Abstract
Behavioral effects resulting from exposure to dietary methylmercury (MeHg) have been reported in studies of several wildlife species. However, quantifying the impact of contaminant exposure on wild populations is complicated by the confounding effects of other environmental stressors. We controlled confounding stressors in a laboratory study to quantify the level of dietary MeHg exposure associated with negative effects on the fitness of captive-reared common loon (Gavia immer) chicks. We evaluated the effect of MeHg on loon chick behavior by employing several assays, including measures of righting reflexes, responsiveness to taped parental calls, reaction to frightening stimuli, and estimates of time activity budgets. Evidence suggested that as chicks aged, those exposed to nominal dietary dose levels of 0.4 and 1.2 μg Hg/g wet-weight in food (average estimated delivered dietary level of 0.55 and 1.94 μg Hg/g, respectively) were less likely (p < 0.01) to right themselves after being positioned on their backs during outdoor trials (≥37 days old) compared to chicks on the control diet. We detected differences (p < 0.05) in several response variables with respect to source of eggs. Chicks from nests on low-pH lakes tended to spend more time on resting platforms, spent less time in the shade, were more likely to walk across a platform upon release and do it quicker, were less responsive to a frightening stimulus, and exhibited less intense response to parental wail calls than did chicks from neutral pH-lakes. Rapid MeHg excretion during feather growth likely provides loon chicks protection from MeHg toxicity and may explain the lack of behavioral differences with dietary intake. Lake source effects suggest that in ovo exposure to MeHg or other factors related to lake pH have consequences on chick behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Consumption of fish is the primary route of exposure in piscivorous wildlife, to which methylmercury (MeHg) can be neurotoxic (Eisler 1987). Common loons (Gavia immer) are thought to be sensitive to the toxic effects of mercury (Hg; Barr 1986; Scheuhammer and Blancher 1994; Nocera and Taylor 1998) and are at elevated risk of Hg exposure relative to other wildlife species on inland (non-marine) North American aquatic systems as they are high trophic level, long-lived, obligate piscivores. Altered adult behavior and egg laying, embryo mortality (reduced hatching rates), increased chick mortality, and altered chick behavior have been associated with elevated Hg exposure in birds (Nocera and Taylor 1998; Heinz 1979; Thompson 1996). Loons nesting on acidic lakes in northern Wisconsin have elevated Hg levels in their blood and eggs and exhibit reduced reproductive performance (Meyer et al. 1995, 1998).
Retarded righting reflexes, impaired swimming ability, impaired maze and avoidance learning, and deficits in operant learning have been observed in domestic rodents exposed to MeHg (Shimai and Satoh 1985). Watanabe and Satoh (1996) summarized effects of prenatal exposure to MeHg on the development of motor, cognitive, and sensory functions; and motivation and arousal behavior in rodents. In avian laboratory dosing studies, effects of MeHg exposure include lower responsiveness to maternal calls and hypersensitivity to fright stimulus in mallard (Anas platyrhynchos) ducklings (Heinz 1975, 1976, 1979); and photosensitivity, alteration in preening activity, and lower motivation to hunt with increasing levels of dietary MeHg exposure in great egret (Ardea alba) nestlings (Bouton et al. 1999). Behavioral field studies of young (<13 days old) common loon chicks documented an increase in time apportioned to preening and a decrease in time spent riding on the backs of parents with increasing chick blood Hg levels (Nocera and Taylor 1998; Counard 2001). Time apportioned to swimming, peering, and begging were positively related to blood Hg in older (>40 days) common loon chicks, whereas diving and frequency of wing flaps of this age group were negatively related to Hg exposure (Counard 2001).
The work reported here was part of a larger study to develop a scientifically defensible ecological risk assessment for mercury for wildlife, based on an at-risk species, using a combination of laboratory and field studies. Many environmental stressors, including Hg exposure, can impact loon productivity. Differing rates of prey availability, nest habitat quality, predation, disease, or human disturbance could conceivably co-vary with loon Hg exposure. We conducted a laboratory experiment to quantify the level of Hg exposure associated with negative effects on loon chick survival and fitness, while controlling for stressors other than Hg exposure. Because Hg concentration in common loon eggs in northern Wisconsin are higher in eggs from low-pH (≤6.3) lakes than from neutral-pH (>6.3) lakes (0.64 ± 0.03 [SE] vs. 0.43 ± 0.05; t = 3.89; p = 0.0006; Fevold et al. 2003), we incorporated source of eggs with respect to lake pH (lake source) into the study design because we suspected that potential in ovo differences in egg-Hg burden or other factors associated with lake source might ultimately impact the response of chicks. Here we report on the effects of chronic dietary MeHg exposure on the behavior of loon chicks reared from hatch to 105 days. We evaluated behavioral effects using a number of measures including assessments of righting reflex, walking response, time activity budgets, reaction to frightening stimuli, and responsiveness to taped parental calls.
Methods
Source and husbandry of common loon chicks
The study was conducted during the summers of 1999, 2000, and 2003. Common loon eggs were collected from a four-county region of northern Wisconsin from lakes where loons are known to have elevated levels of Hg exposure (from low [≤6.3] pH lakes) and at lakes where loons are known to have low levels of Hg exposure (from neutral [>6.3] pH lakes). Details concerning the source, collection, and incubation of eggs are available in Kenow et al. (2003). Hatched chicks were marked with a unique 4-digit web tag (Haramis and Nice 1980), and randomly assigned to one of four dietary MeHg treatment groups.
The experimental design included 83 loon chicks; 24 chicks in 1999, 36 chicks in 2000, and 23 chicks in 2003 (Table 1). Chicks were held in indoor fiberglass fish raceways (190 cm × 70 cm × 36 cm) for the first month. Water depth was maintained at about 26 cm. Each raceway was provided with an elevated brooding platform positioned under a 250-watt infrared heat lamp. Room lighting was maintained at an approximate 16L:8D light cycle. At about 1 month of age, the chicks were moved to 50-m2 outdoor ponds flooded to a depth of about 60 cm and equipped with resting platforms and heat lamps. A constant supply of well water (approximately 12°C) was supplied to the raceways and outdoor ponds. See Kenow et al. (2003) for additional details concerning loon chick husbandry. Handling and care of chicks were approved by the Animal Care and Use Committee of the U. S. Geological Survey’s Upper Midwest Environmental Sciences Center and complied with the Animal Welfare Act.
