Abstract
Seeds of Vicia faba. L were grown in increasing concentrations of lead (Pb)-added soils (0–2,000 mg/kg). After germination of 25 days, roots were harvested to investigate oxidative stress, defense response and indicative biomarkers based upon chemical analyses and biological measurements. The results showed that higher concentrations of Pb-polluted soils led to seedling growth inhibition, indicative of phytotoxicity. O •−2 and lipid peroxidation were increased with the increase of available Pb in soils and Pb contents in roots, displaying a “J”-shaped dose response curve, whereas H2O2 showed a biphasic dose response curve (a consecutive “J”-shaped and inverted “U”-shaped curve). Superoxide dismutase (SOD), guaiacol peroxidase (POD) and ascorbate peroxidase (APX) enzymes were activated by soil Pb, displaying biphasic curves. The upregulated POD and APX enzymes might be major scavengers of excessive H2O2 when CAT activities were drastically reduced with the increasing soil Pb. The enhanced glutathione (GSH) and APX activities suggested that GSH-ascorbate cycle also participated in eliminating H2O2. Moreover, obvious changes were observed in SOD, CAT and POD isoenzyme patterns, but not in APX except increasing intensities of bands. HSP70 synthesis was significantly induced by extraneous Pb from 125 to 1,000 mg/kg and showed a biphasic curve in this experiment. Comparatively, HSP70 and lipid peroxidation might be more sensitive than other parameters in response to Pb stress, suggesting that these two parameters in the roots might be potential biomarkers for early bioassay of Pb-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal polluted soils have severe influence for environmental and human beings as accumulation of heavy metals in crops poses a threat to food safety (Dell’ Amico et al. 2008). The United States Environmental Protection Agency (USEPA) states that Pb is the most common heavy metal contaminant as a result of human, agricultural and industrial activities (Watanabe 1997). As a non-redox-active metal, Pb can substitute essential metals or cofactors at enzyme active sites, leading to an unbalance of essential trace metals and cellular redox status. Pb2+ phytotoxicity has been well documented in many species (Schützendübel and Polle 2002; Verma and Dubey 2003; Pallavi and Rama 2005; Liu et al. 2009), which was generally involved in induction of reactive oxygen species (ROS), resulting in oxidized cell components, inactivated enzymes, damaged DNA and even dead cells.
Enhanced ROS may also act as an elicitor of common stress response in plants (Mittler 2002). To alleviate oxidative stress due to ROS, plants have developed a series of antioxidant defense systems, such as superoxide dismutase (SOD), guaiacol peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and many natural antioxidants, such as α-tocopherol, β-carotene, ascorbate acid (ASC) and glutathione (GSH). Generally, these enzymes have isoenzymes, which may be expressed by distinct regulatory mechanisms in response to various environmental stresses and play the cooperative role in protecting each organelle and minimizing tissue injury (Mittler 2002).
Heat shock proteins (HSPs) are chaperone proteins functioned in maturation of renascent protein peptides in cells, which consist of a series of different molecular weights of proteins. They are also involved in repairing or accelerating disposal of denatured proteins, homeostatic maintenance and adaptation to various stresses. HSP70 induction is regarded as the toxicity criterion of metallic pollution (Aït-Aïssa et al. 2003; Bhargav et al. 2008), especially in response to increase of protein denaturation (Wang et al. 2008). Measurements of these chaperones are biomarkers for the integrity of the superstructure of the organisms (Downs et al. 2001). HSP70 family have been used as a general biomarker for heavy metal toxicity for terrestrial animals in soil (Nadeau et al. 2001; Arts et al. 2004) and aquatic contaminants for aquatic animals (Hallare et al. 2005). This method was also conducted to detect expression of HSPs in plant species, such as HSP70 in Fucus serratus and Lemna minor (Ireland et al. 2004), aquatic moss Fontinalis antipyretica (Rau et al. 2007) and HSP90 in transgenic Physcomitrella patens (Saidi et al. 2007). Based on previous studies of HSP70 with both animals and plants, we speculate that HSP70 could be a sensitive biomarker for Pb phytotoxicity in plants.
