Abstract
The aim of this study was to evaluate the applicability of three bioassays representing multiple trophic levels, for the preliminary ecotoxicological screening of sediments from sites contaminated by mining activities. Of the bioassays used in this study, the ostracod test was the most responsive. Vibrio fischeri luminiscence inhibition assay was less sensitive to the toxicants in the sediments than the phytotoxicity assays. The general trend observed was an increase in toxicity values measured by the bioassays with increasing metal mobilization in sediment samples. Therefore, the test battery can be used as a rapid and sensitive tool to evaluate the heavy metal contamination in sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental persistence of metals in concert with their intensive use by modern society has created a concentration of metals in the biosphere (Kakkar and Jaffery 2005). Determination of the contaminant content is not enough to evaluate the toxic effects or to characterise contaminated sites, because such a measure does not reflect the ecotoxicological danger in the environment and does not provide information on the effects of the chemical compounds. To estimate the risk of contaminants, chemical methods need to be complemented with biological methods (Leitgib et al. 2007). Ecotoxicity tests measure the bioavailability of the contaminants and the effects of the chemically not measured toxic compounds on the members of the soil community (Gruiz 2005). Therefore, ecotoxicological testing may be a useful approach for assessing the toxicity as a complement to chemical analysis (Wilke et al. 2007). Any strategy for assessing properties potentially hazardous to the environment used in a classification system should include test organisms of different trophic levels representing both the terrestrial and aquatic compartments.
Sediment toxicity assessment should involve an integrated approach, which considers contaminant concentrations and biological effects (Nipper et al. 2002; Nendza 2002; Davoren et al. 2005). A multitrophic battery of tests, which incorporates a number of different test species, is therefore advocated to reduce uncertainty in sediment quality assessment (Chapman et al. 2002). In addition, a test strategy which includes the assessment of multiple exposure phases affords a more detailed evaluation of potential sediment toxicity.
Bacteria play a vital role as decomposers in aquatic systems and hence there is justification for their inclusion in a test battery for assessing sediment toxicity. Vibrio fischeri was chosen to assess the acute toxicity of sediment pore-water and water samples. Plants are particularly important components in ecosystems since they are the primary food producers and so it is important to identify the magnitude of the toxicity results in these organisms (Schowanek et al. 2004; Chung et al. 2007). Finally, the crustacean bioassay with Heterocypris incongruens was selected as a representative test of an herbivorous invertebrate species for screening sediment samples.
The aim of this study was to develop a battery of bioassays for the ecotoxicological screening of areas polluted by mining activities. Particularly, the toxicity of water samples, sediments and their pore-water extracts was evaluated by using three assays: bacteria, plants and ostracods. Moreover, the possible relationship between observed toxicity and results of chemical analysis was studied.
Materials and methods
Studied area
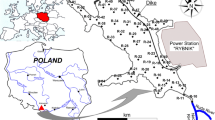
The studied area, Sierra Minera, is close to the mining region of La Unión (Murcia, SE Spain). The entire area is located between Cabo de Palos and Cartagena and was for many centuries subjected to mining. The annual average temperature is 17 °C and precipitation does not exceed 300 mm, with occasional torrential rainfall, which frequently occurs in the period between the end of summer and autumn. Wind is an almost constant atmospheric phenomenon in the littoral zone.
Material and sampling method
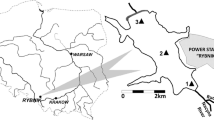
From contamination sources, present in mining areas, a dispersion of polluting agents, as a consequence of torrential rains, takes place. In our area, dispersion routes of these polluting agents are the gullies. Deposition occurs in topographically lower areas where materials are mixed with the surrounding materials, with a carbonated nature. The sampling design has been made to compare gradient of concentration, possible metal mobilization and organism toxicity. The sampling points have been selected according to their proximity to the contamination source, establishing three types of samples: samples located in areas next to the contamination source (primary contamination), samples located in the dispersion routes (secondary contamination) and samples located in areas minimally affected by the contaminants. 26 sediment and water samples were collected (Fig. 1). Water samples were collected at the same site as the sediment after a rain episode (Salminen et al. 1998).