An individual that exhibited three or more behaviors symptomatic of bacterial or viral infection (i.e., lethargy, reduced appetite, mass loss, paralysis of one leg, wing droop, and listing to one side when floating or swimming) was considered likely to have developed an illness from an unidentified pathogen (illness syndrome), and a blood sample was drawn and submitted for complete blood counts and serum protein electrophoresis. Elevated white blood cell counts and presence of globulinopathies were used to confirm the presence of an infectious process.
Delivery of dietary MeHg
Daily MeHg doses were administered via a rainbow trout (Oncorhynchus mykiss) that contained a gelatin capsule with the prescribed daily dose of CH3HgCl. One group served as a control and was fed a fish diet containing no added CH3HgCl; a second group was to receive a fish diet containing 0.08 μg Hg (as CH3HgCl) per g wet fish (representing the Hg level found in loon prey associated with neutral-pH lakes); a third group was to receive a fish diet containing 0.4 μg Hg per g wet fish (the Hg level found in loon prey associated with lakes with low pH [<6.3]); and a fourth group was to receive a diet containing 1.2 μg Hg per g wet fish. Dosing irregularities led to departures from the intended dose levels in 1999 (chicks received higher amounts of CH3HgCl, up to over two-fold than intended due to evaporation of acetone carrier from dose solutions) and 2000 (the purported pure test chemical actually contained 80% MeHg and 20% ethyl mercury). In addition, the 1.2 μg Hg/g exposure group was dropped during the 2000 trial and the 0.08 μg Hg/g group was not included in the 2003 trial. For the purpose of interpretation only, we accounted for differences in dosing regimens among years by inferring the across-year delivered dose from blood Hg exposure data collected at the conclusion of the experiments in each year. The association of dose and blood Hg concentration was estimated from the 2000 and 2003 experiments, where estimated average delivered dose (μg Hg per g wet fish) = (average blood Hg concentration [μg Hg/ml] in chicks at 15 weeks − 0.09837)/6.5017. These estimates yielded across-experiment average estimated doses of control, 0.09, 0.55, and 1.94 μg Hg/g food (1.94 μg represents an extrapolation). Note that the study’s statistical analyses treated dose as categorical and hence did not rely on these calculations of average delivered dose. Observers were blind to the dose regimen for the course of the study to ensure that behavioral data were recorded without bias.
Blood Hg exposure
To document Hg exposure levels over the course of the experiment, blood samples were collected weekly from each chick by venipuncture of the jugular vein and stored in a cryotube for subsequent Hg analysis. The Hg content of whole-blood samples was determined by cold-vapor atomic absorption spectrophotometry (detection limit = 0.01 μg Hg/ml; EnChem, Inc., Madison, WI, USA; any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government) using standard methods (USEPA 1996). Method blanks and standard reference material (certified dog-fish liver) were processed and analyzed concurrently with samples. Because of the limited volume of blood samples, in some cases the parent samples for the duplicates and spike recoveries consisted of reference loon blood or tuna fish.
Righting reflexes and ataxia
Righting reflexes were assessed every 3–5 days throughout the 105-day experiment. Chicks were placed in dorsal recumbency, on the surface of the water and on a platform surface, by a handler, with hands around the thoracic region of the body and wings. A second individual measured the time (±0.01 s) required for the chicks to right themselves. The time keeper directed the handler to release the chick and coincidentally began timing the righting response. The righting response was considered completed when the chick assumed a “normal” posture in the water, or when on the platform, when the chick assumed a “normal” orientation with both feet contacting the platform.
Ataxia was assessed by measuring a walking response across a platform every 5 days once the chicks were 30 days old. Chicks were released on one end of a platform (approximately 104 cm length) by the handler when directed to do so by the timekeeper (loon chicks typically move directly to the water when released in this manner). The timekeeper coincidentally began timing the response. The walking response was considered completed when the chick had crossed the platform, moved off the platform, and was completely in the water.
Time-activity budgets
Time-activity budgets of chicks were recorded every 3–5 days using a scan (instantaneous) sampling procedure (Altmann 1974). Observations of behaviors were recorded at 30-s intervals for a 30-min period following the first morning feeding. Each chick was fitted with a color legband to facilitate identification of individual chicks. To avoid the potential problem of observer bias and interpretation of behaviors, all observers were trained utilizing video footage of captive loon chicks. Observers were located in such a manner so as to have an unobstructed view of the chicks under observation yet remain concealed so as to not influence the behavior of the chicks.
At the 30 s interval, chick behaviors were categorized and recorded as: peering (head partially submerged), begging, locomoting, resting, preening, or agonistic interaction. We also recorded the substrate (platform or water) where the behavior occurred; and when the observations were conducted outdoors (chick >30 days old), we noted whether the chick was in a shaded or lighted area of the pond.
Response to frightening stimulus
The avoidance behavior of loon chicks to a frightening stimulus was determined at 2 and 8 days after hatch. The stimulus was patterned after the methodology of Heinz (1975) and consisted of a rotating wooden axle which produces a flashing black and white pattern and noise caused by the raking of plastic blades against a wire mesh substrate. Testing was conducted in a flooded indoor fiberglass raceway with the stimulus apparatus located on one end of the raceway. Each chick was placed individually in an enclosed holding area of the testing apparatus, and the test begun immediately by lifting the holding area and immediately presenting the frightening stimulus. Observers classified and recorded the response of the chick as (1) not leaving the holding area during axle spin (i.e., approximately 3 s), (2) swimming up to 10 cm, or (3) swimming >10 cm from stimulus. Observers also recorded distance and direction moved at the conclusion of axle rotation, total distance moved including movement post axle rotation, change in posture, and vocalizations made by the chick following the stimulus.