Biomarker response can mirror the stress in organisms and thus act as more precise indicator of the environmental status than that of chemical analysis (Hallare et al. 2005). Therefore, chemical measurements need to be complemented with biochemical assays in a multidisciplinary approach to assess soil contamination.
The previous study showed that Pb-treated soils contributed to oxidative stress and defense response in leaves of Vicia faba seedlings (Wang et al. 2008). As a result, O •−2 , HSP70 and guaiacol peroxidase isoenzymes were proposed as potential biomarkers of Pb-contaminated soils. Heavy metals are absorbed and mainly accumulated in roots. Thus, roots may be primary sufferers and first defense organs against heavy metal stress. In this experiment, roots of V. faba seedlings were employed preferentially as tested material. The objectives are to investigate oxidative stress, defense responses and toxicological effects in the roots of V. faba seedlings grown in Pb-contaminated soils, and further screen and validate the potential biomarkers in the roots for early warning of Pb-polluted soils.
Materials and methods
Soil treatment and plant materials
Soil sample was collected from Huainan district, Anhui Province, China. In the native soil, pH value was 7.6, total Pb content was 20.72 mg/kg dry weight and organic matter was 1.105%. After spiking, concentrations of extraneous Pb in soil were 0, 25, 125, 250, 500, 1000 and 2000 mg/kg, respectively. The soils were equilibrated naturally for nearly 2 months. The pH values were measured according to ISO 10390 (International Organization for Standardization, 1994. Soil Quality-Determination of pH, No. 10390. ISO, Geneva) and ranged from 7.4 to 7.6 before sowing.
The nearly homogeneous seeds of V. faba were selected and sterilized with 0.1% sodium hypochlorite solution for 10 min and then rinsed with distilled water. Six seeds were sown in a pot containing 1.5 kg of Pb-added soil. The seedlings were cultured in a growth chamber under controlled conditions (24–25°C, 70% relative air humidity, a photoperiod of 14/10 h (day/night), photosynthetic active radiation of 200 μmol/m2 s). They were daily watered and supplemented with equal amount of Hoagland nutrient solution (Lucretti et al. 1999) once a week. Roots were harvested after germination of 25 days. A single experiment was performed in 4 replicates in each treatment.
Determination of available Pb in soil and Pb content in the roots
Available Pb in soil was extracted using acetic acid (0.11 M) and then measured by Atomic Absorption Spectrophotomer (Solaar M series equipped with a GF95 graphite furnace and an FS95 autosampler, Thermo Elemental, Cambridge, UK). The detection limits for Pb was 0.07 mg/l.
After being harvested, fresh roots were rinsed with 1 M HCl followed by distilled water, then dried at 60°C. The digestion of dried samples and determination of Pb content were performed in consistent with the previous protocol (Wang et al. 2008). The Pb content was expressed as μg/g dry weight. Certified standard samples (GBW07429) and triplicates of all samples were used to ensure accuracy and precision. The concentrations of Pb in all the samples are above the method detection limits (0.01 μg/l).
Measurement of shoot heights of the seedlings
Length between apical leaf and basal stem was measured and denoted as shoot height. There were four pots in each treatment, and heights of six seedlings were measured in each pot.
Determination of GSH content in the roots
GSH content in roots was determined according to the method described by Hissin and Hilf (1976), with minor modification. 0.4 g of fresh roots were ground in liquid nitrogen and homogenized in 100 mM sodium phosphate buffer (pH 8.0) on ice, containing 5 mM Na2EDTA and 6.0% (m/v) meta-phosphoric acid (HPO3). The homogenate was centrifuged at 13,000×g for 30 min at 4°C. 4.5 ml of the phosphate-Na2EDTA buffer was added to 0.5 ml of the supernatant. The final assay mixture (2.0 ml) contained 0.1 ml of the diluted supernatant, 1.8 ml of phosphate-Na2EDTA buffer and 0.1 ml of O-phthalaldehyde reagent (OPT), including 100 μg of OPT. GSH content was measured fluorometrically after a brief and thorough mixing followed by 15 min of incubation at room temperature. Its fluorescence intensity was recorded at 420 nm after excitation at 350 nm on fluorescence spectrophotometer (Hitachi, model 850). A standard curve of GSH was prepared similarly and used for calibration.
