy metal stress. While the cells in the Pb(II)-exposed biofilm progressively consumed over 96% of the supplied carbon source along the column depth and displayed ATP contents ranging 0.05–0.03% of the total organic matter, Cr(III) induced the inhibition of sucrose uptake and lowered the ATP cell content at all column depths analyzed (Table 1B). These results demonstrate a toxic effect of this metal on the biofilm community. Cr(III) has the potential to damage microbial DNA and proteins, provided its ability to access the intracellular compartment at a sufficient concentration (Bencheik-Latmani et al. 2007; Eastmon et al. 2008). While Cr(VI) in the form of chromate is actively transported across biological membranes in both prokaryotes and eukaryotes by competing for sulfate transporters, most microbial cells are reported impermeable to Cr(III) (Cervantes et al. 2001). Nevertheless, several studies have concluded a similar or higher toxicity of Cr(III) compared to Cr(VI) for pure cultures of bacteria, fungi and algae (Bencheik-Latmani et al. 2007; Murray et al. 2005; Ramana and Sastry 1994; Thompson et al. 2002; Vignati et al. 2010), as well as mixed communities in a nitrifying sludge (Ceçen et al. 2010). The mechanisms by which extracellular binding of Cr(III) exerts toxicity on microorganisms are poorly characterized, although in some studies they have been connected to interference with iron transport and intake (Murray et al. 2005; Ramana and Sastry 1994). Cr(III) toxicity related to its effective uptake and intracellular precipitation was demonstrated in a strain of the iron-reducing bacteria Shewanella (Bencheik-Latmani et al. 2007).
Microbial biofilms are well established in natural habitats and show higher tolerance to heavy metals and other pollutants, compared to planktonic cells. Metal-resistant biofilms often produce large quantities of exopolymers responsible for the biosorption of metals and offering a protective barrier (Harrison et al. 2007). The composition of exopolymers in the biofilms analyzed in this study was similar in response to both Cr(III) and Pb(II), except for the significantly higher content of carbohydrates and acetyl groups in the Cr(III)-exposed biofilm (Table 1B). Modifications of the exopolymer abundance, properties and macromolecular composition are common in biofilms under heavy metal stress, but the changes observed vary widely, according to the conclusions of different studies (Lawrence et al. 2004; Priester et al. 2006; Vílchez et al. 2007). In a Cr(VI)-reducing strain of Pseudomonas putida grown in biofilms, exposure to chromate enhanced production of exopolymers, which were demonstrated involved in the subsequent retention of Cr(III) on the biofilm matrix (Priester et al. 2006). Changes in exopolymer abundance or composition are related to shifts in biofilm community structure or the induction of specific resistance mechanisms, both in response to the heavy metal selective pressure (Lawrence et al. 2004)
SEM–BSE images of the Cr(II) and Pb(II)-removing biofilters coupled to EDX analysis localized the heavy metal ions in the surface of the microbial biomass (Fig. 3), effectively indicating that the retention of the heavy metals takes place mediated by interactions with the extracellular biofilm matrix (biosorption) (Malik 2004). These complex matrixes bear polyionic charges from the chemical functional groups of their organic compounds, which sequester the metal ions to avoid their diffusion across the biofilm (Harrison et al. 2007). Generation of metabolic end-products such as sulfides, carbonates or phosphates by the cells in the biofilm also contribute to the removal of heavy metals by binding or chemically modifying the metal species, resulting in the precipitation of bioinorganic metal complexes (Harrison et al. 2007). In our study, lead and chromium abundantly occurred in the periphery of the cells together with silicate and/or phosphates, according to TEM/EDX analysis. These data suggest that the metal cations sorbed onto cell envelopes, which also served as nucleation centers to promote the precipitation as ternary surface complexes by binding to silicate or phosphate anions, as postulated in other interactions of bacteria with metals (Urrutia-Mera and Beveridge 1993).
In addition, the TEM–EDX analyses of biofilms from the Pb(II) biofilter demonstrated the presence of the heavy metal inside the microbial cells (Fig. 4), showing the involvement of accumulation mechanisms other than external passive biosorption to the biofilm biomass. Bacterial mechanisms for lead resistance are not well characterized and only studied in detail in few strains, but available data evidence several strategies, most often including the already mentioned immobilization by biosorption in combination with the active efflux of the Pb(II) to prevent toxic effects in the cell cytoplasm (Silver and Phung 2005). Nevertheless, known microbial mechanisms of resistance to lead can also involve intracellular sequestration of the heavy metal (Borremans et al. 2001; Levinson et al. 1996). Pb(II) resistance is well characterized in the Betaproteobacterium Cupriavidus metallidurans strain CH34, mediated by the Pb-inducible pbr genes (Borremans et al. 2001). The mechanism involves the active efflux of the metal ions to the periplasmic space via the PbrA ATPase, and their further precipitation in the form of insoluble phosphates, enabled by the inducible phosphatase PbrB (Hyninnen et al. 2009). Homologues of the pbr genes have been found in the genome of several bacterial species, indicating their possible widespread nature as a mechanism of lead resistance (Hyninnen et al. 2009).