Response to taped parental calls
We tested the response of single chicks to recordings of adult common loon two-note wail (McIntyre 1988) and yodel calls. The wail call is believed to serve as a mechanism to reduce distance between loons (McIntyre 1988) such as in cases where an adult becomes separated from its mate or when parents call chicks off the nest or from hiding places. The wail call is also elicited from adult loons when a bald eagle (Halieatus leucocephalus) is sighted and serves as an alarm. In the wild, the typical response of loon chicks to a wail call (in this context) is to pause from their ongoing behavior, flatten themselves on the water surface, swim to an adult, head for the cover of emergent vegetation, and/or dive (A. Lindsay, J. Mager, K. Tischler, N. McCann, and S. Gillum, personal communications).
In the first year of the study (1999) we documented the response of chicks to a recording of a two-note wail call immediately followed by exposure to a moving plywood eagle silhouette that rose and fell in an arc at one end of a 0.2 ha outdoor pond. A single chick was sequestered in the center of the pond, allowed to acclimate for about 30 s in an enclosed holding area, and released by remote mechanism. The taped call was played within about 5 s of the release. An observer recorded any change in posture elicited by the stimuli, movement response, distance and direction moved, and vocalizations made by the chick. Trials were conducted at approximately 2, 10 and 30 days of age. We found it difficult to consistently display the audio and visual stimuli because chicks would often dive upon hearing the wail call, and it was unclear if they observed the eagle silhouette. In 2000 and 2003, we repeated the test but discontinued the use of the eagle silhouette.
We also assessed chick response to the broadcast of a taped yodel call with three repeated phrases. The yodel is an aggressive call that male common loons use in territorial maintenance (Sjolander and Agren 1972; Rummel and Goetzinger 1978; Young 1983). Yodels are often used during territory border confrontations or when intruding loons invade established territories (Barklow 1979, M. W. Meyer, personal observation). In the wild, chicks typically respond to yodel calls by diving (M. W. Meyer, personal observation). The yodel call test was conducted in a similar manner to that described above for the wail call. It was conducted ≥14 min following the wail call test in the same 0.2 ha pond.
Statistical analyses
The physical nature of indoor raceways and outdoor ponds, and indoor and outdoor brooding platforms, differed markedly. Consequently, separate analyses of righting reflexes and time-activity budgets were conducted for indoor (1–27 days old) and outdoor (37–102 days old) measures. We used logistic regression to determine which independent variables (MeHg treatment level, lake source, year of study, gender, age of chick, and interaction terms treatment × age and lake source × age) influenced whether or not a chick completed the righting and walking tests within 60 s. For those cases in which the chick completed the task of righting or walking in <60 s, repeated measures non-linear modeling using generalized estimating equations (GEEs) were used to assess the relations between the independent variables and the time required to complete the task (Lipsitz et al. 1994; Miller et al. 1993; SAS® Proc Genmod, SAS Institute 2003). Generalized estimating equations take into account the dependence of observations that come from the same subject (loon chick). An independent working correlation structure (see SAS® Proc Genmod, SAS Institute 2003) was used. Based on assessments of normal and gamma distributions and log and identity links, we used a gamma distribution and log link for walking and righting response on platforms and gamma distribution and identity link for righting response on water. Chi-square residual plots were used to evaluate distributional assumptions of each model.
Doubly multivariate repeated measures analysis (SAS® Proc GLM, SAS Institute 2003) was used to determine if independent variables had any effect on the way loon chicks apportioned their time for indoor and outdoor observations. Because of the compositional nature of the data (proportion of time occupied by preening, peering, locomotion, agonistic behavior, resting, and other behaviors all sum to 1), log ratios were calculated using ‘other behaviors’ as the denominator and each of the five remaining variables as a numerator (creating 5 log ratios total). Because this transformed ratio is similar to centering ln(proportions) in relation to their mean, the results are independent of the behavioral category selected as the denominator (Aebischer et al. 1993); e.g., results would be the same if ‘locomotion’ were in the denominator). When a particular behavior was not expressed by a chick, the log ratio could not be calculated. To remedy this, we replaced all zero proportions with 0.001 and other activities were adjusted to preserve the unit sum constraint. The data is ‘doubly multivariate’ because there are 5 response variables nested within each of multiple (age) observations on a single subject.
We tested the relations between independent variables and chick responses (percent of time on the platform vs. water, percent of time in direct sunlight vs. shade for outdoor observations) using logistic regression (SAS® Proc GLIMMIX, SAS Institute 2005). For the platform vs. water study, chick effects were addressed by allowing chick effects to vary randomly on the logit link scale. For the sunlight vs. shade study, age effects were presumed quadratic, with random departures from this assumption presumed serially correlated (within birds) as an autoregressive lag 1 process. Linear and quadratic effects of age were presumed constant across study year and chick gender. However, linear (but not quadratic) age effects were allowed to vary by treatment and lake source. Sun vs. shade effects were estimated using residual pseudo-maximum likelihood (SAS® PROC GLIMMIX; SAS Institute 2005). Degrees of freedom were estimated after Kenward and Roger (1997).
The relations between independent variables and the ordinal wail and yodel call responses (no response, swim, dive) and the ordinal frightening stimuli responses (no response, swam up to 10 cm, swam more than 10 cm) were addressed using GEEs. Chicks typically swam away from the frightening stimuli, but some chicks swam toward it. Observations were grouped into categories corresponding to the distance swam from the frightening stimulus regardless of direction. An independent working correlation structure was used. The assumptions of equal slopes across levels of the ordinal responses was assessed (SAS® Proc Logistic, SAS Institute 2003).
Absolute distance traveled in response to frightening stimuli was analyzed using the same independent variables. Standard linear regression and non-linear regression for gamma outcomes were evaluated. Chi-squared residual plots were used to evaluate models. In both cases, the normal and gamma distribution were compared each using a log and identity link.
Our false positive level likely exceeded our nominal level (α = 0.05) because we conducted statistical tests on multiple (eight) models. However, previous findings of mercury effects on bird behavior suggests that this concern is less important than the fact that we conducted tests on multiple predictors contained within that model. Given the mixture of categorical and continuous independent variables, a Bonferroni correction might be used. Unfortunately, this correction may yield conservative tests (i.e., too few significant findings). Bonferroni-corrected confidence intervals may be obtained by multiplying the widths of our reported confidence intervals by 1.42 (for models with nine comparisons), 1.47 (13 comparisons) or 1.50 (15 comparisons).