Determination of antioxidant enzymes’ activities and corresponding isoenzymes in the roots
Enzyme extraction was prepared according to the method described by Romero-Puertas et al. (2004) with supplement of 5 mM ASC. 1 g of fresh roots was ground immediately to be fine powder under liquid nitrogen and homogenized in extraction buffer (0.1 M Tris–HCl (pH 8.0), 10% (v/v) glycerol, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.2% (v/v) Triton X-100, 2 mM DTT, 5 mM ASC and 1 mM phenylmethylsulphonyl fluoride). The extraction was centrifuged at 15,000×g for 30 min to remove precipitation. Soluble protein content was determined by Bradford (1976) with BSA as standard. All operations were performed at 0–4°C.
SOD (EC 1.15.1.1) activity was determined according to previous protocol (Wang et al. 2008). 3 ml of assayed mixture contained 50 mM phosphate buffer (pH 7.8), 50 μM NBT, and 0.03% Triton X-100, 0.1 mM Na2EDTA, 14.5 mM methionine, 1.05 μM riboflavin and 100 μl enzyme extractions. The reaction was started by adding riboflavin and A560 was measured immediately after 12 min illumination with light intensity of 12,000 lux. Control assays with and without the enzyme were also run. SOD activity is expressed as units/min mg protein. One unit of enzyme activity is defined as the amount of enzyme required to inhibit the NBT reduction by 50%.
Guiacol POD (EC 1.11.1.7) activity was assayed according to García-Limones et al. (2002), CAT (EC 1.11.1.6) activity according to Bestwick et al. (2001) and APX (EC 1.11.1.11) activity according to Janda et al. (1999).
Isoenzyme patterns were resolved by native polyacrylamide gel electrophoresis (PAGE) using Bio-Rad Mini-Protein 2 electrophoresis system. Crude enzyme corresponding to 20.0 μg of total soluble protein, mixed with glycerin and bromophenol blue, was loaded into each lane for separation of antioxidant isoenzymes. Electrophoresis was subjected to constant voltage of 70 V reaching resolving gel, and resumed to end by 100 V at ice bath using a 25 mM Tris, 192 mM glycine solution (pH 8.3) as running buffer.
SOD, POD and APX isoenzymes were visualized as described by García-Limones et al. (2002), CAT isoenzymes were visualized as described by Verma and Dubey (2003). Photographs were taken by Bio-Rad gel image system.
Determination of O •−2 , H2O2 and lipid peroxidation in the roots
O •−2 content was determined according to the method described by Ke et al. (2002), with minor modification. 0.4 g of fresh roots were quickly ground in liquid nitrogen and homogenized at a 1:5 (w/v) ratio in a solution containing 100 mM phosphate buffer (pH 8.0), 10 μM pyridoxal phosphate, 1 mM Na2EDTA and 5 mM DTT, then centrifuged at 15,000×g for 20 min at 4°C. The supernatant was collected as crude enzyme extraction and used immediately for investigation of O •−2 production. Detailed operation was specified in our previous protocol (Wang et al. 2008). The specific absorption was assayed at 530 nm. O •−2 content was calculated according to the standard curve, which was prepared with sodium nitrite as discussed above.
H2O2 content was determined by the method of Patterson et al. (1984), with minor modification. 0.5 g of fresh roots were ground in liquid nitrogen, homogenized in 4 ml cold acetone, and centrifuged at 1,500×g for 10 min. 0.1 ml 20% TiCl4 and 0.2 ml concentrated ammonia were added into 1 ml of the supernatant. After 10 min of reaction at 25°C, the mixture was centrifuged at 1,500×g for 5 min. Rinsed twice with cold acetone, the sediment was dissolved in 3 ml of 2 M H2SO4. The absorption was measured at 410 nm, and H2O2 content was calculated according to standard curve.