Sample preparation and chemical analysis
Sediment samples were collected at 0–15 cm depth, air-dried and sieved through a 2 mm screen for general analytical determinations. The pH was determined in a 1:5 (v/v) suspension of sediment in Milli-Q quality water and in a 1 M KCl solution (Peech 1965) using a Crison GLP21 pH meter. Electrical conductivity (EC) (dS m−1 at 25 °C) was measured using a Crison GLP31 meter in the extracts obtained by filtering the 1:5 suspensions through a 0.45 μm cellulose acetate disk filter. Equivalent calcium carbonate (%) was determined by the volumetric method using a Bernard calcimeter previously calibrated against Na2CO3 (Hulseman 1966; Muller and Gatsner 1971). The organic matter content was measured by wet oxidation method (Nelson and Sommers 1982), according to the NF X 31-109 standard. To determine the Pb, Cd and As content, sediments were first ground to a fine powder using an agate ball mill. 0.1 g of sediment samples was placed in Teflon vessels and 5 ml of concentrated HF acid solution, 200 μl of concentrated HNO3 acid solution and 5 ml of water were added. When digestion was complete (15 min at 1,000 W in a Milestone ETHOS PLUS microwave), the samples were transferred to a volumetric flask and brought to 50 ml. The Pb and Cd content were determined by electrothermal atomization atomic absorption spectrometry (ETAAS) using an Unicam 929 AAS spectrometer. The As content was analysed by atomic fluorescence spectrometry using an automated continuous flow hydride generation (As-AFS) spectrometer (PSA Millenium Merlin 10055 for As). The reliability of the results was verified by analysing a standard reference material (SRM 2711 Montana Soil).
After sampling, water samples were filtered and stored for posterior analysis. Finally, 26 pore-water samples were obtained. To extract the pore-water, the soil was subjected to a vacuum pressure (negative pressure) to extract the soil solution through filter paper.
Toxicity tests
Microtox bioassay®
The Microtox® bioassay is based on the inhibition of the bioluminescent marine bacteria Vibrio fischeri and was applied to the water and pore-water samples according to the protocol (Azur Environmental Ltd 1998). The bioluminiscence emitted by the bacteria is reduced in the presence of polluting agents and this reduction is directly related to the toxicity of the sample (Devesa-Rey et al. 2008). Toxicity is expressed as the agent concentration which produces a 50% reduction of the initial luminescence (EC50) after 15 min of contact time. Bioluminescent responses were measured using a Microtox Model 500 analyser. A basic test was conducted with the reference standard for each fresh vial of bacteria opened to ensure the validity of the test method (Azur Environmental Ltd 1998).
Phytotoxkit® and Ostracodtoxkit® method
The Phytotoxkit® test (Phytotoxkit 2004) measures the decrease in (or the absence of) seed germination and of the growth of the young roots after 3 days of exposure of seeds of selected higher plants to a contaminated matrix compared with the controls germinated in a reference soil (Oleszczuk 2007). It is recommended (OECD 1984) that at least one monocotyledon and one dicotyledon species should be used in these tests. The plants selected for the Phytotoxkit® microbiotest were: the monocotyl Sorghum saccharatum (Sorgho) and the dicotyls Lepidium sativum (Garden cress) and Sinapis alba (mustard).
Finally, toxicity determination of the sediment samples was performed in a short term contact test using an Ostracodtoxkit® test (Ostracodtoxkit 2001; Chial Belgis et al. 2003).
Statistical analysis
Descriptive statistics (mean, SD, maximum and minimum) were determined using MINITAB 15. A correlation matrixes analysis was made for the variables and Pearson’s product moment correlation coefficient was used.
GIS methodology
The sampling points, obtained by GPS, were integrated to create a database in which the coordinates were included. With the orthophotos and the Digital Terrain Model (DTM), an hydrography restitution was carried out to study possible relationship between toxicity values and hydrographic net.