Results
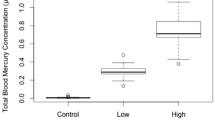
Chick blood-Hg levels generally fell within groups that corresponded to intended treatment categories (Fig. 1). Seventy-four of the 83 chicks concluded the 105-day experiment. One chick died of accidental causes, one was euthanized following a leg injury, one drowned as the result of illness syndrome, five were euthanized after they failed to recover from illness syndrome, and one was euthanized following suspected head trauma. We used data from all 83 chicks for behavior analyses, including birds that were removed from the study before reaching 105 days. Chicks debilitated by illness syndrome were either excluded from assays or observations obtained between the onset of symptoms and recovery were excluded from the analyses.
Blood Hg concentrations (μg/g wet wt) of captive-reared common loon (Gavia immer) chicks by nominal dose group (control [no added Hg], 0.08, 0.4, or 1.2 μg Hg/g wet wt food intake). Data of chicks hatched from eggs from low pH and neutral pH lake sources are included. Box plots represent the median as well as 25th and 75th percentile values, and whiskers represent the high and low extremes. The Hg was delivered as CH3HgCl
Righting reflexes and ataxia
In 83 of 824 (2.79%) righting reflex tests conducted on indoor platforms and 88 of 1,033 (8.52%) assays on outdoor platforms, chicks failed to right themselves within 60 s and the trial was stopped. There was no evidence that dietary MeHg treatment (\( \chi^{2}_{3} = 1. 7 8 \), p = 0.62) or lake source (\( \chi^{2}_{1} = 1. 9 2 \), p = 0.17) influenced the probability that the chick would right itself within 60 s during indoor trials (when chicks were ≤27 days old). However, during the outdoor trials (≥37 days old), age (\( \chi^{2}_{1} = 20. 7 6 \), p < 0.0001) and treatment × age (\( \chi^{2}_{3} = 9. 10 \), p = 0.03) significantly influenced whether a chick righted itself. As chicks aged, those fed the nominal dietary dose levels of 0.4 and 1.2 μg Hg/g (average estimated delivered dietary level of 0.55 and 1.94 μg Hg/g, respectively) were less likely than control chicks to complete the righting test (Table 2). A re-analysis of assay results, dropping 1999 and using only 2000 and 2003 data, also indicate significant treatment × age effects (\( \chi^{2}_{3} = 9.0 7 \), p = 0.03) at the nominal dose levels of 0.4 and 1.2 ug Hg/g food. The conditional odds of righting increased by 20 (95% CI: 10,31)% per day in controls but by only 7 (2,12)% and 4 (1,8)% per day in chicks on the 0.4 μg Hg/g and 1.2 μg Hg/g diets, respectively.
Among loon chicks that completed indoor trials within 60 s, only age (\( \chi^{2}_{1} = 2 9. 6 6 \), p < 0.0001) and year (\( \chi^{2}_{2} = 1 1. 1 5 \), p = 0.004) were significant in predicting the time needed for chicks to right themselves. Age (\( \chi^{2}_{1} = 1 8. 8 8 \), p < 0.0001) and age × lake source (\( \chi^{2}_{1} = 4. 2 5 \), p = 0.04) were significant in predicting righting response time during outdoor trials. The median time to righting in all trials was 1.7 s and ranged from 0.3 to 59.0 s. The predicted mean righting time on indoor platforms increased 6.4 (3.7, 9.2)% per day as they aged (Table 2). During outdoor trials, righting times of chicks from neutral-pH lakes appeared stable (1 [−2.5, 0.7]% per day), while chicks from low-pH lakes declined (2.5 [−3.8, −1.1]% per day). For those chicks that completed the assay, there was no evidence that treatment level (\( \chi^{2}_{3} = 1. 7 2 \), p = 0.63) was influential in predicting the time it took for chicks to right themselves on the platform.
There was no statistical evidence that Hg treatment level (\( \chi^{2}_{3} = 4. 9 7 \), p = 0.17) or lake source (\( \chi^{2}_{1} = 0. 1 2 \), p = 0.73) were influential in predicting the time it took for chicks to right themselves when released on their backs on the water surface. The median time required for loon chicks to right themselves upon being released in water was 0.76 s (range: 0.23 to 11.98 s). Year of study (\( \chi^{2}_{2} = 2 7. 9 1 \), p < 0.0001) and age (age2: \( \chi^{2}_{1} = 9. 7 1 \), p = 0.002) were significant in predicting the time to righting in water. The relation to age was curvilinear, with time to righting increasing with age from hatch to about 55 days post hatch and then decreasing.
We found no evidence to suggest that dietary MeHg treatment influenced the ability of loon chicks to walk across a platform (\( \chi^{2}_{3} = 3. 8 1 \), p = 0.28). In 7% of the trials (70 of 1,018), chicks did not leave the platform within 60 s and the trial was stopped. However, lake source (\( \chi^{2}_{1} = 4.0 4 \), p = 0.04) and lake source × age (\( \chi^{2}_{1} = 6. 9 5 \), p = 0.008) were associated with whether the trials would be completed. For example, at 69 days, the conditional odds of completing the test was 317 (92, 1400)% higher for chicks from low-pH lakes than for chicks from neutral-pH lakes. In addition, the odds of completing the assay appeared stable with age (0.6 [−2.1, 3.5]% per day) for chicks from neutral-pH lakes, but increased by 7.6 (4.0, 11.4)% per day in chicks from low-pH lakes. The mean walking time for chicks that completed the test within 60 s timeframe was 12.8 s (range of 0.4 to 59.2 s) and varied with age (\( \chi^{2}_{1} = 8. 3 3 \), p = 0.004), lake source (\( \chi^{2}_{1} = 5. 2 5 \), p = 0.02) and lake source × age (\( \chi^{2}_{1} = 4. 8 4 \), p = 0.03). On average, chicks from low-pH lakes completed the assay in less time than did chicks from neutral-pH lakes (e.g., least square means at 69 days = 11.1 [9.6, 12.8] and 15.13 [12.6, 18.2] s, respectively). Additionally, chicks from low-pH lakes tended to complete the assay more rapidly as they aged relative to chicks from neutral-pH lakes (platform crossing times of chicks from neutral-pH lakes declined at a rate of 0.5 [−0.8, −0.1]% per day, while chicks from low-pH lakes declined at 1.1 [−1.6, −0.6]% per day).