Immobilization of Pb(II) by bacterial-released phosphates has been often described (Macaskie et al. 1994; Silver and Phung 2005). A PhoN-type phosphatase has been found involved in precipitation of Pb(II) and other heavy metal as phosphates by a Citrobacter spp. strain growing in biofilms (Macaskie and Dean 1987; Macaskie et al. 1994), while in other bacterial strains a connection between resistance to heavy metals and induction of phosphatase activities could not be demonstrated (Levinson and Mahler 1998). In our experiments, we found high values of alkaline phosphatase activity in the Pb(II)-exposed biofilm (Table 1A), compared to an analogous biofilter system operated with groundwater lacking heavy metals (Vílchez et al. 2007). This increment of the alkaline phosphatase activity in response to Pb(II), as well as the detection of P together with Pb(II) by TEM–EDX (Fig. 4), suggest a possible contribution of the released phosphates to the precipitation of Pb(II) in the biofilter, although further studies are required to ascertain if phosphatase activity is induced in biofilms as a response to Pb(II) exposure.
The results of TGGE fingerprinting showed that exposure to each heavy metal led to a different structure of the biofilm bacterial community. However, in both the Cr(III) and Pb(II)-exposed biofilters the bacterial fingerprints remained highly similar throughout the column depth, even though the concentration of heavy metals in water decreased towards the column end, and particularly in the case of Pb(II), the retained heavy metal was mostly accumulated in the upper half of the biofilter fixed-bed. There were also little differences in community composition before and after the back-flushing for both Cr(III) and Pb(II), indicating that the cleaning step effectively removed the excess biofilm material clogging the systems, without disturbing the main species composition of bacteria in the heavy metal-removing communities.
According to sequence analysis of the prevalent bands in TGGE profiles, the bacterial community of Cr(III)-exposed biofilm was dominated by both Alpha and Betaproteobacteria (Fig. 6). OTUs related to the Alphaproteobacteria were mostly close to the Rhizobiales and Sphingomonadales. Sphingomonads have been also reported as prevalent populations in biofilms from a nickel-polluted river (Lawrence et al. 2004), and also dominated the bacterial community in a Cu(II)-removing biofilm analyzed in a previous study in our laboratory (Vílchez et al. 2007). Betaproteobacteria populations identified in the Cr(III) biofilter were related to the genera Curvibacter, Acidovorax, and Variovorax of the Comamonadaceae (Fig. 6). Comamonadaceae are characteristically widespread in continental freshwater habitats as part of the cosmopolitan βI cluster of freshwater bacteria (Brümmer et al. 2003; Glöckner et al. 2000) and are also frequently described as inhabitants of heavy metal polluted environments (Bouskill et al. 2010; Brümmer et al. 2003; Critchley et al. 2004).
Most of the bands identified in the Pb(II)-exposed biofilm had a high sequence similarity, forming a cluster of the Alphaproteobacteria in the periphery of the genus Rhodobacter (Fig. 6). Members of the Rhodobacteraceae are metabolically versatile and common in aquatic habitats, where they can thrive even under poor availability of nutrients (Imhoff 2006). To the best of our knowledge, there are no previous reports in the literature regarding resistance to lead by Rhodobacteraceae, however, it is well known the ability of Rhodobacter spp. to tolerate high concentrations of other divalent metal cations such as Ni (II), Cd (II), Fe(II), Cu (II) or Co (II) (Buccolieri et al. 2006; Giotta et al. 2006; Vílchez et al. 2007; Watanabe et al. 2003). Precipitation of Cd (II) as sulfides has been suggested as the mechanism for removal of this heavy metal by growing cells of R. sphaeroides (Bai et al. 2008). In addition, R. sphaeroides dead biomass was successfully applied to the removal of Cd (II) and Pb(II) from water, by a passive biosorption mechanism (Seki et al. 1998).