Lipid peroxidation was determined by measuring the concentration of 2-thiobarbituric acid reacting substances (TBARS) according to the method described by Verma and Dubey (2003). The content of lipid peroxides was quantified and expressed as total TBARS in terms of μmol/g fresh weight by the extinction coefficient of 155 mM−1 cm−1.
Western blotting and measurement of HSP70 production in the roots
The enzyme extraction was also used as total protein extraction. Lysis buffer consisted of 0.5 M Tris, pH 6.8, 20% (v/v) glycerol, 4% (w/v) SDS, 0.2% (w/v) bromophenol blue and 10% (v/v) β-mercaptoethanol. Aliquots of total protein extraction were mixed with lysis buffer (2:1), boiled for 5 min, cooled on ice. SDS-PAGE and western blotting of HSP70 were conducted according to methods of Arts et al. (2004) with minor modification. 18.0 μg of total protein per lane with prestained molecular weight markers were analyzed by SDS-PAGE (10% separating gel and 5% stacking gel). After electrophoresis, gels were either stained with Coomassie brilliant blue R-250 or transferred onto PVDF membrane (Amersham Pharmacia) (Bio-Rad semi-dry transfer system). The membranes were blocked using 8% (w/v) non-fat milk/TBS-T buffer (50 mM Tris, 150 mM NaCl, pH 7.5, containing 0.05% Tween-20) for 2 h. After washing in TBS-T buffer, mouse anti-HSP70/HSC70 monoclonal antibody (SPA-820, Stressgen, Victoria, B.C., Canada) (dilution 1:5,000 in 1% BSA/TBS), were added and incubated overnight at 4°C. After washing, the membranes were incubated in secondary antibody (anti-mouse IgG conjugated with horseradish peroxidase, Stabizyme, USA) (dilution 1:5,000 in 1% BSA/TBS) at room temperature for 2 h. Bands were visualized by ECL reagent (Amersham) and exposed to X-ray film. Integrated intensities of bands were quantified by Image J software (National Institutes of Health, USA) with background subtraction, and then normalized to the control level. Four replicates in each treatment were performed in this experiment.
Statistical analysis
All the statistical analysis was performed using SPSS version 13.0 for Windows (SPSS, Chicago, IL, USA). The data were all presented as mean ± standard deviations of four replicates. Difference was considered to be significant at p < 0.05(*) and highly significant at p < 0.01(**) using one-way ANOVA by Dunnett’s t test. Representative photographs from each treatment were presented.
Results
Available Pb in soils and Pb content in roots
The results showed that available Pb in soils was increased in parallel with extraneous Pb, increasing 2.9–440 times higher than the control in all the treatments. Pb content in roots was also enhanced with the extraneous Pb, increasing 16.2–125 times higher than that of the control in all the treatments (Table 1). Moreover, the available Pb was well correlated with the Pb content in the roots (r = 0.998, p < 0.01).
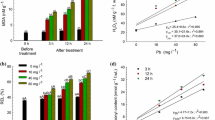
Effect of Pb on shoot heights of the seedlings
As shown in Fig. 1, the shoot heights declined below the control at 25 mg/kg, enhanced from 125 to 250 mg/kg, and then decreased below the control hereafter, displaying a biphasic dose response curve (a consecutive “J”-shaped and inverted “U”-shaped curve) with the increasing extraneous Pb in soils. Significant decline occurred when the concentrations increased above 1,000 mg/kg.
GSH synthesis was induced by Pb in the roots
GSH content in the roots was decreased at 25 mg/kg, increased from 125 to 1,000 mg/kg, and then tended to decline thereafter, displaying a biphasic dose response curve (Fig. 2). The maximal GSH content was approximately 1.3 times higher than that of the control at 1,000 mg/kg. Moreover, the GSH content was well correlated with Pb content in the roots at 25–1,000 mg/kg (r = 0.882, p < 0.05).