Time-activity budgets
Of the 1,768 30-min behavioral observations of the loon chicks conducted in this study, 1,652 (770 indoor and 882 outdoor) observations were used in the compositional analysis. Only data from chicks with a complete set of repeated observations were used in the analysis. Therefore, if a chick was missing an observation, the rest of the information from that chick was excluded.
Indoor observations
While analysis of indoor observation data indicated significant year (Repeated MANOVA; F 2, 69 = 24.67, p < 0.0001) effects, there was no statistical evidence of differences between treatment levels (F 3, 69 = 0.43, p = 0.73), lake source (F 1, 69 = 2.24, p = 0.14), or gender (F 1, 69 = 1.37, p = 0.25) in the proportion of time occupied by the response variables preening, peering, locomotion, agonistic behavior, or resting. Hypothesis test results indicated that three way interactions (response × age × independent variable) were not significant (p > 0.09), suggesting that age of chick was relatively unimportant with respect to how the independent variables affected the responses. A multivariate test of response × age effect, however, was significant (Wilks’ λ = 0.122, F 25, 45 = 3.98, p = 0.0002), suggesting that age is associated with responses. In addition, the no response × year test was significant, suggesting study year effects (Wilks’ λ = 0.312, F 10, 130 = 10.26, p < 0.0001).
We also measured the proportion of time chicks spent on platforms during indoor observations (chicks ≤27 days old). Age (age: F 1,808 = 10021.6, p < 0.0001; age2: F 1,808 = 366.49, p < 0.0001) and lake source (F 1,71 = 6.12, p = 0.02) were significantly associated with whether chicks spent time on the platform or in the water. As chicks aged, they tended to spend less time on the platform. The conditional odds of spending time on the platform was 54 (9, 117)% higher for chicks from low-pH than from neutral-pH lakes. There was no evidence to suggest that treatment level, gender, or year were influential in predicting outcomes.
Outdoor observations
Outdoor time activity budgets did not differ significantly among treatment levels (F 3, 55 = 0.43, p = 0.73) or lake sources (F 1, 55 = 0.29, p = 0.59). However, there was a significant year (F 2, 55 = 4.49, p = 0.02) effect. Because of a reduced number of useable observations due to incomplete data (reduced to 63 subjects), three way interactions (response × age × independent variable) could not be assessed.
Lake source was significantly associated with whether a chick spent time in shade or sunlight during outdoor time activity observations (F 1,133 = 5.64, p = 0.02). The average proportion of time that chicks were in sunlight was 0.53 ± 0.02 (SE) for low-pH chicks and 0.46 ± 0.02 for neutral pH chicks. Conditional odds indicated that chicks from low-pH lakes were 75% (95% CI: 10, 178) more likely to be in the sunlight than were chicks from neutral-pH lakes. Treatment level was not associated with proportion of time spent in sunlight or shade (F 3,134 = 0.01, p = 0.99).
Frightening stimulus response
Treatment level (\( \chi^{2}_{3} = 4. 1 3 \), p = 0.25) and lake source (\( \chi^{2}_{1} = 0.0 3 \), p = 0.86) were not significantly associated with the categorical response of loon chicks to the frightening stimulus. However, we did find that year of study had a significant influence on the response of loon chicks to the frightening stimulus (\( \chi^{2}_{2} = 1 6. 5 3 \), p = 0.0003). Distance traveled after response to the frightening stimulus was associated with year (\( \chi^{2}_{2} = 1 1. 4 5 \), p = 0.003) and lake source (\( \chi^{2}_{1} = 3. 9 8 \), p = 0.046), with lake source possibly depending on age (\( \chi^{2}_{2} = 3. 7 3 \), p = 0.053). Of chicks that moved following the stimulus, those from low-pH lakes tended to travel a shorter distance than did chicks from neutral-pH lakes (Table 3; LSM: 27.1 [95% CI: 21.7, 32.5] vs. 34.6 [95% CI: 28.1, 41.1] cm). Treatment level (\( \chi^{2}_{3} = 3. 8 9 \), p = 0.27) was not associated with distance traveled after exposure to the frightening stimulus.
Response to taped parental calls
Lake source (\( \chi^{2}_{1} = 7. 8 8 \), p = 0.005), year, (\( \chi^{2}_{2} = 1 9. 9 5 \), p < 0.0001), and age (\( \chi^{2}_{2} = 1 1. 2 4 \), p = 0.004) were significantly associated with response of loon chicks to wail calls. The odds of a higher level of response (e.g., dive or swim vs. none) were lower for chicks from low-pH lakes than from neutral-pH lakes (odds = 0.45; 95% CI: 0.27, 0.76; Table 3).
Chicks studied in 1999 were much more likely to have a higher response compared to those in 2003 (conditional odds = 6.0; 95% CI: 2.8, 12.7), whereas those studied in 2000 did not differ significantly from the other 2 years (p = 0.69). In addition to the standard parameter estimates, a contrast used to test differences between chicks studied in 1999 and 2000 indicated a significant difference (odds = 5.32; 95% CI: 2.39, 11.86) between these years as well. We conclude that chicks studied in 1999 were more likely to have a higher response than those chicks studied in 2000 or 2003. This result is likely due to the use of the eagle silhouette in 1999. In 1999, chicks responded to the stimuli by diving in 70.1% of the trials and swam on 22.4% of the occasions. By contrast, in 2000 and 2003 the chicks dove in response on 32.9% of occasions and swam 31.8% of the time. Treatment level was not associated with the response of loon chicks to the wail call (\( \chi^{ 2}_{ 3} = 0. 3 2 \), p = 0.96).
The response of chicks to yodel calls was generally more intense than the response to wail calls (59.3% of yodel trials resulted in a dive response compared to a 43.5% dive response for the wail trials). The response of chicks to yodel calls was significantly associated with age (\( \chi^{2}_{2} = 1 9. 4 8 \), p < 0.0001) but not with lake source (\( \chi^{2}_{1} = 1.0 6 \), p = 0.30) or MeHg treatment level (\( \chi^{2}_{3} = 5. 5 6 \), p = 0.13; Table 3).