To sum up, the results presented in this work further support previous studies regarding the suitability of biofilm-based biofilters as a low-cost method for the removal of heavy metals from groundwater (Vílchez et al. 2007), and broaden the knowledge regarding the physiological responses and community shifting experienced in the aquatic biofilms following exposure to the heavy metals chromium and lead. These data will be valuable for the future design and operation of full-scale systems aiming for the wide application of this remediation technology.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Alessio L, Campagna M, Lucchini R (2007) From lead to manganese through mercury: mythology, science and lessons for prevention. Am J Ind Med 50:779–787
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
APHA (2001). Standard methods for the examination of water and wastewater, 20th edn. LS Clesceri, AE Greenberg and AD Eaton (eds) American Public Health Association, Washington DC, USA
Bai HJ, Zhang ZM, Yang GE, Li BZ (2008) Bioremediation of cadmium by growing Rhodobacter sphaeroides: kinetic characteristic and mechanism studies. Bioresour Technol 99:7716–7722
Bencheikh-Latmani R, Obraztsova A, Mackey M, Ellisman M, Tebo BM (2007) Toxicity of Cr(III) to Shewanella sp. strain MR-4 during Cr(VI) reduction. Environ Sci Technol 41:214–220
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Borremans B, Hobman JL, Provoost A, Brown NL, Van der Lelie D (2001) Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J Bacteriol 183:5651–5658
Bouskill NJ, Barker-Finkel J, Galloway TS, Handy RD, Ford TE (2010) Temporal bacterial diversity associated with metal-contaminated river sediments. Ecotoxicology 19:317–328
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Brümmer IHM, Felske A, Wagner-Döbler I (2003) Diversity and seasonal variability of β-Proteobacteria in biofilms of polluted rivers: analysis by temperature-gradient gel electrophoresis and cloning. Appl Environ Microbiol 69:4463–4473
Buccolieri A, Italiano F, Dell’Atti A, Buccolieri G, Giotta L, Agostiano A, Milano F, Trotta M (2006) Testing the photosynthetic bacterium Rhodobacter sphaeroides as heavy metal removal tool. Ann Chem 96:195–203
Ceçen F, Semerci N, Geyik AG (2010) Inhibition of respiration and distribution of Cd, Pb, Hg, Ag and Cr species in a nitrifying sludge. J Hazard Mater 178:619–627
Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Critchley MM, Pasetto R, O’Halloran RJ (2004) Microbiological influences in “blue water” copper corrosion. J Appl Microbiol 97:590–597
Dean WE (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sed Petrol 44:242–248
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eastmon DA, MacGregor JT, Slesinski RS (2008) Trivalent chromium: assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit Rev Toxicol 38:173–190
Eccles H (1999) Treatment of metal-contaminated wastes: why select a biological process? Trends Biotechnol 17:462–465
Esmaeili A, Mesdaghi A, Vazirinejad R (2005) Chromium (III) removal and recovery from tannery wastewater by precipitation process. Am J Appl Sci 2:1471–1473
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med 26:235–244
Friedmann EI, Wierzchos J, Ascaso C, Winklhoferi M (2001) Chains of magnetite crystals in the meteorite ALH84001: evidence of biological origin. P Nat Acad Sci USA 98:2176–2181
Giotta L, Agostiano A, Italiano F, Milano F, Trotta M (2006) Heavy metal ion influence on the photosynthetic growth of Rhodobacter sphaeroides. Chemosphere 62:1490–1499
Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann R (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including and abundant group of actinobacteria. Appl Environ Microbiol 66:5053–5065
Gómez MA, Hontoria E, González-López J (2002) Effect of dissolved oxygen concentration on nitrate removal from groundwater using a denitrifying submerged filter. J Hazard Mater 90:267–278
Harrison JJ, Ceri H, Turner RJ (2007) Multimetal resistance and tolerance in microbial biofilms. Nat Rev Microbiol 5:928–938
Hynninen A, Touzé T, Pitkänen L, Mengin-Lecreulx D, Virta M (2009) An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol Microbiol 74:384–394
Imhoff JF (2006) The photoprophic beta-proteobacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes: a handbook on the biology of bacteria, 3rd Edn., vol 5: proteobacteria: alpha and beta subclasses. Springer, New York, pp 41–64
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with clustal X. Trends Biochem Sci 23:403–405
Karl DM (1980) Cellular nucleotide measurements and application in microbial ecology. Microbiol Rev 44:739–796
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Arizona State University, Arizona,