Results and discussion
Chemical analysis
Average heavy metal content in contamination sources in the studied area were: 41 mg kg−1 for Cd, 8,000 mg kg−1 for Pb and 315 mg kg−1 for As (Navarro et al. 2008). Table 1 summarises the chemical characteristics and trace element content of sediments and Tables 2 and 3 show the chemical characteristics and the Pb, Cd and As content of water and pore-water samples, respectively. Sediment samples showed a mean pH value of 6.0 in water and 5.7 in KCl. The EC varied from 1.0 dS m−1 to 56.2 dS m−1, with a mean value of 17.7 dS m−1. The mean value for Pb was 7,157 mg kg−1, 36 mg kg−1 for Cd and 1,452 mg kg−1 for As in sediment samples. The water samples presented a mean pH value of 5.1 and the pore-water extracts 5.9 (Tables 2 and 3). Pore-water and water samples showed similar values of EC (17.7 dS m−1 and 14.1 dS m−1 respectively) and the mean Eh value was 294 mV in pore-water extracts and 388 in water samples (Tables 2 and 3). The Pb content was similar in pore-water extracts and in water samples: 0.84 mg l−1 in pore-water extracts and 1.3 mg l−1 in water samples. However, the Cd content was much higher in pore-water extracts (23.18 mg l−1) than in water samples (6.1 mg l−1). Finally, the As content was low and similar, the average concentration was 0.16 mg l−1 in pore-water extracts and 0.6 mg l−1 in water samples (Tables 2 and 3).
Microtox® bioassay
The test with V. fischeri pointed to a significant toxic response following exposure of bacteria to water and pore-waters from the Sierra Minera. The EC50 results showed an average value of 27.7% for water samples and 40.8% for pore-water extracts. The waters and pore-waters of the sampling points S1, S2, S5, S6, S8, S9, S10, S11, S13, S21, S22, S23, S24, S25 and S26 showed the lowest EC50 values, being therefore the most toxic samples for this bioassay. Some water samples were toxic for the bacteria but not the pore-water extracts (S4, S12, S16, S20 and S30), while S3 pore-water was toxic but not the water sample. Finally, S14, S17, S18, S19 and S27 showed no toxicity for this bioassay (Table 4). Linear correlation could be established between EC50 values and pH (r = 0.723) and Eh values (r = −0.658), high pH values correspond to high EC50 values. This should be borne in mind when the pH exceeds 7.0 because As in then in its soluble form as arseniate (Al-Abed et al. 2007). Cd may also be in its soluble form especially if the carbonate content is low. In this last case, Cd is associated with sulphate (Zn SO4. 6H2O, like Bianchite) that admits variable quantities of divalent metals such as Mg, Zn, Cd, etc., forming mixed crystals (García-Rizo et al. 1999). Of the samples, only S16, S17, S18, S19 and S30 contained Cd and Pb at values above pH 7. These samples were taken from a littoral area so that the soluble Cd and Pb contents are related with the formation of soluble complexes with chlorides. Although the EC50 values showed no linear correlation with the soluble trace element content, these variable were related (Fig. 2). In general, a high content of the elements under consideration corresponds to high toxicity levels. The bioassay applied showed greater sensitivity to As followed by Pb and, lastly Cd.
Phytotoxicity bioassay
As regards the phytotoxicity test, an influence on seed germination (SG) was observed (Fig. 3a). In S9, S10, S18, S21, S22, S25 and S26 no seeds germinated for any plant species. In samples S5, S20, and S23 the seed germination inhibition percentage ranged from 60 to 90% and samples S1, S3, S4, S12, S14, S16, S17, S19, S24, S27 and S30 showed percentages of seed germination inhibition lower than 20%.