The assumption of equal effects across wail and yodel call response categories may be invalid (wail call response, \( \chi^{2}_{17} = 2 8.0 2 \), p = 0.04; yodel call response, \( \chi^{2}_{17} = 2 8. 5 4 \), p = 0.04; modeled under independence assumption). We addressed this concern by regrouping response categories into two sets of repeated binomial outcomes (dived vs. did not dive, no response vs. swam or dived). Subsequent repeated measures binomial regression analysis indicated that large changes in parameter estimates were limited to variables that were non-significant under the ordinal models.
Discussion
Dietary MeHg effects
The Hg exposure levels achieved with MeHgCl dietary treatments in this study bracket the ecologically relevant range. Evers et al. (1998) reported that blood Hg levels in loon chicks from across North America ranged from 0.03 to 0.78 μg/g (mean = 0.16 μg/g) at 3 to 6 weeks of age. For comparison, blood Hg levels at 5 weeks of age in this study averaged 0.02 ± 0.003 (SE) μg/g in controls, 0.12 ± 0.01 μg/g in the 0.08 μg Hg/g nominal treatment group, 0.66 ± 0.06 μg/g in the 0.4 μg Hg/g nominal treatment group, and 2.26 ± 0.28 μg/g in the 1.2 μg Hg/g nominal treatment group. Despite the high levels of Hg exposure achieved in our study, we observed no overt behavioral abnormalities. Among the behavioral assessments conducted in this experiment (time activity budgets, righting reflexes, walking response, response to frightening stimulus, response to two types of parental calls), effects related to the level of Hg in the diet were limited to performance differences in righting reflex on the platform in older (>37 day old) chicks. This assay requires a high level of motor coordination. Adapted for an aquatic life, common loons are clumsy on land (McIntyre 1988). The chicks typically kicked their feet in combination with lifting their backs off the platform to generate enough momentum to roll over to an upright position. In most cases, common loon chicks were able to right themselves relatively quickly (median time of 1.7 s). Failure to perform this task may be the result of reduced motor function, which has been associated with lesions in the cerebellum and cerebrum induced by MeHg exposure (Wolfe et al. 1998).
We observed no shift in activity patterns of chicks receiving high levels of dietary MeHg. This contrasts with the findings of Bouton et al. (1999), who observed a shift away from active, energetic, and maintenance behaviors in juvenile great egrets exposed to ecologically relevant exposure levels (0.5 μg MeHgCl/g dietary intake). However, while one of the target dose levels was comparable (0.4 μg Hg/g) in the studies, it appears that mercury exposure (based on blood mercury concentrations in similar-aged juveniles at the conclusion of the studies) was 3.2-times higher in the juvenile great egrets (Spalding et al. 2000) than in our loon chicks. It is also possible egret chicks absorb, metabolize, or sequester methylmercury differently than common loon chicks. Bouten et al. (1999) also observed a shift to activities that were less energy demanding or required fewer motor skills in birds that received high levels of MeHg (5.0 μg MeHgCl/g in food). The apparent higher mercury exposure levels in the great egrets may have contributed to the differences in findings between the two studies relative to activity patterns. Nocera and Taylor (1998) and Counard (2001) observed that downy common loon young in the wild spend more time preening and less time riding on the backs of adults with increased blood Hg concentrations. Counard (2001) also observed a negative relation between frequency of wing flaps and diving, and a corresponding increase in swimming, peering, and begging, with blood mercury concentration in older (>40 days) chicks. While we were unable to assess tendencies for back riding or begging in our artificial setting, we did tally time apportioned to preening and locomotion (swimming) and did not observe differences in these activities among dietary Hg treatment groups.
We suspect that our lack of behavioral effects in common loon chicks is likely the result of protection afforded by rapid MeHg elimination rates during feather development (Fournier et al. 2002; Kenow et al. 2007b). We observed no negative effects of dietary MeHg on growth or survival of common loon chicks (Kenow et al. 2003), however we did document negative effects of dietary MeHg on immune function (Kenow et al. 2007a) and effects related to oxidative stress and altered glutathione metabolism (Kenow et al. 2008) at and/or above the 0.4 μg Hg/g nominal treatment group (with an average estimated delivered dietary level of 0.55 μg Hg/g). We have concluded that the high rate of MeHg excretion measured in our study provided a large measure of protection from MeHg toxicity. These results should not be extrapolated beyond the 105-day trial period, since blood mercury levels were on an upward trajectory and tissue mercury levels may increase considerably.
Year effects on response means were detected in several of the assays we conducted. We suspect that these differences probably arose from subtleties attributed to various observers over the 3-year study or slight differences in rearing conditions among years (e.g., 4 chicks per experimental block in 1999 vs 3 chicks per block in the other years, weather differences among years). Also, with respect to chick response to taped paternal calls, addition of a visual stimulus in 1999 likely contributed to the year effects measured in these assays (see Results). In the case of the single assay for which we observed treatment effects (Righting outdoor platform: completed with 60 s), there was no indication of year effects (Table 2). The observed year effects in our study point to the necessity of maintaining consistency among observers and rearing conditions during multi-year studies.
Lake source effects
An important finding in our study is that we detected differences in several response variables with respect to source of eggs. Chicks hatched from eggs collected from nests on low-pH lakes tended to spend more time on resting platforms, were less responsive to a frightening stimulus, and exhibited less intense response to parental wail calls than did chicks from neutral-pH lakes. These results suggest that chicks from low-pH lakes may be generally less motivated or more lethargic.