Antioxidant enzymes and corresponding isoenzymes were activated by Pb in the roots
The results showed that SOD activities slightly declined at 25 mg/kg, increased with the increasing extraneous Pb from 25 to 500 mg/kg, and then tended to decrease thereafter, displaying a biphasic curve in all the treatments. Significant enhancement of SOD activities was observed at 500 and 1,000 mg/kg (Fig. 3a). The results also showed that the total activities of CAT enzymes decreased along with the increasing extraneous Pb, and significant inhibition was showed when the added Pb increased above 250 mg/kg (p < 0.05, p < 0.01) (Fig. 3b). A biphasic curve was observed along with the increase of extraneous Pb from 25 to 2,000 mg/kg (Fig. 3c, d) when POD or APX activities were concerned.
Electrophotograms of SOD, CAT, POD and APX isoenzymes were resolved by native PAGE, respectively. Obvious changes were found in SOD and CAT patterns, of which, band-1 was completely lost at 2,000 mg/kg (Fig. 4a, b). In POD patterns, band-3 was hardly visible at 125–250 mg/kg and blurry in the other treatments (Fig. 4c). APX isoenzymes did not show any changes in their patterns but integrated intensities of bands compared with the control (Fig. 4d). In addition, the intensities of SOD, CAT, POD and APX bands were generally consistent with their respective enzyme activities.
Changes in the patterns of antioxidant isoenzymes in roots of V. faba seedlings grown in Pb-treated soils after germination of 25 days. a–d represent the patterns of SOD, CAT, POD and APX isoenzymes, respectively. 0 represents the corresponding standard of antioxidant enzymes; 1–7 represent the control, 25, 125, 250, 500, 1000 and 2000 mg/kg of Pb-treated soils, respectively
Production of O •−2 , H2O2 and lipid peroxidation were induced by Pb in the roots
A “J”-shaped curve of O •−2 production was detected in the roots exposed to 0–2,000 mg/kg of Pb-treated soils for 25 days. The O •−2 production was well correlated with Pb contents in the roots at 25–2,000 mg/kg (r = 0.919, p < 0.01), and significant enhancement was detected at 1,000–2,000 mg/kg (Fig. 5a).
H2O2 production was fluctuated as a biphasic curve along with the increase of extraneous Pb in soils. It decreased below the control at 25 mg/kg, enhanced slightly until 250 mg/kg, and then tended to decline thereafter. The significant reduction occurred when the extraneous Pb was more than 500 mg/kg (Fig. 5b). By comparison, the trend of H2O2 production was approximately opposite to that of the O •−2 in the roots.
Lipid peroxide levels were quantified and expressed as total TBARS in terms of μmol/g fresh weight in the roots. As shown in Fig. 6, TBARS production was slightly decreased at 25 mg/kg, and then significantly enhanced thereafter (p < 0.01), showing a “J”-shaped curve. The maximum of TBARS increased up to nearly 5.5-fold of the control at 2,000 mg/kg.
Pb induced HSP70 expression in the roots
HSP70 production in the roots was investigated by SDS-PAGE and western blotting (Fig. 7a). Integrated intensities of HSP70 bands were measured and transformed into relative integrated intensities versus the control, denoted as HSP70 levels. The results showed that HSP70 levels were slightly decreased at 25 mg/kg, significantly increased at 125–1,000 mg/kg (p < 0.05, p < 0.01), and then fell below the control at 2,000 mg/kg (Fig. 7b), displaying a biphasic curve. The maximum of HSP70 reached nearly 3 times as high as that of the control.
HSP70 was induced in roots of V. faba seedlings grown in Pb-treated soils after germination of 25 days; a represents SDS-PAGE and Western blotting of HSP70; b represents relative integrated intensities of HSP70 compared with the control. 1–8 represent standard mass of protein markers, 25, 125, 250, 500, 1000 and 2000 mg/kg of Pb-treated soil, respectively. Values are denoted as mean ± SD (n = 4). Significant differences from the control are indicated as follows: * p < 0.05, ** p < 0.01
Discussion
Heavy metals in soil can be absorbed by roots and further transported to shoots. The results showed that Pb absorbed by V. faba seedlings was mainly accumulated in the roots (Table 1), and only a small proportion transferred to the leaves (Wang et al. 2008). This result agrees with the findings of Liu et al. (2009). Thus, roots are primary sufferers from heavy metal-polluted soils. Much attention was focused on roots of V. faba seedlings in the present study.