When root length was evaluated, a significant inhibition of root growth (RI) was noticed. In the case of S9, S10, S18, S21, S22, S23, S25 and S26, inhibition ranged from 80 to 100%. In the case of S5, S11, S12, S13, S16, S20 and S30 it ranged from 30 to 60% while the other sediment samples showed a root length inhibition percentage lower than 30%. Samples S17 and S27 were an exception and had a stimulating effect on root growth (Fig. 3b). Correlation analysis showed that growth inhibition in the plant species was negatively correlated with pH and calcium carbonate content and was positively correlated with Eh, EC and total As content in sediment samples (Table 5). Lower germination inhibition rates were measured for the samples with low trace element content, basic pH values and high calcium carbonate content. Moreover, mortality in plant species is positively correlated with Pb and Cd content in pore-water samples (Table 6). All three species chosen for this assay showed greater sensitivity to Cd than As and Pb (in that order) (Fig. 4).
Ostracod bioassay
The mortality data for H. incongruens for the 26 sediment samples is plotted in Fig. 5a. From this figure it is clear that the majority of the samples induced 100% mortality in the ostracods. For the three sediment samples (S6, S12 and S17) the mortality scores ranged from more than 50–70%. Only five sediment samples (S4, S14, S16, S19 and S27) killed less than 20% of ostracods during the 6-day exposure period.
The percent of growth inhibition for the ostracod test can be seen in Fig. 5b. The bar graph reveals that for the samples in which mortality was lower than 100%, significant growth inhibition was noted. In S3, S14, S19 and S27, mortality was lower than 20%. Samples S4 and S16 showed the highest mortality percentage (28–50%). Correlation analysis showed that the mortality of ostracods was positively correlated with Eh and EC and negatively correlated with pH and CaCO3 content (Table 5). Moreover, ostracods mortality is positively correlated with Pb content in pore-water samples (Table 6). As in the case of plants, the sensitivity of ostracods was greatest for Cd, followed by As and Pb (Fig. 6).
Conclusion
The various bioassays showed different sensitivities to the target elements. Of the bioassays employed, the ostracod test was the most responsive to the toxicants. Moreover, the Vibrio fischeri luminescence inhibition assay showed less sensitivity to the toxicants than phytotoxicity assay. The assays carried out in plants and ostracods showed greater sensitivity to Cd followed by As and, lastly Pb, while the Microtox® assay showed greater sensitivity to As, followed by Pb and, lastly, Cd. According to our results it is highly advisable to complement chemical analyses with environmental toxicity testing to characterise the risks presented by contaminated sediments. Knowing trace element concentration in contaminations sources, we can deduce that the application of one bioassay does not discriminate all the contamination sources. However, the application of a test battery allows the identification of contamination sources and non contaminated areas, corresponding to decreasing toxicity values (Fig. 7).
Finally, these methods satisfy the requirements of environmental toxicology in their reliability, sensitivity, reproducibility, rapidity and low cost.
References
Al-Abed SR, Jegadadeesan G, Purandare J, Allen D (2007) Arsenic leaching from iron rich mineral processing waste: influence of pH and redox potential. Chemosphere 66:775–782
Azur Environmental Ltd (1998) Microtox® acute toxicity basic test procedures. Carlsbad, USA
Chapman PM, Ho KT, Munns WR, Soloman K, Weinstein MP (2002) Issues in sediment toxicity and ecological risk assessment. Mar Pollut Bull 44:271–278
Chial Belgis Z, Persoone G, Blaise C (2003) Cyst-based toxicity tests XVI-sensitivity comparison of the solid phase Heterocypris incongruens microbiotest with the Hyalella azteca and Chironomus riparius contact assays on freshwater sediments from Peninsula Harbour (Ontario, Canada). Chemosphere 52:95–101