Chicks from low-pH lakes exhibited higher blood mercury levels at hatch compared to chicks from neutral-pH lakes (based on samples collected from 26 chicks at hatch in 2003: 2.05 ± 0.15 [SE] vs 1.46 ± 0.13 μg/g blood; t 24 = 2.99, p = 0.006) and it may be that the lake source effects were related to in ovo exposure to MeHg. Bouton et al. (1999) attributed similar characteristics of lethargy and reduced motivation to hunt in juvenile great egrets with elevated levels of dietary MeHgCl. Also, common loon chicks from low-pH lakes spent more time on indoor platforms and more time in the shade than did neutral-pH chicks, suggesting that chicks from low-pH lakes may have had higher thermoregulatory needs (water temp was 12°C). Juvenile great egrets dosed with MeHg spent more time in the shade, believed to represent a decreased tolerance to heat and suggested an effect of mercury on thermoregulation (Bouton et al. 1999; although the authors found the results to be only partially supportive of the thermoregulation hypothesis).
Earlier we reported growth effects related to chick lake source (Kenow et al. 2003). Mass of chicks from low-pH lakes at hatch during the 3-year study averaged 3.8% (95% CI: −7.72, 0.12) lower and exhibited a 7% lower asymptotic mass (200 g [95% CI: 68.8, 331.2]) reduction from baseline asymptotic mass in chicks from low-pH compared to neutral-pH chicks. With respect to immune function, we observed that chicks originating from low-pH lakes were more likely to have higher levels of lymphoid depletion compared to chicks from neutral-pH lakes (Kenow et al. 2007a). Embryo mortality, reduction in growth, and developmental abnormalities were reported in mallard eggs externally treated with MeHg (Hoffman and Moore 1979). Studies by Heinz (1975, 1976, 1979) demonstrated the effects of in ovo MeHg exposure persist beyond the embryo to affect behavior and survival, and resulted in brain lesions (Heinz and Locke 1976) in mallard ducklings. Mallard ducklings from adults provided with MeHg in their diet were less responsive than controls to maternal calls. Similarly, loon chicks from low-pH lakes in our study were less responsive to adult wail calls.
The Frightening Stimulus Response we performed was patterned after the avoidance response test used by Heinz (1975) to assess the effects of in ovo Hg exposure to mallard ducklings. Mallard ducklings from adults provided mercury in their diet ran further in response to the stimulus than did control ducklings (Heinz 1979). We found that loon chicks from low-pH lakes traveled a shorter distance in response to the stimulus. This was inconsistent with previous findings in mallards. However, we did find a hyper-reactive response in chicks from low-pH lakes with respect to the walking response. Chicks from low-pH lakes were more likely to complete the assay and as they aged, of those chicks that did complete the assay, chicks from low-pH lakes completed the assay in less time than did chicks from neutral-pH lakes.
At this point, we can only speculate that the lake source effects may have been related to in ovo exposure to MeHg. A significant inverse relation between lake pH and egg Hg exposure has been reported in common loons in our study area in northern Wisconsin (Fevold et al. 2003) and the same pattern was found in blood Hg levels in chicks at hatch in our study. However, there are a couple of alternative hypotheses that should be considered. First, other factors related to lake pH, such as altered availability of toxic metals (Weiner 1987; Scheuhammer 1991) or essential elements (Scheuhammer 1991) may have had persistent consequences on development of chicks from eggs collected on low-pH lakes. For instance, in a related study we found that shells from loon eggs from low-pH lakes were an average of 3% thinner than the shells of eggs from neutral-pH lakes, and may be related to lower levels of calcium in the diet of breeding loons on low-pH lakes (Pollentier et al. 2007). Meyer et al. (1995) reported that adult female loon blood cell calcium levels averaged 43% lower in samples collected on acidic lakes compared to samples collected on neutral-pH lakes. Second, there may be some difference in overall fitness of parents nesting on low-pH vs. neutral-pH lakes. Consequently, lake source differences in chick quality observed in our study may be rooted in parental quality.
Because chick blood Hg in the wild co-varies with lake pH, effects arising from lake source (e.g., in ovo exposure to MeHg) need to be considered when interpreting field studies linking observed differences in chick behavior and fitness to blood Hg level alone. Counard (2001) acknowledges that behavioral differences associated with blood Hg in wild common loons may be explained by forage base differences with lake pH. Merrill et al. (2005) found loon adults on low-pH lakes were less efficient at foraging and chicks received less prey. Again, lake pH was also associated with elevated mercury exposure in adults and chicks. The direct causal relation between loon chick fitness and in ovo exposure to MeHg requires further investigation. Carefully designed studies are needed to address the mechanism(s) leading to lake source effects.
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Barklow WE (1979) The function of variations in the vocalizations of the Common Loon (Gavia immer). Ph.D. Dissertation., Tufts University, Boston, MA, USA
Barr JF (1986) Population dynamics of the common loon (Gavia immer) associated with mercury-contaminated waters in north-western Ontario. Can Wildl Serv Occas Pap 56, 25 pp
Bouton SN, Frederick PC, Spalding MG, McGill H (1999) Effects of chronic, low concentrations of dietary methylmercury on the behavior of juvenile great egrets. Environ Toxicol Chem 18:1934–1939
Counard CJ (2001) Mercury exposure and effects on common loon (Gavia immer) behavior in the Upper Midwestern United States. M.S. Thesis, University of Minnesota, St. Paul, MN, USA
Eisler R (1987) Mercury hazards to fish, wildlife, and invertebrates: a synoptic review. US Fish Wildl Serv Biol Rep 85(1.10)
Evers DC, Kaplan JD, Meyer MW, Reaman PS, Braselton WE, Major A, Burgess N, Scheuhammer AM (1998) Geographic trend in mercury measured in common loon feathers and blood. Environ Toxicol Chem 17:173–183