Lawrence JR, Chenier MR, Roy R, Beaumier D, Fortin N, Swerhorne GDW, Neu TR, Greer CW (2004) Microscale and molecular assessment of impacts of nickel, nutrients and oxygen level on structure and function of river biofilm communities. Appl Environ Microbiol 70:4326–4339
Levinson HS, Mahler I (1998) Phosphatase activity and lead resistance in Citrobacter freundii and Staphylococcus aureus. FEMS Microbiol Lett 161:135–138
Levinson HS, Mahler I, Blackwelder P, Hood T (1996) Lead resistance and sensitivity in Staphylococcus aureus. FEMS Microbiol Lett 145:421–425
Ludwig RD, Su C, Lee TR, Wilkin RT, Acree SD, Ross RR, Keeley A (2007) In situ chemical reduction of Cr(VI) in groundwater using a combination of ferrous sulfate and sodium ditionate: a field investigation. Environ Sci Technol 41:5299–5305
Macaskie LE, Dean ACR (1987) Use of immobilized biofilm of Citrobacter sp. for the removal of uranium and lead from aqueous flows. Enzyme Microb Tech 9:2–4
Macaskie LE, Bonthronea KM, Rouch DA (1994) Phosphatase-mediated heavy metal accumulation by a Citrobacter sp. and related enterobacteria. FEMS Microbiol Lett 121:141–146
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278
Matlock MM, Howerton B, Atwood D (2002) Chemical precipitation of lead from lead battery recycling plant wastewater. Ind Eng Chem Res 41:1579–1582
McComb EA, McCready RM (1957) Determination of acetyl in pectin and acetylated carbohydrate polymers. Anal Chem 29:819–821
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Murray KJ, Mozafarzadeh ML, Tebo BM (2005) Cr(III) oxidation and Cr toxicity in cultures of the manganese(II)-oxidizing Pseudomonas putida strain GB-1. Geomicrobiol J 22:151–159
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S RNA. Appl Environ Microbiol 59:695–700
Priester JH, Olson SG, Webb SM, Neu MP, Hersman LE, Holden PA (2006) Enhanced exopolymer production and chromium stabilization in Pseudomonas putida unsaturated biofilms. Appl Environ Microbiol 72:1988–1996
Ramana VV, Sastry KS (1994) Chromium toxicity in Neurospora crassa. J Inorg Biochem 56:87–95
Roe JH, Papadopoulos NM (1954) The determination of fructose-6-phosphate and fructose-1,6-diphosphate. J Biol Chem 210:703–707
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Seki H, Suzuki A, Mitsueda SI (1998) Biosorption of heavy metal ions on Rhodobacter sphaeroides and Alcaligenes eutrophus H16. J Colloid Interf Sci 15:185–190
Silver S, Phung LT (2005) A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol 32:587–605
Sokal RR, Rohlf FJ (1962) The comparison of dendrograms by objective methods. Taxon 11:33–40
Thompson SL, Manning FCR, McColl SM (2002) Comparison of the toxicity of chromium III and chromium VI to cyanobacteria. Bull Environ Contam Toxicol 69:286–293
Urrutia-Mera MU, Beveridge TJ (1993) Mechanism of silicate binding to the bacterial cell wall in Bacillus subtilis. J Bacteriol 175:1936–1945
Vignati DAL, Dominik J, Beye ML, Pettine M, Ferrari BJD (2010) Chromium (VI) is more toxic than chromium (III) to freshwater algae: a paradigm to revise? Ecotoxicol Environ Safe 73:743–749
Vílchez R, Pozo C, Gómez MA, Rodelas B, Gonzalez-Lopez J (2007) Dominance of sphingomonads in a copper-exposed biofilm community for groundwater treatment. Microbiol SGM 153:325–337
Watanabe M, Kawahara K, Sasaki K, Noparatnaraporn N (2003) Biosorption of cadmium ions using a photosynthetic bacterium, Rhodobacter sphaeroides S and a marine photosynthetic bacterium, Rhodovulum sp. and their biosorption kinetics. J Biosci Bioeng 95:374–378
Weisburg WG, Barn SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Bashan R (2007) New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharm 225:1–27
Wu Y, Zhang S, Guo X, Huang H (2008) Adsorption of Cr(III) on lignin. Bioresour Technol 99:7709–7715
Yu Z, Morrison M (2004) Comparison of different hypervariable regions of rrs genes fro use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol 70:4800–4806
Zayed A, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Acknowledgments
The authors thank the Confederación Hidrográfica del Guadalquivir (CHG, Sevilla, Spain) for their financial support for this research, and a Ph.D. scholarship to Dr. R. Vílchez. We thank the DNA Sequencing Service of Instituto de Parasitología y Bioquímica Lopez-Neyra (CSIC, Granada). Ms. C. Hernández Castillo, Ms. I. Guerra Tschuschke, and Ms. M. Abad Ortega (Centro de Instrumentación Científica, Universidad de Granada) are acknowledged for their invaluable technical assistance with SEM, TEM and EDX.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vílchez, R., Gómez-Silván, C., Purswani, J. et al. Characterization of bacterial communities exposed to Cr(III) and Pb(II) in submerged fixed-bed biofilms for groundwater treatment. Ecotoxicology 20, 779–792 (2011). https://doi.org/10.1007/s10646-011-0629-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0629-x