The results showed that soil Pb caused phytotoxicity to the seedlings, which was evidenced by the significant decrease of shoot heights at concentrations of 1,000–2,000 mg/kg (Fig. 1). For the same treatments, Pb contents in the roots were much higher than in the leaves, resulting in the levels of O •−2 and lipid peroxidation in roots (Figs. 5a, 6) were higher than those in the leaves (Figs. 1a, 2a in Wang et al. 2008). Moreover, the levels of O •−2 in roots and leaves were well correlated with the respective Pb contents in roots (r = 0.919, p < 0.01) and leaves (r = 0.969, p < 0.01) (Wang et al. 2008). Thus, Pb, as a non-redox-active metal, was possibly involved in mediating the production of O •−2 in the seedlings.
Oxidative stress occurs only if ROS levels exceed the antioxidative capacity of organisms (Wiegand and Pflugmacher 2005). To counteract adverse effects of ROS, plants have developed a series of antioxidative enzymes and antioxidants, employed in eliminating ROS. Generally, these enzymes have multiple isoenzymes, which may be expressed by distinct regulatory mechanisms in response to various environmental stresses and play the cooperative role in protecting each organelle and minimizing tissue injury (Mittler 2002). The results showed that SOD, POD and APX isoenzymes and corresponding activities in the roots were activated by concentrations of soil Pb and displayed biphasic dose response curves. CAT activities in the roots were decreased drastically with the increase of soil Pb and that in the leaves showed an inverted U-shaped dose response curve. It is the hormetic effect, which was attributed to higher Pb contents in the roots than in the leaves.
H2O2 levels in the roots and leaves were displayed as a biphasic curve or an inverted “U”-shaped curve in all the treatments, respectively. H2O2 levels in the roots were much lower than those in the leaves (Fig. 5b) due to lower SOD activities in the roots. H2O2 ought to be overproduced when SOD activites and O •−2 production were markedly enhanced at 1,000 mg/kg (Figs. 3a, 5a). However, the H2O2 levels in the roots were significantly decreased at 500–2,000 mg/kg. Similar trend was also observed in the leaves at 1,000–2,000 mg/kg (Wang et al. 2008). The enhanced POD and APX activities might be acted as major scavengers of excessive H2O2 when CAT activities were drastically reduced (Fig. 3), which might be responsible for the declined H2O2 in the roots exposed to higher concentrations of Pb. These results were consistent with the previous report (Wang et al. 2008).
The mechanism of heavy metal detoxification in plant species may also involve in chelating metals by phytochelatins (PCs). GSH is not only an effective antioxidant in GSH-ASC cycle, but also a precursor for biosynthesis of PCs. The results showed that GSH production was positively correlated with the available Pb in soil (r = 0.987, p < 0.01) and Pb contents in roots (r = 0.997, p < 0.01) at 25–1,000 mg/kg. GSH synthesis was induced by Pb in the roots. Moreover, the enhancement of GSH production and APX activities indicated that GSH-ASC cycle also participated in H2O2 elimination in the roots.
Organisms tend to increase HSPs’ synthesis besides enhancement of a series of antioxidative enzymes and antioxidants under environmental stressors. HSP70 family are highly conserved, whose induction has been observed in a wide range of organisms. HSP70 accumulates due to stress, especially in response to increased protein synthesis and denaturation. Investigation of HSP70 expression will be useful in determining whether the organism is stressed by a particular environmental condition and the degrees to be stressed in the organism. Therefore, HSP70 family have been conducted as potential biomarkers for environmental stressors, such as heavy metal toxicity for earthworm Lumbricus terrestris (Nadeau et al. 2001), isopods and nematodes (Arts et al. 2004) and transgenic Drosophila melanogaster (Bhargav et al. 2008), as well as contaminants for zebrafish (Hallare et al. 2005) and Mediterranean mussel (Franzellitti and Fabbri 2005). But there were only a few studies focused on HSPs expression in plant species by means of western blotting method (Ireland et al. 2004; Rau et al. 2007; Wang et al. 2008). In the results, HSP70 was significantly induced at 125–1,000 mg/kg of Pb-treated groups, suggesting that HSP70 could potentially be a biomarker of Pb-polluted soils.