Fevold BM, Meyer MW, Rasmussen PW, Temple SA (2003) Bioaccumulation patterns and temporal trends in mercury exposure in Wisconsin common loons. Ecotoxicol 12:83–93
Fournier F, Karasov WH, Kenow KP, Meyer MW, Hines RK (2002) The oral bioavailability and toxicokinetics of methylmercury in common loon (Gavia immer) chicks. Comp Biochem Physiol A 133:703–714
Haramis GM, Nice AD (1980) An improved web tagging technique for waterfowl. J Wildl Manag 44:898–899
Heinz GH (1975) Effects of methylmercury on approach and avoidance behavior of mallard ducklings. Bull Environ Contam Toxicol 13:554–564
Heinz GH (1976) Methylmercury: second-year feeding effects on mallard reproduction and duckling behavior. J Wildl Manag 40:82–90
Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manag 43:394–401
Heinz GH, Locke LN (1976) Brain lesions in mallard ducklings from parents fed methylmercury. Avian Dis 20:9–17
Hoffman DJ, Moore JM (1979) Teratogenic effects of external egg applications of methyl mercury in the mallard, Anas platyrhynchos. Teratology 20:453–462
Kenow KP, Gutreuter S, Hines RK, Meyer MW, Fournier F, Karasov WH (2003) Effects of methyl mercury exposure on the growth of juvenile common loons. Ecotoxicol 12:171–181
Kenow KP, Grasman KA, Hines RK, Meyer MW, Gendron-Fitzpatrick A, Spalding MG, Gray BR (2007a) Effects of methylmercury exposure on the immune function of juvenile common loons. Environ Toxicol Chem 26:1460–1469
Kenow KP, Meyer MW, Hines RK, Karasov WH (2007b) Distribution and accumulation of mercury in tissues and organs of captive-reared common loon (Gavia immer) chicks. Environ Toxicol Chem 26:1047–1055
Kenow KP, Hoffman DJ, Hines RK, Meyer MW, Bickham JW, Matson CW, Stebbins KR, Montagna P, Elfessi A (2008) Effects of methylmercury exposure on glutathione metabolism, oxidative stress, and chromosomal damage in captive-reared common loon (Gavia immer) chicks. Environ Pollut 156:732–738
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Lipsitz SH, Kim K, Zhao L (1994) Analysis of repeated categorical data using generalized estimating equations. Stat Med 13:1149–1163
McIntyre JW (1988) The Common Loon: spirit of northern lakes. University of Minnesota Press, Minneapolis, MN, USA
Merrill EH, Hartigan JJ, Meyer MW (2005) Does prey biomass or mercury exposure affect loon chick survival in Wisconsin? J Wildl Manag 69:57–67
Meyer MW, Evers DC, Daulton T, Braselton WE (1995) Common loons (Gavia immer) nesting on low pH lakes in northern Wisconsin have elevated blood mercury content. Water Air Soil Pollut 80:871–880
Meyer MW, Evers DC, Hartigan JJ, Rasmussen PS (1998) Patterns of Common Loon (Gavia immer) mercury exposure, reproduction, and survival in Wisconsin, USA. Environ Toxicol Chem 17:184–190
Miller ME, Davis CS, Landis JR (1993) The analysis of longitudinal polytomous data: generalized estimating equations and connections with weighted least squares. Biometrics 49:1033–1044
Nocera JJ, Taylor PD (1998) In situ behavioral response of common loons associated with elevated mercury (Hg) exposure. Conserv Ecol. 2: article 10. http://www.consecol.org/vol2/iss2/art10/
Pollentier CD, Kenow KP, Meyer MW (2007) Common loon (Gavia immer) eggshell thickness and egg volume vary with acidity of nest lake in northern Wisconsin. Waterbirds 30:367–374
Rummel L, Goetzinger C (1978) Aggressive display in the Common Loon. Auk 95:183–196
SAS Institute (2003) SAS OnlineDoc® 9.1. Cary, NC, USA
SAS Institute (2005) SAS OnlineDoc® 9.1 for the GLIMMIX Procedure. Cary, NC, USA
Scheuhammer AM (1991) Effects of acidification on the availability of toxic metals and calcium to wild birds and mammals. Environ Pollut 71:329–375
Scheuhammer AM, Blancher P (1994) Potential risk to common loons (Gavia immer) from methylmercury exposure in acidified lakes. Hydrobiologia 279/280:445–455
Shimai S, Satoh H (1985) Behavioral teratology of methylmercury. J Toxicol Sci 10:199–216
Sjolander S, Agren G (1972) Reproductive behavior of the Common Loon. Wilson Bul 84:296–308
Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR (2000) Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis 36:411–422
Thompson DR (1996) Mercury in birds and mammals. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. Lewis, Boca Raton, FL, USA, pp 341–356
US Environmental Protection Agency (1996) Mercury in solid or semisolid waste (manual cold-vapor technique). Method 7471A. Test methods for evaluating solid waste, physical/chemical methods. SW846. Office of Solid Waste, Washington, DC
Watanabe C, Satoh H (1996) Evolution of our understanding of methylmercury as a health threat. Environ Health Perspect 104:367–379
Weiner JG (1987) Metal contamination of fish in low-pH lakes and potential implications for piscivorous wildlife. Trans N Am Wildl Nat Resour Conf 52:645–657
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Young KE (1983) Seasonal and temporal patterning of Common Loon (Gavia immer) vocalizations. M.S. Thesis, Syracuse University, Syracuse, NY, USA
Acknowledgements
Financial support for this project was provided by the Electric Power Research Institute, the Wisconsin Utilities Association, the Wisconsin Department of Administration Wisconsin Focus on Energy Environmental Research Program, the Wisconsin Department of Natural Resources, and the U.S. Geological Survey. We thank the following individuals for their assistance: F. Fournier, J.E. Lyon, K.A. Kroc, M.S. Meier, A.J. Lindo, M.L. Weinandt, R.L. Beckmann, L.E. McColl, S.M. Strom, E.R. Deppe, D.D. King, C.M. Lipke, H.L. O’Brien, S.T. Troxell, R.M. Kreiling, A. Stone, T.N. Willers, K.A. Zinszer, E.A. Kurth, K.M. McColl, A.M. Hankee, K.A. DuBois, E.L. Strom, L.L Meek, S.C. Houdek, C.D. Pollentier, A.J. Kimball, J.L. Inglish, J.A. Homyack, B.A. Rycyzyn, C.R. Gonczy, T. Daulton, B. Fevold, S. Gillum, M. Parrara, S. Weick, L.A. Lee, J. Luoma, C.A. Berg, D.M. Kennedy, T.D. Hubert, L.G. Johnson, E. Lavoie, and K. Day.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kenow, K.P., Hines, R.K., Meyer, M.W. et al. Effects of methylmercury exposure on the behavior of captive-reared common loon (Gavia immer) chicks. Ecotoxicology 19, 933–944 (2010). https://doi.org/10.1007/s10646-010-0475-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0475-2