Chung MK, Hu R, Wong MH, Chung KC (2007) Comparative toxicity of hydrophobic contaminants to microalgae and higher plants. Ecotoxicol 16:393–402
Devesa-Rey R, Moldes AB, Díaz-Fierros F, Barral MT (2008) Toxicity of Anllóns river sediment extracts using Microtox and the Zucconi phytotoxicity test. Bull Environ Contam Toxicol 80:225–230
Davoren M, Shúilleabháin SN, Halloran JO, Hartl MGJ, Sheehan D, O′Brien NM, Van Pelt FNAM, Mothershill C (2005) A test battery approach for the ecotoxicological evaluation of estuarine sediments. Ecotoxicol 14:741–755
García-Rizo C, Martínez-Sánchez MJ, Pérez-Sirvent C (1999) Environmental transfer of zinc in calcareous soils in zones near old mining sites with semi-aridic climate. Chemosphere 39:209–227
Gruiz K (2005) Soil testing triad and interactive ecotoxicity tests for contaminated soil. In: Fava F, Canepa P (eds.) Soil remediation series no. 6. INCA, Venice, pp 45–70
Hulseman J (1966) An inventory of marine carbonate materials. J Sediment Petrol 36:622–625
Kakkar P, Jaffery FN (2005) Biological markers for metal toxicity. Environ Toxicol Health 19:335–349
Leitgib L, Kálmán J, Gruiz K (2007) Comparison of bioassays by testing whole soil and their water extract from contaminated sites. Chemosphere 66:428–434
Muller G, Gatsner M (1971) Chemical analysis. Neues Jb Miner Monat 10:466–469
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96:183–193
Nendza M (2002) Inventory of marine biotest methods for the evaluation of dredged material and sediments. Chemosphere 48:865–883
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis. Part 2. American Society of Agronomy and Soil Science Society of America, Madison, Wisconsin, pp 539–580
Nipper M, Carr RS, Biedenbach JM, Hooten RL, Miller K (2002) Toxicological and chemical assessment of ordnance compounds in marine sediments and pore-waters. Mar Pollut Bull 44:789–806
Norme française. NF X 31-109, 1069. In: Qualité des sols. Méthodes chimiques. Détermination du carbone organique par oxydation sulfochromique, AFNOR, p. 7
OECD (1984) Guideline of the OECD for testing chemical products-terrestrial plants. Growth Test Method 208, OECD, Paris
Oleszczuk P (2007) The toxicity of composts from sewage sludges evaluated by the direct contact tests phytotoxkit and ostracodtoxkit. Waste Manag 28:1645–1653
Ostracodtoxkit FTM (2001) Chronic direct contact toxicity test for freshwater sediments. Standard operational procedure. Microbiotest, Creasel, Deinze, Belgium
Peech M (1965) Hidrogen-ion activity. In: Black CA, Evans DD, White JL, Ensminger LE, Clark FE (eds) Methods of soils analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Wisconsin, pp 914–926
Phytotoxkit (2004) Seed germination and early growth microbiotest with higher plants. Standard operation procedure. Microbiotest, Nazareth, Belgium
Salminen R, Tarvainen T, Demetriades A, Duris M, Fordyce FM, Gregorauskiene V, Kahelin H, Kivisilla J, Klaver G, Klin H, Larson JO, Lis J, Locutura J, Marsina K, Mjartanova H, Mouvet C, O’connor P, Odor L, Ottonello G, Paukola T, Plant JA, Reimann C, Schermann O, Siewers U, Steenfelt A, Van der Sluys J, De Vivo B, Wiliams L (1998) Foregs geochemical mapping field manual. Geological survey of Finland, Espoo, p 36
Schowanek D, Carr R, David H, Douben P, Hall J, Kirchmann H, Patria L, Sequi P, Smith S, Webb S (2004) A risk-based methodology for deriving quality standards for organic contaminants in sewage sludge for use in agriculture-conceptual framework. Regul Toxicol Pharm 40:227–251
Wilke BM, Riepert F, Koch C, Kühne T (2007) Ecotoxicological characterization of hazardous wastes. Ecotoxicol Environ Saf 70:283–293
Acknowledgments
The authors are grateful to the Spanish MMA (Project A299/2007/1-01.1) and MICINN (Project CTM2008-04567) for financial support. Mari Luz García-Lorenzo also acknowledges a fellowship from Fundación Séneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Lorenzo, M.L., Martínez-Sánchez, M.J., Pérez-Sirvent, C. et al. Ecotoxicological evaluation for the screening of areas polluted by mining activities. Ecotoxicology 18, 1077–1086 (2009). https://doi.org/10.1007/s10646-009-0362-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0362-x