It is widely accepted that biomonitoring is necessary for a reliable environmental risk assessment in addition to chemical monitoring (Van der Oost et al. 2003). For instance, increased SOD activity was considered as a sensitive biomarker for oxidative stress, such as Cd (Sun and Zhou 2008). In the previous study, the change in POD patterns of the leaves could be indicative of Pb-treated soils (Wang et al. 2008). In the roots, band-3 became hardly visible at 125–250 mg/kg, and blurry at the control and other treatments (Fig. 4c), which might not be used as a qualitative indicator of Pb-contaminated soils in the roots.
Since a single biomarker alone may not always provide a proper assessment of an unknown environmental stressor, multiple biological markers at different biological levels are necessary to properly assess the level of ecological integrity (Franco et al. 2002). Furthermore, choice of a suite of biomarkers combined with chemical analysis has been proposed as a more appropriate approach to monitor effects of pollutants in aquatic ecosystems (Cajaraville et al. 2000). Thus, a set of physiological and biochemical parameters were investigated combined with chemical analysis of available Pb in soil and total Pb in roots. Comparatively, HSP70 and lipid peroxidation were more sensitive than other parameters in response to soil Pb stress, suggesting that these two parameters could be used in early bioassay of Pb-polluted soils.
Conclusions
O •−2 , H2O2, lipid peroxidation and shoot heights changed as a “J”-shaped or biphasic dose response curve along with the increase of soil extraneous Pb from 0 to 2,000 mg/kg, which indicated that higher concentrations of soil Pb caused oxidative stress in roots of V. faba seedlings. As defense responses, SOD, CAT, POD and APX enzymes including respective isoenzymes were activated, while GSH and HSP70 production were also enhanced for Pb detoxification in the roots. The increased GSH and APX activities suggested that GSH-ASC cycle also functioned in clearance of excessive H2O2. In SOD and CAT isoenzyme patterns, band-1 was completely lost at 2,000 mg/kg, which could be only used as a qualitative indicator of higher doses of Pb-contaminated soils. In addition, the total intensities in SOD, CAT, POD or APX isoenzymes were generally isochronous with their respective enzyme activities. Our results appeared to suggest that HSP70 and TBARS production could be used as potential biomarkers for early bioassay of Pb-polluted soils. It takes more than one study to firmly prove that HSP 70 can be used as a reliable biomarker of heavy metals in plants.
References
Aït-Aïssa S, Pandard P, Magaud H, Arrigo A-P, Thybaud E, Porcher J-M (2003) Evaluation of an in vitro hsp70 induction test for toxicity assessment of complex mixtures: comparison with chemical analyses and ecotoxicity tests. Ecotoxicol Environ Saf 54:92–104
Arts MJSJ, Schill RO, Knigge T, Eckwert H, Kammenga JE, Kohler HR (2004) Stress proteins (HSP70, HSP60) induced in isopods and nematodes by field exposure to metals in a gradient near Avonmouth, UK. Ecotoxicology 13:739–755
Bestwick CS, Adam AL, Puri N, Mansfield JW (2001) Characterization of and changes to pro- and anti-oxidant enzyme activities during the hypersensitive reaction in lettuce (Lactuca satia L.). Plant Sci 161:497–506
Bhargav D, Singh MP, Murthy RC, Mathur N, Misra D, Saxena DK, Chowdhuri DK (2008) Toxic potential of municipal solid waste leachates in transgenic Drosophila melanogaster (hsp70-lacZ): hsp70 as a marker of cellular damage. Ecotoxicol Environ Saf 69:233–245
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:201–212
Dell’ Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium resistant rhizobacteria. Soil Biol Biochem 40:74–84
Downs CA, Fauth JE, Woodley CM (2001) Assessing the health of grass Shrimp (Palaeomonetes pugio) exposed to natural and anthropogenic stressors: a molecular biomarker system. Mar Biotechnol 3:370–397
Franco A, Malavasi S, Pranovi F, Naci C, Torricelli P (2002) Ethoxyresorufin O-deethylase (EROD) activity and fluctuating asymmetry (FA) in Zosterisesssor ophiocephalus (Teleostei, Gobiidae) as indicators of environmental stress in the Venice lagoon. J Aquat Ecosyst Stress Recovery 9:239–247
Franzellitti S, Fabbri E (2005) Differential HSP70 gene expression in the Mediterranean mussel exposed to various stressors. Biochem Biophys Res Commun 336:1157–1163
García-Limones C, Hervás A, Navas-Cortés JA, Jiménez-Diaz RM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol Mol Plant Pathol 61:325–337
Hallare AV, Pagulayan R, Lacdan N, Köhler HR, Triebskorn R (2005) Assessing water quality in a tropical lake using biomarkers in zebrafish embryos: developmental toxicity and stress protein response. Environ Monit Assess 104:171–187
Hissin PJ, Hilf RA (1976) Fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Ireland HE, Harding SJ, Bonwick GA, Jones M, Smith CJ, Williams JHH (2004) Evaluation of heat shock protein 70 as a biomarker of environmental stress in Fucus serratus and Lemna minor. Biomarkers 9:139–155
Janda T, Szalai G, Tari I, Páldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208:175–180
Ke DS, Wang AG, Sun GC, Dong LF (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot Sin 44:551–556 (in Chinese)
Liu DH, Zou J, Meng QM, Zou JH, Jiang WS (2009) Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology 18:134–143
Lucretti S, Nardi L, Nisini PT, Moretti F, Gualberti G, Doležel J (1999) Bivariate flow cytometry DNA/BrdUrd analysis of plant cell cycle. Methods Cell Sci 21:155–166
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nadeau D, Corneau S, Plante I, Morrow G, Tanguay RM (2001) Evaluation for HSP70 as a biomarker of effects of pollutants on the earthworm Lumbricus terrestris. Cell Stress Chaperon 6:153–163
Pallavi S, Rama SD (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Patterson BD, Mackae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Rau S, Miersch J, Neumann D, Weber E, Krauss G-J (2007) Biochemical responses of the aquatic moss Fontinalis antipyretica to Cd, Cu, Pb and Zn determined by chlorophyll fluorescence and protein levels. Environ Exp Bot 59:299–306
Romero-Puertas MC, Mccarthy I, Gómez M, Sandalio LM, Corpas FJ, Del Río LA, Palma JM (2004) Reactive oxygen species-mediated enzymatic systems involved in the oxidative action of 2,4-dichlorophenoxyacetic acid. Plant Cell Environ 27:1135–1148
Saidi Y, Domini M, Choy F, Jean-Pierre Z, Jean-Paul S, Goloubinoff P (2007) Activation of the heat shock response in plants by chlorophenols: transgenic Physcomitrella patens as a sensitive biosensor for organic pollutants. Plant Cell Environ 30:753–763
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sun FH, Zhou QX (2008) Oxidative stress biomarkers of the polychaete Nereis diversicolor exposed to cadmium and petroleum hydrocarbons. Ecotoxicol Environ Saf 70(1):106–114
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang CR, Wang XR, Tian Y, Yu HX, Gu XY, Du WC, Zhou H (2008) Oxidative stress, defense response, and early biomarkers for lead-contaminated soil in Vicia faba seedlings. Environ Toxicol Chem 27:970–977
Watanabe ME (1997) Phytoremediation on the brink of commercialization. Environ Sci Technol 31:182–186
Wiegand C, Pflugmacher S (2005) Ecotoxicological effects of selected cyanobacterial secondary metabolites: a short review. Toxicol Appl Pharmacol 203:201–218
Acknowledgments
We appreciate the National Nature Science Foundations of China (grant No. 20577021 and No. 20877032). We also appreciate the Foundation of State Key Laboratory of Pollution Control and Resources Reuse of China (grant No. PCRRF 08011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Tian, Y., Wang, X. et al. Lead-contaminated soil induced oxidative stress, defense response and its indicative biomarkers in roots of Vicia faba seedlings. Ecotoxicology 19, 1130–1139 (2010). https://doi.org/10.1007/s10646-010-0496-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0496-x